Abstract
CEACAM1 is a cell adhesion molecule that has been implicated in a number of physiological processes (eg, tumor suppressor in epithelial tissues, potent angiogenic factor in microvessel formation, microbial receptor in human granulocytes and epithelial cells). The mechanism of CEACAM1 action is still largely unresolved but recent findings demonstrated that the cytoplasmic CEACAM1 domain is linked indirectly to the actin-based cytoskeleton. We have isolated integrin β3 as an associated protein using CEACAM1 tail affinity purification. This association depends on phosphorylation of Tyr-488 in the CEACAM1 cytoplasmic domain. Confocal laser scanning microscopy confirmed in vivo colocalization of both molecules in human granulocytes and epithelial cells. Furthermore, the concentrated colocalization at the tumor-stroma interface of invading melanoma masses suggests a functional role of CEACAM1-integrin β3 interaction in melanoma invasion. Moreover, colocalization of the two adhesion molecules is also found at the apical surface of glandular cells of pregnancy endometrium. Colocalization of CEACAM1 and integrin β3 at the transitional zone from proliferative to invasive extravillous trophoblast of the maternal-fetal interface supports a role for CEACAM1/integrin β3 complexes in cell invasion.
Formerly known as biliary glycoprotein and recently designated as CEACAM1, 1 the human homologue of Cell-CAM is an established cell adhesion molecule in the rat. 2,3 This cell-surface immunoglobulin-like glycoprotein belongs to the carcinoembryonic antigen (CEA) family and is expressed in a wide range of human tissues such as liver, endometrium, mammary ducts as well as epithelial cells of the gastrointestinal tract, endothelial cells, and hemopoietic cells. 4
Like several other cell adhesion molecules, CEACAM1 also functions as a microbial receptor. Thus, the mouse hepatitis virus utilizes mouse CEACAM1 (Ceacam1) as its receptor 5 and in human granulocytes and epithelial cells CEACAM1 is a receptor for bacterial proteins from Escherichia coli, Salmonella typhimurium, Neisseria gonorrhoeae, and Haemophilus influenzae. 6-10 A number of physiological functions have been ascribed to CEACAM1 in different tissues. In endothelial cells, CEACAM1 exhibits properties of an angiogenic factor and acts as a major effector of vascular endothelial growth factor (VEGF). 11 In epithelial cells it is generally believed, that CEACAM1 acts as a growth suppressor, since the expression of CEACAM1 was shown to be lost or significantly down- or dysregulated in carcinomas of the liver, 12,13 prostate, 14 endometrium, 15 breast, 16 and colon. 17-19 Consistent with this notion it has been shown that CEACAM1 acts as a negative regulator of tumor cell growth in human prostate 20 and breast carcinoma models. 21 Furthermore, CEACAM1 is strongly expressed by the extravillous trophoblast during the invasion of the maternal endometrium, and might, as a tumor suppressor, be involved in the molecular mechanisms controlling this process and for differentiating them from those implicated in tumor progression. 22
The growth-suppressive effect of CEACAM1 depends on the presence of its cytoplasmic domain. Although the mechanism of action is largely unresolved, several reports suggest that CEACAM1 participates in signal transduction by interacting with other membrane or cytoplasmic proteins via its cytoplasmic domain. The cytoplasmic domain (CEACAM1cyt) is phosphorylated by multiple protein kinases 23 and the phosphorylation on one or both of its two tyrosine residues (Tyr-488 and Tyr-515) is triggered by several physiological events. 24 One or both of these tyrosine-phosphorylated residues are involved in the association with protein kinases of the Src family, 25 paxillin, 26 the protein-tyrosine phosphatases SHP-1 27 and SHP-2. 28
In the present study, we used differentially phosphorylated recombinant expressed CEACAM cytoplasmic domains and mutants to isolate integrin β3 as an associated protein with the tyrosine phosphorylated cytoplasmic CEACAM1 domain. In vitro, this association depends on the phosphorylation of Tyr-488 in CEACAM1cyt. Using coprecipitation studies and laser scanning confocal microscopy analyses, we demonstrate the association of both molecules in granulocytes and the colonic carcinoma cell line HT29. Furthermore, our findings of CEACAM1/integrin β3 co-expression and colocalization at the maternal-fetal interface during placental development plus at the invading front of primary melanoma suggest a functional role of CEACAM1-integrin β3 association in cell migration.
Materials and Methods
Purification of Antigens on Ni-NTA Immobilized CEACAM1 and CEACAM3 Domains
Cloning of the cDNA coding for the wild-type cytoplasmic domain of CEACAM1 and CEACAM3 and generation of the mutant domains CEACAM1cytY488F, CEACAM1cytY515Fwas performed as described. 25,26
Protein kinases PKC, CK II (Biomol, Hamburg, Germany), and Src (Upstate Biotech, Lake Placid, NY) were diluted in kinase dilution buffer according to manufacturers’ instructions and added to 500 μg of purified cytoplasmic CEACAM1 and CEACAM3 domains in kinase assay buffer. For radioactive labeling 50 μCi [γ-32P]ATP (30 Ci/mmol; 1 Ci = 37 GBq) was added. After incubation at 30°C for 1 hour, phosphotyrosyl proteins were purified using the PY Immunoaffinity system (Oncogene Science, Manhasset, NY).
The purified phosphorylated or unphosphorylated CEACAM1 and CEACAM3 domains were adjusted to 250 μg/ml with a 50% slurry of Ni-nitrilotriacetic acid (NTA) resin (Qiagen, Hilden, Germany). After incubation for 2 hours at 4°C 2 ml of this suspension were transferred into a column and washed three times in 1 ml of wash buffer (300 mmol/L NaCl, 50 mmol/L Na-phosphate, pH 7.5). Granulocytes were isolated from buffy coat of normal donors by Ficoll-Paque gradient centrifugation (d = 1.119). Freshly isolated granulocytes (5.5 × 1020) were extracted with 1% Nonidet P-40 diluted in PBS containing proteinase inhibitors. After centrifugation (10,000 × g, 30 minutes) 1 ml of the Nonidet P-40-soluble supernatant containing 1.25 g/L protein was passed over the Ni-NTA immobilized CEACAM1 and CEACAM3 domains. The column was washed with 0.3 mol/L NaCl containing 0.05 mol/L phosphate (pH 6.0) and eluted using 0.3 mol/L NaCl, 0.05 mol/L phosphate (pH 3,0). SDS-PAGE, silver staining, and Western blots were performed as described. 29
Microsequencing of Proteins
Bound proteins from granulocyte extracts were separated by SDS-PAGE and transferred to PVDF membrane. Protein bands were visualized by Serva blue R staining and excised. N-terminal sequences were determined by automated Edman degradation (Richter AG, Hamburg, Germany). Sequence library searches were performed against SWISS-PROT and PIR databases.
