Abstract
Secreted protein acidic and rich in cysteine (SPARC) is an extracellular Ca2+-binding matricellular glycoprotein that associates with cell populations undergoing migration, morphogenesis, and differentiation. Studies on endothelial cells have established that its principal functions in vitro are counteradhesion and antiproliferation. The mechanism(s) underlying these antitumor effects is unknown. In this study, we showed that SPARC expression in ovarian cancer cells is inversely correlated with the degree of malignancy. The immunohistochemical data presented here confirmed the importance of diminished SPARC expression in ovarian cancer development. Treating human ovarian surface epithelial cells and ovarian cancer cells with SPARC revealed that as SPARC inhibits the proliferation of both normal and cancer cells, it induces apoptosis only in cancer cells. This observation indicates that down-regulation of SPARC is essential for ovarian carcinogenesis as cancer cells become sensitized to the apoptotic activity of SPARC during malignant transformation. We also showed here the first direct evidence that putative SPARC receptors are present on ovarian epithelial cells. Their levels are higher in human ovarian surface epithelial cells than cancer cells. Binding of SPARC to its receptor is likely to trigger tissue-specific signaling pathways that mediate its tumor suppressing functions. Decrease in ligand-receptor interaction by the down-regulation of SPARC and/or its receptor is essential for ovarian carcinogenesis.
Ovarian carcinoma is the major cause of death among all gynecological malignancies. It is the seventh most common cancer in women worldwide and is the fourth leading cause of death from cancer among American women, following lung, breast, and colorectal cancers. 1 The overall 5-year survival rate is only ∼30%. 2 More than 90% of human ovarian cancers are thought to arise from the ovarian surface epithelium, which shares the same developmental origin (coelomic epithelium) with the general pelvic and abdominal peritoneum. Ovarian carcinogenesis is a multistep process involving multiple genetic changes. Although several oncogenes (eg, c-ErbB1, HER-2/neu, c-fms, K-ras) and tumor suppressor genes (eg, BRCA1, p53, DOC-2) have been implicated to be involved in ovarian tumor formation, 3 the pathogenic mechanisms by which normal ovarian epithelial cells become malignant remain poorly understood.
SPARC (secreted protein acidic and rich in cysteine), also termed osteonectin, BM-40, and 43K protein, is a calcium-binding matricellular glycoprotein that displays a high degree of interspecies sequence conservation. 4-8 This consistency indicates that SPARC performs a basic and important function in animal tissues. Localization by immunohistochemistry and in situ hybridization have demonstrated that SPARC is spatially and temporally regulated during development. It is transiently expressed in derivatives of the three primitive germ layers in mouse embryos. 9,10 High levels of SPARC mRNA and protein have been found in developing bones and teeth, principally osteoblasts, odontoblasts, perichondrial fibroblasts, and differentiating chondrocytes in murine, bovine, and human embryos. 11 SPARC also plays important roles in cell-matrix interactions during tissue remodeling, wound repair, morphogenesis, cellular differentiation, cell migration, and angiogenesis. 12
In fetal and newborn ovaries, highest SPARC expression has been found in granulosa cell precursors and the early stages of oocytes. In the ovaries of 2-week-old immature female mice, the thecal cells around developing follicles showed the highest levels of SPARC expression, whereas low levels are detected in follicular cells and oocytes. In ovaries of pregnant females, the thecal expression is maintained, whereas high levels of SPARC are also seen throughout the corpora lutea. 11,13 As for adult human ovary, SPARC is expressed at high levels in ovarian surface epithelium 14 and in the fibrous stroma associated with ovarian carcinomas. 15-17
Although SPARC is associated with the extracellular matrix, it does not support cell attachment in vitro. 18 Exogenous SPARC has been shown to induce cell rounding and to inhibit endothelial cells, smooth muscle cells, and fibroblasts from spreading on collagen-coated surface. 19 SPARC also disrupts focal adhesions and alters the distribution of cytoskeletal elements and permeability of endothelial cells. 20 It has been proposed that SPARC interacts with cell surface protein(s) to facilitate changes in cell shape and cell migration. 19,21 Apart from these counteradhesive activities, SPARC also inhibits proliferation of endothelial cells. 22,23 Recent studies have suggested that the counteradhesive effect of SPARC on endothelial cells is mediated through a tyrosine phosphorylation-dependent pathway, whereas its antiproliferative function is dependent on signal transduction via a G-protein-coupled receptor. 24 Nevertheless, neither the SPARC receptor nor the intracellular signaling events that it triggered have been characterized.
Our earlier study demonstrated that SPARC is down-regulated in ovarian carcinoma cells. 25 Restoring SPARC expression in stable transfectants of ovarian cancer cells leads to reduced growth and tumorigenicity. 14 To investigate the unknown mechanism(s) underlying the antitumor activities of SPARC, we have treated normal ovarian epithelial cells and ovarian cancer cells with exogenous SPARC and studied their growth and induction of apoptosis. We have also examined the presence of putative SPARC receptors on ovarian cell surfaces.
Materials and Methods
Human Ovarian Specimens
A total of 12 cases of benign epithelial ovarian tumors, 16 cases of borderline epithelial ovarian tumors, and 58 cases of malignant ovarian carcinomas were studied. These patients were diagnosed and treated for ovarian tumors at Brigham and Women’s Hospital, Boston, MA. Patient’s consent was obtained before the collection of surgical materials. All tissues were fixed in 10% buffered formalin and embedded in paraffin blocks. The histopathological diagnosis was confirmed by two gynecological pathologists at Brigham and Women’s Hospital and Massachusetts General Hospital. The tumors were classified and graded according to the International Federation of Gynecology and Obstetrics (FIGO, 1987) criteria for ovarian tumors. Among those malignant ovarian carcinomas, 21 cases are grade 1; 13 cases are grade 2; and 24 cases are grade 3. Twenty-five normal ovarian tissue samples obtained from patients with other nonneoplastic gynecological diseases were also included in this study for comparison.
Immunohistochemical Staining of SPARC Protein
SPARC was detected on paraffin-embedded tissue sections by the avidin-biotin peroxidase complex (ABC) method using a rabbit polyclonal antibody, LF-54, which was kindly provided by Dr. Larry W. Fisher at the National Institutes of Health (Bone Research Branch), Bethesda, MD. For comparison, some of the specimens were immunostained using a mouse monoclonal antibody generated against the N-terminal region of SPARC (AON-5031; Hematologic Technologies Inc., Essex Junction, VT). Seven-μm-thick tissue sections on gelatin-coated slides were baked at 60°C for more than 2 hours and were deparaffinized in xylene and rehydrated in graded ethanol. For antigen unmasking, sections were immersed in antigen unmasking solution (Vector Laboratories, Inc., Burlingame, CA) and boiled in microwave oven for 10 minutes. Antigen retrieval was done only for procedures using the monoclonal antibody AON-5031. The tissue sections were then washed in phosphate-buffered saline (PBS) for 15 minutes and the endogenous peroxidase activity was blocked by soaking the sections in 0.3% hydrogen peroxide in methanol for 15 minutes. After washing in PBS for 15 minutes, the nonspecific serum binding sites were blocked by incubating the sections in normal goat or horse serum (1:20, Vector Laboratories). Excess serum was then removed and the tissue sections were incubated overnight with LF-54 at a dilution of 1:200 or AON-5031 at 1 μg/ml. After washing the slides in PBS for 30 minutes, the sections were incubated with biotinylated goat anti-rabbit antibody or horse anti-mouse antibody (1:200) for 45 minutes, followed by a 15-minute washing in PBS. For the negative control, PBS instead of the goat or horse antibody was used in the incubation. The sections were subsequently incubated with ABC reagent [ABC reagent contains reagent A (avidin DH) and reagent B (biotinylated horseradish peroxidase H)] for 30 minutes. After washing the tissue sections with Tris buffer for 15 minutes, color was developed by incubating the sections in peroxidase substrate solution (3,3′-diaminobenzidine tetrahydrochloride) for 30 seconds. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted. The specificity of staining was confirmed by preabsorbing the antibody with purified SPARC protein (Hematologic Technologies Inc.) at 37°C for 2 hours before applying to the sections (1 μg protein per 1 μg IgG).
Semiquantitative Evaluation of Immunoreactivity
The tissue sections immunostained with anti-SPARC antibody were examined by two independent observers unaware of the clinical data. At least five sections were randomly chosen from each specimen for scoring. The intensity of immunoreactivity was graded by a 12-point weighted score that was computed by multiplying the intensity score with the percentage of positive cells score on a section. The intensity score was denoted by a semiquantitative scale: 1, low; 2, medium; and 3, high-staining intensity. The percentage of positive cells score was estimated by the average number of positive cells out of the total number of epithelial cells seen on a section: 0, no positive cells; 1, 1 to 25%; 2, 26 to 50%; 3, 51 to 75% and 4, >75% of positive cells. Therefore, the weighted scores that range from 0 to 12 represent both the intensity and the percentage of immunopositive cells. The data obtained were analyzed by one-tailed one-sample t-test. The staining of a tumor sample is considered as significantly different from that of normal ovaries when P < 0.05.
