Abstract
Anaplastic large-cell lymphoma (ALCL) of T- or null-cell lineage, as defined in the revised European-American lymphoma classification, includes a subset of tumors that carry the t(2;5)(p23;q35) resulting in overexpression of anaplastic lymphoma kinase (ALK). Patients with ALK+ ALCL are reported to have a better prognosis than patients with ALK− ALCL. Because the mechanisms for this survival difference are unknown, we investigated the hypothesis that apoptotic pathways may be involved. We therefore assessed expression levels of the anti-apoptotic proteins BCL-2 and BCL-XL and the pro-apoptotic proteins BAX and BCL-XS in T/null-cell ALCL using immunohistochemical methods and correlated the findings with ALK expression and apoptotic rate (AR), the latter assessed by a modified Tdt-mediated dUTP nick-end labeling assay. ALK was detected in 21 of 66 (31.8%) ALCLs. BCL-2 was not detected in 21 ALK+ ALCLs but was present in 26 of 45 (57.8%) ALK− ALCLs (P < 0.0001). ALK+ and ALK− ALCLs also showed significant differences in expression of BCL-XL, BAX, and BCL-XS. ALK+ tumors less commonly had a high level of BCL-XL (1 of 17 versus 14 of 35, P = 0.01), and more commonly had high levels of BAX (13 of 18 versus 15 of 36, P = 0.05), and BCL-XS (11 of 16 versus 12 of 31, P = 0.05) compared with ALK− tumors. ALK+ tumors also had a higher mean AR than ALK− tumors (3.4% versus 1.1%, P = 0.0002). Differential expression of BCL-2 family proteins may be responsible for the higher AR observed in ALK+ ALCL and provides a possible biological explanation for the better prognosis reported for patients with ALK+ ALCL.
Anaplastic large-cell lymphoma (ALCL) of T- or null-cell lineage, as defined in the revised European-American lymphoma classification, includes a subset of tumors that carry the t(2;5)(p23;q35). 1,2 The t(2;5) disrupts the nucleophosmin (NPM) gene at 5q35 and the anaplastic lymphoma kinase (ALK) gene at 2p23, generating a novel NPM-ALK gene consisting of the N-terminal portion of NPM fused to the cytoplasmic catalytic domain of ALK. 3,4 The t(2;5), its chimeric transcripts, and resulting overexpression of ALK protein have been detected in a variable proportion of ALCL, ranging from 12 to 80% in different series. 5-10 Variant translocations involving 2p23 also can cause overexpression of ALK. 11-14 Several studies have reported that patients with ALK+ ALCL have better overall survival than patients with ALK− ALCL. 15-17 However, the underlying biological mechanisms for this survival difference are unknown.
A number of proteins are involved in apoptosis, of which the BCL-2 protein family is best known. BCL-2, first identified by its involvement in the t(14;18) (q32;q21), 18,19 is a major negative regulator of apoptosis. The t(14;18) in follicular lymphoma results in BCL-2 overexpression, and represents the first example of oncogenesis mediated by decreased cell death. 20,21 A number of proteins have since been identified that share homology with conserved regions of BCL-2, the BCL-2 homology (BH) domains, and these proteins have either pro-apoptotic or anti-apoptotic activity in mammalian cells. 22 BAX, a 21-kd protein with significant homology clustered in the BH1 and BH2 regions, was the first of these proteins identified and is an important cell death agonist. 23 BCL-X, another gene homologous with BCL-2, gives rise to two alternatively spliced forms with opposite functions, long (BCL-XL) and short (BCL-XS). BCL-XL, like BCL-2, inhibits apoptosis, whereas BCL-XS is a cell death promoter. 24-26 Many other BCL-2 family members have been described. 22 Pro-apoptotic and anti-apoptotic members of the BCL-2 family are capable of forming homodimers or heterodimers and their relative ratio acts as a rheostat for susceptibility to programmed cell death. 22,27
BCL-2 expression has been studied extensively in aggressive non-Hodgkin’s lymphomas, principally diffuse large B-cell lymphoma, and has been correlated with worse prognosis. 28-31 In contrast, BAX, BCL-XL, and BCL-XS expression and their clinical significance, are less well studied with discordant results. 32-34 Relatively little is known about expression of BCL-2 family member proteins in aggressive non-Hodgkin’s lymphomas of T-cell lineage. 35
In this study, we hypothesized that alterations of apoptotic pathways may be responsible for the different prognosis of patients with ALK+ and ALK− ALCL. We therefore assessed the expression levels of BCL-2, BAX, BCL-XL, and BCL-XS in a series of ALCL of T/null phenotype. We correlated these findings with ALK expression and apoptotic rate (AR), the latter evaluated by a modified Tdt-mediated dUTP nick-end labeling (TUNEL) assay. Our results indicate that there are striking differences in expression of BCL-2 family proteins between the ALK+ and ALK− tumors.