Co-Immunoprecipitation
Extracts from cells containing 250 μg of protein were incubated with approximately 5 μg of monoclonal antibody for 1 hour at 4°C. Subsequently, Protein G PLUS/Protein A-agarose (50 μl) was added. After incubation on a rocker platform at 4°C for 24 hours, the precipitates were washed four times with antibody (Ab)-wash buffer (1% Triton X-100, 10 mmol/L Tris-HCl pH 7.6, 5 mmol/L EDTA, 50 mmol/L NaCl, 30 mmol/L sodium pyrophosphate, 50 mmol/L sodium fluoride, 100 μmol/L sodium orthovanadate, 0.1% azide). Precipitated proteins were boiled in sample buffer, separated by SDS-PAGE electrophoresis and visualized by immunoblotting.
In Vitro Complex Formation Studies
Extracts from cells containing 250 μg of protein precleared by rotating at 4°C with 30 μl of a 50% slurry of Protein G PLUS/Protein A-agarose (Dianova, Hamburg, Germany) for 30 minutes and beads were removed by centrifugation (5000 rpm). Supernatants were incubated with approximately 5 μg of human integrin β3 mAb (Chemicon International Inc., Temecula, CA) for 1 hour at 4°C. Subsequently, Protein G PLUS/Protein A-agarose (50 μl) and radioactively labeled CEACAM domains adjusted to 50 μg/ml were added. After incubation on a rocker platform at 4°C for 2 hours, the precipitates were washed four times with antibody (Ab)-wash buffer. Precipitated proteins were boiled in sample buffer, separated by SDS-PAGE electrophoresis and visualized by autoradiography.
Cell Culture
Cells of the colonic carcinoma cell line HT29-D4 were grown in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum for 6 days. After washing (PBS) cells were lysed in Triton X-100 lysis buffer (50 mmol/L Hepes pH 7,5, 150 mmol/L NaCl, 1% Triton-X-100, 2% aprotinin, 2 mmol/L EDTA, 50 mmol/L NaF, 10 mmol/L Na-pyruvate, 10% glycerine, 1 mmol/L Na-orthovanadate, 1 mmol/L PMSF). Lysis was carried out on ice for 30 minutes followed by clarification in a microfuge (13,000 rpm for 30 minutes at 4°C). Endometrial epithelial cells were prepared from endometrium of cycling women undergoing hysterectomies for leiomyomas. Briefly, endometrial tissue was minced thoroughly and digested in DMEM with 0.5 mg/ml collagenase-dispase (Boehringer-Mannheim, Mannheim, Germany) and 2.5 mg/ml desoxyribonuclease (Sigma, Deisenhofen, Germany) for up to 2 hours, with gentle pipetting every 20–30 minutes. The suspension was filtered through a nylon stocking to remove nondispersed tissue fragments. Cells were harvested by centrifugation at 400 × g for 6 minutes, and resuspended in plating media, a 1:1 mixture of DMEM and HF-12 containing 10% FCSDCC (FCS depleted of steroids by treatment with dextran-coated charcoal), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 μg/ml insulin (Sigma), and 10−9 mol/L 17β-estradiol (E2; Sigma). Stromal and epithelial cells were separated by sieving the cell suspension through a steal sieve (38 μm pore size). The retentate containing the epithelial glands was washed out by inverting the sieve and rinsing it with plating media. Endometrial stromal cells were collected in the filtrate. After centrifugation at 400 g for 6 minutes, epithelial glands were plated in six-well plates. Medium was changed every 48–72 hours.
Tissue Collection
For confocal laser scanning microscopy tissue material, routinely fixed in 4% buffered formalin and embedded in paraffin, was selected after histological review from the files of the Department of Gynecopathology and the Department of Neuroanatomy, University Hospital Eppendorf, Hamburg. For the present study three CEACAM1 positive primary melanomas along with three first trimester placentas were investigated.
Confocal Laser Scanning Microscopy
Processing of cells for CLSM was performed as described. 26 From the tissues material used serial sections of 4 to 6 μm were cut from the paraffin blocks and mounted on 3-aminopropyl-triethoxysilan (APES)-coated slides, deparaffinized in Rotihistol, and rehydrated in graded alcohol to PBS-buffered saline. The slides were microwaved five times for 2 minutes in 10 mmol/L citrate pH 6.0. After cooling down for 20 minutes, the slides were washed three times in PBS for 5 minutes. Unspecific binding was blocked by incubating the slides in 20% FCS/3% BSA in PBS for 30 minutes and the first antibody −4D1/C2 against CEACAM1 was added in adjusted dilution (1:25). After incubation overnight slides were washed three times in PBS and incubated with goat anti-mouse IgG (H+L, GaM Dianova) TRITC (1:50). After washing three times for 5 minutes with PBS the samples were further processed by blocking any remaining epitopes on the mouse Fc fragment with a goat anti-mouse IgG (H+L, Dianova) F(ab′) fragment for 30 minutes. After three washing steps with PBS the second primary antibody (anti-integrin β3 of DAKO, Glostrup, Denmark) was added, incubated for 60 minutes at room temperature.
Integrin signals were detected by a second FITC-conjugated antibody (goat anti-mouse IgG H+L, FITC labeled, Dianova). To rule out any cross-reactions between the two primary antibodies we performed immunostaining even with an anti-integrin β3-antibody (Chemicon International) raised in rabbits. In this case, the integrin β3-signals were detected using a sheep-anti-rabbit (Fc)-FITC secondary antibody (Dianova). Since we obtained the same results with respect to colocalization even using this protocol as a specificity control, these data are not presented separately in the results section or as figures.
To avoid photobleaching of the fluorochrome during fluorescence microscopy, the slides were embedded in anti-fade solution (BiomedDia, Zweibrücken, Germany). The double-fluorescent stained specimens were analyzed with a confocal laser scanning microscope equipped with an external argon laser (Zeiss Invers 410, Zeiss, Oberkochen, Germany). The cells were x/y scanned in the reflecting mode with a simultaneous excitation wavelength of 488 and 543 nm. Using the confocal mode, the pinhole was closed to approximately 25. To visualize the distribution of the fluorescence signals the specimens were monitored using the extended depth of focus mode (EDF).
