Abstract
The proteasome mediates pathways associated with oxidative stress and inflammation, two pathogenic events correlated with age-related macular degeneration (AMD). In human donor eyes corresponding to four stages of AMD, we found the proteasomal chymotrypsin-like activity increased in neurosensory retina with disease progression. Increased activity correlated with a dramatic increase in the inducible subunits of the immunoproteasome that was not due to an increase in CD45 positive immune cells in the retina. The novel observation of proteasome transformation may reflect retinal response to local inflammation or oxidative stress with AMD.
1. Introduction
The proteasome is a key intracellular protease that regulates pathways that are critical for cell survival. Different oligomeric forms of the proteasome exist, defined by both the composition of the catalytic subunits in the 20S core and associated regulatory complexes [1]. The 20S catalytic core is composed of four seven-member rings; constitutively expressed α-subunits form the outer rings and β-subunits form the inner rings. The β-subunits contain three pairs of active sites (β1, β2, β5) that perform distinct proteolytic activities [2]. The active sites have been identified as caspase-like, trypsin-like, and chymotrypsin-like for cleavage after acidic, basic, and hydrophobic amino acids, respectively.
Following exposure to cytokines, such as interferon γ (IFNγ), the constitutive catalytic subunits, β1, β2, and β5 can be replaced in nascent proteasomes by the inducible subunits β1i, β2i, and β5i [3]. The exchange of catalytic subunits to the inducible subunits forms the catalytic core of the immunoproteasome. Association of the 20S core containing inducible subunits with the proteasome activator PA28 forms the immunoproteasome, a specialized form of the proteasome known to generate immunogenic peptides [4].
Age-related macular degeneration (AMD) is the leading cause of vision loss and blindness in individuals over the age of 65 [5, 6]. Considerable evidence implicates retinal oxidative stress [7] and inflammation [8] as critical pathogenic events with AMD, both of which are partially mediated by the proteasome [9, 10]. We have previously utilized the Minnesota Grading System (MGS) for eyebank eyes [11] to track molecular changes in the neurosensory retina proteome over the course of the disease [12, 13]. We found altered expression of proteins linked to the proteasome [12], thus prompting a more in-depth analysis to investigate how this essential protein changes with the disease.
2. Materials and Methods
2.1 Materials
Peptide substrates were purchased from Sigma, Calbiochem, and BioMol. Proteasome inhibitors were from Peptides International. Antibodies and their respective companies are as follows: proteasomal subunits and activators (Affinity Bioreagents and Affiniti), HSP90 (Santa Cruz), CD45 and jurkat cell lysate (Epitomics), goat anti-rabbit or -mouse, donkey anti-goat or -rabbit alkaline phosphatase and HRP conjugated secondary antibodies (BioRad and Pierce).
2.2 Grading Donor Eyes
Eyes obtained from the Minnesota Lions Eye Bank were acquired with consent of the donor or donor family to be used for medical research in accordance with the principals outlined in the Declaration of Helsinki. Criteria established by the MGS [11] were used to determine the stage of AMD according to established guidelines. MGS4 is subdivided into individuals with (exudative AMD) or without (atrophic AMD) neovascularization. All MGS4 donors used in this study had exudative AMD. Eyes from Caucasian donors were screened for and excluded if other retinal diseases were observed.
2.3 Preparation of retinal homogenates
Dissection of the sensory retina was performed as reported previously [13]. The macula was harvested using a 6mm trephine punch. The periphery includes retina outside this 6mm macular region.
2.4 Rat retinal protein stability
Five-month-old Fischer 344 rats were purchased from the Veterinary Medical Unit at the Minneapolis Veterans Affairs Medical Center’s aging rodent colony, which is maintained by the University of Minnesota. An animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota. In these experiments, the post-mortem conditions of human donor eyes were replicated using rat eyes. In brief, one eye from each rat was immediately enucleated and served as a control for the paired eyes that were dissected two to twenty-four hours postmortem. Bodies were maintained at room temperature for 2.5 hours then refrigerated until enucleation at 4.5 hours post-mortem. After enucleation, eyes were stored in a moist chamber at 4°C until retinal dissection was performed. Retinas were dissected and frozen at −80° C and were later processed as outlined above for human retinas [13].