Materials and Methods
Study Group
This group included 66 cases of systemic ALCL of T- or null-cell lineage accessioned at The University of Texas MD Anderson Cancer Center between 1984 and 2000. No patients had received therapy before biopsy. The clinicopathological features of these patients are shown in Table 1 ▶ . The median age of patients with ALK+ tumors was 35 years compared with 49 years for patients with ALK− tumors (P = 0.005). All other clinical parameters including Ann Arbor stage were comparable. The histopathological diagnosis of ALCL was based on both morphological and immunohistological criteria according to the revised European-American lymphoma and World Health Organization 36 classifications and all specimens were reviewed at the time of immunohistochemical analysis. All cases were routinely processed, fixed in 10% buffered formalin, and embedded in paraffin. Immunohistochemically, all ALCL cases expressed CD30 and were negative for B-cell antigens (CD20 and/or CD79a). All T-cell tumors were positive for one or more T-cell antigens (CD3, CD5, CD43, or CD45RO). Tumors negative for CD3 and CD5 and positive for CD43 or CD45RO were considered to be of T-cell lineage in this study, although null-cell lineage cannot be excluded as CD43 and CD45RO react with histiocytes. Null-cell cases were negative for all T-cell antigens.
Table 1.
Clinical Characteristics of Patients with ALK-Positive and ALK-Negative Anaplastic Large-Cell Lymphoma of T/Null Lineage
| ALK Positive n = 21 | ALK Negative n = 45 | P | |
|---|---|---|---|
| Age | |||
| Median (years) | 35 | 49 | 0.005 |
| Range (years) | 3–60 | 17–79 | |
| Gender | |||
| Male | 11 (52%) | 33 (73%) | 0.1 |
| Female | 10 (48%) | 12 (27%) | |
| Skin involvement | 4/21 (19%) | 13/45 (29%) | 0.5 |
| Ann Arbor Stage: | |||
| I–II | 7 (33%) | 20 (44%) | 0.4 |
| III–IV | 14 (67%) | 25 (56%) | |
| Serum LDH >1.5× normal | 12/21 (57%) | 21/45 (47%) | 0.4 |
| Serum β2-microglobulin >2.5 mg/L | 13/21 (62%) | 25/45 (55%) | 0.6 |
| Albumin <3.5 g/dL | 5/21 (24%) | 15/45 (33%) | 0.7 |
| WBC (mean± SD) | 12.1 ± 13.4 | 9.9 ± 4.9 | 0.4 |
| Anemia | 11/21 (52%) | 21/45 (47%) | 0.8 |
Immunohistochemical Methods
Tissue sections (3 or 4 μm thick) were deparaffinized in xylene and rehydrated in a graded series of ethanols.
The panel of antibodies used included: ALK-1 (1:30) and BCL-2 (1:40) (DAKO, Carpinteria, CA); BAX (1:40) and BCL-XL (1:25) (Zymed, South San Francisco, CA); BCL-XS (1:50) (Calbiochem, San Diego, CA) and MIB-1 (1:120) (Immunotech, Westbrook, ME). 37 For all antibodies, heat-induced epitope retrieval was performed using a modification of the method described previously by Shi and colleagues. 38 Tissue sections were placed in plastic Coplin jars containing preheated target retrieval solution (DAKO), heated in a household vegetable steamer (Sunbeam-Oster, Model Sunbeam 4713/5710, 900 W) for 35 minutes, and allowed to cool at room temperature for at least 15 minutes. Subsequent steps of the immunostaining procedure were performed using the DAKO Autostainer at room temperature, and included the following: 1) blocking of endogenous peroxidase in 3% H2O2 in phosphate buffered saline (PBS), pH 7.4, for 5 minutes; 2) blocking of nonspecific protein-binding sites using protein blocking solution (DAKO) for 5 minutes; 3) incubation with the primary antibody for 1 hour; and 4) detection using the streptavidin-biotin-peroxidase based LSAB+ kit (DAKO) for 2 × 15 minutes. We used 3,3′ diaminobenzidine/H2O2 (Biogenex, San Ramon, CA) or 3-amino-9-ethyl carbazol as the chromogen and hematoxylin as the counterstain.
