Abstract
Mycobacterium avium complex (MAC) is the most common disseminated bacterial disease in patients infected by the human immunodeficiency virus. Although murine models of disseminated MAC exist, they are primarily based on underlying genetic susceptibilities and cannot adequately address the complex interactions that occur between host, mycobacteria, and immunosuppressive lentivirus. To address this problem we have developed an experimental system to co-inoculate rhesus macaques with the simian immunodeficiency virus (SIV) and a clinical M. avium isolate that results in a disease virtually identical to that observed in human cases. Using this experimental system we have found that the development of disseminated MAC is dependent on viral strain. Animals co-infected with SIVmac251 and M. avium developed progressive disease, whereas control animals and animals inoculated with closely related viruses (SIVmac239 and SIVmac239MER) developed self-limiting infections. The ability of animals infected with SIVmac239 or SIVmac239MER to eliminate mycobacterial disease was independent of viral load and CD4 T-cell number but was correlated with the size and composition of microgranulomas. This work establishes a novel primate model of disseminated MAC in the context of immunosuppressive lentiviral infection and advances our understanding of why human immunodeficiency virus-infected patients are remarkably sensitive to the development of mycobacterial disease.
Mycobacterium avium complex (MAC) is the most common disseminated bacterial disease in patients infected by the human immunodeficiency virus (HIV) and is diagnosed in 30 to 40% of untreated patients dying with the acquired immune deficiency syndrome (AIDS). 1,2 MAC organisms are found ubiquitously in the environment and yet rarely cause disease in non-HIV-infected individuals. Furthermore, although the occurrence of MAC is associated with severe CD4 T-lymphocyte depletion in HIV-infected patients, it is uncommon in humans with other immunosuppressive disorders. 1 The reason AIDS patients are uniquely sensitive to infection by these organisms is unknown but evidence suggests that in addition to depletion of CD4 T lymphocytes, viral proteins may have an adverse effect on the host’s ability to eliminate MAC organisms. 3-10
Current animal models of disseminated MAC have used different strains of laboratory mice to investigate factors associated with disease progression and the host’s immune response to mycobacterial infection. Extensive work has been conducted with the beige mouse, a strain that contains a homozygous mutation in the natural resistance associated macrophage protein-1 (Nramp-1) gene. 11-13 The Nramp-1 gene encodes a transmembrane cationic transport protein that seems to play a fundamental role in the elimination of intracellular pathogens. More recently, knockout mice have been used to examine the role of specific cytokine pathways and cellular immune responses in mycobacterial disease pathogenesis. 14-22 Although these models have been useful in elucidating many factors associated with disseminated disease, they are primarily dependent on underlying genetic susceptibilities and cannot reproduce the complex interactions between host, mycobacteria, and lentiviral proteins that are likely responsible for the full manifestation of disease in human AIDS patients. For these reasons alternative animal models may prove useful for investigating the pathogenesis of disseminated mycobacterial disease.
Disseminated MAC also occurs in simian immunodeficiency virus (SIV)-inoculated rhesus macaques (Macaca mulatta) as a spontaneous disease in the terminal stages of AIDS and this process shares extensive similarities with the condition in human patients. 23-26 SIV-infected animals acquire M. avium from environmental sources and develop spontaneous disease at severe CD4 T-lymphocyte depletion. 25 As in man, these animals develop debilitating diarrhea and wasting with progressive disease. In disseminated disease, tissue distribution and histomorphology closely approximates that seen in HIV-infected humans. Retrospective analysis of spontaneous disseminated MAC in rhesus macaques has revealed that the risk of mycobacterial infection is greater after inoculation with the wild-type SIV isolate SIVmac251 than with a closely related molecular clone, SIVmac239. 23 No significant difference in CD4 count near death, survival, or the occurrence of other opportunistic infections have been found between SIVmac251- and SIVmac239-inoculated macaques, eliminating these as confounding variables.
Such findings suggest that independent of CD4 T-lymphocyte depletion viral determinants play a critical role in promoting mycobacterial disease. This hypothesis is supported by in vitro work using HIV and MAC, which argues that viral proteins such as gp 120 and tat may adversely impact macrophage function. 3-10 Here we describe an experimental system to co-inoculate rhesus macaques with SIV and M. avium and reproduce disseminated disease. This model has proven useful to study the effect of viral inoculum on progressive disease and the immunopathogenesis of early mycobacterial infection.
Materials and Methods
Study Design
Experimental co-infection of rhesus macaques was undertaken to investigate the pathogenesis of mycobacterial disease during simian AIDS. Immunologically normal animals (n = 4) were compared to animals infected with SIVmac251 (n = 3), SIVmac239 (n = 2), and SIVmac239MER (n = 2). M. avium was administered 2 weeks after SIV infection to coincide with the period of peak viremia. All animals were bled and had biopsies of peripheral lymph node, colon, and duodenum before mycobacterial inoculation and at 2, 4, 6, 8, and 10 weeks after inoculation. Tissues were processed for routine histopathology, acid-fast stains, immunohistochemistry, and lymphocyte isolation and subset analysis. Blood samples were processed for complete blood counts, serum chemistries, mycobacterial culture, viral isolation, and lymphocyte subset analysis. All three SIVmac251 co-infected animals were euthanized between 45 and 70 days after M. avium challenge because of progressive wasting and diarrhea. To allow comparisons, one animal in each of the normal, SIVmac239, and SIVmac239MER groups was euthanized and necropsied on day 45. Complete postmortem examinations were performed on all animals, and representative samples of tissue were taken for formalin fixation, and freezing. All sections were stained with hematoxylin and eosin (H&E) for routine evaluation and with Ziehl-Neelson acid-fast stain to determine the distribution of mycobacterial organisms.
Animals and Housing
Rhesus macaques (M. mulatta) were housed at the New England Regional Primate Research Center in a centralized animal biolevel 3 containment facility in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and Harvard Medical School’s Animal Care and Use Committee. Animals were tested and found free of simian retrovirus type-D, SIV, simian T lymphotropic virus-1, and herpes B virus before assignment to experimental protocols. Lymph node, gastrointestinal, and hepatic biopsies were performed as previously described. 27 Animals received commercial monkey chow and to reduce exposure to environmental mycobacteria autoclaved water ad libitum. The monkeys were monitored closely and euthanized when moribund or deemed necessary by the veterinary staff.