Cell Cultures
The primary human mesothelial (MESO) and ovarian surface epithelial (HOSE) cell cultures were established as previously described. 26 The immortalized HOSE cell line HOSE1-15 was obtained by infecting HOSE cells with a replication-defective retroviral construct, LXSN16E6E7, and positive clones were selected using 0.3 mg/ml G418 for 10 days as described. 26 The ovarian carcinoma cell line SKOV3 was purchased from ATCC (Rockville, MD), and all of the other ovarian cancer cell lines used in this study were either established in our laboratory or obtained elsewhere. They were cultured in Medium 199 and MCDB 105 (1:1) supplemented with 10% fetal bovine serum (GIBCO BRL, Rockville, MD).
SPARC Secretion Assay
Secretion of SPARC into culture medium was studied using a primary culture of normal ovarian epithelial cells (HOSE713), an immortalized ovarian surface epithelial cell line (HOSE1-15), and four ovarian carcinoma cell lines (SKOV3, OVCA420, OVCA429, and DOV13). A total of 5 × 10 4 cells from each cell line were seeded into separate 25-cm 2 tissue culture flasks containing 5 ml of culture medium. Culture medium alone in a flask was also set up as control. After 4 days, the culture medium from each cell line was collected and the cells were counted. The culture medium (2 ml) from each cell line was loaded in separate Centricon-100 centrifugal concentrators and spun in a Sorvall RC-5B refrigerated centrifuge at 2600 rpm for 30 minutes at room temperature. The filtrate from each vial was then collected and loaded in a Centricon-10 concentrator and spun at 5700 rpm for 1 hour at room temperature. After spinning, the retentate was collected and the protein concentration of each sample was determined by the Micro BCA protein assay kit (Pierce, Rockford, IL). To determine the amount of secreted SPARC for each cell line, we also spiked serum-free culture medium with 1, 5, 10, or 25 μg of purified human platelet SPARC proteins (Hematologic Technologies Inc.), and they were concentrated from the medium the same way as described for the cell lines. Western blotting was performed and the SPARC signal intensity was quantified by densitometric analysis.
Western Blot Analysis
Protein samples (25 μg) were mixed with equal volumes of 2× sodium dodecyl sulfate (SDS) sample buffer (125 mmol/L Tris, 2.2 mol/L glycerol, 1.42 mol/L β-mercaptoethanol, 160 mmol/L SDS, 10 mg/L bromophenol blue, pH 6.8), boiled for 10 minutes, and resolved by SDS-polyacrylamide gel electrophoresis (10%). They were then transferred to polyvinylidene difluoride membrane (NEN, Boston, MA) and incubated overnight at 4°C in TBST (10 mmol/L Tris, pH 7.5, 100 mmol/L NaCl, 0.1% Tween-20) containing 5% nonfat dry milk. After washing with TBST, the membrane was incubated in 5% milk containing 2 μg/ml of the monoclonal anti-SPARC antibody AON-5031 (Hematologic Technologies Inc.) at room temperature for 1 hour. The membrane was then washed six times with TBST, each for 5 minutes, and incubated at room temperature with horseradish peroxidase-conjugated donkey anti-mouse IgG (Amersham, Piscataway, NJ) for 1 hour. After washing with TBST, signals were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce).
Growth Kinetics
Cell proliferation was studied using MTT assays and BrdU incorporation enzyme-linked immunosorbent assay (ELISA) (Roche Molecular Biochemicals, Indianapolis, IN). MTT assay is based on the conversion of MTT (a tetrazolium salt) by viable cells into a violet formazan dye that can be quantified by measuring absorbance at 550 nm. HOSE 1-15 or SKOV3 cells (1 × 104/well) were seeded in a 96-well plate in 0.1 ml of culture medium supplemented with different amounts of SPARC. After 72 hours, MTT-labeling reagent was added to 0.5 mg/ml and the cells were incubated at 37°C for another 4 hours. The cells were then lysed by adding 0.1 ml of solubilization solution (10% SDS in 0.01 mol/L hydrochloric acid). After an overnight incubation at 37°C, absorbance at 550 nm (with a reference wavelength of 655 nm) was determined using a Benchmark microplate reader (Bio-Rad, Hercules, CA).
For BrdU ELISA, SKOV3 cells, or HOSE1-15 cells (5 × 10 3 cells/well) were treated for 96 hours at 37°C with different concentrations of exogenous SPARC in a 96-well plate. After incubation, the cells were labeled at 37°C with the pyrimidine analogue BrdU (10 μmol/L) for 6 hours. DNA synthesis was monitored based on the incorporation of BrdU into DNA, which was detected by immunoassay according to the manufacturer’s instruction. Cellular proliferation was determined by measuring absorbance at 370 nm (reference wavelength 490 nm).
Analysis of Apoptosis
Induction of apoptosis in SKOV3 cells and HOSE1-15 cells after exogenous SPARC treatment was examined using in situ cell death detection [terminal dUTP nick-end labeling (TUNEL)] assay and cell death detection ELISA (Roche Molecular Biochemicals). SKOV3 cells or HOSE1-15 cells (1 × 10 4 cells/well) were treated with different concentrations of SPARC (0.5 to 20 μg/ml) for 48 hours at 37°C in 8-well chamber slides or 96-well plates. In the TUNEL assay, SPARC-treated or untreated SKOV3 cells on a chamber slide were fixed with 4% paraformaldehyde at room temperature for 30 minutes, and the cells were permeabilized with 0.1% Triton X-100 (in 0.1% sodium citrate) solution for 3 minutes at 4°C. DNA strand breaks induced by apoptosis were detected by the incorporation of fluorescein-labeled nucleotides to free 3′-OH DNA ends using terminal deoxynucleotidyl transferase. The number and the staining intensity of the apoptotic cells indicate the extent of apoptotic induction. SKOV3 cells were also stained with DAPI (0.5 μg/ml) for 5 to 10 minutes at room temperature. The early apoptotic cells and DAPI-stained cells were seen under fluorescence microscope.
Cell death ELISA was performed according to the manufacturer’s protocol. Briefly, SPARC-treated or untreated SKOV3 and HOSE1-15 cells were lysed in 200 μl of lysis buffer at room temperature for 30 minutes. The supernatant collected after centrifugation was transferred to a streptavidin-coated microtiter plate. After 2 hours of incubation at room temperature with the immunoreagent mix, the bound mono- and oligonucleosomes on the microtiter plate were washed three times with incubation buffer. After the washes, 100 μl of substrate solution was added to each well. Absorbance at 405 nm (reference wavelength 490 nm) was determined after 10 to 20 minutes of incubation with shaking at room temperature.
Receptor Binding Assay
To prepare the SPARC-AP fusion protein used in the receptor binding assay, cDNA encoding SPARC was polymerase chain reaction-amplified from a SPARC expression vector 14 using T7 primer and a SPARC-coding region reverse primer that contains a BglII restriction site (5′-GAA GAT CTT CCG ATC ACA AGA TCC TTG-3′). The amplified DNA fragment was digested with HindIII and BglII, which was subsequently inserted in-frame upstream of the coding sequence of a thermostable human placental alkaline phosphatase (AP) in the expression vector pAPtag-2 (GenHunter Corp., Nashville, TN). This SPARC-AP construct was co-transfected with the pTK-Hyg plasmid, which contains the hygromycin B resistance gene, into 293T cells. After hygromycin B selection (0.2 mg/ml), stable transfectants that secrete high levels of SPARC-AP proteins were selected and grown to confluence. After 3 days, the culture medium containing the secreted proteins were collected and filtered. As a negative control, AP proteins were similarly prepared from a 293T/pAPtag-4 stable cell line that secretes AP alone (GenHunter Corp.). The secreted proteins in culture medium were used in the receptor-binding assay described below.
In receptor-binding assays, ovarian cancer cells and HOSE cells were grown to ∼80% confluent in 60-mm culture dishes. They were rinsed once with HBHA buffer (Hanks’ balanced salt solution with 0.5 mg/ml bovine serum albumin and 20 mmol/L HEPES, pH 7.0) and incubated with SPARC-AP or AP proteins (as negative control) at room temperature for 90 minutes. After six washes with HBHA buffer for 10 minutes, the cells were lysed and the endogenous alkaline-phosphatase activities in the lysates were heat-inactivated at 65°C for 20 minutes. AP activities were determined by using an AP assay reagent containing p-nitrophenyl phosphate. Increases in AP activities, and hence ligand-receptor binding, were indicated by the increases in absorbance at 405 nm.