We used two cell lines as positive controls for ALK, SU-DHL1 and KARPAS 299, known to express ALK as a result of the t(2;5)(p23;q35). These cell lines were grown to mid-logarithmic phase, fixed overnight in buffered formalin, and embedded in paraffin. A case of follicular lymphoma bearing the t(14;18) served as positive control for BCL-2. Control slides from prostate tissue were used as positive controls for the BAX, BCL-XL, and BCL-XS as described elsewhere. 39 In addition, reactive small lymphocytes in tissue sections served as internal positive controls. The K562 cell line was used as a negative control in all experiments. A lymph node with follicular hyperplasia was used as a positive control for MIB-1. Another internal control was the application of normal rabbit serum (DAKO) without primary antibody to the control slides, to exclude nonspecific cross-reactions with the primary antibody.
All immunostained slides were evaluated by two of us (GZR and LJM). Any cytoplasmic or nuclear staining of ALCL cells was considered positive, irrespective of intensity. Expression levels for BAX, BCL-XL, and BCL-XS were determined by counting at least 1000 neoplastic cells in each case. Based on the distribution of data (histograms), and for the purpose of statistical analysis, we considered BAX expression as high when the percentage of positive neoplastic cells was >50%. BCL-XL or BCL-XS expression was considered to be high when the percentage of BCL-XL or BCL-XS-positive lymphoma cells was >25%. No statistical cutoffs were used for the BCL-2 or ALK results as these were all-or-none phenomena. Proliferation index (PI), assessed in a subset of 10 ALK+ and 10 ALK− tumors, was determined by counting the percentage of MIB1-positive nuclei.
Modified TUNEL Assay
Tissue sections were mounted on coated slides, deparaffinized in a graded series of ethanols, rehydrated, and pretreated with proteinase K (20 μg/ml) for 25 minutes at 37°C. Slides were then incubated for 5 minutes in 3% H2O2 in PBS at pH 7.4, to block endogenous peroxidase activity. Terminal deoxynucleotidyl transferase (TdT) (New England Biolabs, Beverly, MA) was subsequently applied (15 U/slide) for 1 hour, at 37°C, in 20 mmol/L Tris-acetate, pH 7.9, 50 mmol/L potassium acetate, 10 mmol/L magnesium acetate, 1 mmol/L dithiothreitol, 0.25 mmol/L CoCl2, 24 μmol/L biotin-dATP (Life Technologies, Gaithersburg, MD). Terminal deoxynucleotidyl transferase catalyzes the addition of deoxynucleotides to the 3′ hydroxy terminus of DNA molecules. 40 The TUNEL assay was modified by substituting dUTP for dATP. For the detection of labeled termini, streptavidin-biotin-horseradish peroxidase complex (LSAB+ kit) and 3,3′-diamino-benzidine (both from DAKO) were used, according to the manufacturer’s instructions. The slides were counterstained with hematoxylin.
After quenching endogenous peroxidase activity, positive control sections from K562 cells were incubated with DNase I (2.5 μg in 50 μl/slide in Tris-buffered saline containing 6 mmol/L MgCl2) for 30 minutes at 37°C. Tissue sections from the same cell line incubated with a reaction mixture lacking TdT served as negative controls in each experiment.
At least 2000 nuclei from ALCL cells were evaluated in each case and the percentage of positively stained nuclei was considered to be the AR.
Statistical Analysis
Statistical comparison of patient age between ALK+ and ALK− groups was based on the one-way analysis of variance test. The nonparametric Spearman’s rank correlation coefficient was applied to evaluate the strength of the relationship between BAX, BCL-XL, BCL-XS, AR, and PI. The chi-square and Fisher’s exact tests were used to compare the expression of all proteins as groups (positive versus negative, low versus high) with various clinicopathological parameters. The Mann-Whitney U test was chosen for the nonparametric correlation of BAX, BCL-XL, BCL-XS, AR, and PI between ALK+ and ALK− ALCL.
Progression-free survival (PFS) was chosen to evaluate the clinical outcome of the patients because various factors after relapse might impact overall survival of the patients. PFS was defined as time from initiation of therapy to last follow-up, primary treatment failure, or relapse. Analysis was based on the method of Kaplan and Meier with Mantel-Cox log-rank test. All computations were performed using StatView statistical program (SAS Institute, Cary, NC).
Results
ALK Expression
Twenty-one of 66 (31.8%) ALCL of T/null lineage were positive for ALK-1 with a nuclear and cytoplasmic pattern of staining (Figure 1) ▶ . Seventeen of 21 ALK+ cases were reported to carry the t(2;5)(p23;q35) by reverse transcriptase-polymerase chain reaction (cases referred from other institutions) or long-range genomic DNA polymerase chain reaction, as reported previously. 8 Seventeen (81%) of 21 ALK+ and 31 (68.9%) of 45 ALK− tumors expressed one or more T-cell antigens. Histological subtypes of the ALK+ cases included: 12 classical pleomorphic, 7 monomorphic, 1 sarcomatoid, and 1 lymphohistiocytic.