Antibodies and Other Reagents
Details on the production and characterization of the monoclonal CEACAM1 4D1/C2 have been published. 30,31 The CEACAM mAb 12-140-4 was a kind gift from Ole P. Børmer (Norwegian Radium Hospital, Oslo, Norway). Monoclonal antibodies to human integrin β3 (Chemicon International and DAKO) were purchased and used according to the manufacturers’ specifications.
Results
The Tyrosine Phosphorylated Cytoplasmic Domain of CEACAM1 Binds Integrin β3 from Granulocyte Extracts
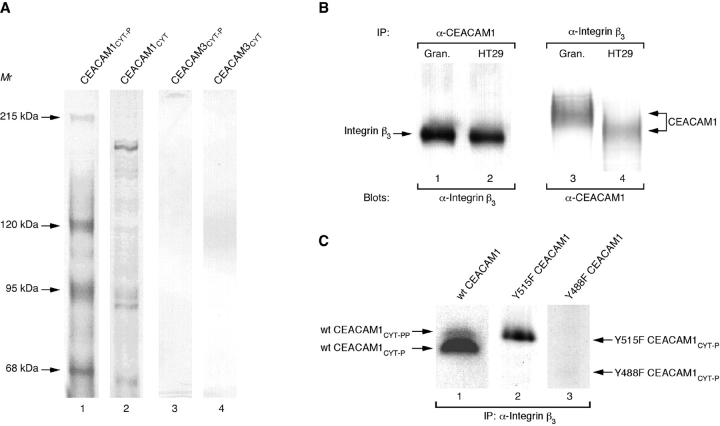
As shown in Figure 1A ▶ , lane 1, a defined set of proteins was purified using the in vitro tyrosine phosphorylated cytoplasmic CEACAM1 domain. These bands were not detectable in the control lanes using unphosphorylated domain of CEACAM1 (Figure 1A ▶ , lane 2), the tyrosine phosphorylated (Figure 1A ▶ , lane 3) or unphosphorylated (Figure 1A ▶ , lane 4) cytoplasmic domain of CEACAM3. Here we report on the band of approximately 95 kd, which was identified unanimously as the human integrin β3 subunit by N-terminal sequencing. In Western blots performed with the eluted antigens, a monoclonal anti-integrin β3 antibody detected the 95 kd protein only in the tyrosine phosphorylated CEACAM1cyt eluate (not shown). In conclusion, the purification of integrin β3 was observed with the tyrosine phosphorylated cytoplasmic domain of CEACAM1 only.
Figure 1.
Purification of CEACAM1cyt -associated proteins from granulocyte extracts. A: SDS-PAGE and silver staining of eluates from immobilized CEACAM domains. Extracts from granulocytes were subjected to purification on adjusted amounts of immobilized in vitro tyrosine phosphorylated CEACAM1cyt (lane 1), unphosphorylated CEACAM1cyt (lane 2), in vitro tyrosine phosphorylated CEACAM3cyt (lane 3), and unphosphorylated CEACAM3cyt (lane 4). Positions of the purified proteins mentioned in the text and apparent molecular masses are indicated on the left (in kilodaltons). B: Coprecipitation of integrin β3 and CEACAM1. Extracts from granulocytes (lanes 1 and 3) and HT29 cells (lanes 2 and 4) were subjected to immunoprecipitation with CEACAM1 mAb 12-140-4 (lanes 1 and 2) or anti-integrin β3 mAb (lanes 3 and 4). Bound proteins were resolved by 7.5% SDS-polyacrylamide gel electrophoresis and immunoblotted with anti-integrin β3 mAb (lanes 1 and 2) or anti-CEACAM1 mAb 4D1/C2 (lanes 3 and 4). The positions of integrin β3 and CEACAM1 are indicated on the left or right. C: Coprecipitation of tyrosine phosphorylated wild-type and mutant CEACAM1cyt with anti-integrin β3 mAb. Following in vitro tyrosinephosphorylation, the radioactively labeled phosphoproteins (wild-type CEACAM1cyt (lane 1), Y515F CEACAM1cyt (lane 2), and Y488F CEACAM1cyt (lane 3)) and Protein G PLUS/Protein A-agarose were incubated with precleared granulocyte extracts containing integrin β3 mAb at 4°C for 2 hours. After immunoprecipitation, precipitates were resolved with 17.5% SDS-PAGE followed by autoradiography. Positions of labeled phosphoproteins areindicated on the left or right.
Coprecipitation of Integrin β3 and CEACAM1 from Granulocytes and HT29 Cells
To further investigate an association of CEACAM1 with integrin β3, co-immunoprecipitation experiments were performed from membrane extracts of granulocytes. Extracts were prepared and incubated with monoclonal antibodies against integrin β3 or CEACAM1 or polyclonal mouse IgG (control). Immunoprecipitated complexes were analyzed by immunoblotting with the monoclonal antibodies against either integrin β3 or CEACAM1. Complexes containing CEACAM1 and integrin β3 could be immunoprecipitated with both anti-CEACAM1 (Figure 1B ▶ , lane 1) and anti-integrin β3 antibodies (Figure 1B ▶ , lane 3). Furthermore, co-immunoprecipitation of CEACAM1-integrin β3 complexes was also observed in the epithelial colonic carcinoma cell line HT29 (Figure 1B ▶ , lanes 2 and 4). Differences of the apparent molecular weight of CEACAM1 in different cell types are established and probably due to glycosylation-differences. 11,15,26,32 Complexes containing detectable CEACAM1 or integrin β3 could be not immunoprecipitated using polyclonal mouse IgG and vinculin as controls (not shown).
Association of CEACAM1 with Integrin β3 Requires the Phosphorylated CEACAM1 Tyr-488 Residue
The in vitro tyrosine phosphorylated wild-type cytoplasmic CEACAM1 domain is, after incubation with granulocyte extracts, co-immunoprecipitated with anti-integrin β3 mAb (Figure 1C ▶ , lane 1). We used different CEACAM1cyt mutant domains to determine whether phosphorylated tyrosine residues within CEACAM1cyt are required for binding to integrin β3. The (Y515F) mutation (CEACAM1cytY515F-P) did not interfere with the observed interaction (Figure 1C ▶ , lane 2), but the (Y488F) mutation (CEACAM1cytY488F-P) did almost completely abolish coprecipitation (Figure 1C ▶ , lane 3). The tyrosine phosphorylated CEACAM3 domain as well as serine/threonine phosphorylated wild-type and mutant (Y488F and Y515F) CEACAM1cyt did not co-immunoprecipitate with integrin β3 mAb (not shown).