Results
SPARC Expression is Down-Regulated in Ovarian Cancer
Using Northern and Western blotting, we have previously shown that SPARC is highly expressed in HOSE cells. Its expression is reduced in ovarian cancer cell lines and tissues. 14 Recent studies examining the immunoreactivity of SPARC in ovarian tumor tissues revealed different patterns of SPARC expression. 15-17 To investigate the inconsistent pattern of SPARC deregulation in ovarian cancers, we have performed immunostaining for SPARC protein using a polyclonal SPARC antibody LF-54 on 111 paraffin-embedded ovarian tissue samples. Consistent with our RNA and protein data, 14 strong immunoreactivity with a high mean weighted score of 11.5 ± 1.3 was found in the surface epithelial cells of all of the 25 normal ovaries examined (Table 1) ▶ . Positive staining appeared as dark brown granules spreading throughout their cytoplasm (Figure 1a) ▶ . No staining was observed in the stromal cells. Strong immunoreactivity, as indicated by the high mean weighted score (10.8 ± 2.4, Table 1 ▶ ), was also detected in all of the benign tumors studied. The staining pattern is similar to that of the normal ovaries, in which most of the surface epithelial cells were positively stained (Figure 1b) ▶ .
Table 1.
Weighted Scores for the Immunohistochemical Staining of SPARC in Ovarian Tissues*
| Ovarian tissues | Sample number | Mean weighted score† | Specimens with positive staining, % |
|---|---|---|---|
| Normal | 25 | 11.5 ± 1.3 | 100 |
| Benign | 12 | 10.8 ± 2.4 | 100 |
| Borderline | 16 | 5.8 ± 0.5* | 100 |
| Invasive | |||
| Grade I | 21 | 3.7 ± 2.2* | 100 |
| Grade II | 13 | 1.3 ± 1.6* | 85 |
| Grade III | 24 | 0.9 ± 0.1* | 87 |
*Weighted scores ranging from 0 to 12 represent both the intensity of the immunostaining and the percentage of positive cells.
†Values are given as means ± SEM. Asterisks behind the values denote P < 0.05 as determined by one-tailed t-test. These values are significantly different from the mean value obtained from the staining of normal ovaries.
Figure 1.
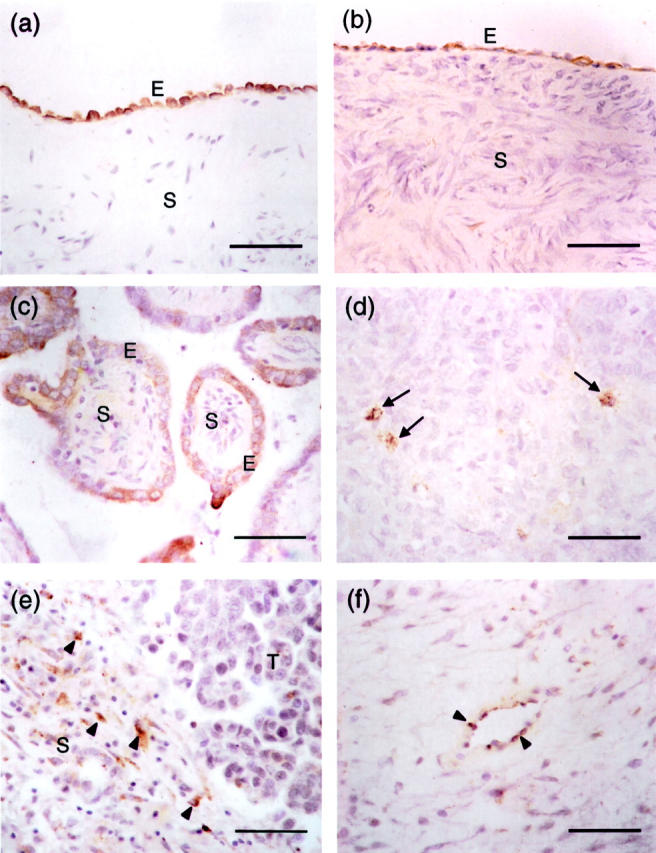
Immunohistochemical analysis and localization of SPARC expression in normal ovary and ovarian tumors. Photomicrographs are taken from sections of normal ovary (a), serous benign ovarian adenoma (b), borderline ovarian tumor (c), and grade III serous ovarian carcinoma (d, e, and f). All sections were stained with a rabbit polyclonal antibody specific to SPARC. Immunoreactive cells, which are stained brown in the cytoplasm, are found in the epithelium (E) of normal ovary, benign ovarian adenoma, and borderline ovarian tumor (a, b, and c). No staining is observed in the underlying stroma (S) of these tissue samples. In grade III serous ovarian carcinoma (d), immunoreactivity is significantly reduced in which only a few scattered cancer cells are stained brown (arrows). e: In contrast to normal ovary, benign and borderline ovarian tumors, scattered positively stained cells (arrowheads) can be found in the stroma (S) of ovarian cancer adjacent to the tumor (T). Endothelial cells in ovarian carcinoma also show occasional positivity to SPARC staining (arrowheads; f). Scale bars, 50 μm.
A reduction of immunoreactivity was seen in the cytoplasm of borderline and invasive serous carcinoma cells. In all of the borderline cases examined, <50% of the surface epithelial cells were stained positive (Figure 1c) ▶ . The cytoplasmic staining appeared light brown, and the intensity was weaker than that of normal ovaries and benign tumors (compare Figure 1; a, b, and c ▶ ). Although positive cells were detected in all of the borderline tumors examined, they showed a significantly lower (P < 0.05) mean weighted score (5.8 ± 0.5, Table 1 ▶ ). Grade I and grade II invasive ovarian tumors also showed significantly decreased staining with mean weighted scores of 3.7 ± 2.2 and 1.3 ± 1.6, respectively (P < 0.05, Table 1 ▶ ). Positively stained epithelial cells were scattered and only occasionally found (data not shown). Two (15%) of the 13 grade II carcinomas examined did not show any positive epithelial cells. The lowest mean weighted score (0.9 ± 0.1, Table 1 ▶ ) was found in grade III invasive tumors. Light brown cytoplasmic staining was only found in scattered positive epithelial cells (Figure 1d) ▶ . No stained cells were seen in 13% (3 of 24) of the grade III invasive tumors examined. In addition to the normal and cancerous ovarian epithelial cells, we also observed low levels of immunoreactivity in scattered stromal cells (Figure 1e) ▶ and endothelial cells (Figure 1f) ▶ of high-grade carcinomas. No signal was seen when the SPARC antibody was incubated with purified blood platelet SPARC protein before applying to the tissue sections, indicating that the immunostainings observed are specific to SPARC.
As different results were reported using the monoclonal antibody AON-5031 for immunohistochemical studies of paraffin-embedded ovarian cancer tissues, 15,17 we have also immunostained some of the paraffin sections to investigate whether similar observation can be obtained using this antibody. Because using high concentrations (>5.4 μg/ml) of AON-5031 for immunostaining have been reported to result in ubiquitous staining throughout the ovary, 17 we used a lower concentration (1 μg/ml) of the antibody in this study. Inconsistent with our results obtained from Northern blotting, Western blotting, and immunostaining, not much positive staining was detected in the surface epithelial cells. However, we found strong immunoreactivity in the stroma underlying the surface epithelium of normal ovary (Figure 2a) ▶ . On antigen retrieval, the stromal signal was intensified, but the HOSE cells were still faintly stained (Figure 2b) ▶ . As for ovarian cancer samples, strong immunoreactivity was seen in scattered stromal cells. Although a few epithelial cells were occasionally stained, no immunostaining was evident in the vast majority of cancer cells (Figure 2, c and d) ▶ . No difference in staining pattern was found in the cancer tissue sections with or without antigen unmasking. We also did not find noticeable difference in staining when 0.5 μg/ml of the antibody was used.
Figure 2.
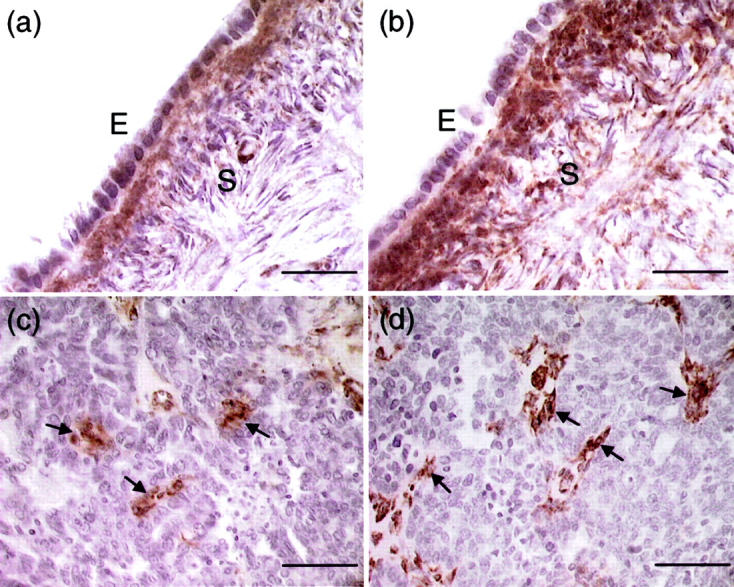
Localization of SPARC immunoreactivity in normal ovary (a and b) and ovarian cancer (c and d) using the monoclonal antibody AON-5031. a: Very weak staining was observed in the normal ovarian epithelium (E). In contrast, cells in the underlying stroma (S) showed strong SPARC immunoreactivity. b: The staining of stromal cells was intensified after heating with antigen-unmasking solution. However, the normal ovarian epithelial cells remain faintly stained. c: Positive staining was seen in a few cancer cells and some scattered stromal cells (arrows). No immunoreactivity was evident in the vast majority of cancer cells. d: Stromal cells (arrows) showed strong SPARC staining after antigen retrieval, whereas cancer cells remain unstained. Scale bars, 50 μm (a and b); 100 μm (c and d).