Animal Inoculations
Animals were inoculated intravenously with equivalent doses of SIVmac251, SIVmac239, or SIVmac239MER. The in vitro growth properties and in vivo disease characteristics of these viruses have been described extensively. 28-33 Briefly, SIVmac251 is a pathogenic wild-type viral isolate that replicates to high titer in cells of lymphocyte and monocyte lineage. SIVmac239 is a pathogenic molecular clone derived from a SIVmac251-infected animal and replicates well in lymphocytes. 34 T-cell tropism in SIVmac239 results from the decreased efficiency at which this virus utilizes the CD4 receptor present on tissue macrophages at low density. 35 SIVmac239MER is derived from the SIVmac239 parental clone and has three amino acid changes in the envelope protein that confers the ability of the virus to replicate in tissue macrophages and monocytes. 36,37
To develop a pathogenic M. avium isolate, a SIVmac251-infected rhesus macaque (155-84) was injected intravenously with a splenic tissue homogenate from a second SIV-infected animal (31-83) that had died with massive splenomegaly and mesenteric lymphadenopathy. M. avium was confirmed in the donor animal by histopathology, mycobacterial culture, and DNA probe (Gene-Probe, San Diego, CA). 38 Rhesus macaque 155-84 subsequently developed severe diarrhea and wasting and died from disseminated M. avium 52 days later. Clinical signs, tissue distribution, and histopathology were identical to spontaneous cases recognized in SIV-infected macaques. A smooth transparent M. avium clone (clinical isolate no. 88415) was recovered from frozen mesenteric lymph node and used to inoculate 50 ml of 7H9 broth (Difco, Detroit, MI). The broth was harvested during log-phase growth and aliquots of 10 8 colony-forming units (cfu) were stored at −70°C. For inoculation, aliquots were rapidly thawed at 37°C and sonicated for 10 seconds before administration intravenously.
Cell Isolation and Flow Cytometry
Peripheral blood, lymph node, and intestinal lymphocytes were stained and analyzed by flow cytometry as previously described. 27,39 Briefly, jejunal and colonic endoscopic biopsies were incubated with 1 mmol/L ethylenediaminetetraacetic acid in Hanks’ balanced salt solution for 30 minutes, followed by 1 hour in RPMI containing 20 U of collagenase per ml while rapidly shaking at 37°C. Biopsies were further disrupted, and single-cell suspensions were prepared by pipetting 5 to 10 times with a 16-gauge feeding needle. Lymphocytes were enriched by Percoll density centrifugation. Lymph node biopsies were processed similarly without ethylenediaminetetraacetic acid. Cells were stained by incubating 10 6 cells from each of the above-described samples with excess amounts of monoclonal antibodies at 4°C for 30 minutes, followed by a wash (400 × g, 7 minutes) and fixation in 2% paraformaldehyde. Blood was stained by incubating 100 μl of whole blood with monoclonal antibodies for 30 minutes at 4°C, followed by a 7-minute lyse with FACS lysing solution (Becton Dickinson, San Jose, CA). Cells were then washed (400 × g, 7 minutes) and resuspended in 2% paraformaldehyde. All antibodies were directly conjugated to either fluorescein isothiocyanate, phycoerythrin, or peridinin chlorophyll protein. Monoclonal antibodies directed at CD2 (Leu-5b, Becton Dickinson), CD3 (6G12; provided by J. Wong, Massachusetts General Hospital, Boston, MA), CD4 (Leu3a, Becton Dickinson), CD8 (Leu-2a, Becton Dickinson), CD20 (Leu-16, Becton Dickinson), CD45RA (Leu-18, Becton Dickinson), CD56 (Leu19, Becton Dickinson), and HLA-DR (Becton Dickinson) were used. All antibodies have been used extensively in rhesus macaques. 27,40 Samples were acquired on a FACS Calibur flow cytometer and analyzed with Cell Quest software (Becton Dickinson).
Immunohistochemistry and in Situ Hybridization
To examine the immunophenotype of cells within tissues, immunohistochemistry was performed on formalin-fixed paraffin-embedded tissues. Tissue sections were cut at 5 μm and immunostained using an avidin-biotin-horseradish peroxidase complex technique with diaminobenzidine chromogen as previously described. 41 Sections were stained for CD68 (EBM-11; DAKO, Carpinteria, CA), CD3 (A0452, DAKO), CD20 (L26, DAKO), HLA-DR (CR3/43, DAKO), CD4 (Nu/Th1; Nicheri Research Institute, Tokyo, Japan) and CD8 (DK25, DAKO) and examined with an Olympus Vanox-S microscope interfaced with a Quantimet image analyzer (Leica, Cambridge, UK) via a DEI 750 charge-coupled device camera (Optronics, Goleta, CA). For quantitative measurements, images were captured and analyzed with Quantimet software as previously described. 27,42 Briefly 5-μm sections of liver were stained for CD3, CD4, CD8, and HLA-DR. Twenty random hepatic microgranulomas from two animals in each group were identified in tissue sections and their images captured. Based on diaminobenzidine chromogen staining the total number of positive and negative cells for CD3, CD4, and CD8 were detected within individual microgranulomas and used to calculate relative (%) cell number. To obtain density, the area of each granuloma in a section was concurrently measured with data expressed as positive cells/10 3 μm2. Sections stained with irrelevant isotype-matched antibodies (DAKO) were uniformly below background. For HLA-DR, diaminobenzidine-staining intensity was measured by microdensitometry for individual microgranulomas and expressed as mean gray scale intensity/granuloma. In situ hybridization was performed on formalin-fixed, paraffin-embedded tissues to demonstrate SIVmac-infected cells as previously described. 39
Virus Isolation and Quantitation
Peripheral blood was collected for viral isolation before inoculation and at 3, 7, 14, 28, 42, 56, and 70 days after SIV infection. Quantitative viral cultures were performed on each blood sample as previously described. 29 Briefly serial threefold dilutions beginning with 10 6 peripheral blood mononuclear cells were co-cultured with 10 5 CEMx174 cells in a volume of 1 ml and viral production assayed by enzyme-linked immunosorbent assay for SIV p27 (Coulter, Hialeah, FL). Results are expressed as the number of SIV-positive cells/10 6 peripheral blood mononuclear cells.
Statistical Analysis
Groups were compared statistically using commercially available software (Sigma Stat, Jandel Scientific) by t-test, one-way analysis of variance with multiple comparisons (Dunn’s method or Tukey test) where appropriate.
Results
Co-Inoculation of Rhesus Macaques Produces Progressive Mycobacterial Disease
Three rhesus macaques were inoculated with SIVmac251 and challenged intravenously with 10 8 cfu of M. avium 2 weeks after primary SIV infection (Table 1) ▶ . Peak viral load (729 to 2188/10 6 peripheral blood mononuclear cells) occurred at 14 days after inoculation and coincided with M. avium administration. As has been previously described during acute SIV infection, there was a temporary reduction in the relative and absolute number of CD3+CD4+ lymphocytes detected in blood and peripheral lymph node that reached it’s nadir at 2 to 4 weeks after inoculation and then rebounded to near normal values (Figure 1, A and B) ▶ . Despite nearly normal peripheral CD4 T-lymphocyte values, all SIVmac251-co-infected animals developed severe clinical signs requiring euthanasia 45 to 70 days after mycobacterial challenge. The syndrome paralleled the course observed in spontaneous M. avium disease seen in simian AIDS and was characterized by anorexia, diarrhea, and progressive wasting. These clinical signs were accompanied by peripheral lymphadenopathy, a febrile response, and marked hepatosplenomegaly. Complete blood counts revealed a modest leukopenia (mean, 3100 cells/μl) 7 days after mycobacterial infection but values returned to normal by 14 days. Serum chemistries indicated a gradual rise in serum globulin and marked elevations in liver enzymes (alkaline phosphatase and alanine aminotransferase) by 4 weeks after inoculation.