2.5 Measurement of proteasome activity
The fluorogenic peptides LLE-AMC (200 μM), LLVY-AMC (75 μM), and VGR-AMC (150 μM) were used as model substrates to test the caspase-like, chymotrypsin-like, and trypsin-like activities of the proteasome, respectively [14]. Retinal homogenates (3 μg) were pre-incubated in the absence or presence of proteasome inhibitor MG132 (200 μM), or lactacystin (50 μM) in reaction buffer prior to the addition of substrate. Proteasome activity was measured as described previously [15]. All samples were assayed in triplicate.
2.6 Western Immunoblotting of 1D gels
Following 6% or 13% SDS-PAGE, retinal proteins were transferred to PVDF membranes as described [12]. For each antibody, 35 μg of protein was resolved, which was within the linear range of response. PVDF membranes were probed with the monoclonal antibodies (HSP90 and α7) or polyclonal antibodies that recognize proteasomal subunits (C2, β5, β5i, β1, and β1i) or regulators (PA28α, PA200, PA700-S4). The monoclonal antibody, CD45, was used to test for evidence of infiltration of immune cells. Jurkat cell lysates were used as a positive control. Appropriate secondaries were used in conjunction with BCIP-NBT or chemiluminescence to visualize the immunoreaction. Membranes were imaged with a GS800 Densitometer or ChemiDoc (BioRad), followed by quantification with Quantity One (BioRad). All 1D densities were normalized to a standard used on all blots [13].
2.7 Proteasome content
The immune reactions of the α7 subunit for 20S proteasome purified from rat liver [16] and human retinal homogenates were compared to determine the relative amount of proteasome in retinal homogenates.
2.8 Statistical Analysis
Data are reported as mean ± standard error of the mean (SEM). The SEM for ratios β5i/β5, β1i/β1, and β5i/CD45 were corrected for the propagation of errors. The relationship between MGS stage and either activity or immunoblot density were compared using linear regression. Outliers were removed if they were > 2.5 standard deviations (SD) from the mean. All statistical tests were two-sided with α = 0.05.
3. Results
3.1 Experimental Design
The MGS [11] was used to classify donor eyes into four progressive stages (MGS1-4) of AMD. MGS1 serves as the control group. MGS2 represents early stage AMD, MGS3 is an intermediate stage, and MGS4 is considered end stage (late) AMD. The macula and periphery of the neurosensory retina from human donor eyes were analyzed separately to determine if the preferential deterioration of the macula observed clinically is manifested at the molecular level.
3.2 Proteasome Stability Post-mortem
Demographic and clinical information for donor eyes obtained from the Minnesota Lions Eye Bank is similar to those published previously [12, 13]. Post-mortem conditions prior to tissue preparation did not differ between the four MGS groups, including the time from death to tissue freezing (average ± SD= 16.6 ± 4.0). Due to the broad range in tissue freezing times among donors in our study (range= 7.5–22.0 hrs), we tested proteasome activity and content in rats that replicated the average handling conditions for donor eyes (see Methods). Using fluorogenic peptides, we found proteasome chymotrypsin-like, trypsin-like, and caspase-like activity decreased with time to freezing up to eight hours (Fig 1). Activity remained stable from eight to twenty-four hours post-mortem, which corresponds to the time range for donor eyes. Furthermore, the content of α7 remained constant during this time, implying there was no appreciable degradation of 20S (data not shown). Based on these results, differences in freezing times for donor eyes should not influence our experimental results.
Figure 1. Post-mortem proteasome activity.
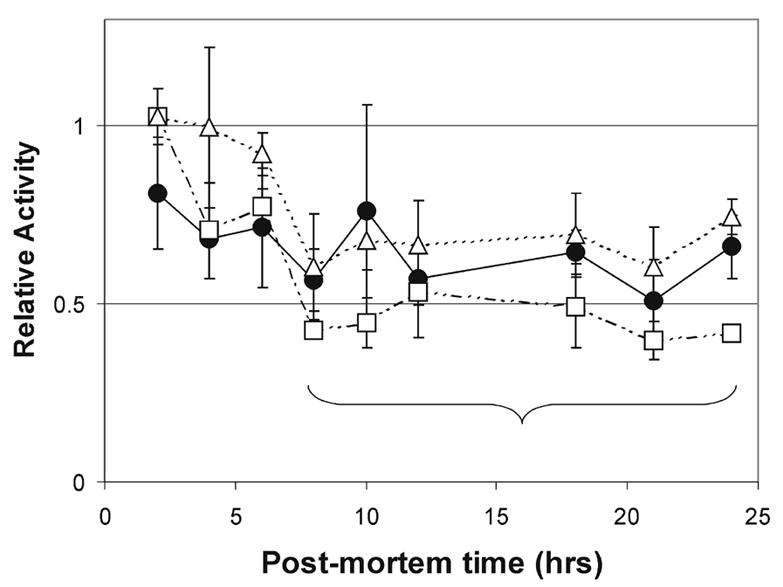
One eye from each F344 rat was immediately enucleated and served as a control for the paired eye; paired eyes replicated eye bank conditions. Retinal homogenates were assayed for proteasome activity by measuring the hydrolysis of fluorogenic peptides LLE-AMC (circles & solid line), VGR-AMC (triangles & dashed line), and LLVY-AMC (squares & uneven dashed line). The bracket represents the time range for human donor eyes in this study. Data are activity relative to the control eye. Values are the mean ± SEM. n= 2–3 rats per time point.