Co-Expression and Colocalization of CEACAM1 with Integrin β3 on Granulocytes and HT29 Cells
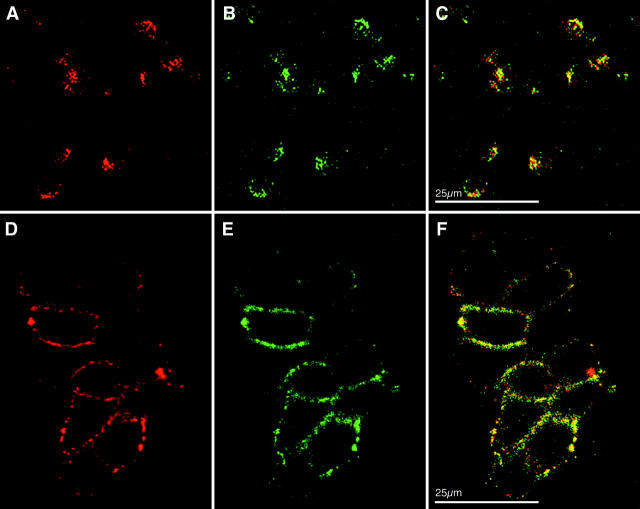
To substantiate an association of CEACAM1 and integrin β3 in vivo, we conducted double-fluorescent confocal laser scanning microscopy on human granulocytes. First, the cellular distribution and the relative intensity of fluorescence on the cells were assessed qualitatively. Immunofluorescence of TRITC localizing CEACAM1 (Figure 2A ▶ , red) and of FITC localizing anti-integrin β3 mAb (Figure 2B ▶ , green) revealed a strong membrane-associated granular pattern for each of the molecules. Using double-fluorescent labeling colocalization of CEACAM1 and integrin β3 in the same cells was indicated by yellow fluorescence (Figure 2C) ▶ .
Figure 2.
Co-expression and colocalization of CEACAM1 and integrin β3 on granulocytes and HT29 cells. On granulocytes (A–C) and cells of the colonic carcinoma cell line HT29 (D–F), immunofluorescence of TRITC, localizing CEACAM1 (A and D, red), and FITC, localizing integrin β3 (B and E, green), was examined. Double-fluorescent staining (C and F) revealed colocalization indicated by yellow color.
In addition to granulocytes, immunoreactivity of 4D1/C2 (Figure 2D) ▶ and integrin β3 mAb (Figure 2E) ▶ were localized on cells of the colonic carcinoma cell line HT29. Double-fluorescent labeling correspondingly displayed a close association of CEACAM1 with integrin β3 in HT29 cells (Figure 2F) ▶ .
Co-Expression and Colocalization of CEACAM1 with Integrin β3 in Primary Melanomas
Since integrin β3 has a well established role in the invasion and metastasis of malignant melanomas 33,34 and since CEACAM1 expression is clinically positively correlated with metastasis of melanomas (P < 0.0001; U. Schumacher, submitted), we investigated primary melanomas for CEACAM1/integrin β3 expression. Representative results of CEACAM1 and integrin β3 expression in CEACAM1 positive primary melanomas are displayed Figure 3 ▶ . Whereas CEACAM1/integrin β3 colocalization is heterogeneous in the center of primary melanoma (Figure 3A) ▶ . In contrast, horizontal (Figure 3B) ▶ and longitudinal (Figure 3, C–E) ▶ sections revealed concentrated co-expression and colocalization at the tumor-stroma interface of invading tumor masses. Interestingly the strongest co-expression and colocalization is detected at the invading front (Figure 3E) ▶ . Colocalization appears to be restrained by the spatially more restricted expression of CEACAM1 (Figure 3, C and E ▶ ; partial magnification in F). In contrast a different staining pattern of paxillin, with absent CEACAM1 colocalization at the invasion front, was observed (not shown).
Figure 3.
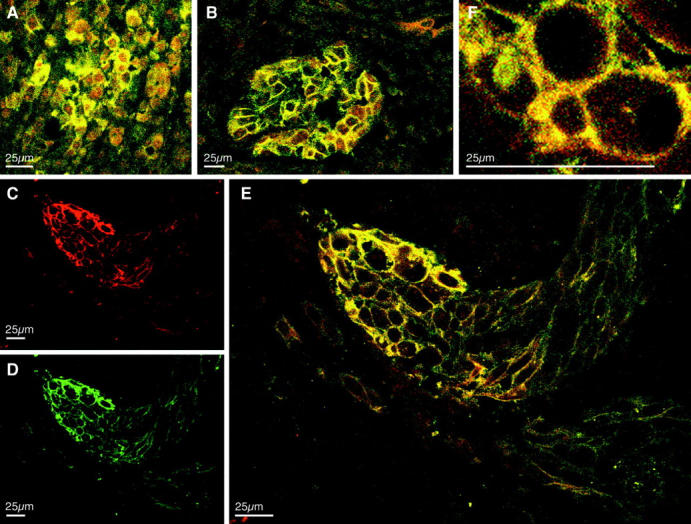
Co-expression and colocalization of CEACAM1 with integrin β3 in primary melanoma. Immunofluorescent staining in primary melanoma using the fluorochromes TRITC, localizing CEACAM1 mAb 4D1/C2 (C, red), and FITC, for the detection of anti-integrin β3 mAb (D, green). Simultaneous detection of integrin β3 and CEACAM1 reveals heterogeneous colocalization of both signals (yellow) in the center of primary melanoma (A) and concentrated colocalization at the invading front (B, E, and F).
Co-Expression and Colocalization of CEACAM1 with Integrin β3 in Endometrial and Placental Structures
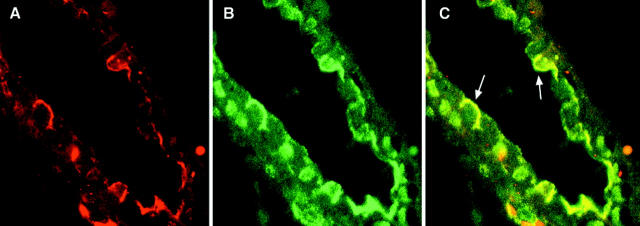
Western blot analysis with anti-CEACAM1 mAb 4D1/C2 and anti-integrin β3 mAb with protein extracts of isolated and cultured epithelial endometrial cells revealed expression of CEACAM1 only, with no detectable integrin β3 expression (not shown). To investigate the expression and colocalization of CEACAM1 and integrin β3 in endometrium of pregnancy, we conducted double-fluorescent confocal laser scanning microscopy. Double-fluorescent microscopy using the fluorochrome TRITC for localizing CEACAM1 (Figure 4A) ▶ and FITC labeling to follow integrin β3 expression (Figure 4B) ▶ revealed colocalization of CEACAM1 and integrin β3 (Figure 4C) ▶ at the apical poles of surface epithelial cells in endometrial glands in pregnancy, whereas stromal (decidual) cells were negative for CEACAM1 and integrin β3 (not shown).