Table 1.
Viral Inoculum, Survival, and Clinical Findings in Rhesus Macaques Inoculated with Mycobacterium avium
| Animal | Viral inoculum | Survival days | Clinical findings |
|---|---|---|---|
| 51-97 | SIVmac251 | 45 | Diarrhea, hepatosplenomegaly, peripheral lymphadenopathy, dermatitis |
| 271-96 | SIVmac251 | 70 | Diarrhea, hepatosplenomegaly, peripheral lymphadenopathy |
| 85-96 | SIVmac251 | 45 | Diarrhea, hepatosplenomegaly, peripheral lymphadenopathy; dyspnea |
| 372-90 | None | +365 | None |
| 318-90 | None | +365 | None |
| 109-96 | None | 45 | None; euthanized as control |
| 192-96 | None | +365 | None |
| 65-97 | SIVmac239 | 45 | None; euthanized as control |
| 355-96 | SIVmac239 | 304 | Euthanized when moribund with AIDS; no evidence of M. avium |
| 154-96 | SIVmac239MER | 45 | None; euthanized as control |
| 221-96 | SIVmac239MER | 410 | Euthanized when moribund with AIDS; no evidence of M. avium |
Figure 1.
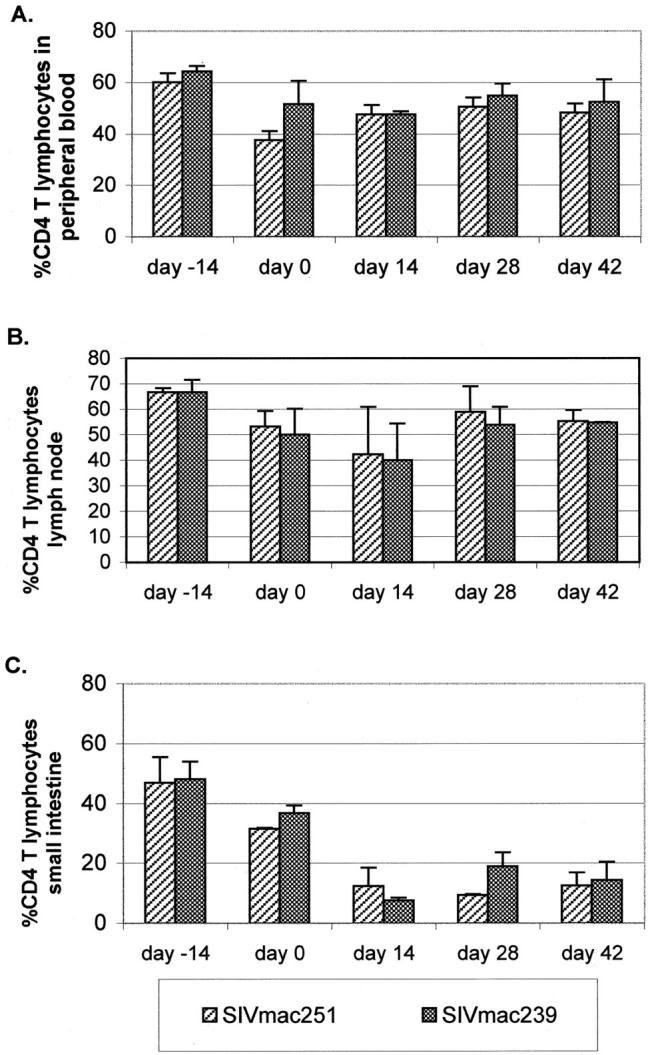
Temporal alterations in CD3CD4-positive lymphocytes. Changes observed after M. avium challenge in SIVmac251 (51-97 and 271-96) and SIVmac239-infected (65-97 and 355-96) rhesus macaques. A: Blood; B: peripheral lymph node; and C: duodenum. Each bar represents the mean values from two infected animals after mycobacterial challenge (day 0).
Sequential biopsies of peripheral lymph node, liver, duodenum, and colon were performed to determine the temporal changes in mycobacterial distribution. Acid-fast bacilli were first detected in scattered macrophages within lymph nodes and the lamina propria of the small intestine at 28 days after mycobacterial challenge. Mycobacterial blood cultures became positive at this time and subsequent biopsies indicated an increase in the number of infected cells and mycobacterial tissue load (Table 2) ▶ . By 6 weeks after M. avium inoculation morphological features typical of disseminated MAC were apparent.
Table 2.
Distribution of M. avium in Biopsy Sections of Peripheral Lymph Node and Small Intestine after Intravenous Mycobacterial Challenge in Normal, SIVmac251-Infected, and SIVmac239-Infected Animals
| 51-97 SIVmac251 LN/SI* | 271-96 SIVmac251 LN/SI | 109-96 Normal LN/SI | 192-96 Normal LN/SI | 65-97 SIVmac239 LN/SI | 355-96 SIVmac239 LN/SI | |
|---|---|---|---|---|---|---|
| −2 wk PI† | − /−‡ | − /− | − /− | − /− | − /− | − /− |
| 2 wk PI | − /− | − /− | − /− | − /− | − /− | − /− |
| 4 wk PI | + /+ | + /− | + /− | + /− | + /− | + /− |
| 6 wk PI | +++ /++ | ++ /+ | − /− | − /− | − /− | − /− |
| 8 wk PI | +++ /++ | − /− | − /− | |||
| 10 wk PI | ++++ /++ | − /− | − /− | |||
| 12 wk PI | − /− | − /− |
*LN/SI, lymph node/small intestine.
†PI, Weeks after mycobacterial challenge.
‡−, No acid fast bacteria evident; +, <3 infected cells per high-power field; ++, >3 infected cells per high-power field; +++, 10–50% of cells contain acid-fast bacilli; ++++, >50% of cells contain acid-fast bacilli.
At necropsy acid-fast bacilli were found in numerous tissues including peripheral and mesenteric lymph nodes, small and large intestine, liver, spleen, lung, kidneys, and bone marrow. Tissue distribution and morphological findings were identical to that seen in spontaneous cases of disseminated MAC found in human and simian AIDS. In lymph nodes, sheets of pale eosinophilic histiocytes effaced normal architecture and contained large numbers of acid-fast bacilli (Figure 2, A and B) ▶ . Similar histiocytic infiltrates were found throughout the spleen, mesenteric lymph nodes, and lamina propria of the small intestine (Figure 2C) ▶ . Typical microgranulomas, all containing bacilli, were found in liver, spleen, lung, and bone marrow and consisted of small aggregates of CD68-positive histiocytes and variable numbers of CD4- and CD8-positive lymphocytes. Microgranulomas rarely contained CD20-positive B lymphocytes and expressed low levels of MHC II antigen. As previously described, 24 co-infection of macrophages by SIVmac and M. avium as detected by in situ hybridization and acid-fast stains was rarely identified. Other opportunistic infections were not identified at necropsy.