3.3 Increased retinal proteasome activity with AMD
Using fluorogenic peptide substrates, activity of the three catalytic sites of the proteasome was measured in human retinal homogenates from both the macula and periphery at four stages of AMD. The chymotrypsin-like activity was significantly increased with disease progression in both the macula (p=0.038) and the periphery (p=0.031), while the trypsin-like and caspase-like activities remained unchanged (Fig 2). Since it is primarily the chymotryptic-like activity that determines the rate of protein breakdown [17], the increased activity likely results in an increased rate of protein degradation as AMD progresses.
Figure 2. Proteasome activity in donor retinas with AMD.
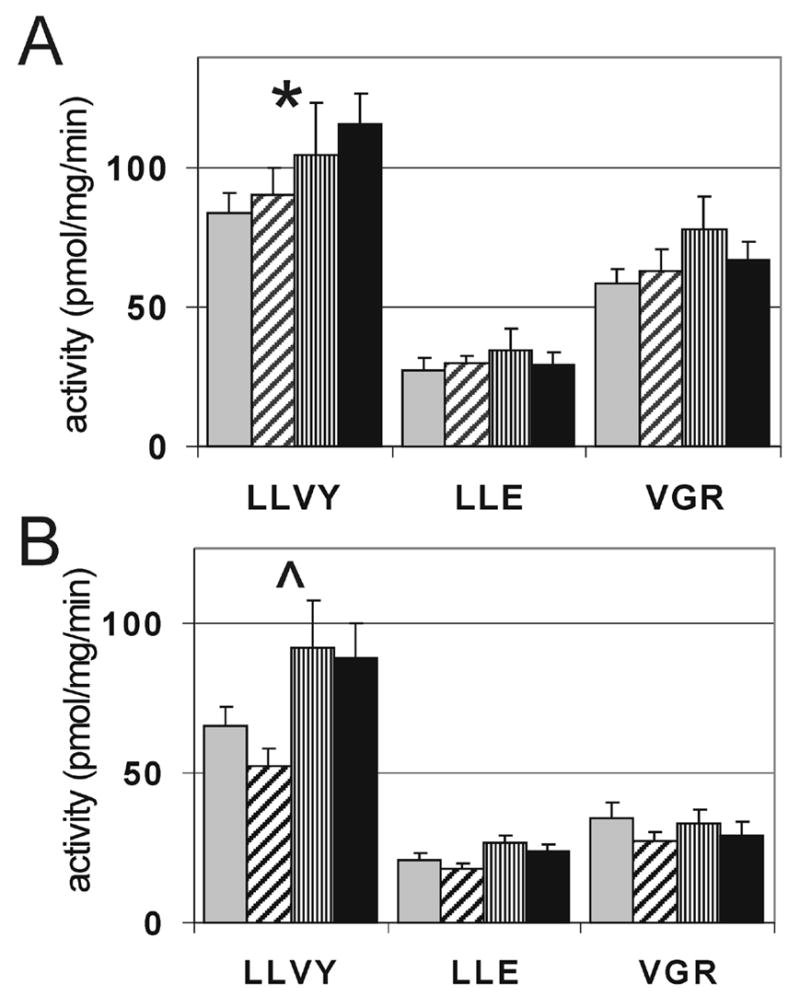
Hydrolysis of fluorogenic peptides measuring the caspase-like (LLE), trypsin-like (VGR), and chymotrypsin-like (LLVY) activities, were determined in the macula (A) and periphery (B) from donors at MGS1 (grey), MGS2 (diagonal), MGS3 (vertical), MGS4 (black). *P = 0.038, ^P = 0.031, n = 6–9 for each group tested in both the macula and periphery.