Figure 4.
Colocalization of CEACAM1 and integrin β3 in isolated endometrial glands. Immunofluorescence of TRITC (localizing CEACAM1 in A, red), and FITC (localizing anti-integrin β3 mAb in B, green) in isolated endometrial glands. Double-fluorescent staining is shown in C.
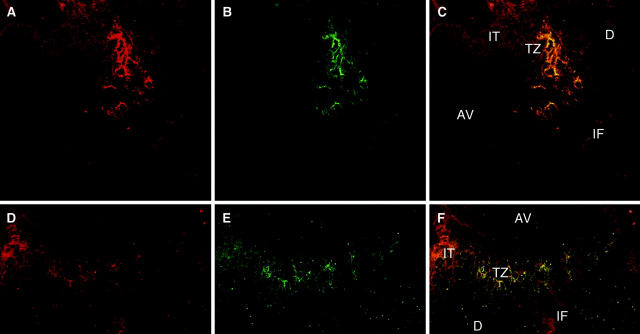
Since CEACAM1 is specifically expressed by the extravillous trophoblast in the human placenta 22 and since integrin β3 also has been shown to be expressed in the human endometrium during the so-called implantation window, 35,36 we investigated CEACAM1 and integrin β3 expression at the maternal-fetal interface. Representative results of double-fluorescent CLSM in two different implantation sites are displayed in Figure 5 ▶ . Since, in both implantation sites the β3-positive cells (Figure 5, B and E) ▶ are still in contact with each other it appears that integrin β3 is not expressed at the invasion front (IF), but rather at the transitional zone (TZ) from the proliferative to invasive extravillous trophoblast. In this transitional zone strong co-expression and colocalization of CEACAM1 and integrin β3 is observed, but in contrast to integrin β3 CEACAM1 (Figure 5, A and D) ▶ is expressed at the invasion front, where the cells are isolated from each other and at least partly in contact with decidual components, which they invade. At the invasion front, which can be seen at the bottom of the figures (IF, Figure 5, C and F ▶ ), cells are CEACAM1-positive, but integrin β3-negative.
Figure 5.
Co-expression and colocalization of CEACAM1 and integrin β3 at the maternal-fetal interface. Immunofluorescent staining in endometrium of first trimester pregnancy using TRITC (localizing CEACAM1 in A and D, red) and FITC (localizing anti-integrin β3 mAb in B and E, green). Simultaneous detection of integrin β3 and CEACAM1 (C and F) reveals colocalization of both signals at the transitional zone (TZ) from proliferative to invasive trophoblast, with no co-expression or colocalization in the anchoring villous (AV) or decidua (d). Note broader expression of CEACAM1 in the extravillous trophoblast (IT) and invasion front (IF).
Discussion
Accumulating data suggest that CEACAM1 participates in signal transduction, which involves interaction of the cytoplasmic CEACAM1 domain with other proteins. Alternative splicing gives rise to two forms of CEACAM1 cytoplasmic domains containing either 10 or 73 amino acids. The long cytoplasmic domain of CEACAM1 is essential for its function as a tumor suppressor 20,21,37,38 and contains two tyrosine residues. Binding of protein tyrosine kinases as well as protein tyrosine phosphatases to CEACAM1 depending on tyrosine phosphorylation of CEACAM1cyt has been shown. 24,25,28 In addition CEACAM1cyt associates with paxillin, 26 a key protein of focal adhesions. Recent reports link CEACAM1 to the actin-based cytoskeleton. 39,40 Another signal-regulating protein that binds to CEACAM1cyt is calmodulin. 41 This association is regulated by the intracellular calcium concentration and leads to down-regulation of CEACAM1 self-association. 42
CEACAM3, formerly known as carcinoembryonic gene member 1 or CD66d, is a cell-surface immunoglobulin-like glycoprotein of the carcinoembryonic antigen (CEA) gene family, which is exclusively expressed in the granulocytic lineage. 43 The cytoplasmic domain of CEACAM3 (CEACAM3cyt) is structurally homologous to CEACAM1 containing 71 amino acids including two tyrosine residues and multiple serine/threonine residues, which are phosphorylated by multiple protein kinases in vitro. 23 Using phosphorylated and unphosphorylated CEACAM3cyt as a specificity control, we have demonstrated a specific interaction between integrin β3 and a fusion protein containing the cytoplasmic domain of CEACAM1 cloned behind a poly-histadine tag. Since CEACAM1cyt-mutants in which Tyr-488 was replaced with Phe within the cytoplasmic domain were not coprecipitated with integrin β3, it appears that phosphorylation of Tyr-488 is required for the observed association.
Since we 23,25 and others 44,45 have shown that CEACAM1 is partially tyrosine phosphorylated in quiescent cells, we investigated CEACAM1-integrin β3 interaction in intact cells. Several lines of evidence allocate a relevant in vivo interaction. First, CEACAM1-integrin β3 complexes are coprecipitated from cell extracts of granulocytes and epithelial cells. Second, on cultured epithelial cells as well as on granulocytes CEACAM1 and integrin β3 are co-expressed and colocalized at the plasma membrane. Third, we demonstrated co-expression and colocalization of CEACAM1 and integrin β3 in primary melanomas. Here, we observed a predominant co-expression and colocalization of CEACAM1 and integrin β3 at the invasion front of the primary tumor. Finally, colocalization of the two molecules was also observed at the apical surface of endometrial glandular cells of endometrium in pregnancy and at the transitional zone from proliferative to invasive extravillous trophoblast of the maternal-fetal interface.
Integrins are αβ heterodimers and function in cell adhesion and signaling by interaction with extracellular matrix proteins or cellular counter receptors as well as with intracellular proteins. 46,47 Integrins may also form cis associations with other transmembrane receptors on the same cell, which may well modulate integrin affinity either by altering integrin conformation directly or indirectly via clustering of the associated integrin. 48 Several transmembrane proteins, which associate with integrins and modulate their function, have been described. 48 CEACAM1 has been reported as a ligand for E-selectin 49 and implicated in integrin affinity up-regulation 50 in granulocytes. However, this is the first report of an association of CEACAM1 with integrin β3. Although the exact molecular mapping of this interaction has to further elucidated, especially direct versus indirect binding eg, via an integrin β3 binding putative adapter protein, we speculate that the complex of integrin β3 and CEACAM1 acts as a functional package facilitating cell migration. For integrin β3 a promoting effect in cell migration during angiogenesis, 51 tumor invasion, 34,52 and invasion of the maternal uterus by fetal extravillous cytotrophoblast 35,36 is well documented.