Decreased SPARC Secretion in Ovarian Cancers
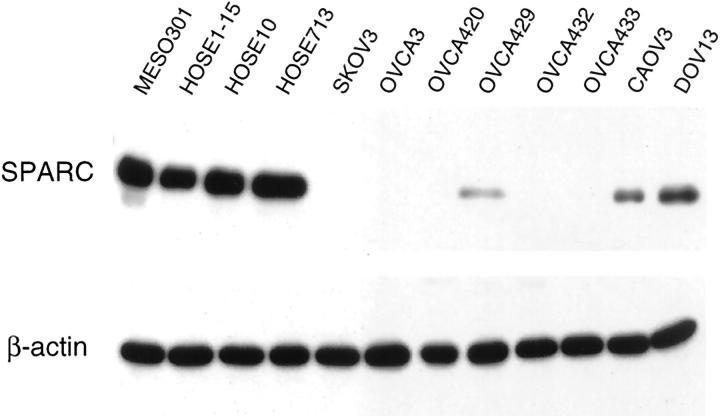
Because SPARC is a secreted protein, it may affect ovarian cancer growth in an autocrine and/or paracrine manner. Before we analyzed the levels of SPARC secretion in normal and malignant ovarian cell lines, we first confirmed our earlier findings by Western blot analysis showing that SPARC expression is greatly reduced in ovarian cancer. As normal mesothelial and HOSE cells express high levels of SPARC, it was not detected in the cell lysates of most of the cancer cell lines. Among them, DOV13 has the highest SPARC expression level, followed by CAOV3 and OVCA429 (Figure 3) ▶ . Based on the expression pattern, we anticipate that SPARC is secreted at higher levels in normal ovarian epithelial cells than in ovarian cancer cells. Confirming and estimating the levels of SPARC secretion by normal and malignant ovarian cells are important for validating our subsequent experiments in which the cells were treated with exogenous SPARC.
Figure 3.
Western blot analysis of total protein lysates prepared from one mesothelial cell line (MESO301), one immortalized HOSE cell line (HOSE1-15), two primary HOSE cell cultures (HOSE10 and HOSE713), and eight ovarian cancer cell lines. The protein lysates (25 μg) were resolved on 10% SDS-polyacrylamide gel, transferred to polyvinylidene difluoride membrane, and analyzed with the monoclonal anti-SPARC antibody AON-5031. Detection of β-actin signal at the bottom served as a control of the quality of the protein lysates.
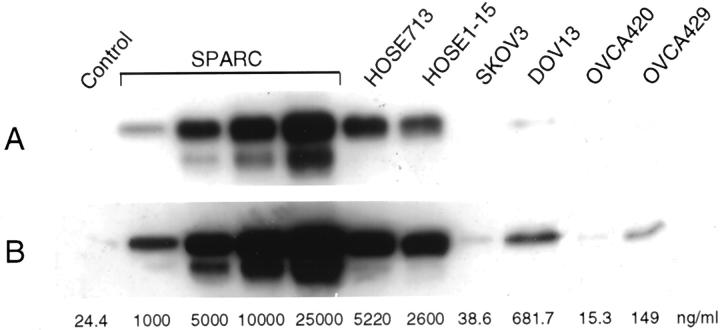
Culture media from separate cultures of HOSE cells or ovarian cancer cells were collected and concentrated. Equivalent amounts of proteins were run on polyacrylamide gels and analyzed by Western blotting. We found that the culture medium containing 10% fetal bovine serum has ∼24.4 ng/ml of the 43-kd SPARC protein (Figure 4) ▶ . As expected, high levels of SPARC secretion were seen for primary cell culture HOSE713 (5220 ng/ml) and immortalized HOSE1-15 cells (2600 ng/ml). Significantly reduced levels of secreted SPARC were detected for ovarian cancer cell lines SKOV3 (38.6 ng/ml), OVCA420 (15.3 ng/ml), and OVCA429 (149 ng/ml). Although still much lower than that of HOSE cells, DOV13 has the highest level of SPARC secretion (681.7 ng/ml). The amounts of SPARC secreted by the cell cultures directly correlate with their SPARC expression levels as detected by Western blot analysis of their total cell lysates (compare Figure 3 and 4 ▶ ▶ ).
Figure 4.
Western blot analysis of SPARC in the culture medium of normal HOSE cells and ovarian cancer cells. Culture medium was collected and concentrated from separate cultures of a primary HOSE cell culture (HOSE713), an immortalized HOSE cell line (HOSE1-15), and four ovarian cancer cell lines (SKOV3, DOV13, OVCA420, and OVCA429). For quantification, culture medium alone (control) and culture medium spiked with different amounts of SPARC (1 μg/ml, 5 μg/ml, 10 μg/ml, and 25 μg/ml) were also included. The estimated concentrations of SPARC in the culture medium of the various cell cultures are indicated at the bottom of the respective lanes. A: Shorter exposure to show the signals obtained from purified human blood platelet SPARC spiked into culture medium. B: Longer exposure to show the signals detected from the culture media of ovarian cancer cells.
Exogenous SPARC Inhibits the Proliferation of Ovarian Cancer Cells
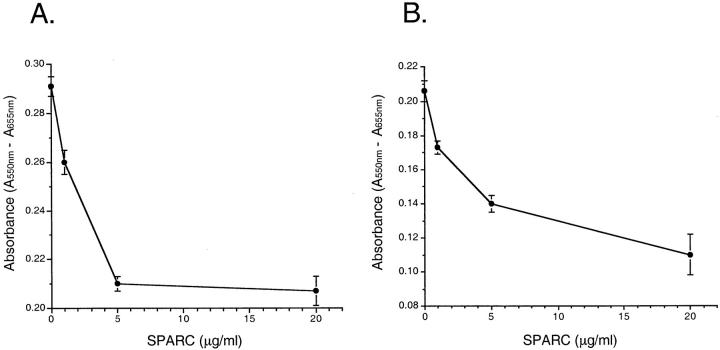
To investigate the autocrine/paracrine effects of SPARC on ovarian cell growth, we have cultured HOSE1-15 and SKOV3 cells for 3 days in culture medium containing different concentrations of exogenous human platelet SPARC and monitored the amount of viable cells after incubation by MTT assay. This result is important, as it will indicate whether the platelet SPARC we used in this study produces the same growth inhibitory effect as the endogenous SPARC synthesized by HOSE cells. MTT assay showed that the number of viable HOSE 1-15 cells decreased to ∼70% of the control when SPARC was added to the culture medium (Figure 5A) ▶ . As for SKOV3 cells, the number of living cells decreased to ∼68% of the control in the presence of 5 μg/ml of SPARC, and reduced to ∼54% of the control after being treated with 20 μg/ml of SPARC (Figure 5B) ▶ . These results are consistent with the data obtained from BrdU incorporation ELISA. In this assay, SKOV3 and HOSE cells (5 × 10 3 cells/well) were incubated with different concentrations of SPARC in separate wells in a 96-well plate. After incubation at 37°C for 96 hours, the cells were labeled with the pyrimidine analogue BrdU for 6 hours. DNA synthesis was monitored based on the incorporation of BrdU into DNA, which is detected by immunoassay. Cellular proliferation of both HOSE cells and SKOV3 cells was reduced when treated with increasing amounts of SPARC (Figure 6) ▶ , as indicated by the decrease in absorbance at 370 nm. The inhibitory effect of SPARC on SKOV3 cells is obviously greater than that on HOSE cells. Moreover, the overall BrdU incorporation is less in HOSE cells than in SKOV3 cells, probably because of the slower growth rate of the normal ovarian epithelial cells. Similar antiproliferative activity of SPARC was also observed for another two ovarian cancer cell lines OVCA433 and DOV13 (data not shown).
Figure 5.
Exogenous SPARC inhibits the growth of HOSE1-15 and SKOV3 cells. Growth of the normal ovarian epithelial cell line HOSE1-15 (A) and the ovarian cancer cell line SKOV3 (B) treated with different concentrations of human blood platelet SPARC protein was monitored by MTT assay. HOSE1-15 or SKOV3 cells were labeled with MTT after being treated with SPARC for 72 hours. They were then lysed and absorbance at 550 nm was measured. The decrease in absorbance with increasing concentrations of SPARC indicates that SPARC inhibits cell growth. This experiment was repeated three times with comparable results. The numbers shown represent the values from four wells in a representative experiment. Scale bars, SEM.
Figure 6.