Figure 2.
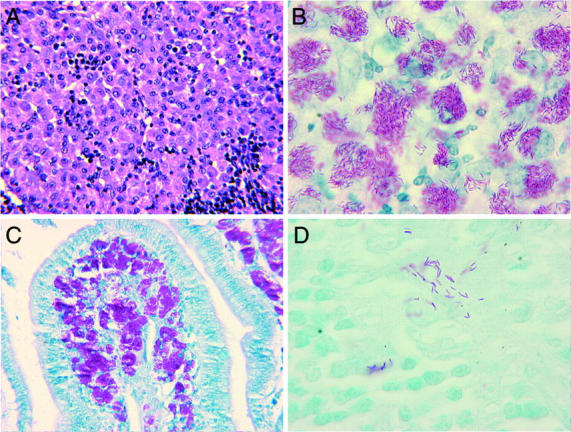
Morphological features of disseminated Mycobacterium avium after experimental inoculation in simian AIDS. A: Mesenteric lymph node from an SIVmac251-infected animal 6 weeks after M. avium challenge reveals characteristic histiocytic infiltrates (H&E stain; original magnification, ×140). B: Ziehl-Neelson stain reveals acid-fast bacilli within an infected mesenteric lymph node (Ziehl-Neelson acid-fast stain; original magnification, ×424). C: M. avium found within lamina propria of ileum (Ziehl-Neelson acid-fast stain; original magnification, ×140). Animals were challenged intravenously with M. avium and disseminated infection resulted including extensive involvement of the gastrointestinal tract. Localization here likely resulted in severe diarrhea noted clinically. D: Scattered acid-fast bacilli were noted in the peripheral lymph node of immunologically normal animals 4 weeks after M. avium challenge (axillary lymph node, Ziehl-Neelson acid-fast stain; original magnification, ×424). As in SIVmac239 and SIVmac239MER co-infected animals, disseminated infection resolved by 6 to 8 weeks after M. avium challenge.
Immunologically Normal Animals Develop Disseminated Disease but Resolve Mycobacterial Infection
To investigate host responses in immunologically normal animals, 10 8 cfu of M. avium was administered to four non-SIV-infected rhesus macaques. These non-SIV-infected animals remained clinically normal throughout the study period (Table 1) ▶ . Appetite, activity, and stool consistency remained unchanged during acute infection and long-term follow-up conducted in three animals for >365 days has been unremarkable. As in SIVmac251-infected animals, complete blood counts performed biweekly revealed minimal changes. Likewise lymphocyte subset analysis performed on peripheral blood was unremarkable. Lymph node biopsies were obtained at 2-week intervals from these animals and scattered acid-fast bacilli were recognized in histiocytes within medullary sinuses at 4 weeks after mycobacterial challenge but not at later time points (Figure 2D ▶ and Table 2 ▶ ). Bacilli were not detected in gastrointestinal biopsies and mycobacterial blood cultures remained negative.
Serum chemistry profiles on these normal animals revealed a transient increase in serum globulin and hepatic enzymes. Serum alkaline phosphatase values peaked 4 weeks after M. avium inoculation and were significantly higher than base-line values (5667 IU/L versus 1099 IU/L, respectively). H&E-stained sections of liver obtained at necropsy and by hepatic biopsy revealed large numbers of hepatic microgranulomas at 4, 6, and 8 weeks after challenge. Although morphologically similar to those seen in disseminated M. avium arising spontaneously in simian AIDS, Ziehl-Neelson stains uniformly failed to demonstrate acid-fast organisms within histiocytes. In three animals followed long term, elevations in hepatic enzymes returned to pre-inoculation values and hepatic microgranulomas resolved. Hepatic morphology was normal by 6 months after M. avium administration. Similarly, although lymph node biopsies obtained at 4 weeks after inoculation contained acid-fast bacilli, subsequent biopsies evaluated at 6, 8, and 10 weeks failed to demonstrate M. avium indicating resolution of mycobacterial infection.
As a control and to investigate tissue distribution of M. avium, one normal animal was euthanized and a complete necropsy performed on day 45 after challenge. Typical microgranulomas were identified in multiple organs including liver, spleen, lung, and bone marrow, however as with hepatic lesions these they were uniformly devoid of acid-fast bacilli. Necropsy findings confirmed that although normal animals developed disseminated infection, a vigorous host response developed that eliminated mycobacterial organisms and prevented the establishment of progressive disease and death.
SIVmac Strain Differences Exist in Production of Progressive Disease
Retrospective analysis of spontaneous disseminated MAC in simian AIDS has revealed a strong association between SIVmac251 and the occurrence of mycobacterial disease as compared to SIVmac239 and its macrophage-tropic derivative. 23 The mechanism behind this association is unknown but it seems to be independent of viral load and CD4 T-lymphocyte count at death. To investigate whether a similar association could be reproduced in a more tightly controlled experimental setting, animals were infected with SIVmac239 (n = 2) and SIVmac239MER (n = 2) and challenged with M. avium 2 weeks later. After SIV inoculation, parameters of viral infection including peripheral viral load and alterations in lymphocyte subsets were similar to those seen in SIVmac251-infected animals. As with immunologically normal animals, these macaques failed to develop overt clinical signs after M. avium challenge.
Sequential biopsies of peripheral lymph node revealed scattered mycobacterial organisms at 4 weeks after infection (Table 2) ▶ . Blood samples and gastrointestinal biopsies remained negative. However in contrast to SIVmac251-infected animals and paralleling our observations made in the challenged normal macaques described above, biopsies of peripheral lymph node and gastrointestinal tract revealed resolution of mycobacterial infection. Acid-fast bacilli could not be detected in tissues obtained at 6, 8, and 10 weeks after mycobacterial challenge (Table 2 ▶ and Table 3 ▶ ). To serve as controls, SIVmac239-infected animal 65-97 and SIVmac239MER-infected animal 154-96 were euthanized at 45 days after M. avium administration and a complete necropsy performed. As in immunologically normal animals challenged with M. avium, microgranulomas were present in multiple organs including spleen, lung, liver, kidney, and bone marrow but these were uniformly devoid of acid-fast bacilli. Small numbers of acid-fast bacilli were evident focally within colonic sections from the SIVmac239-infected animal 65-97.
Table 3.