3.4 Proteasome content remains constant
One possible explanation for the increased activity is that proteasome content is upregulated. Content was estimated from the levels of the α7 subunit, which is a constitutive component of the 20S catalytic core and therefore a good estimate of the total content [15]. Densitometric analysis following immunoblotting showed no change in total content of proteasome with progression of the disease (Fig 3A) in either the macula (p=0.65) or periphery (p=0.33). These results were confirmed using another α-subunit, α6 (data not shown). Comparing the immune reactions of the α7 subunit in a standard of 20S proteasome purified from liver with the retinal homogenates, we estimated the content of proteasome to be 2.02 ± 0.08 μg/mg protein (mean ± SEM, n=40). Thus, the uniform content through disease progression suggests that the increase in activity is due to an increase in proteasome specific activity.
Figure 3. Content of 20S proteasomal subunits in AMD retinal tissue.
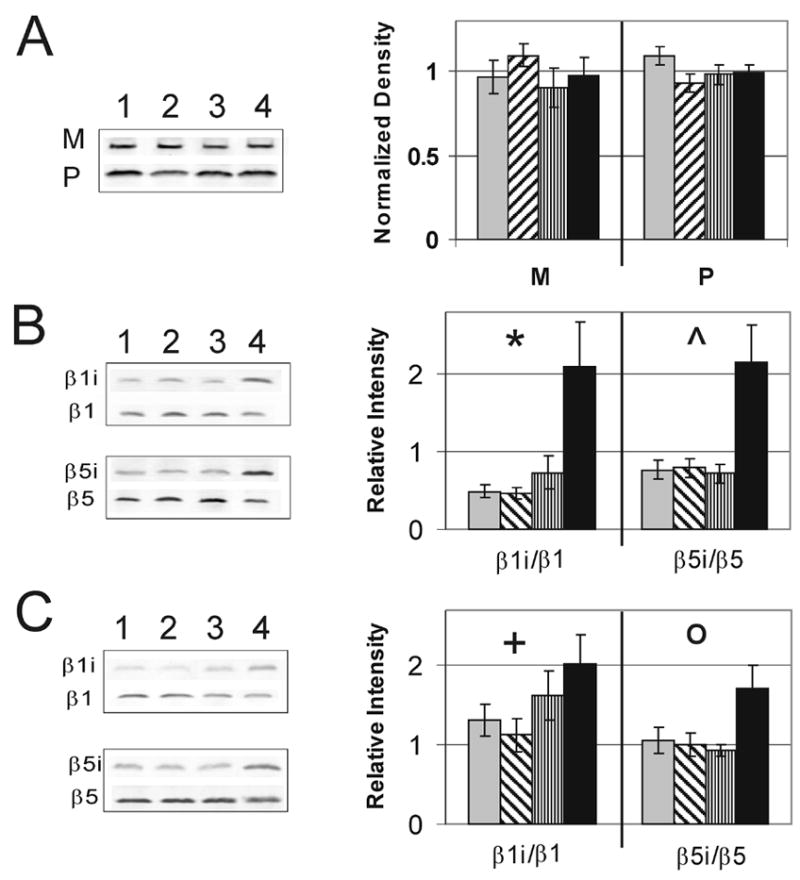
Relative content of proteasome subunit α7 and representative immunoblot (A) in the macula (M) and periphery (P). Exchange of constitutive subunits β1 and β5 for inducible subunits β1i and β5i in the macula (B) and periphery (C) at MGS1 (grey), MGS2 (diagonal), MGS3 (vertical), MGS4 (black). Corresponding representative immunoblots from donors at MGS1–4 are shown to the left. *P = 0.001, ^P = 0.007, +P = 0.047, OP = 0.037, n = 6–10 for each group tested in both the macula and periphery.
3.5 Increased cytokine-induced catalytic subunits with AMD
It has been previously shown that the inducible β-subunits of the 20S core can alter the proteolytic activity of the proteasome [18, 19]. Therefore, we examined the levels of both the constitutive β1 and β5 subunits along with their inducible counterparts, β1i and β5i, to determine whether a change in subunit composition could explain the increased specific activity with disease progression. Because the total level of proteasome is not altered with disease, we expect that if inducible subunits increased, there would be a corresponding decrease in constitutive subunits. We found that the exchange of constitutive β-subunits for their inducible counterparts occurs between MGS1 and MGS4 (Fig 3B and 3C) in the both the macula and periphery. These results are consistent with higher expression of the immunoproteasome with AMD progression.