Much less is known about a possible promoting role of CEACAM1 in cell migration or cell spreading. In epithelial tissues it was generally believed 53 that CEACAM1 contributes to contact inhibition of cell proliferation, and evidence in favor of this assumption was reported recently. 54 However, CEACAM1 is not only expressed in organized epithelial tissues but also found on migrating cells such as activated T cells 55 and endothelial cells. 4,56 In contrast to epithelial cells, CEACAM1 enhances proliferation in activated T cells 57 and endothelial cells, 11 indicating a specialized CEACAM1 function.
Colocalization of the two molecules was also observed at the apical surface of endometrial glandular cells of endometrium in pregnancy. In endometrial epithelial cells in primary culture, only expression of CEACAM1 was observed, while integrin β3 is not expressed. Thus, colocalization of the two molecules appears to be a feature of the endometrial glandular structure that is spatially as well as temporally tightly regulated. It has been shown that integrin β3 is only expressed in the glandular endometrial epithelial compartment during the so-called “implantation window” 35,36 and that this expression is delayed in infertile patients with discordant luteal phase biopsies. 58 For CEACAM1, expression had been previously described in endometrial epithelial cells in the rat uterus, where differential expression in the luminal and glandular epithelia as a function of estrogen and progesterone environment was observed. 59 In the human endometrium expression at the luminal (apical) poles of endometrial glandular cells and in the luminal epithelium has been observed throughout the cycle. 15
Colocalization of CEACAM1 and integrin β3 at the transitional zone of proliferative to invasive extravillous trophoblast indicates that CEACAM1-integrin β3 complexes might play a role in trophoblast invasion and implantation. For integrin β3, previous studies have shown expression on the surface of the attachment-competent trophoblast at the time of implantation in mouse embryos in the form of integrin αvβ3. 60 In vitro studies have shown expression of αvβ3 by trophoblast cells with an invasive phenotype. 61,62 For CEACAM1, expression had been described on the surface of trophectoderm on rat blastocysts, 63 where it appeared to be lost or masked from the surface of the mural trophoblast cells of adhesive-stage blastocysts. In human placenta we could recently demonstrate expression of CEACAM1 in the extravillous trophoblast, 22 with expression present as soon as the extravillous trophoblast differentiates and seen in both capping masses and cell columns. The present study shows that, at the human maternal-fetal interface, CEACAM1 and integrin β3 are co-expressed in extravillous trophoblast cells at the transitional zone, while colocalization appears to be restricted by the spatially more restricted expression of integrin β3 in invasive trophoblast.
A recent study demonstrated that CEACAM1 is also expressed on a subset of primary melanomas, whereas melanocytes do not express CEACAM1 (U. Schumacher, submitted). Using CLSM, we could demonstrate colocalization of CEACAM1 and integrin β3 at the invasion front of malignant melanomas, indicating that CEACAM1-integrin β3 complexes might play a role in melanoma cell migration and invasion. Interestingly, in a recent study by Hsu et al, 64 ectopic E-cadherin expression in a skin reconstruction model was shown to inhibit invasion of melanoma cells into dermis by down-regulating integrin β3.
Integrin family members are heterodimers that contain a larger α subunit that is unique to each individual receptor and a smaller β subunit that can be shared by several receptors. 46 The β3 subunit associates with two α subunits: αIIb in platelets or αv in a variety of cells. 47 Although melanoma cells 33,34 and extravillous trophoblasts 35,36 normally express αvβ3 integrin, additional CLSM studies with staining for αvβ3 are necessary to formally prove the interaction of CEACAM1 with αvβ3. Since we demonstrated that CEACAM1 and integrin β3 are colocalized on invading cells (ie, melanoma cells, extravillous trophoblasts), we speculate that, at least in situations in which association with integrin β3 is granted, CEACAM1, in the form of CEACAM1-integrin β3 complexes, may play a role in cellular invasion. To be determined are the specific conditions under which an adhesion molecule like CEACAM1 can function as both a tumor suppressor with an expression pattern indicating a role in maintaining tissue architecture, as well as a tumor promoter, with a role in processes such as angiogenesis 11 and invasion.
Footnotes
Address reprint requests to Dr. Jens Brümmer, Abteilung für Klinische Chemie, Klinik und Poliklinik für Innere Medizin, Universitätsklinikum Hamburg-Eppendorf, Martinistrasse 52, 20251 Hamburg, Germany. E-mail: bruemmer@uke.uni-hamburg.de.
Supported by grant Br 1741/1–1 from the Deutsche Forschungsgemeinschaft to J. B. and C. W.