Figure 1.
ALK expression in ALCL of T/null lineage. Strong cytoplasmic and nuclear staining for ALK protein is present (AEC, hematoxylin counterstain; original magnification, ×400).
Expression of BCL-2, BAX, BCL-XL, and BCL-XS
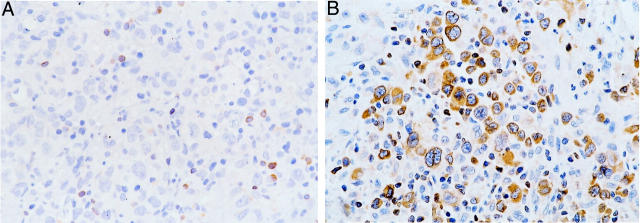
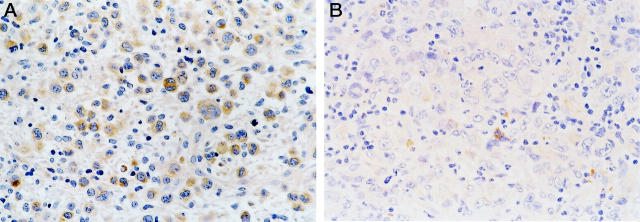
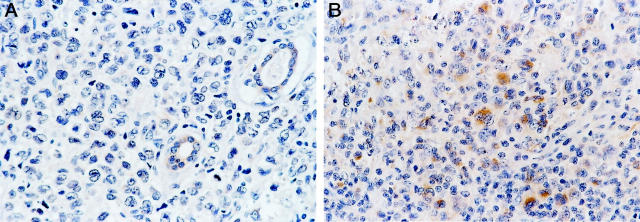
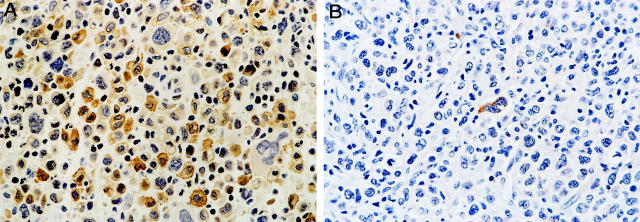
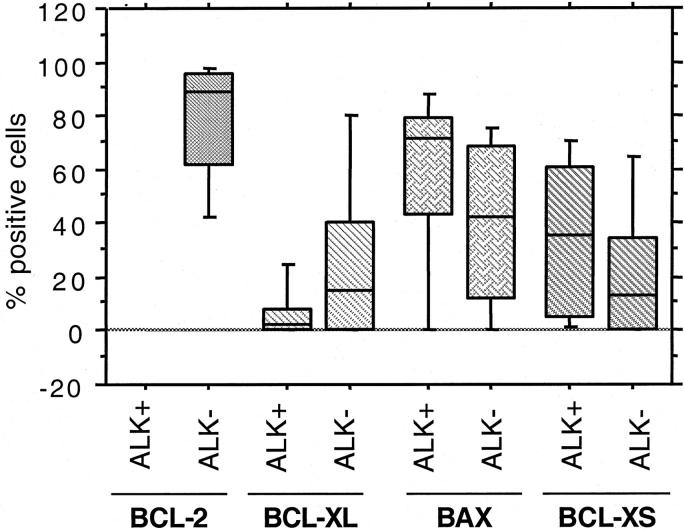
BCL-2 was not detected in any of the 21 ALK+ ALCL, whereas 26 of 45 (57.8%) ALK− tumors expressed BCL-2 (P < 0.0001, Fisher’s exact test) (Figure 2) ▶ . The percentage of BCL-2-positive cells ranged from 14.3 to 98%, but most cases expressed high levels of BCL-2 (mean, 78.1%; median, 88.5%). BAX immunoreactivity was observed in 43 of 54 (79.6%) ALCL assessed (Figure 3) ▶ . The proportion of BAX-positive cells ranged from 4 to 93% (mean, 46.2%; median, 54%). Using a 50% cutoff, 13 of 18 (72.2%) ALK+ tumors had high BAX expression compared with 15 of 36 (41.7%) ALK− tumors (P = 0.05) (Table 2) ▶ . BCL-XL-positive cells were detected in 23 of 52 (44.2%) ALCL assessed. The percentage of BCL-XL-positive cells ranged from 2 to 96% (mean, 19.8%; median, 7%) (Figure 4) ▶ . Using a 25% cutoff, 1 of 17 (5.9%) ALK+ tumors had high BCL-XL compared with 14 of 35 (40%) ALK− tumors (P = 0.01) (Table 2) ▶ . Twenty-eight (59.6%) of 47 ALCL assessed were BCL-XS-positive. The percentage of BCL-XS-positive cells ranged from 1 to 82% (mean, 26.8%; median, 20%) (Figure 5) ▶ . Using a 25% cutoff, 11 of 16 (68.8%) ALK+ tumors had high BCL-XS compared with 12 of 31 (38.7%) ALK− tumors (P = 0.05). All BCL-2 family proteins were immunolocalized principally in the cytoplasm of neoplastic cells.