Immunophenotypic Composition of Hepatic Microgranulomas after Intravenous Mycobacterial Challenge in Normal, SIVmac251-Infected, and SIVmac239-Infected Animals
| Mean granuloma size (μm2 × 103) | %CD3 cells | No. CD3 cells/103 μm2 | %CD4 cells | No. CD4 cells/103 μm2 | %CD8 cells | No. CD8 cells/103 μm2 | |
|---|---|---|---|---|---|---|---|
| Normal | 93.0 | 50.1 | 0.79 | 32.4 | 0.40 | 33.6 | 0.47 |
| SIVmac239 | 80.9 | 54.5 | 0.95 | 39.5 | 0.56 | 33.9 | 0.54 |
| SIVmac239MER | 91.2 | 47.6 | 0.94 | 33.1 | 0.56 | 22.8 | 0.39 |
| SIVmac251 | 31.1* | 33.5* | 0.50† | 3.0* | 0.05* | 25.2 | 0.36 |
*P < 0.001 Kruskal-Wallis one-way analysis of variance on ranks with multiple comparisons versus control (normal) group (Dunn’s method, P < 0.05).
†P < 0.001 one-way analysis of variance with multiple comparisons versus control (normal) group (Tukey test, P < 0.05).
Although animals remained clinically normal, elevations in alkaline phosphatase and alanine aminotransferase were detected as early as 2 weeks after M. avium challenge. Histological examination of liver sections obtained during weeks 6, 8, and 10 revealed numerous microgranulomas. In striking contrast to SIVmac251-infected macaques and similar to immunologically normal animals, these microgranulomas contained no acid-fast bacilli (Figure 3) ▶ . One SIVmac239 (355-96) and one SIVmac239MER-infected (221-96) animal were followed long term and euthanized 304 and 410 days after M. avium challenge. Both animals died with opportunistic infections including Pneumocytis pneumonia and Cryptosporidial enteritis. Evaluation of tissues obtained at necropsy revealed complete resolution of granulomatous hepatitis and absence of acid-fast bacilli in all tissues examined.
Figure 3.
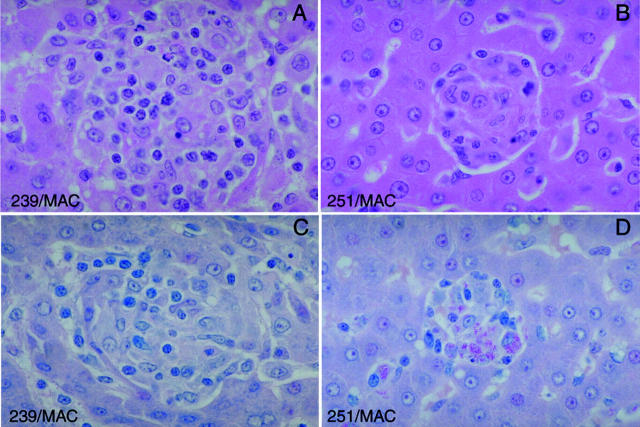
Comparison of microgranulomas from animals experimentally co-infected with SIVmac and M. avium (original magnification, ×140). Although animals co-infected with SIVmac239 or SIVmac251 and M. avium developed hepatic microgranulomas, only granulomas from SIVmac251-infected animals contained acid-fast bacilli. A: Rhesus macaque 65-97 infected with SIVmac239 followed by 10 8 cfu of M. avium was euthanized on day 45 and hepatic tissue collected. Typical microgranulomas were found scattered throughout the liver (liver, H&E). B: Rhesus macaque 51-97 infected with SIVmac251 followed by 10 8 cfu of M. avium was euthanized on day 45 and hepatic tissue collected. Typical microgranulomas were found scattered throughout the liver (H&E stain). In comparison to the SIVmac239-co-infected animals, granulomas were smaller in size and involved less of the hepatic parenchyma. C: Granulomas from rhesus macaque 65-97 co-infected with SIVmac239 and M. avium did not contain acid-fast bacilli (liver, Ziehl-Neelson acid-fast stain). D: Granulomas from rhesus macaque 51-97 co-infected with SIVmac251 and M. avium contained large numbers of acid-fast bacilli (liver, Ziehl-Neelson acid-fast stain).
Co-inoculation experiments revealed that all SIV-infected and immunologically normal animals developed disseminated disease as evidenced by the presence of widespread microgranulomas. However only animals infected with SIVmac251 developed progressive infection characterized by continued mycobacterial growth, severe clinical signs, and death. The ability of SIVmac239- and SIVmac239MER-infected animals to eliminate mycobacterial disease in a manner similar to non-SIV-infected controls seems to be independent of viral load and CD4 T-lymphocyte number present in blood, peripheral lymph node, and gastrointestinal tract as these did not differ among groups.
Failure to Recruit CD3-, CD4-, and CD8-Positive Lymphocytes Predicts Disease Progression
Microgranulomas are commonly found in hepatic tissue of macaques with AIDS and spontaneous disseminated MAC. To determine whether there were immunomorphological correlates of protection after experimental inoculation, immunohistochemistry was performed on liver sections obtained at 6 weeks after M. avium challenge to identify the immunophenotype of cells within newly formed microgranulomas. Quantitative image analysis revealed fundamental differences between microgranulomas from SIVmac251-infected animals compared to normal and SIVmac239 and SIVmac239MER-infected animals (Table 3) ▶ . Microgranulomas from SIVmac251-inoculated animals were significantly smaller than microgranulomas from immunologically normal and SIVmac239/SIVmac239MER-infected animals. Furthermore, histiocytes within such granuloma expressed decreased levels of the MHC II antigen compared to normal animals (127.2 versus 159.5 mean gray scale intensity/granuloma, P < 0.001 t-test) suggesting a lesser degree of macrophage activation.
The percentage of CD3-positive cells within granulomas and the number of CD3-positive lymphocytes/10 3 μm 2 within microgranulomas in SIVmac251-infected animals was significantly less than those recruited in normal and SIVmac239/SIVmac239MER-infected animals (Figure 4 ▶ and Table 3 ▶ ). Image analysis also confirmed a striking reduction in both the percent and density of CD4-positive cells within hepatic microgranulomas from SIVmac251-infected animals (Table 3) ▶ . This difference did not represent a global phenomenon as little difference was observed between groups in CD4 relative number in blood, peripheral lymph node, or gastrointestinal tract (Figure 1) ▶ . Rather, the decreased number of CD4 cells reflects a decreased recruitment of such cells to microgranulomas in animals with progressive M. avium disease.
Figure 4.