Antiproliferative effects of SPARC on SKOV3 and HOSE cells. Incorporation of BrdU into the DNA of SKOV3 and HOSE1-15 cells after incubation with SPARC for 96 hours was detected by immunoassay. The reaction product, which directly correlates to the amount of DNA synthesis, was quantified by measuring absorbance at 370 nm. Reduction in cellular proliferation of both SKOV3 and HOSE cells after treatment with increasing amounts of SPARC is indicated by the decrease in absorbance at 370 nm (reference wavelength at 490 nm). The values shown are the means ± SEM (bars) of four measurements from one representative experiment. Filled circles, SKOV3 cells; open circles, HOSE1-15 cells.
SPARC Induces Apoptosis in Ovarian Cancer Cells
Studies on transgenic and knockout mice have provided direct evidence that the disruption of apoptotic pathways in cells can lead to tumor development. To investigate the mechanism(s) that drives the antitumor activities of SPARC in ovarian cancer cells, we have examined whether SPARC can induce apoptosis in SKOV3 and HOSE cells. Induction of apoptosis after exogenous SPARC treatment was examined using the in situ cell death detection (TUNEL) assay and cell death detection ELISA (Roche Molecular Biochemicals).
In the TUNEL assay, SKOV3 cells (1 × 10 4 cells/well) were incubated with different amounts of SPARC at 37°C in 8-well chamber slides for 48 hours. After incubation, DNA strand breaks in the cells induced by apoptosis were detected by labeling the free 3′-OH DNA ends with fluorescein-labeled nucleotides using terminal deoxynucleotidyl transferase. For comparison, cell nuclei were also stained with DAPI regardless of the apoptotic status of the cells. Signals were visualized under fluorescence microscope. Our results showed that untreated SKOV3 cells exhibit very low levels of spontaneous apoptosis frequently observed in cultured cells, which are barely visible in the figure (Figure 7A) ▶ . When they were treated with 1 μg/ml of blood platelet SPARC, more apoptosis was detected (Figure 7C) ▶ . Increased amounts of DNA strand breaks after treating SKOV3 cells with 5 μg/ml of SPARC were indicated by the increase in number and labeling intensity of the nuclei (Figure 7E) ▶ . Cell nuclei stained with DAPI were detected as blue dots in all assays (Figure 7; B, D, and F ▶ ). To clearly demonstrate the apoptotic effects of SPARC, we have selected areas on the slides in which more apoptotic cells were congregated. The estimated numbers of apoptotic SKOV3 cells detected after treating with SPARC were ∼50 to 60% of the entire cell population examined. Our results showed that SPARC induces apoptosis in ovarian cancer cells.
Figure 7.
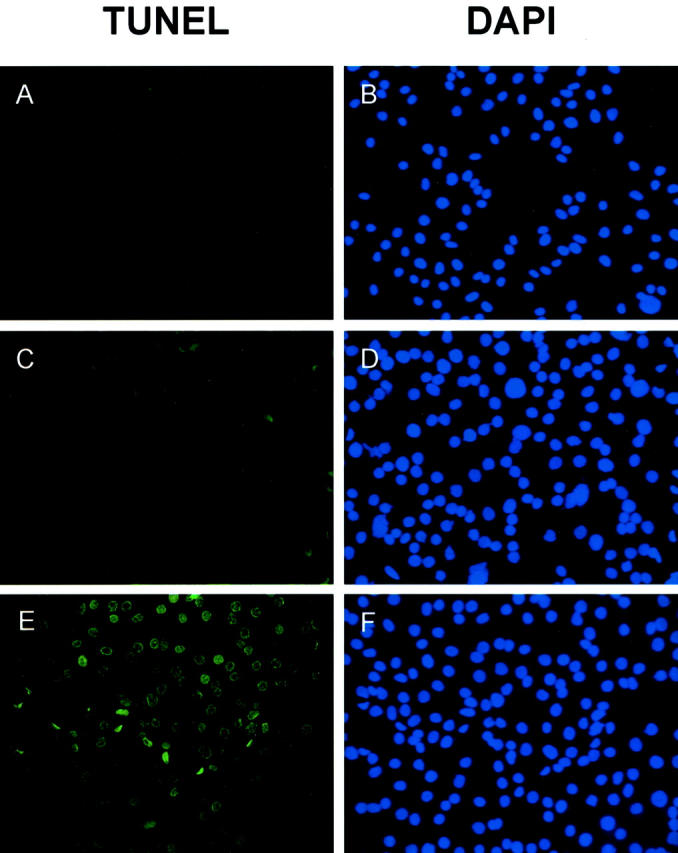
Detection of apoptotic ovarian cancer cells by TUNEL assay. SKOV3 cells seeded in chamber slides were treated with different concentrations of human blood platelet SPARC proteins for 48 hours (A and B, untreated; C and D, 1 μg/ml; E and F, 5 μg/ml). After incubation, TUNEL assays were performed (A, C, and E). To visualize the cell nuclei regardless of apoptosis, SKOV3 cells were also stained by DAPI (0.5 μg/ml) at room temperature for 10 minutes (B, D, and F). Early apoptotic cells and DAPI-stained cells were detected by fluorescence microscopy. DAPI-stained cell nuclei were seen as blue dots whereas the nuclei of apoptotic cells were stained green. There is a significant increase in the number and staining intensity of apoptotic cell nuclei when SKOV3 cells were treated with increasing amounts of SPARC, indicating more DNA strand breaks.
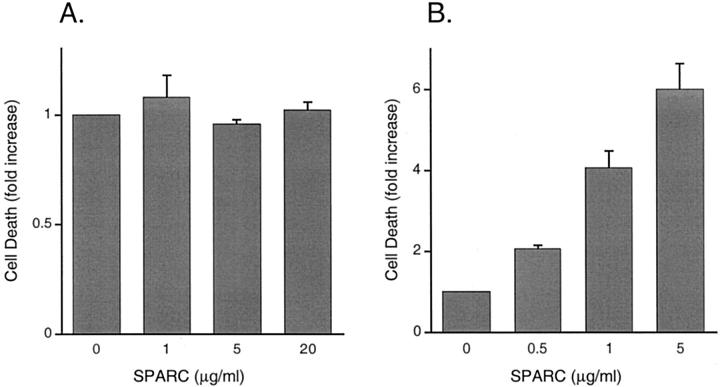
To determine the apoptotic effects of SPARC on HOSE cells, cell death detection ELISA was used as a sensitive and quantitative assay for detecting DNA fragmentation. SKOV3 cells were also analyzed for comparison. In this assay, SPARC-treated or -untreated cells were lysed after incubation and the lysates were assayed for cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes). Increase in DNA fragmentation is indicated by the increase in absorbance at 405 nm. Consistent with the results obtained from TUNEL assays, SKOV3 cells showed increase in DNA fragmentation when treated with increasing amounts of SPARC (Figure 8B) ▶ . When treated with 1 μg/ml of SPARC, a level much higher than that secreted by ovarian cancer cells (see Figure 4 ▶ ), SKOV3 cells showed a significant increase in DNA fragmentation when compared to the untreated control. In the presence of 5 μg/ml of SPARC, a concentration close to the normal range of SPARC secretion by primary HOSE cells (see Figure 4 ▶ ), DNA fragmentation is six times more than that of the control (Figure 8B) ▶ . On the contrary, no significant increase in DNA fragmentation was seen when HOSE1-15 cells were treated with increasing amounts of SPARC (Figure 8A) ▶ . No apoptotic effect was observed even after HOSE cells had been incubated with 20 μg/ml of SPARC (Figure 8A) ▶ , which is much higher than their normal amounts of SPARC secretion (see Figure 4 ▶ ). Various extents of SPARC-induced apoptosis were also detected in four other ovarian cancer cell lines DOV13, OVCA3, OVCA420, and OVCA429 (data not shown).
Figure 8.
Detection of apoptosis in SPARC-treated HOSE cells and SKOV3 cells. A quantitative ELISA that detects mono- and oligo-nucleosomes was used to assay the whole cell lysates prepared from HOSE1-15 cells (A) and SKOV3 cells (B) treated with different amounts of exogenous SPARC. Increase in apoptosis is indicated by the increase in absorbance at 405 nm (reference wavelength 490 nm). SPARC effectively induces apoptosis in SKOV3 cells, but not in HOSE cells. The values shown are the means ± SEM (bars) of duplicate measurements in two independent experiments.
SPARC Binds to Normal and Cancerous Ovarian Cell Surface
The antiproliferative effects of SPARC on bovine aortic endothelial cells have been suggested to depend, in part, on signal transduction via a G protein-coupled receptor. 24 Together with the results we obtained from the treatment of HOSE and ovarian cancer cells with exogenous human blood platelet SPARC, we sought to find out whether the antiproliferative and apoptotic effects of SPARC on ovarian cells are also mediated through a cell surface receptor.