Figure 2.
BCL-2 expression in ALCL of T/null lineage. A: Absence of BCL-2 immunoreactivity in an ALK+ ALCL. Reactive small lymphocytes stained positively and served as internal positive controls. B: A case of ALK− ALCL with strong cytoplasmic staining for BCL-2 in the majority of large tumor cells (DAB, hematoxylin counterstain; original magnification, ×400).
Figure 3.
BAX expression in ALCL of T/null lineage. A: High expression levels of BAX in an ALK+ ALCL. B: Occasional BAX-positive neoplastic cells in a case of ALK− ALCL (DAB, hematoxylin counterstain; original magnification, ×400).
Table 2.
Association between Expression of BCL-2 Family Proteins, Apoptotic Rate, and ALK Expression in Anaplastic Large-Cell Lymphoma of T/Null Lineage
| BCL-2 (Positive) (n = 66) | BCL-XL (High) (n = 52) | BAX (High) (n = 54) | BCL-XS (High) (n = 47) | Apoptotic rate (%) (n = 53) | Proliferation index (%) (n = 20) | |
|---|---|---|---|---|---|---|
| ALK+ (n = 21) | 0/21 (0%) | 1 /17 (5.9%) | 13 /18 (72.2%) | 11 /16 (68.8%) | 3.4 | 71.0 |
| ALK− (n = 45) | 26/45 (57.8%) | 14 /35 (40%) | 15 /36 (41.7%) | 12 /31 (38.7%) | 1.1 | 67.5 |
| P value | <0.0001 | 0.01 | 0.05 | 0.05 | 0.0002 | 0.7 |
The statistical comparisons between BCL-2, BCL-XL, BAX, BCL-XS, and ALK status were based on Fisher’s exact test. The statistical difference of apoptotic rate and proliferation index between ALK + and ALK − tumors was calculated using the nonparametrical Mann-Whitney test.
Figure 4.
BCL-XL expression in ALCL of T/null lineage. A: Absence of BCL-XL expression in a case of ALK+ ALCL with skin involvement. Co-existing skin adnexal structures are weakly positive for BCL-XL. B: High expression levels of BCL-XL in an ALK− ALCL. A small number of reactive small lymphocytes show weak cytoplasmic immunoreactivity for BCL-XL (DAB, hematoxylin counterstain; original magnification, ×400).
Figure 5.
BCL-XS expression in ALCL of T/null lineage. A: Many BCL-XS-positive tumor cells in an ALK+ ALCL. However, a small number of large anaplastic cells are BCL-XS-negative. B: Rare BCL-XS-positive tumor cells in a case of ALK− ALCL (DAB, hematoxylin counterstain; original magnification, ×400).
Apoptotic Rate
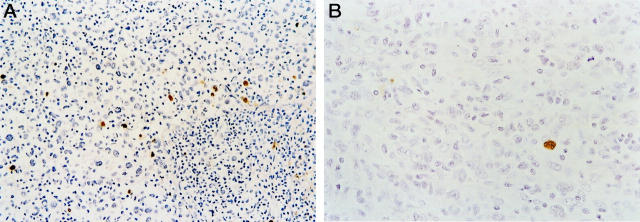
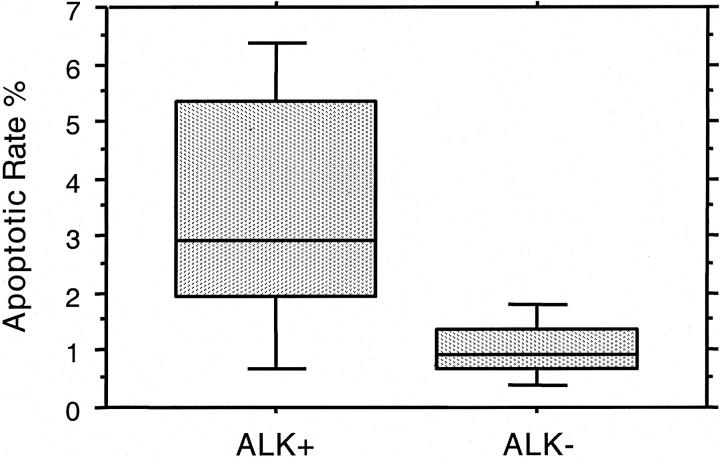
The mean percentage of apoptotic neoplastic cells was 1.9 ± 1.8% for the entire study group, ranging from 0.1 to 7.9%. Staining was restricted to the nucleus of apoptotic cells (Figures 6 and 7) ▶ ▶ . Fragmented nuclei and nuclear debris near apoptotic cells were also positively stained. The mean AR was 3.4% in ALK+ tumors, threefold higher than ALK− tumors (P = 0.0002, Mann-Whitney U test) (Table 2 ▶ , Figure 8 ▶ ). AR also correlated with BCL-2 expression. For 53 tumors with both AR and BCL-2 results, the mean AR was 2.5% in BCL-2-negative cases compared with 0.9% for BCL-2-positive cases (P = 0.0016).