To address the possibility that the observed increase of inducible subunits is due to infiltrating immune cells containing high levels of immunoproteasome, we assayed the level of CD45 in retinal tissue. CD45 is expressed in all bone-marrow derived immune cells including resident microglia and infiltrating immune cells in the retina. If there was significant infiltration of immune cells into the retinal tissue, we should see a concomitant increase in levels of CD45 and immunoproteasome subunits. In samples from the macula and periphery, we observed that although there was no increase in CD45 (Fig 4A), there was a dramatic increase in content of immunoproteasome subunit β5i (Fig 4B). To account for the level of CD45 in each donor, we calculated the ratio of β5i and CD45 immune density (Fig 4C). These results closely replicate the graph of β5i content alone. Although we cannot definitively say whether immune cells have entered the neurosensory retina, given the limitations of the sensitivity of this assay, we feel confidant that the increased content of immunoproteasome subunits at late stage AMD is not simply due to the contribution from infiltrating immune cells but rather reflects changes in retinal proteasome composition.
Figure 4. CD45 and immunoproteasome subunit levels.
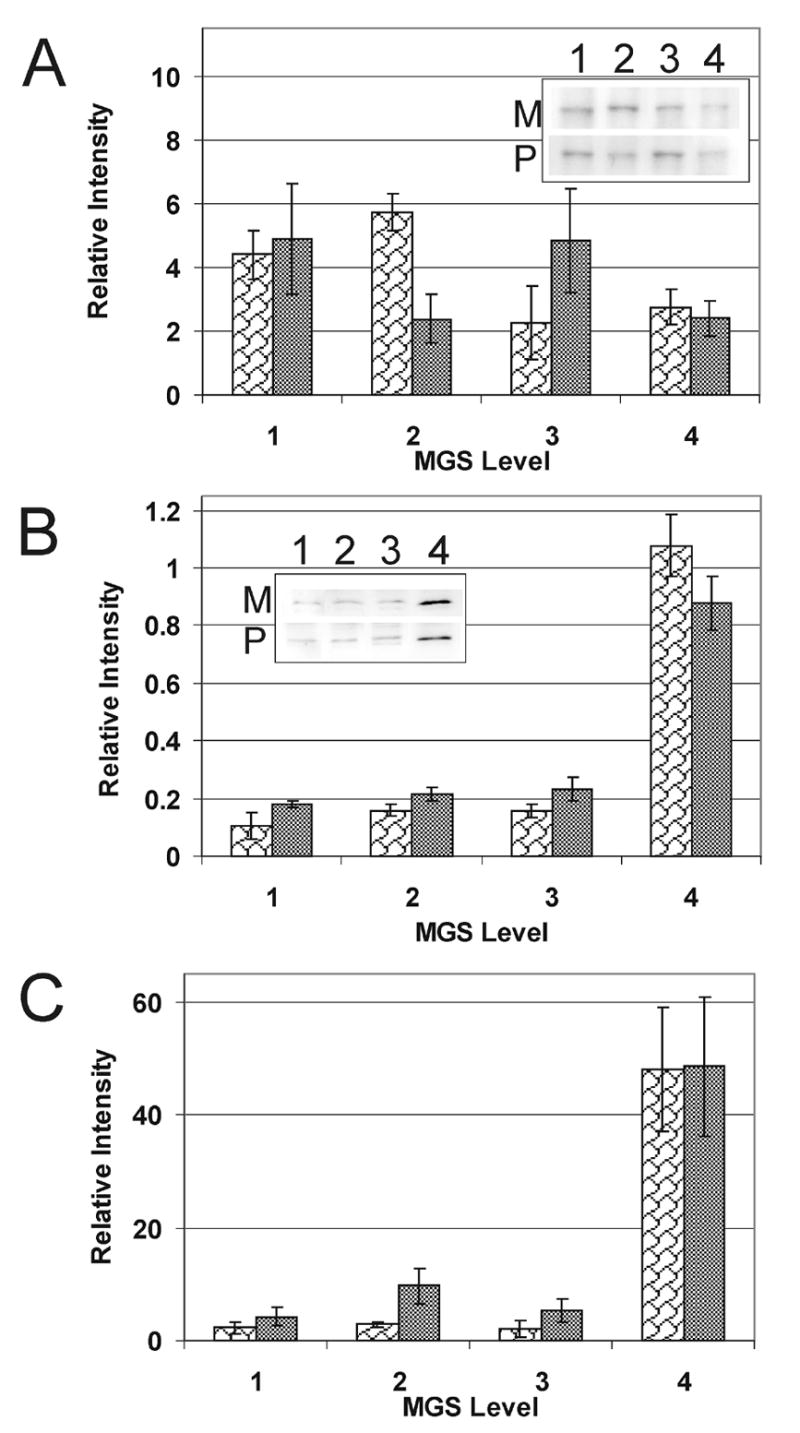
(A) Relative content of CD45, present in bone-marrow derived immune cells, was measured at progressive levels of MGS from the macular (M, white wavy bars) and peripheral (P, gray bars) regions of the retina. The inset shows representative immunoblots. (B) Relative content of β5i from donor tissue used in (A). The inset shows representative immunoblots. (C) β5i levels normalized to the content of CD45 were calculated as the ratio of the densities (β5i/CD45) for each donor. n=2–4 for each group tested in both the macula and periphery.