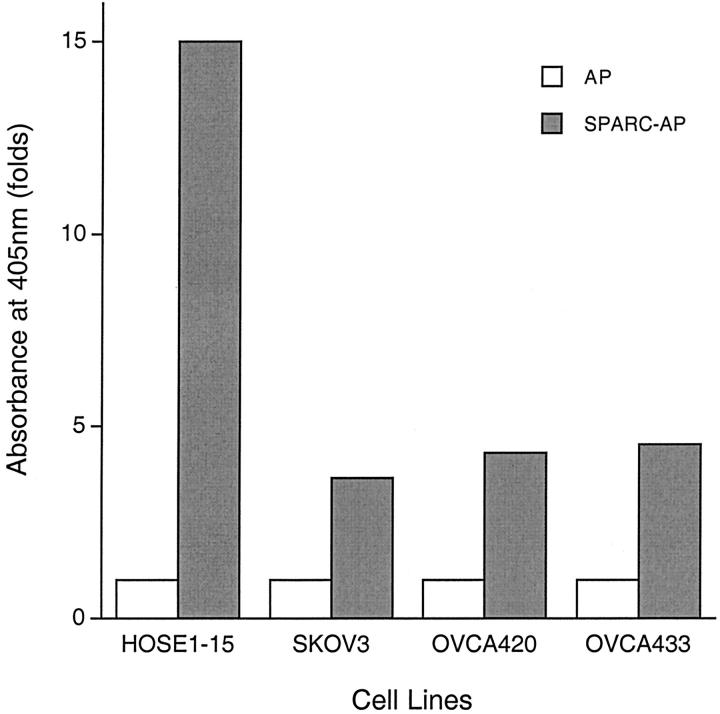
To examine whether putative SPARC receptors are present on ovarian cells, a secretory fusion protein containing full-length human SPARC and a thermostable human placental AP was made using a human embryonic kidney cell line (293T). As a negative control, culture medium containing high levels of secreted AP protein was also prepared from a 293T/pAPtag-4 stable cell line. Receptor binding assays using AP and SPARC-AP proteins revealed that putative SPARC receptors are present on both HOSE cells and ovarian cancer cells (Figure 9) ▶ . The AP activities detected when the cells were incubated with the control AP protein are probably because of nonspecific binding of AP to cell surfaces and/or incomplete denaturation of the endogenous AP activities in the cells. Our results showed that in addition to the higher levels of SPARC expression, HOSE cells also have much more putative SPARC receptors on their cell surfaces (Figure 9) ▶ .
Figure 9.
Cell surface receptor binding assays of HOSE cells and ovarian cancer cells. A secreted SPARC-AP fusion protein consists of human SPARC and a thermostable human placental alkaline phosphatase (AP) was used to examine the presence of putative SPARC receptors in ovarian cells. As background control, a secretory AP protein produced by the 293T/pAPtag-4 stable cell line was also used in this assay. Cells incubated with culture medium containing SPARC-AP or AP proteins were washed, lysed, and assayed for AP activities. Increase in ligand binding is indicated by the increase in AP activity, which is quantified by measuring absorbance at 405 nm. HOSE1-15 cells showed high levels of binding, whereas the three ovarian cancer cell lines studied have less binding to the SPARC-AP fusion protein.
Discussion
Using arbitrarily designed primers to generate differential RNA fingerprints from normal HOSE and ovarian carcinoma cells, we previously showed that SPARC is down-regulated in ovarian cancer. 25 Stable transfectants of ovarian cancer cells expressing high levels of SPARC grow slower and have greatly reduced ability to induce tumor formation in nude mice. 14 Nevertheless, the underlying mechanism(s) of these tumor-suppressing activities of SPARC is still unknown. Recent studies examining the immunoreactivity of SPARC in ovarian cancer tissues showed increased SPARC expression in ovarian cancer, 15,17 which are different from our earlier finding. To investigate this inconsistent pattern of SPARC deregulation in ovarian cancer, we studied a large number of ovarian tumor tissues of different grades and stages by immunohistochemistry using a rabbit polyclonal antibody to SPARC. This antibody, LF-54, has been tested to confirm its specificity and reactivity to bovine and human SPARC. 27 Our results revealed strong cytoplasmic immunoreactivity in the surface epithelial cells of human normal ovaries and benign epithelial tumors, which is progressively decreased in borderline epithelial tumors, and is significantly reduced or absent in invasive ovarian epithelial cancers. This strong direct correlation indicates that repression of SPARC expression is important in ovarian cancer development.
The strong immunoreactivity of SPARC in the germinal epithelium of normal ovary suggests that SPARC is important in maintaining the normal functions of ovarian surface epithelial cells. Its high expression in normal ovarian epithelial cells has also been detected by immunostaining using a monoclonal antibody, mAb SSP2, against a Ca2+-binding region of murine SPARC. 16 Although SPARC was reported to be detected in 8 of 10 cases of ovarian carcinomas examined in that study, 16 no comparison of SPARC immunoreactivity of normal ovarian cells to that of benign, borderline, or invasive ovarian tumor was described. Our results presented here showed that although SPARC can still be detected in ovarian cancers, its expression is significantly down-regulated in high-grade ovarian cancers. The levels of expression are actually inversely correlated with the degree of malignancy.
In contrast to our findings, a recent study reported that SPARC can be detected in ovarian adenocarcinoma but not in ovarian surface epithelial cells by immunostaining using a monoclonal antibody generated against the N-terminal region of bovine SPARC (AON-5031). 17 Because SPARC expression could not be detected in the same cancer tissues by in situ hybridization, the authors speculated that the SPARC found in cancer cells might be originated from stromal cells, as stromal cells in ovarian cancers have been shown to express SPARC (Porter et al, 16 Brown et al, 17 and this study). Although this hypothesis is interesting, it remains possible that the differences in SPARC immunoreactivity observed are because of the use of different antibodies and staining procedures. This is well illustrated by our immunostaining results using two different antibodies that showed distinctive patterns of SPARC immunoreactivity in ovarian tissues (compare Figures 1 and 2 ▶ ▶ ). Although the normal ovarian epithelium was strongly stained by LF-54, it was weakly stained with AON-5031. To the contrary, strong immunoreactivity was seen in the stroma of normal ovaries stained with AON-5031, but not in the sections stained with LF-54. In ovarian cancers, SPARC immunostaining was evident in the stromal cells, but rarely noted in the cancer cells with both the antibodies. This observation for ovarian tumor tissues agrees well with that previously reported by Paley and colleagues. 15 Based on these findings, the polyclonal antibody LF-54 might be more specific in human SPARC immunostaining. The immunohistochemical data we obtained with it are summarized in Table 1 ▶ , which are consistent with our published results from Northern and Western blotting. 14
The biological functions of SPARC seem to be variable in human cancers. Different tumors exhibit different patterns of SPARC expression. High levels of SPARC have been detected in several human cancers including melanoma, 28 breast cancer, 29 colorectal cancer, 30 hepatocellular carcinoma, 31 invasive meningioma, 32 and prostate cancer. 33 Moreover, it has been reported that SPARC promotes cell migration and invasion in prostate cancer and glioblastoma. 34,35 Suppression of SPARC expression by antisense RNA results in a significant decrease in the tumorigenicity of melanoma cells. 36 In contrast, no SPARC expression was found in chondrosarcoma, Ewing’s sarcoma, fibrosarcoma, malignant fibrous histiocytoma, and brown tumor from hyperparathyroidism. 37 Decreased SPARC expression has also been found in ovarian cancer. 14 Additional evidence showing the tumor-suppressing activity of SPARC came from the studies of cultured cells. Significant down-regulation of SPARC was demonstrated in vSrc-transformed chicken embryo fibroblasts, 38 c-Jun-transformed primary rat embryo fibroblasts, 39 and Ha-Ras-, v-Abl-, v-Src-, or Ki-Ras-transformed rodent fibroblasts. 39-42 A recent study has also shown that SPARC strongly inhibits the growth of vJun-m1 and v-Src-transformed chicken embryo fibroblasts. 42 As different tumors are probably developed through different multistep carcinogenic pathways, these findings suggested that SPARC might play a tissue-specific role in cancer development.
The recent characterization of another SPARC-like counteradhesive extracellular matrix protein called Hevin/MAST9 further demonstrates the possible tumor-suppressing activities of matricellular proteins in human carcinogenesis. Hevin was found to be down-regulated in metastatic prostate adenocarcinoma and non-small cell lung cancer. 43,44 SPARC and Hevin are 62% identical in sequence and are highly homologous in the SPARC coding region. 43 Similar to SPARC, Hevin is also a secreted acidic cysteine-rich calcium-binding glycoprotein and has been shown to inhibit cell attachment and spreading. 45 The importance of these SPARC-like proteins in ovarian oncogenesis remains to be elucidated.