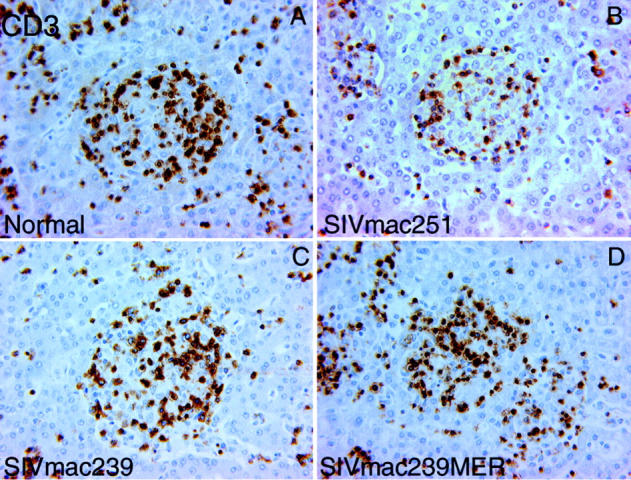
CD3-positive lymphocytes within hepatic microgranulomas from rhesus macaques 45 days after M. avium challenge (original magnification, ×140). The relative and absolute number of CD3-positive lymphocytes recruited to microgranulomas in SIVmac251-infected animals was significantly less than those recruited in normal and SIVmac239/SIVmac239MER-infected animals. A: Hepatic microgranuloma from normal rhesus macaque 109-96. B: Hepatic microgranuloma from SIVmac251-infected rhesus macaque 51-97. C: Hepatic microgranuloma from SIVmac239-infected rhesus macaque 65-97. D: Hepatic microgranuloma from SIVmac239MER-infected rhesus macaque 154-96.
CD8-positive lymphocytes constitute the principal cytotoxic T-cell population in defense against many intracellular pathogens. Although most experimental work has indicated a dominant role for CD4 T cells in protection against mycobacterial infection, murine studies have suggested a role for cytotoxic T lymphocytes as well. 21,43 There was no significant difference in the percent or density of CD8-positive cells within hepatic microgranulomas. Thus, microgranulomas differed in the composition of specific T-lymphocyte subsets and these differences correlated with the presence of acid-fast bacilli and the development of progressive disease.
Discussion
In this study, we describe an experimental system to co-inoculate rhesus macaques with M. avium and SIV to reproduce disseminated mycobacterial disease. We have used this model system to demonstrate the complex interactions that occur between host, mycobacteria, and immunosuppressive lentiviruses. We demonstrate that the development of progressive disease with continued mycobacterial growth is dependent on the strain of SIV. Specifically, co-infection with uncloned SIVmac251 resulted in progressive infection whereas co-infection with closely related T-cell tropic SIVmac239 or macrophage tropic SIVmac239MER did not. This occurred despite an absence of differences between the groups with respect to viral load or CD4 T-cell number but was correlated with significant differences in the composition of microgranulomas. These data suggest that viral determinants play a critical role in facilitating MAC infection through modulation of monocyte/macrophage effector function.
The clinical and morphological features of this process closely parallels that seen in humans and rhesus macaques with AIDS. SIVmac251-co-infected animals developed persistent diarrhea and progressive wasting. Physical examination revealed a febrile response, peripheral lymphadenopathy, and massive hepatosplenomegaly. At death mycobacterial infection was widespread with acid-fast bacilli found in multiple organs. Despite intravenous challenge tissue distribution was nearly identical to that found in spontaneous disease including significant involvement of the gastrointestinal tract. This suggests that the sites of MAC infection are determined by factors other than route of infection. Furthermore, histomorphology revealed typical histiocytic infiltrates and microgranulomas characteristic of disseminated MAC in AIDS.
In human patients and rhesus macaques, spontaneous disseminated MAC develops at severe CD4 T-lymphocyte depletion. In this model, animals were challenged intravenously with 10 8 cfu of M. avium 2 weeks after primary SIV infection and developed disseminated disease despite preservation of CD4 T lymphocytes in blood and peripheral lymph node. An alternative experimental approach would be to wait until CD4 T-lymphocyte count dropped below a critical level before mycobacterial challenge. We have reproduced disease in such severely immunodeficient animals (CD4 T lymphocytes <100 cells/dL) after oral and intravenous inoculation of M. avium (data not shown). Although this alternative approach will undoubtedly have potential applications, the long interval between primary SIV infection and the occurrence of a suitable level of immunodeficiency poses a serious drawback. Mean survival to AIDS in rhesus macaques infected with either SIVmac251 or SIVmac239 is 18 months. Furthermore, the occurrence of spontaneous opportunistic infections during the course of progressive immunodeficiency could confound interpretation of experimental mycobacterial inoculation. Our interests are focused on elucidating interactions between the virus, host, and mycobacteria that may promote disseminated disease. A 2-week time frame was chosen to coincide with the period of peak viremia and to decrease the degree of genetic variation that would occur in SIV with time and disease progression.
Granuloma formation likely represents a critical event in the orchestration of an effective host immune response to mycobacterial infection and is dependent on the recruitment of inflammatory cells and macrophage activation. In our studies morphological evaluation of hepatic microgranulomas revealed differences in granuloma composition that predicted progressive mycobacterial disease. In SIVmac251-infected animals, fewer T lymphocytes were recruited to microgranulomas primarily reflecting a decrease in the CD4-positive subtype. CD4 T lymphocytes have been shown to play a critical role in the elimination of intracellular mycobacteria through the elaboration of cytokines that result in macrophage activation and increased mycobacteriocidal activity. Decreased staining of microgranulomas for MHC-II supports the presence of ineffective macrophage activation in SIVmac251-infected animals.
Although variability is noted in the number of acid-fast bacilli and granuloma composition in spontaneous cases of disseminated MAC, this likely represents differences in disease stage. In contrast to experimental inoculation, granulomas in spontaneous cases do not resolve and are therefore fundamentally different. Although staining for acid-fast bacilli lacks the sensitivity of mycobacterial culture, complete resolution of granulomas in normal, SIVmac239-inoculated, and SIVmac239MER-inoculated animals indicates that these granulomas were effective in eliminating mycobacterial organisms.
Recent evidence has also suggested a role for CD8-positive cytotoxic T lymphocytes in host defenses against disseminated mycobacterial infection. 21,43 The preservation of CD4- and CD8-positive T lymphocytes in blood and peripheral lymph node and the absence of significant differences between SIVmac251- and SIVmac239-infected animals at these sites suggests that the decreased number of T lymphocytes within hepatic microgranulomas did not simply represent a global phenomenon. Rather, mycobacterial-infected macrophages in SIVmac251-inoculated animals failed to recruit an effective T-cell response.