3.6 Proteasome regulatory proteins
Additional mechanisms for increased proteasome specific activity is through the association of the 20S core with regulatory complexes such as PA28 and PA700 [20]. Using antibodies that recognize the α-subunit of PA28 and the S4 subunit of PA700, we measured the relative concentration of these proteasome activators (Fig 5). No change in PA700 S4 was observed in the macula (p=0.54) or periphery (p=0.83). PA28α content was uniform in the periphery (p=0.42), but was increased (p=0.07) by MGS4 in the macula. Thus, in the macula, increased PA28α content is consistent with higher content of immunoproteasome subunits [19].
Figure 5. Content of proteasomal regulatory proteins.
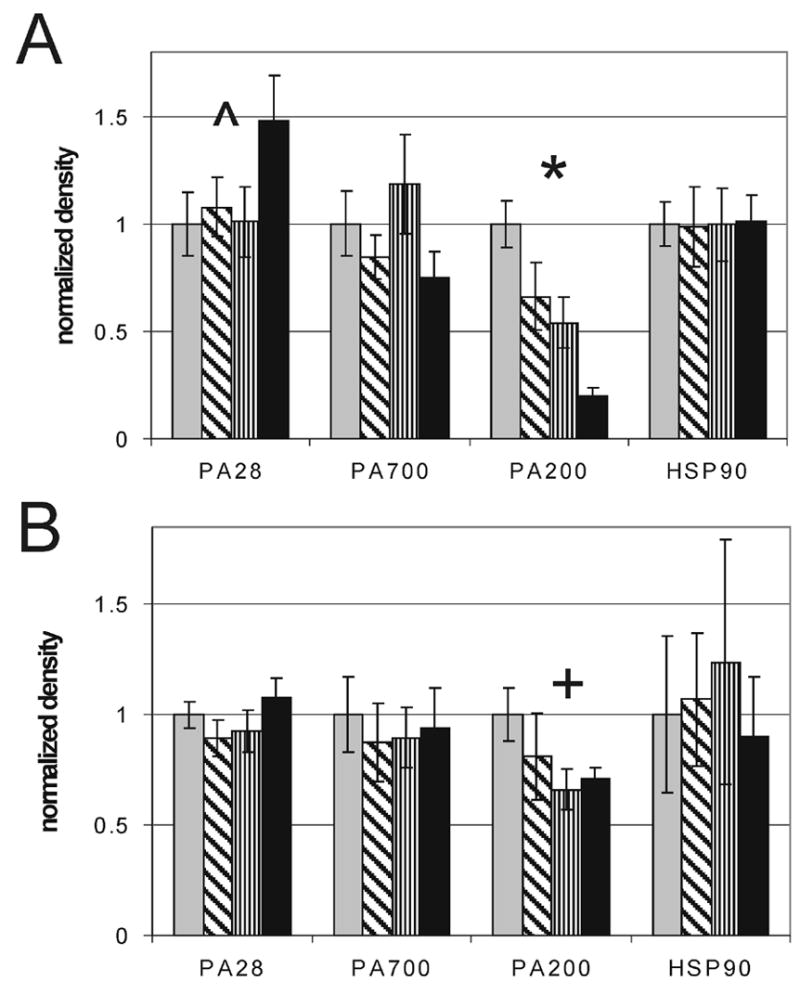
Relative content of regulatory complexes PA28, PA700, PA200 and HSP90 were measured in the macula (A) and periphery (B) from donors at MGS1 (grey), MGS2 (diagonal), MGS3 (vertical), MGS4 (black). *P = 0.0001, ^P = 0.071, +P = 0.058, n = 6–10 for each group tested in both the macula and periphery.