The antiproliferative activity of SPARC has been demonstrated in endothelial cells. Growth of normal endothelial cells is inhibited when cultured with medium conditioned by endothelioma cells that secrete high levels of SPARC. 22 Exogenous SPARC has also been shown to suppress DNA synthesis in bovine aortic endothelial cells and human microvascular endothelial cells stimulated by vascular endothelial growth factor. 46,47 The SPARC domain IV that contains an EF-hand-like loop and has a high-affinity Ca2+-binding site is sufficient for the observed growth inhibitory functions. 23 The antiproliferative activity of SPARC was further illustrated by a recent study showing that the mesangial cells, fibroblasts, and smooth muscle cells isolated from SPARC-null mice grew faster than their respective wild-type counterparts. 48 Despite all these findings, no direct evidence is available showing the antiproliferative effects of SPARC on cancer cells. Here we show that exogenous SPARC can reduce the proliferation of both HOSE and ovarian cancer cells in a concentration-dependent manner. As HOSE cells secrete high levels of SPARC, the paracrine and/or autocrine antiproliferative activities of SPARC may play an important role in the precise regulation of normal HOSE cell growth. Diminished SPARC expression in ovarian cancer cells, together with other oncogenic factors, may lead to uncontrolled cell growth and cancer development.
Recent advances in basic cancer research have established that human cancers are the results of deregulation of not only the factors that control cellular proliferation and differentiation, but also those that influence apoptosis. 49 As a possible mechanism that contributes to the antitumor activities of SPARC, we presented here direct evidence that SPARC could induce apoptosis in ovarian cancer cells, but not HOSE cells. HOSE cells, which secrete high levels of SPARC, seem to have some mechanisms that protect themselves from the apoptotic activities of SPARC. This hypothesis is supported by a recent study showing that apoptosis can be inhibited in HOSE cells by the up-regulation of insulin-like growth factor-1, as mediated by luteinizing hormone/human chorionic gonadotropin signaling. 50 Follicle-stimulating hormone and human chorionic gonadotropin can both stimulate HOSE cell proliferation, but not SKOV3 cells. 51 As both follicle-stimulating hormone and luteinizing hormone receptors are highly expressed in HOSE cells, they were not detected in the gonadotropin insensitive SKOV3 cells. 51 A recent study has also reported that whereas HOSE cells show consistent expression of luteinizing hormone receptors, ovarian cancers exhibit a steady decrease in luteinizing hormone receptor expression from low-grade to high-grade cancer. 52
As the growth rates of both HOSE cells and ovarian cancer cells were reduced by SPARC, our findings suggest that distinct signal transduction pathways are used to mediate the antiproliferative and apoptotic effects of SPARC. Our preliminary results showed that SPARC could transiently trigger significant increases of intracellular Ca2+ levels in ovarian cancer cells, but not in HOSE cells, indicating that the apoptotic pathway induced by SPARC might be calcium-dependent. In addition, immunoblotting revealed that the expression of a pro-apoptotic protein was induced or significantly increased in SPARC-treated SKOV3 cells, but not HOSE cells (our unpublished data). We hypothesize that although down-regulation of SPARC in ovarian cancer cells may not be directly involved in oncogenesis, it is coupled to other oncogenic pathways that drive the development of ovarian cancer. Repression of SPARC expression may be essential to facilitate ovarian tumorigenesis, as cancer cells are sensitized to the apoptotic effects of SPARC. This concept is supported by the study showing that low levels of SPARC expression in v-Jun-m1 and v-Src-transformed chicken embryo fibroblasts favor their induction of local, primary fibrosarcomas after being subcutaneously injected into the wing web of chicken. 42
Platinum derivatives such as cisplatin are routinely used in the chemotherapy of ovarian epithelial cancer. Studies of cisplatin-resistant ovarian cancer cells revealed that the observed chemoresistance might be caused by the expression of an apoptosis suppressor protein known as X-linked inhibitor of apoptosis protein (Xiap). 53 Cisplatin induces apoptosis in cisplatin-sensitive, but not cisplatin-resistant cells by decreasing Xiap expression. 54 Although it has yet to be determined, the apoptotic effect of SPARC on SKOV3 cells is unlikely mediated by the down-regulation of Xiap because SKOV3 cells have null p53 mutation. It has been shown that decreased Xiap expression could not induce apoptosis in SKOV3 cells because Xiap down-regulation triggers apoptosis only in ovarian cancer cells that have wild-type p53, but not in the cells with no or mutated p53 protein. 53
The antiproliferative and apoptotic effects of exogenous SPARC on ovarian cancer cells indicate the presence of cell surface receptors for SPARC. This notion is supported by the results obtained from studying the mediators through which exogenous SPARC exerts its counteradhesive and antiproliferative effects on endothelial cells. Pretreating endothelial cells with protein tyrosine kinase inhibitors protected them against the inhibitory effect of SPARC on cell spreading. Moreover, inhibition of cell cycle progression by SPARC on these cells was found to be reversible by treating them with inhibitors for heterotrimeric G proteins such as pertussis toxin and cholera toxin. 24 Our preliminary data also showed that cholera toxin (1 μg/ml) can reverse the growth inhibitory effect of SPARC by 41.5%, indicating the involvement of G-protein in SPARC signaling in ovarian cells (our unpublished data). To date, neither the putative SPARC receptor nor the intracellular signaling pathway(s) triggered by SPARC has been identified.
Using a fusion protein containing SPARC and human placental AP, we presented here the first direct evidence that SPARC binds to putative SPARC receptors on the cell surfaces of HOSE and ovarian cancer cells. This AP-TAG technology has been used to successfully clone the receptors for leptin and Semaphorin III. 55,56 Our results showed that in addition to the down-regulation of SPARC expression, ovarian cancer cells have lower levels of SPARC receptor than HOSE cells. The binding of SPARC to its receptor may be important for mediating its antitumor effects. The diminished ligand-receptor interaction in ovarian cancer cells may explain why the growth of ovarian cancer is not influenced by the presence of low levels of SPARC produced by the adjacent stromal cells. Although HOSE cells have more putative SPARC receptors than ovarian cancer cells, their differential response to SPARC is probably caused by the distinctive downstream signaling events triggered by the binding of SPARC to its receptor as cancer cells have undergone numerous genetic changes during oncogenesis.
In this study, we showed that exogenous SPARC could reduce proliferation and induce apoptosis in ovarian cancer cells. Although distinct signaling pathways may mediate these tumor-suppressing effects, they probably are dependent on the binding of SPARC to its cell surface receptor. Down-regulation of SPARC and/or SPARC receptor in ovarian cancer cells will decrease and interrupt normal SPARC ligand-receptor interaction, which in turn affects the downstream signaling events that are important for controlling the growth and differentiation of HOSE cells. Interaction of SPARC and its putative receptor, in addition to the various posttranslational modification of SPARC, may also contribute to the tissue- and cell-specific biological functions of SPARC in different normal and cancerous cells.
Acknowledgments
We thank Cristiano V. Colitti for his excellent technical support and Dr. Larry W. Fisher for kindly providing the rabbit polyclonal SPARC antibody LF-54.
Footnotes
Address reprint requests to Dr. Samuel C. Mok, Brigham and Women’s Hospital, Laboratory of Gynecologic Oncology, 221 Longwood Ave., Rm BLI 449B, Boston, MA 02115. E-mail: scmok@rics.bwh.harvard.edu.
Supported by grants CA69453 and CA63381 from the National Institutes of Health (to S. C. M.) and grant CUHK4218/97M from the Research Grants Council of the Hong Kong Special Administrative Region (to W. Y. C.)