Figure 6.
AR in ALCL of T/null lineage. A: An ALK+ ALCL case with AR of 6.5%. The positive signal is restricted to the nuclei of apoptotic cells (DAB, hematoxylin counterstain; original magnification, ×200). B: An ALK− ALCL with AR of 0.9%. Only one apoptotic tumor cell is found in this field (DAB, hematoxylin counterstain; original magnification, ×400).
Figure 7.
Box plot showing the significant difference in AR between ALK+ and ALK− ALCL.
Figure 8.
Expression levels of BCL-2 family proteins in ALK+ and ALK− ALCL. Box plots represent the expression levels of BCL-2, BAX, BCL-XL, and BCL-XS and allow comparison between ALK+ and ALK− tumors. Because BCL-2 was not detected in any ALK+ tumors, no box plot is shown.
Proliferation Index
Tumor PI, evaluated in a subset of 20 cases of ALCL, ranged from 20.4 to 94.6% (mean, 69.2 ± 18.9%; median, 75%). These results are similar to those reported in other types of diffuse aggressive non-Hodgkin’s lymphomas. 37 No statistical difference in PI was observed between ALK+ and ALK− tumors (Table 2) ▶ .
Association between BCL-2 Family Proteins, AR, and PI
Box plots demonstrating the distribution of BCL-2, BAX, BCL-XL, and BCL-XS expression between the ALK+ and ALK− groups are shown in Figure 8 ▶ . For the entire study group, correlation between the continuous variables BAX, BCL-XL, BCL-XS, AR, and PI showed a slight statistical association between PI and BAX (r = 0.45, P = 0.07) and between PI and BCL-XS (r = 0.55, P = 0.04) expression (Spearman’s rank R correlation coefficient). The ratio BCL-XS/BCL-XL was also found to be significantly higher in ALK+ compared with ALK− tumors (mean, 6.5 versus 1.6). More specifically, a BCL-XS/Bxl-xL ratio >3 was observed in 56% of the ALK+ tumors compared with 17% of the ALK− tumors (P = 0.03, Fisher’s exact test).
Clinical Outcome
BCL-2-positive cases of ALCL showed a statistical trend toward worse PFS although this did not reach statistical significance (P = 0.13, by log rank; Table 3 ▶ ). Expression of BAX, BCL-XL, and BCL-XS, did not correlate significantly with PFS (Table 3) ▶ . Using a cutoff of 1.9% (median AR of the entire study group), AR did not significantly impact PFS (P = 0.5 by log rank). However, using the median AR (2.9%) of the ALK+ ALCL group as a cutoff, none of 10 patients with AR higher than 2.9% failed therapy with a median follow-up period of 39 months.
Table 3.
Five-Year Progression-Free Survival of Patients With Anaplastic Large-Cell Lymphoma of T/Null Lineage According to Expression of BCL-2, BAX, BCL-XL, and BCL-XS
| 5-year PFS ± SE | P value* | |
|---|---|---|
| BCL-2 | ||
| Positive | 46 ± 11 | 0.13 |
| Negative | 67 ± 9 | |
| BAX | ||
| High† | 52 ± 12 | 0.79 |
| Low | 42 ± 19 | |
| BCL-XL | ||
| High† | 53 ± 13 | 0.54 |
| Low | 63 ± 10 | |
| BCL-XS | ||
| High† | 71 ± 11 | 0.61 |
| Low | 52 ± 13 |
*By log rank (Mantel-Cox).
†High BAX: >50% of tumor cells positive. High BCL-XL, BCL-XS: >25% of tumor cells positive.
Discussion
In this study we investigated the hypothesis that ALK expression may correlate with expression of BCL-2 family proteins in ALCL. Our series included 66 biopsy specimens obtained from untreated patients with ALCL of T- or null-cell lineage. Twenty-one tumors were ALK+ and 45 tumors were ALK−.