The reason SIVmac251-infected animals are more susceptible to spontaneous M. avium disease compared to SIVmac239-infected animals is unknown. 23 This conclusion was based on a retrospective analysis of animals with AIDS and was independent of CD4 count at death. In this report we reproduce this phenomenon through inoculation of animals with M. avium during the acute phase of SIV infection. During this period severe depletion of CD4 T cells does not occur and opportunistic infections are not seen. We suspect that viral determinants present within the SIVmac251 wild-type inoculum are absent from the SIVmac239 molecular clone and that these differences promote pathogen survival through adverse effects on the host’s ability to eliminate mycobacteria. The in vitro and in vivo characteristics of SIVmac251 and SIVmac239 have been described extensively. 33 SIVmac251 represents the first SIV wild-type isolate and replicates and causes cytopathology in lymphocytes and macrophages. SIVmac239 was the first pathogenic molecular clone and was derived from a SIVmac251-inoculated animal. This virus grows to high titer in lymphocytes but decreased utilization of the CD4 receptor present at low density in tissue macrophages restricts its ability to replicate in cells of monocyte/macrophage lineage. 35,44 Genetic differences between the consensus sequence of SIVmac251 and the SIVmac239 molecular clone are primarily concentrated within the envelope region; overall they share 98% nucleotide identity. 34 Although the in vitro tropism of SIVmac239 and SIVmac251 differ significantly, the clinical disease course with these two viruses is nearly identical with no significant difference in mean survival and CD4 T-lymphocyte count at death. 23,45 With the exception of disseminated MAC the incidence of other spontaneously occurring opportunistic infections including Pneumocystis carnii, cytomegalovirus, Cryptosporidium parvum, Enterocytozoon bieneusi, Adenovirus, simian virus 40, and lymphocryptovirus is similar. 46
It is tempting to speculate that in comparison to SIVmac239 the ability of SIVmac251 to replicate in macrophages may have been responsible for its association with mycobacterial disease. However, macrophage tropic variants arise frequently in SIVmac239-infected animals 29 and SIVmac239 infects macrophages in vivo within 2 to 3 weeks of primary inoculation. 39,42 Furthermore, we could find no evidence that the macrophage tropic clone, SIVmac239MER, altered disease course from that seen in the parental strain. SIVmac239MER is a variant of SIVmac239 containing three amino acid differences in the envelope protein that confer the ability to enter and replicate within macrophages presumably through changes in CD4-receptor affinity. Therefore it is unlikely that macrophage tropism in-and-of-itself can account for the differences in mycobacterial disease pathogenesis observed between SIVmac251- and SIVmac239-infected animals.
In vitro evidence suggests direct and indirect growth-promoting effects of viral proteins on MAC organisms. 3-5,8-10 At physiological concentrations recombinant gp120 has been shown to enhance growth of M. avium within human monocytes and macrophages. 5,10 Recombinant gp120 induces phenotypic changes in human monocytes including decreased expression of HLA-DR, CD64, and interferon-γ-receptor 1 and causes alterations in tumor necrosis factor production that may adversely impact the ability of macrophages to eliminate M. avium. 47 Impaired phagolysomal fusion has been identified in peripheral blood monocytes from HIV-1-infected patients, a finding that could be reproduced by the addition of recombinant gp 120 to cells from normal individuals. 48 The in vivo relevance of these findings have been difficult to ascertain. We suspect that in addition to the destructive effect of HIV on CD4 T lymphocytes, that viral determinants may play a critical role in facilitating infection by MAC through modulating monocyte/macrophage effector function. Such interactions are likely to be most relevant during the late stages of HIV infection when CD4 T lymphocytes are depleted and can no longer orchestrate an effective immune response. The rhesus macaque model of disseminated M. avium provides the opportunity to investigate these in vitro observations and evaluate the in vivo consequences of differences in viral determinants on mycobacterial disease.
Footnotes
Address reprint requests to Keith G. Mansfield, Harvard Medical School, New England Regional Primate Research Center, PO Box 9102, Southborough, MA 01772-9012. E-mail: keith_mansfield@hms.harvard.edu.
Supported by Public Health Service grants RR 00168, DK50550, DK55510, and AI41889. A. Lackner is the recipient of an Elizabeth Glaser Scientist Award.
References
- 1.Inderlied CB, Kemper CA, Bermudez LE: The Mycobacterium avium complex. Clin Microbiol Rev 1993, 6:266-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horsburgh CR: Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med 1991, 324:1332-1338 [DOI] [PubMed] [Google Scholar]
- 3.Ghassemi M, Andersen BR, Reddy VM, Gangadharam PRJ, Spear GT, Novak RM: Human immunodeficiency virus and Mycobacterium avium complex coinfection of monocytoid cells results in reciprocal enhancement of multiplication. J Infect Dis 1995, 171:68-73 [DOI] [PubMed] [Google Scholar]
- 4.Denis M: Tat protein from HIV-1 binds to Mycobacterium avium via a bacterial integrin. J Immunol 1994, 153:2072-2081 [PubMed] [Google Scholar]
- 5.Shiratsuchi H, Johnson JL, Toossi Z, Ellner JJ: Modulation of the effector function of human monocytes for Mycobacterium avium by HIV-1 envelope glycoprotein gp120. J Clin Invest 1994, 93:885-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallis RS, Ellner JJ, Shiratsuchi H: Macrophages, mycobacteria and HIV: the role of cytokines in determining mycobacterial virulence and regulating viral replication. Forum Microbiol 1994, 8:398-405 [DOI] [PubMed] [Google Scholar]
- 7.Gibellini D, Zauli G, Re ME, Milani D, Furlini G, Caramelli E, Capitini S, La Placa M: Recombinant human immunodeficiency virus type 1 (HIV-1) Tat protein sequentially up-regulates IL-6 and TGF mRNA expression and protein synthesis in peripheral blood monocytes. Br J Haematol 1994, 88:261-267 [DOI] [PubMed] [Google Scholar]
- 8.Kallenius G, Kovula T, Rydgard KJ: Human immunodeficiency virus type 1 enhances intracellular growth of Mycobacterium avium in human macrophages. Infect Immun 1995, 60:2453-2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis M, Ghadirian E: Interaction between Mycobacterium avium and human immunodeficiency virus type 1 (HIV-1) in bronchoalveolar macrophages of normal and HIV-1-infected subjects. Am J Respir Cell Mol Biol 1994, 11:487-495 [DOI] [PubMed] [Google Scholar]
- 10.Denis M: Envelope glycoprotein (gp120) from HIV-1 enhances Mycobacterium avium growth in human bronchoalveolar macrophages; an effect mediated by enhanced prostaglandin synthesis. Clin Exp Immunol 1994, 98:123-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal S, Gros P, Skamene E: Natural resistance to infection with intracellular parasites: molecular genetics identifies nRAMP1 as the BCG/ITY/LSH locus. J Leukoc Biol 1995, 58:382-390 [DOI] [PubMed] [Google Scholar]
- 12.Blackwell JM, Barton CH, White JK, Searle S, Baker A, Williams H, Shaw M: Genomic organization and sequence of the human Nramp gene: identification and mapping of promoter region polymorphism. Mol Med 1995, 1:194-205 [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal S, Malo D, Vogan K, Skamene E, Gros P: Natural resistance to infection with intracellular parasites: isolation of candidate for Bcg. Cell 1993, 73:469-485 [DOI] [PubMed] [Google Scholar]