References
- 1.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK: Cancer incidence and mortality, 1973–1995, a report card for the U.S. Cancer 1998, 82:1197-1207 [DOI] [PubMed] [Google Scholar]
- 2.Piver MS, Fanning J, Crag KA: Cancer of the ovary. Knapp RC Berkowitz RS eds. Gynecologic Oncology. 1992, :pp 250-291 McGraw-Hill, New York [Google Scholar]
- 3.Auersperg N, Edelson MI, Mok SC, Johnson SW, Hamilton TC: The biology of ovarian cancer. Semin Oncol 1998, 25:281-304 [PubMed] [Google Scholar]
- 4.Bornstein P: Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 1995, 130:503-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankat-Buttgereit B, Mann K, Deutzmann R, Timpl R, Krieg T: Cloning and complete amino acid sequences of human and murine basement membrane protein BM-40 (SPARC, osteonectin). FEBS Lett 1988, 236:352-356 [DOI] [PubMed] [Google Scholar]
- 6.Sage H, Johnson C, Bornstein P: Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem 1984, 259:3993-4007 [PubMed] [Google Scholar]
- 7.Bolander ME, Young MF, Fisher LW, Yamada Y, Termine JD: Osteonectin cDNA sequence reveals potential binding regions for calcium and hydroxyapatite and shows homologies with both a basement membrane protein (SPARC) and a serine proteinase inhibitor (ovomucoid). Proc Natl Acad Sci USA 1988, 85:2919-2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarreal XC, Mann KG, Long GL: Structure of human osteonectin based upon analysis of cDNA and genomic sequences. Biochemistry 1989, 28:6483-6491 [DOI] [PubMed] [Google Scholar]
- 9.Nomura S, Wills AJ, Edwards DR, Heath JK, Hogan BL: Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol 1988, 106:441-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sage H, Vernon RB, Decker J, Funk S, Iruela-Arispe ML: Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem 1989, 37:819-829 [DOI] [PubMed] [Google Scholar]
- 11.Mundlos S, Schwahn B, Reichert T, Zabel B: Distribution of osteonectin mRNA and protein during human embryonic and fetal development. J Histochem Cytochem 1992, 40:283-291 [DOI] [PubMed] [Google Scholar]
- 12.Lane TF, Sage EH: The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J 1994, 8:163-173 [PubMed] [Google Scholar]
- 13.Holland PW, Harper SJ, McVey JH, Hogan BL: In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol 1987, 105:473-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok SC, Chan WY, Wong KK, Muto MG, Berkowitz RS: SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene 1996, 12:1895-1901 [PubMed] [Google Scholar]
- 15.Paley PJ, Goff BA, Gown AM, Greer BE, Sage EH: Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol Oncol 2000, 78:336-341 [DOI] [PubMed] [Google Scholar]
- 16.Porter PL, Sage EH, Lane TF, Funk SE, Gown AM: Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 1995, 43:791-800 [DOI] [PubMed] [Google Scholar]
- 17.Brown TJ, Shaw PA, Karp X, Huynh MH, Begley H, Ringuette MJ: Activation of SPARC expression in reactive stroma associated with human epithelial ovarian cancer. Gynecol Oncol 1999, 75:25-33 [DOI] [PubMed] [Google Scholar]
- 18.Sage EH, Bornstein P: Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem 1991, 266:14831-14834 [PubMed] [Google Scholar]
- 19.Sage H, Vernon RB, Funk SE, Everitt EA, Angello J: SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol 1989, 109:341-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldblum SE, Ding X, Funk SE, Sage EH: SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci USA 1994, 91:3448-3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everitt EA, Sage EH: Overexpression of SPARC in stably transfected F9 cells mediates attachment and spreading in Ca(2+)-deficient medium. Biochem Cell Biol 1992, 70:1368-1379 [DOI] [PubMed] [Google Scholar]
- 22.Sage EH: Secretion of SPARC by endothelial cells transformed by polyoma middle T oncogene inhibits the growth of normal endothelial cells in vitro. Biochem Cell Biol 1992, 70:579-592 [DOI] [PubMed] [Google Scholar]
- 23.Kupprion C, Motamed K, Sage EH: SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem 1998, 273:29635-29640 [DOI] [PubMed] [Google Scholar]
- 24.Motamed K, Sage EH: SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem 1998, 70:543-552 [DOI] [PubMed] [Google Scholar]
- 25.Mok SC, Wong KK, Chan RK, Lau CC, Tsao SW, Knapp RC, Berkowitz RS: Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol Oncol 1994, 52:247-252 [DOI] [PubMed] [Google Scholar]
- 26.Tsao SW, Mok SC, Fey EG, Fletcher JA, Wan TS, Chew EC, Muto MG, Knapp RC, Berkowitz RS: Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs). Exp Cell Res 1995, 218:499-507 [DOI] [PubMed] [Google Scholar]
- 27.Fisher LW, Stubbs JT, Young MF: Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand 1995, 266(Suppl):S61-S65 [PubMed] [Google Scholar]
- 28.Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL: The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol 1997, 108:210-214 [DOI] [PubMed] [Google Scholar]
- 29.Bellahcene A, Castronovo V: Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol 1995, 146:95-100 [PMC free article] [PubMed] [Google Scholar]
- 30.Porte H, Chastre E, Prevot S, Nordlinger B, Empereur S, Basset P, Chambon P, Gespach C: Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int J Cancer 1995, 64:70-75 [DOI] [PubMed] [Google Scholar]
- 31.Le Bail B, Faouzi S, Boussarie L, Guirouilh J, Blanc JF, Carles J, Bioulac-Sage P, Balabaud C, Rosenbaum J: Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J Pathol 1999, 189:46-52 [DOI] [PubMed] [Google Scholar]
- 32.Rempel SA, Ge S, Gutierrez JA: SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res 1999, 5:237-241 [PubMed] [Google Scholar]
- 33.Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL: Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res 2000, 6:1140-1149 [PubMed] [Google Scholar]
- 34.Jacob K, Webber M, Benayahu D, Kleinman HK: Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res 1999, 59:4453-4457 [PubMed] [Google Scholar]
- 35.Golembieski WA, Ge S, Nelson K, Mikkelsen T, Rempel SA: Increased SPARC expression promotes U87 glioblastoma invasion in vitro. Int J Dev Neurosci 1999, 17:463-472 [DOI] [PubMed] [Google Scholar]
- 36.Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, Mordoh J, Podhajcer OL: Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med 1997, 3:171-176 [DOI] [PubMed] [Google Scholar]
- 37.Serra M, Morini MC, Scotlandi K, Fisher LW, Zini N, Colombo MP, Campanacci M, Maraldi NM, Olivari S, Baldini N: Evaluation of osteonectin as a diagnostic marker of osteogenic bone tumors. Hum Pathol 1992, 23:1326-1331 [DOI] [PubMed] [Google Scholar]
- 38.Young MF, Bolander ME, Day AA, Ramis CI, Robey PG, Yamada Y, Termine JD: Osteonectin mRNA: distribution in normal and transformed cells. Nucleic Acids Res 1986, 14:4483-4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mettouchi A, Cabon F, Montreau N, Vernier P, Mercier G, Blangy D, Tricoire H, Vigier P, Binetruy B: SPARC and thrombospondin genes are repressed by the c-jun oncogene in rat embryo fibroblasts. EMBO J 1994, 13:5668-5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason IJ, Taylor A, Williams JG, Sage H, Hogan BL: Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell ‘culture shock’ glycoprotein of Mr 43,000. EMBO J , 5:1465-1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo MP, Ferrari G, Biondi G, Galasso D, Howe CC, Parmiani G: Down-regulation of SPARC/osteonectin/BM-40 expression in methylcholanthrene-induced fibrosarcomas and in Kirsten-MSV transformed fibroblasts. Eur J Cancer 1991, 27:58-62 [DOI] [PubMed] [Google Scholar]
- 42.Vial E, Castellazzi M: Down-regulation of the extracellular matrix protein SPARC in vSrc- and vJun-transformed chick embryo fibroblasts contributes to tumor formation in vivo. Oncogene 2000, 19:1772-1782 [DOI] [PubMed] [Google Scholar]
- 43.Nelson PS, Plymate SR, Wang K, True LD, Ware JL, Gan L, Liu AY, Hood L: Hevin, an antiadhesive extracellular matrix protein, is down-regulated in metastatic prostate adenocarcinoma. Cancer Res 1998, 58:232-236 [PubMed] [Google Scholar]
- 44.Bendik I, Schraml P, Ludwig CU: Characterization of MAST9/Hevin, a SPARC-like protein, that is down-regulated in non-small cell lung cancer. Cancer Res 1998, 58:626-629 [PubMed] [Google Scholar]
- 45.Girard JP, Springer TA: Modulation of endothelial cell adhesion by hevin, an acidic protein associated with high endothelial venules. J Biol Chem 1996, 271:4511-4517 [DOI] [PubMed] [Google Scholar]
- 46.Funk SE, Sage EH: The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA 1991, 88:2648-2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funk SE, Sage EH: Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol 1993, 154:53-63 [DOI] [PubMed] [Google Scholar]
- 48.Bradshaw AD, Francki A, Motamed K, Howe C, Sage EH: Primary mesenchymal cells isolated from SPARC-null mice exhibit altered morphology and rates of proliferation. Mol Biol Cell 1999, 10:1569-1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267:1456-1462 [DOI] [PubMed] [Google Scholar]
- 50.Kuroda H, Mandai M, Konishi I, Tsuruta Y, Kusakari T, Kariya M, Fujii S: Human ovarian surface epithelial (OSE) cells express LH/hCG receptors, and hCG inhibits apoptosis of OSE cells via up-regulation of insulin-like growth factor-1. Int J Cancer 2001, 92:309-315 [DOI] [PubMed] [Google Scholar]
- 51.Parrott JA, Doraiswamy VV, Kim G, Mosher R, Skinner MK: Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol 2001, 172:213-222 [DOI] [PubMed] [Google Scholar]
- 52.Lu JJ, Zheng Y, Kang X, Yuan JM, Lauchlan SC, Pike MC, Zheng W: Decreased luteinizing hormone receptor mRNA expression in human ovarian epithelial cancer. Gynecol Oncol 2000, 79:158-168 [DOI] [PubMed] [Google Scholar]
- 53.Sasaki H, Sheng Y, Kotsuji F, Tsang BK: Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res 2000, 60:5659-5666 [PubMed] [Google Scholar]
- 54.Li J, Feng Q, Kim J, Schneiderman D, Liston P, Li M, Vanderhyden B, Faught W, Fung MFK, Senterman M, Korneluk RG, Tsang BK: Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology 2001, 142:370-380 [DOI] [PubMed] [Google Scholar]
- 55.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI: Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83:1263-1271 [DOI] [PubMed] [Google Scholar]
- 56.He Z, Tessier-Lavigne M: Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90:739-751 [DOI] [PubMed] [Google Scholar]