BCL-2 expression was not detected in any of the ALK+ tumors. Because BCL-2 immunoreactivity was found in ∼60% of the ALK− cases in this series, it is possible that NPM-ALK down-regulates BCL-2 gene expression in vivo. Approximately 80% of ALCL cases were immunoreactive with BAX in our study. Notably, the majority of the ALK+ tumors showed high levels of BAX expression (Table 2) ▶ . This imbalance between BAX and BCL-2 favors apoptosis, and in part may explain the susceptibility of ALK+ ALCL to chemotherapy.
In previous studies, BCL-2 and BAX have been shown to be expressed in diffuse large B-cell lymphoma, in levels comparable with those presented here for the ALK− ALCL subgroup. In addition, these previous studies have correlated BCL-2 immunoreactivity with poorer prognosis in diffuse large B-cell lymphoma. 31-33 In the present study, patients with BCL-2-positive ALCL showed a statistical trend toward unfavorable prognosis, but this did not reach statistical significance (P = 0.13). BCL-2 and BAX expression also have been reported in T-cell lymphoid malignancies. 35 However, detailed correlation between expression of BCL-2-related proteins and various types of T-cell lymphoma has not been reported.
The anti-apoptotic protein BCL-XL was not detected, or was expressed at lower levels, in ALK+ cases compared with the ALK− cases of ALCL. In contrast, the pro-apoptotic partner, BCL-XS, was more frequently detected in ALK+ ALCL (Table 2) ▶ . In one previous study, Xerri and colleagues 34 detected BCL-XL and BCL-XS in a variety of lymphoma types. However, the number of cases examined is limited. 34 As far as we are aware, our study provides novel information concerning the expression of both spliced forms of the BCL-X gene in ALCL.
The mitogenic effect of NPM-ALK has been shown by previous in vitro studies. 41 Recently, Bai and colleagues 42 also showed that NPM-ALK activates an anti-apoptotic signaling pathway via activation of phosphatidylinositol 3-kinase, which in turn activates the serine-threonine kinase Akt/PKB. The latter kinase is capable of phosphorylating the pro-apoptotic BCL-2 family protein BAD, thus preventing BAD from binding to and inhibiting the anti-apoptotic proteins BCL-2 and BCL-XL, resulting in tumor cell survival. 42 The impact of this pathway in the molecular pathogenesis of ALK+ ALCL in vivo needs to be further investigated. It seems likely that this pathway has a limited role in ALK+ ALCL in vivo because levels of BCL-2 and BCL-XL are undetectable or low. However, our data do not allow an assessment of the functional status of these molecules (bound or unbound to BAD or other BCL-2 family proteins). Other mechanisms involving aberrant ALK tyrosine kinase signaling 43 need to be further investigated at the molecular level in relation to cell proliferation and apoptosis in ALCL.
The AR, as determined by modified TUNEL assay, was found to be threefold higher in ALK+ compared with ALK− tumors (P = 0.0002 by Mann-Whitney U test). The undetectable or low levels of the anti-apoptotic proteins BCL-2 and BCL-XL, and the statistically higher expression of the pro-apoptotic homologues BAX and BCL-XS 22 provide a biological explanation for the higher AR observed in ALK+ ALCL. Moreover, the high BCL-XS/BCL-XL ratio noticed in ALK+ cases strengthens these associations. It is also possible that down-regulation of BCL-XL might be associated with decreased cell survival in a manner independent of BCL-2 expression levels, similar to that shown in cell cultures derived from follicular lymphomas 44
However, we do not mean to imply that differential expression of BCL-2 family proteins is the only explanation for the significant difference in AR between ALK+ and ALK− ALCL. Other potential explanations include the Fas-mediated apoptotic machinery. Previous in vitro studies using cell lines derived from systemic ALCL cases have provided evidence that t(2;5)-positive ALCL cells express CD95 and undergo CD95-induced apoptosis. 45 Clusterin, recently reported to be specifically expressed in ALCL cell lines and tumors, also may be involved. 46 Clusterin (also known as sulfated glycoprotein 2, human apolipoprotein A-I, J) is a highly conserved glycoprotein, which among its other functions, promotes cell death. 47,48 Although Wellmann and colleagues 46 reported that clusterin was not related to ALK expression, it remains unclear whether overexpression of this cell death promoter might contribute to increase AR in a subset of ALK+ ALCL. The NPM gene, the second partner of the NPM-ALK hybrid, also can be translocated to chromosomal regions other than 2p23. For instance, NPM-MLF1 is the chimeric product of t(3;5)(q25.1;q34), which is associated with myelodysplastic syndromes. In a recent paper by Yoneda-Kato and colleagues, 49 overexpression of NPM-MLF-1 was capable of inducing apoptosis in NIH3T3 mouse fibroblasts. This induction required the presence of an intact NPM dimerization domain. Notably, co-expression of BCL-2 rescued the fibroblasts from NPM-MLF1-mediated cell death. 49
In summary, we have shown that expression of BCL-2 family proteins and AR differ significantly between ALK+ and ALK− ALCL of T/null lineage, and are not obviously related to PI. These findings further support the concept that ALK+ ALCL of T/null lineage is a clinicopathological entity, distinct from ALK− ALCL, as has been suggested by others. 50 The absence or low levels of anti-apoptotic proteins, such as BCL-2 and BCL-XL, probably plays a role in the good response to chemotherapy observed in patients with ALK+ ALCL.