Two additional auxillary proteins, PA200 and HSP90, have been shown to regulate proteasome activity. PA200 is a proteasomal activator whose biological roles are currently poorly understood [20]. PA200 content was significantly decreased in the macula (p=0.0001) and demonstrated a strong trend in the periphery (p=0.058). HSP90 has been shown to have the dual function of inhibiting the constitutive proteasome and activating the immunoproteasome [20]. Although HSP90 was highly variable between donors, no significant correlation with disease stage in either the macula (p=0.95) or periphery (p=0.89) was detected.
4. Discussion
Most aging studies focused on the proteasome report an age-related decline in activity, which has been suggested as an explanation for accumulation of oxidized proteins in aged tissue [21]. In the current study, MGS4 donors were statistically older (p=0.01) (86 ± 13 yrs, mean ± SD, n=10) than donors in MGS1-3 (71 ± 6 yrs, mean ± SD, n=30). Since the increase in chymotrypsin-like activity by MGS4 is contrary to the expected age-related decrease in activity, we concluded the increased activity reflects alterations due to the disease.
Inflammation has been proposed to play an important role in AMD pathology based upon proteomic [22] and immunohistochemical [8] evidence that detected complement proteins in drusen and by the identification of a risk-conferring complement factor H polymorphism [23–26]. The complement system is a key element of the innate immune response. Local inflammation leads to the production of cytokines [27] that can upregulate both immunoproteasome subunits and the proteasome activator PA28 [3]. Therefore, the dramatic upregulation of immunoproteasome subunits β1i and β5i observed in both the macular and peripheral neurosensory retina, as well as increased PA28 in the macular region, supports the presence of local inflammation at end stage of AMD. However, the uncoordinated regulation of PA28 and inducible subunit expression suggests other mechanisms may be involved.
The expression of inducible subunits in disease-free MGS1 immune-priviledged retina implies that the immunoproteasome may participate in non-immune functions. This idea is further supported by the presence of non-cytokine response elements in the promoter region in the mouse β5i gene [28]. An emerging hypothesis is that immunoproteasome plays a protective role during oxidative stress [29, 30]. Data in support of this hypothesis includes upregulation of inducible subunits by nitric oxide [31] and an increase in oxidized proteins with a β1i knockout model [30]. A major finding with AMD is the accumulation of oxidized proteins in retinal tissue [7]. Therefore, increased immunoproteasome may reflect a compensatory, albeit insufficient, response to the oxidative stress since oxidized proteins accumulate with the disease.
The prominent increase of immunoproteasome at end stage does not explain the gradual disease-related increase in chymotryptic activity. This prompted further investigation of cellular regulators of proteasome function. Levels of proteasome activators measured in this study revealed increased PA28 and decreased PA200, although the physiological significance of the latter remains uncertain [20]. Several inhibitors that we did not investigate may also play a role in regulating proteasome activity. Some of the recently discovered inhibitors PI31, Tat, HBx, and PR39 compete with PA28 for the same binding sites on α-subunits of the 20S core [20]. PR39 is reported to stimulate angiogenesis and suppress inflammation by inhibiting proteasome mediated degradation of HIF1α [20, 27] and will be of interest for future studies.
Regulation of the proteasome is of profound importance for cellular function. We interpret the presence of immunoproteasome at later stages of AMD as an indicator of local inflammation or increased oxidative stress in the neurosensory retina. Further studies are required to understand the underlying mechanisms behind immunoproteasome upregulation and the functional consequences of proteasome transformation in AMD pathology.
Acknowledgments
The authors thank the Minnesota Lions Eye Bank for their assistance in procuring eyes for this study and Curt Nordgaard for careful review of the manuscript.