References
- 1.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlsson A, Kuroki M, Lin S-H, Lucka L, Najjar SM, Neumaier M, Obrink B, Shively JE, Skubitz KM, Stanners CP, Thomas P, Virji M, Von Kleist S, Wagener C, Watt S, Zimmermann W: Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res 1999, 25:243-249 [DOI] [PubMed] [Google Scholar]
- 2.Aurivillius M, Hansen OC, Lazrek MB, Bock E, Öbrink B: The cell adhesion molecule Cell-CAM 105 is an ecto-ATPase and a member of the immunoglobulin superfamily. FEBS Lett 1990, 264:267-269 [DOI] [PubMed] [Google Scholar]
- 3.Lin SH, Giudotti G: Cloning and expression of a cDNA coding for a rat liver plasma ecto-ATPase: the primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem 1989, 264:14408-14414 [PubMed] [Google Scholar]
- 4.Prall F, Nollau P, Neumaier M, Haubeck HD, Drzeniek Z, Helmchen U, Löning T, Wagener C: CEACAM1 (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem 1996, 44:35-41 [DOI] [PubMed] [Google Scholar]
- 5.Dveksler GS, Dieffenbach CW, Cardellichio CB, McCuaig K, Pensiero MN, Jiang GS, Beauchemin N, Holmes KV: Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J Virol 1993, 67:1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virji M, Watt SM, Barker S, Makepeace K, Doyonnas R: The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitides and Neisseria gonorrhoeae. Mol Microbiol 1996, 22:929-939 [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Grunert F, Medina-Marino A, Gotschlich EC: Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med 1997, 185:1557-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF: CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J 1997, 16:3435-3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leusch HG, Drzeniek Z, Markos-Pusztai Z, Wagener C: Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic α-glycosides of mannose. Infect Immun 1991, 59:2051-2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virji M, Evans D, Hadfield A, Grunert F, Teixeira AM, Watt SM: Critical determinants of host receptor targeting by Neisseria meningitides and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol 1999, 34:538-551 [DOI] [PubMed] [Google Scholar]
- 11.Ergün S, Kilic N, Ziegeler G, Hansen A, Nollau P, Götze J, Wurmbach J-H, Horst A, Weil J, Malkanthi F, Wagener C: CEA-related cell adhesion molecule 1 (CEACAM1): a potent angiogenic factor and a major effector of vascular endothelial growth factor (VEGF). Mol Cell 2000, 5:311-320 [DOI] [PubMed] [Google Scholar]
- 12.Hixson DC, McEntire KD: Detection of an altered form of cell-CAM 105 on rat transplantable and primary hepatocellular carcinomas. Cancer Res 1989, 49:6788-6794 [PubMed] [Google Scholar]
- 13.Hixson DC, McEntire KD, Obrink B: Alterations in the expression of a hepatocyte cell adhesion molecule by transplantable rat hepatocellular carcinomas. Cancer Res 1985, 45:3742-3749 [PubMed] [Google Scholar]
- 14.Kleinerman DI, Troncoso P, Lin SH, Pisters LL, Sherwood ER, Brooks T, von Eschenbach AC, Hsieh JT: Consistent expression of an epithelial cell adhesion molecule (C-CAM) during human prostate development and loss of expression in prostate cancer: implication as a tumor suppressor. Cancer Res 1995, 55:1215-1220 [PubMed] [Google Scholar]
- 15.Bamberger A, Riethdorf L, Nollau P, Naumann M, Erdmann I, Götze J, Brümmer J, Schulte HM, Wagener C, Löning T: Dysregulated expression of CEACAM1 (BGP, C-CAM), an adhesion molecule of the CEA family, in endometrial cancer. Am J Pathol 1998, 152:1401-1406 [PMC free article] [PubMed] [Google Scholar]
- 16.Riethdorf L, Lisboa BW, Henkel U, Naumann M, Wagener C, Löning T: Differential expression of CEACAM1 (BGP), a cell adhesion molecule of the carcinoembryonic antigen family, in benign, premalignant, and malignant lesions of the human mammary gland. J Histochem Cytochem 1997, 45:957-963 [DOI] [PubMed] [Google Scholar]
- 17.Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C: Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci USA 1993, 90:10744-10748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmüller F, Wagener C, Neumaier M: Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen gene family of adhesion molecules in human colorectal adenomas. Cancer Res 1997, 57:2354-2357 [PubMed] [Google Scholar]
- 19.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW: Gene expression profiles in normal and cancer cells. Science 1999, 276:1268-1272 [DOI] [PubMed] [Google Scholar]
- 20.Hsieh J-T, Luo W, Song W, Wang Y, Kleinerman DI, Van NT, Lin S-H: Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res 1995, 55:190-197 [PubMed] [Google Scholar]
- 21.Luo W, Wood CG, Earley K, Hung M-C, Lin S-H: Suppression of tumorigenicity of breast cancer cells by an epithelial cell adhesion molecule (C-CAM1): the adhesion and growth suppression are mediated by different domains. Oncogene 1997, 14:1697-1704 [DOI] [PubMed] [Google Scholar]
- 22.Bamberger A-M, Sudahl S, Löning T, Wagener C, Bamberger C, Drakakis P, Coutifaris C, Makrigiannakis A: The adhesion molecule CEACAM1 (CD66a, C-CAM, BGP) is specifically expressed by the extravillous intermediate trophoblast. Am J Pathol 2000, 156:1165-1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brümmer J, Ganzer S, Ebrahimnejad A, Flayeh R, Streichert T, Wagener C: The CD66 complex: activation of tyrosine kinases of the Src family. Leucocyte Typing VI. Edited by T Kishimoto, H Kikutani, A von dem Borne, SM Goyert, DY Mason, M Miyasaka, L Moretta, K Okumara, S Shaw, TA Springer, K Sugamura, H Zola. Garland Publishing New York, 1997, pp 1011–1012
- 24.Skubitz KM, Campbell KD, Ahmed K, Skubitz AP: CD66 family members are associated with tyrosine kinase activity in human neutrophils. J. Immunol 1995, 155:5382-5390 [PubMed] [Google Scholar]
- 25.Brümmer J, Neumaier M, Göpfert C, Wagener C: Association of pp60c-src with biliary glycoprotein (CEACAM1), an adhesion molecule of the carcinoembryonic antigen family down regulated in colorectal carcinomas. Oncogene 1995, 11:1649-1655 [PubMed] [Google Scholar]
- 26.Ebrahimnejad A, Flayeh R, Unteregger G, Wagener C, Brümmer J: The cell adhesion molecule CEACAM1 associates with paxillin in granulocytes, epithelial and endothelial Cells. Exp Cell Res 2000, 260:365-373 [DOI] [PubMed] [Google Scholar]
- 27.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veilette A: Association of biliary glycoprotein with protein tyrosine phosphatases SHP-1 in malignant colon epithelial cells. Oncogene 1997, 14:783-790 [DOI] [PubMed] [Google Scholar]
- 28.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N: The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem 1999, 274:335-344 [DOI] [PubMed] [Google Scholar]
- 29.Stoffel A, Neumaier M, Gaida F-J, Fenger U, Drzeniek Z, Haubeck H-D, Wagener C: Monoclonal, anti-domain and anti-peptide antibodies assign the molecular weight 160,000 granulocyte membrane antigen of the CD66 cluster to a mRNA species encoded by the biliary glycoprotein gene, a member of the carcinoembryonic antigen gene family. J Immunol 1993, 150:4978-4984 [PubMed] [Google Scholar]