- 14.Benini J, Ehlers EM, Ehlers S: Different types of pulmonary granuloma necrosis in immunocompetent vs. TNFRp55-gene-deficient mice aerogenically infected with highly virulent Mycobacterium avium. J Pathol 1999, 189:127-137 [DOI] [PubMed] [Google Scholar]
- 15.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel ET, Pfeffer K: Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun 1999, 67:3571-3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers S, Kutsch S, Ehlers EM, Benini J, Pfeffer K: Lethal granuloma disintegration in mycobacteria-infected TNFRp55−/− mice is dependent on T cells and IL-12. J Immunol 2000, 165:483-492 [DOI] [PubMed] [Google Scholar]
- 17.Tanaka S, Itohara S, Sato M, Taniguchi T, Yokomizo Y: Reduced formation of granulomata in gamma(delta) T cell knockout BALB/c mice inoculated with Mycobacterium avium subsp. paratuberculosis. Vet Pathol 2000, 37:415-421 [DOI] [PubMed] [Google Scholar]
- 18.Fratazzi C, Manjunath N, Arbeit RD, Carini C, Gerken TA, Ardman B, Remold-O’Donnell E, Remold HG: A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of CD43. J Exp Med 2000, 192:183-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bermudez LE, Petrofsky M: Host defense against Mycobacterium avium does not have an absolute requirement for major histocompatibility complex class I-restricted T cells. Infect Immun 1999, 67:3108-3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannering SI, Zhan Y, Gilbertson B, Lieschke GJ, Cheers C: T lymphocytes from granulocyte colony-stimulating factor−/− mice produce large quantities of interferon-gamma in a chronic infection model. Immunology 2000, 101:132-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR: Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 1992, 89:12013-12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Lafuse WP, Zwilling BS: Regulation of toll-like receptor 2 expression by macrophages following mycobacterium avium infection. J Immunol 2000, 165:6308-6313 [DOI] [PubMed] [Google Scholar]
- 23.Mansfield KG, Pauley D, Young HL, Lackner AA: Mycobacterium avium complex in macaques with AIDS is associated with a specific strain of simian immunodeficiency virus and prolonged survival after primary infection. J Infect Dis 1995, 172:1149-1152 [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Mansfield KG, Lackner AA, Haase AT: Quantitative image analysis of simian immunodeficiency virus replication in macrophages coinfected with Mycobacterium avium complex. J Infect Dis 2000, 181:867-871 [DOI] [PubMed] [Google Scholar]
- 25.Mansfield KG, Lackner AA: Simian immunodeficiency virus-inoculated macaques acquire Mycobacterium avium from potable water during AIDS. J Infect Dis 1997, 175:184-187 [DOI] [PubMed] [Google Scholar]
- 26.Baskin GB, Murphey-Corb M, Watson EA, Martin LN: Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol 1988, 25:456-467 [DOI] [PubMed] [Google Scholar]
- 27.Veazey RS, Tham IC, Mansfield KG, Demaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA: Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol 2000, 74:57-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW: Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science 1985, 230:71-73 [DOI] [PubMed] [Google Scholar]
- 29.Desrosiers RC, Hansen-Moosa A, Mori K, Bouvier DP, King NW, Daniel MD, Ringler DJ: Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol 1991, 139:29-35 [PMC free article] [PubMed] [Google Scholar]
- 30.Kestler HW, Li Y, Naidu YM, Butler CV, Ochs MF, Jaenel G, King NW, Daniel MD, Desrosiers RC: Comparison of simian immunodeficiency isolates. Nature 1988, 331:619-621 [DOI] [PubMed] [Google Scholar]
- 31.Kirchhoff F, Pohlmann S, Hamacher M, Means RE, Kraus T, Uberla K, Di Marzio P: Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol 1997, 71:6509-6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers RC: Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 1990, 248:1109-1112 [DOI] [PubMed] [Google Scholar]
- 33.Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD: Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 1985, 228:1201-1204 [DOI] [PubMed] [Google Scholar]
- 34.Regier DA, Desrosiers RC: The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses 1990, 6:1221-1231 [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Rosenzwajg M, Desrosiers RC: Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol 2000, 74:10852-10859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori K, Ringler DJ, Desrosiers RC: Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol 1993, 67:2807-2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori K, Ringler DJ, Kodoma T, Desrosiers RC: Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol 1991, 66:2067-2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake TA, Hindler JA, Berlin OGW, Brukner DA: Rapid identification of Mycobacterium avium complex in culture by using DNA probes. J Clin Microbiol 1987, 25:1442-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veazey RS, Demaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA: Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280:427-431 [DOI] [PubMed] [Google Scholar]
- 40.Reimann KA, Waite BCD, Lee-Parritz DE, Lin W, Uchanska-Ziegler B, O’Connell MJ, Letvin N: Use of human leukocyte-specific monoclonal antibodies for clinically immunophenotyping lymphocytes of rhesus monkeys. Cytometry 1994, 17:102-108 [DOI] [PubMed] [Google Scholar]
- 41.Horvath CJ, Hunt RD, Simon MA, Sehgal PK, Ringler DJ: An immunohistologic study of granulomatous inflammation in SIV-infected rhesus monkeys. J Leukoc Biol 1993, 53:532-540 [DOI] [PubMed] [Google Scholar]
- 42.Wykrzykowska JJ, Rosenzweig M, Veazey RS, Simon MA, Halvorsen K, Desrosiers RC, Johnson RP, Lackner AA: Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J Exp Med 1998, 187:1767-1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith SM, Brookes R, Klein MR, Malin AS, Lukey PT, King AS, Ogg GS, Hill AV, Dockrell HM: Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J Immunol 2000, 165:7088-7095 [DOI] [PubMed] [Google Scholar]
- 44.Bannert N, Schenten D, Craig S, Sodroski J: The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophage tropic human immunodeficiency viruses. J Virol 2000, 74:10984-10993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westmoreland SV, Halpern E, Lackner AA: Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol 1998, 4:260-268 [DOI] [PubMed] [Google Scholar]
- 46.Mansfield KG, Carville A, Shvetz D, MacKey J, Tzipori S, Lackner AA: Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian immunodeficiency virus inoculated macaques with hepatobiliary disease. Am J Pathol 1997, 150:1395-1405 [PMC free article] [PubMed] [Google Scholar]
- 47.Tyring SK, Cauda R, Tumbarello M, Ortona L, Kennedy RC, Chanh TC, Kanda P: Synthetic peptides corresponding to sequences in HIV envelope gp41 and gp120 enhance in vitro production of interleukin-1 and tumor necrosis factor but depress production of interferon-alpha, interferon-gamma and interleukin-2. Viral Immunol 1991, 4:33-42 [DOI] [PubMed] [Google Scholar]
- 48.Pittis MG, Sternik G, Sen L, Diez RA, Planes N, Pirola D, Estevez ME: Impaired phagosomal lysosomal fusion of peripheral blood monocytes from HIV-infected subjects. Scand J Immunol 1993, 38:423-427 [DOI] [PubMed] [Google Scholar]