Footnotes
Financial Disclosure: None
Financial Support: This work was supported in part by grants from the National Institutes of Health EY014176, EY013623 (DAF) and AG025392 (TWO), a Career Development Award from the American Federation for Aging Research and Foundation Fighting Blindness (DAF), American Health Assistance Foundation, Minnesota Medical Foundation, the University of Minnesota Academic Health Center and Graduate School, the Fesler-Lampert Foundation, and an unrestricted grant to the Department of Ophthalmology from the Research to Prevent Blindness Foundation. CME was supported with a Training Grant for Vision Science (T32-EY07133).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–47. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 2.Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, et al. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci U S A. 1999;96(20):10976–83. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloetzel PM, Soza A, Stohwasser R. The role of the proteasome system and the proteasome activator PA28 complex in the cellular immune response. Biol Chem. 1999;380(3):293–7. doi: 10.1515/BC.1999.040. [DOI] [PubMed] [Google Scholar]
- 4.Rivett AJ, Hearn AR. Proteasome function in antigen presentation: immunoproteasome complexes, Peptide production, and interactions with viral proteins. Curr Protein Pept Sci. 2004;5(3):153–61. doi: 10.2174/1389203043379774. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study Ophthalmology. 1992;99(6):933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 6.Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116(5):653–8. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 7.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 8.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51(2):137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305(3):709–18. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- 10.Di Napoli M, Papa F. The proteasome system and proteasome inhibitors in stroke: controlling the inflammatory response. Curr Opin Investig Drugs. 2003;4(11):1333–42. [PubMed] [Google Scholar]
- 11.Olsen TW, Feng X. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(12):4484–90. doi: 10.1167/iovs.04-0342. [DOI] [PubMed] [Google Scholar]
- 12.Ethen CM, Reilly C, Feng X, Olsen TW, Ferrington DA. The proteome of central and peripheral retina with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(6):2280–90. doi: 10.1167/iovs.05-1395. [DOI] [PubMed] [Google Scholar]
- 13.Ethen CM, Feng X, Olsen TW, Ferrington DA. Declines in arrestin and rhodopsin in the macula with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46(3):769–75. doi: 10.1167/iovs.04-0810. [DOI] [PubMed] [Google Scholar]
- 14.Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578(3):217–23. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421(1):67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Ferrington DA, Sun H, Murray KK, Costa J, Williams TD, Bigelow DJ, et al. Selective degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2001;276(2):937–43. doi: 10.1074/jbc.M005356200. [DOI] [PubMed] [Google Scholar]
- 17.Kisselev AF, Akopian TN, Castillo V, Goldberg AL. Proteasome active sites allosterically regulate each other, suggesting a cyclical bite-chew mechanism for protein breakdown. Mol Cell. 1999;4(3):395–402. doi: 10.1016/s1097-2765(00)80341-x. [DOI] [PubMed] [Google Scholar]
- 18.Ehring B, Meyer TH, Eckerskorn C, Lottspeich F, Tampe R. Effects of major-histocompatibility-complex-encoded subunits on the peptidase and proteolytic activities of human 20S proteasomes. Cleavage of proteins and antigenic peptides. Eur J Biochem. 1996;235(1–2):404–15. doi: 10.1111/j.1432-1033.1996.00404.x. [DOI] [PubMed] [Google Scholar]
- 19.Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, et al. The interferon-gamma-inducible 11 S regulator (PA28) and the LMP2/LMP7 subunits govern the peptide production by the 20 S proteasome in vitro. J Biol Chem. 1995;270(40):23808–15. doi: 10.1074/jbc.270.40.23808. [DOI] [PubMed] [Google Scholar]
- 20.Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15(1):27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Carrard G, Bulteau AL, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. Int J Biochem Cell Biol. 2002;34(11):1461–74. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 22.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(23):14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 24.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 26.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58(3):353–63. [PubMed] [Google Scholar]
- 28.Zanelli E, Zhou P, Cao H, Smart MK, David CS. Genomic organization and tissue expression of the mouse proteasome gene Lmp-7. Immunogenetics. 1993;38(6):400–7. doi: 10.1007/BF00184520. [DOI] [PubMed] [Google Scholar]
- 29.Teoh CY, Davies KJ. Potential roles of protein oxidation and the immunoproteasome in MHC class I antigen presentation: the 'PrOxI' hypothesis. Arch Biochem Biophys. 2004;423(1):88–96. doi: 10.1016/j.abb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Ding Q, Martin S, Dimayuga E, Bruce-Keller AJ, Keller JN. LMP2 knock-out mice have reduced proteasome activities and increased levels of oxidatively damaged proteins. Antioxid Redox Signal. 2006;8(1–2):130–5. doi: 10.1089/ars.2006.8.130. [DOI] [PubMed] [Google Scholar]
- 31.Kotamraju S, Matalon S, Matsunaga T, Shang T, Hickman-Davis JM, Kalyanaraman B. Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells. Free Radic Biol Med. 2006;40(6):1034–44. doi: 10.1016/j.freeradbiomed.2005.10.052. [DOI] [PubMed] [Google Scholar]