- 30.Wagener C, Clark BR, Rickard KJ, Shively JE: Monoclonal antibodies for carcinoembryonic antigen and related antigens as a model system: determination of affinities and specificities of monoclonal antibodies by using biotin-labeled antibodies and avidin as precipitating agent in a solution phase immunoassay. J. Immunol 1983, 130:2302-2307 [PubMed] [Google Scholar]
- 31.Neumaier M, Fenger U, Wagener C: Monoclonal antibodies for carcinoembryonic antigen (CEA) as a model system: identification of two novel CEA-related antigens in meconium and colorectal carcinoma tissue by Western blots and differential immunoaffinity chromatography. J. Immunol 1985, 135:3604-3609 [PubMed] [Google Scholar]
- 32.Drzeniek Z, Lamerz R, Fenger U, Wagener C, Haubeck H-D: Identification of membrane antigen in granulocytes and colonic carcinoma cells by a monoclonal antibody specific for biliary glycoprotein, a member of the carcinoembryonic antigen family. Cancer Lett 1991, 56:173-179 [DOI] [PubMed] [Google Scholar]
- 33.Hsu MY, Shih DT, Meier FE, Van Belle P, Hsu JY, Elder DE, Buck CA, Herlyn M: Adenoviral gene transfer of β3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am J Pathol 1998, 153:1435-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, Giannarelli D, Temponi M, Ferrone S: Clinical significance of α (v) β3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res 1997, 57:1554-1560 [PubMed] [Google Scholar]
- 35.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ: Integrin switching regulates normal trophoblast invasion. Development 1996, 120:3657-3666 [DOI] [PubMed] [Google Scholar]
- 36.Damsky CH, Fisher SJ: Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998, 10:660-666 [DOI] [PubMed] [Google Scholar]
- 37.Izzi L, Turbide C, Houde C, Kunath T, Beauchemin N: Cis-determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene 1999, 18:5563-5572 [DOI] [PubMed] [Google Scholar]
- 38.Kunath T, Ordonez-Garcia C, Turbide C, Beauchemin N: Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene 1995, 11:2375-2382 [PubMed] [Google Scholar]
- 39.Huang J, Hardy JD, Sun Y, Shively JE: Essential role of biliary glycoprotein (CD66a) in morphogenesis of the human mammary epithelial cell line MCF10F. J Cell Sci 1999, 112:4193-4205 [DOI] [PubMed] [Google Scholar]
- 40.Sadekova S, Lamarche-Vane N, Li X, Beauchemin N: The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell-cell contact through activation of Rho-like GTPases. Mol Biol Cell 2000, 11:65-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edlund M, Öbrink B: Evidence for calmodulin binding to the cytoplasmic domains of two C-CAM isoforms. FEBS Lett 1994, 327:90-94 [DOI] [PubMed] [Google Scholar]
- 42.Edlund M, Blikstad I, Obrink B: Calmodulin binds to specific sequences in the cytoplasmic domain of C-CAM and down-regulates C-CAM self-association. J Biol Chem 1996, 19:1393-1399 [DOI] [PubMed] [Google Scholar]
- 43.Nagel G, Grunert F, Kuijpers TW, Watt SM, Thompson J, Zimmermann W: Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcino-embryonic antigen family. Eur J Biochem 1993, 214:27-35 [DOI] [PubMed] [Google Scholar]
- 44.Afar DE, Stanners CP, Bell JC: Tyrosine phosphorylation of biliary glycoprotein, a cell adhesion molecule related to carcinoembryonic antigen. Biochim Biophys Acta 1992, 1134:46-52 [DOI] [PubMed] [Google Scholar]
- 45.Skubitz KM, Ducker TP, Skubitz AP, Goueli SA: Antiserum to carcinoembryonic antigen recognizes a phosphotyrosine-containing protein in human colon cancer cell lines. FEBS Lett 1993, 318:200-204 [DOI] [PubMed] [Google Scholar]
- 46.Hynes RO: Integrins: versatility, modulation and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 47.Clark YP, Brugge JS: integrins and signal transduction pathways: the road taken. Science 1995, 268:233-239 [DOI] [PubMed] [Google Scholar]
- 48.Porter JC, Hogg N: Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol 1998, 8:390-396 [DOI] [PubMed] [Google Scholar]
- 49.Kuijpers TW, Hoogerwerf M, van der Laan LJ, Nagel G, van der Schoot CE, Grunert F, Roos D: CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol 1992, 118:457-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stocks SC, Ruchaud-Sparagano MH, Kerr MA, Grunert F, Haslett C, Dransfield I: CD66: role in the regulation of neutrophil effector function. Eur J Immunol 1996, 26:2924-2932 [DOI] [PubMed] [Google Scholar]
- 51.Petitclerc E, Strömblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AM, Cheresh DA, Brooks PC: Integrin α (v) β3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res 1999, 59:2724-2730 [PubMed] [Google Scholar]
- 52.Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA: Anti-integrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 1995, 96:1815-1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Öbrink B: CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol 1997, 9:616-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singer BB, Scheffrahn I, Öbrink B: The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res 2000, 60:1236-1244 [PubMed] [Google Scholar]
- 55.Möller M, Kammerer R, Grunert F, von Kleist S: Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell subpopulation. Int J Cancer 1996, 65:740-745 [DOI] [PubMed] [Google Scholar]
- 56.Sawa H, Kamada K, Sato H, Sendo S, Kondo A, Saito I, Edlund M, Öbrink B: C-CAM expression in the developing rat central nervous system. Dev Brain Res 1994, 78:35-43 [DOI] [PubMed] [Google Scholar]
- 57.Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S: Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol 1998, 28:3664-3674 [DOI] [PubMed] [Google Scholar]
- 58.Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA: Integrin adhesion molecules in the human endometrium: correlation with the normal and abnormal menstrual cycle. J Clin Invest 1992, 90:188-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svalander PC, Odin P, Nilsson BO, Öbrink B: Expression of cellCAM-105 in the apical surface of rat uterine epithelium is controlled by ovarian steroid hormones. J Reprod Fertil 1990, 88:213-221 [DOI] [PubMed] [Google Scholar]
- 60.Damsky CH, Sutherland A, Fisher SJ: Extracellular matrix 5: adhesive interactions in early mammalian embryogenesis, implantation and placentation. FASEB J 1993, 7:1320-1329 [DOI] [PubMed] [Google Scholar]
- 61.Irving JA, Lysiak JJ, Graham CH, Hearn S, Han VKM, Lala PK: Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta 1995, 16:413-433 [DOI] [PubMed] [Google Scholar]
- 62.Frank HG, Kaufmann P: Nonvillous parts and trophoblast invasion. Pathology of the Human Placenta, 4th ed. Edited by K Bernischke, P Kaufmann. New York, Springer Verlag, 2000
- 63.Svalander PC, Odin P, Nilsson BO, Öbrink B: Trophectoderm surface expression of the cell adhesion molecule cell-CAM 105 on rat blastocysts. Development 1987, 100:653-660 [DOI] [PubMed] [Google Scholar]
- 64.Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M: E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol 2000, 156:1515-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]