Footnotes
Address reprint requests to L. Jeffrey Medeiros, MD, Department of Hematopathology, Box 72, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, Texas 77030. E-mail: jmedeiro@mail.mdanderson.org.
Supported in part by an Alexander S. Onassis Foundation scholarship (to G. Z. R.).
References
- 1.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 2.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, Schwarting R, Lennert K: The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66:848-858 [PubMed] [Google Scholar]
- 3.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263:1281-1284 [DOI] [PubMed] [Google Scholar]
- 4.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP: ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene 1997, 14:2175-2188 [DOI] [PubMed] [Google Scholar]
- 5.Bullrich F, Morris SW, Hummel M, Pileri S, Stein H, Croce CM: Nucleophosmin (NPM) gene rearrangements in Ki-1-positive lymphomas. Cancer Res 1994, 54:2873-2877 [PubMed] [Google Scholar]
- 6.Downing JR, Shurtleff SA, Zielenska M, Curcio-Brint AM, Behm FG, Head DR, Sandlund JT, Weisenburger DD, Kossakowska AE, Thorner P, Lorenzana A, Ladanyi M, Morris SW: Molecular detection of the (2;5) translocation of non-Hodgkin’s lymphoma by reverse transcriptase-polymerase chain reaction. Blood 1995, 85:3416-3422 [PubMed] [Google Scholar]
- 7.Lamant L, Meggetto F, al Saati T, Brugieres L, de Paillerets BB, Dastugue N, Bernheim A, Rubie H, Terrier-Lacombe MJ, Robert A, Rigal F, Schlaifer D, Shiuta M, Mori S, Delsol G: High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood 1996, 87:284-291 [PubMed] [Google Scholar]
- 8.Sarris AH, Luthra R, Papadimitracopoulou V, Waasdorp M, Dimopoulos MA, McBride JA, Cabanillas F, Duvic M, Deisseroth A, Morris SW, Pugh WC: Amplification of genomic DNA demonstrates the presence of the t(2;5)(p23;q35) in anaplastic large cell lymphoma, but not in other non-Hodgkin’s lymphomas, Hodgkin’s disease, or lymphomatoid papulosis. Blood 1996, 88:1771-1779 [PubMed] [Google Scholar]
- 9.Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, Delsol G, Mason DY: Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997, 89:1394-1404 [PubMed] [Google Scholar]
- 10.Pittaluga S, Wlodarska I, Pulford K, Campo E, Morris SW, Van den Berghe H, De Wolf-Peeters C: The monoclonal antibody ALK1 identifies a distinct morphological subtype of anaplastic large cell lymphoma associated with 2p23/ALK rearrangements. Am J Pathol 1997, 151:343-351 [PMC free article] [PubMed] [Google Scholar]
- 11.Lamant L, Dastugue N, Pulford K, Delsol G, Mariame B: A new fusion gene TPM3-ALK in anaplastic large cell lymphoma created by a (1;2)(q25;p23) translocation. Blood 1999, 93:3088-3095 [PubMed] [Google Scholar]
- 12.Hernandez L, Pinyol M, Hernandez S, Bea S, Pulford K, Rosenwald A, Lamant L, Falini B, Ott G, Mason DY, Delsol G, Campo E: TPK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood 1999, 94:3265-3268 [PubMed] [Google Scholar]
- 13.Ma Z, Cools J, Marynen P, Cui X, Siebert R, Gesk S, Schlegelberger B, Peeters B, De Wolf-Peeters C, Wlodarska I, Morris SW: Inv(2)(p23q35) in anaplastic large-cell lymphoma induces constitutive anaplastic lymphoma kinase (ALK) tyrosine kinase activation by fusion to ATIC, an enzyme involved in purine nucleotide biosynthesis. Blood 2000, 95:2144-2149 [PubMed] [Google Scholar]
- 14.Touriol C, Greenland C, Lamant L, Pulford K, Bernard F, Rousset T, Mason DY, Delsol G: Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood 2000, 95:3204-3207 [PubMed] [Google Scholar]
- 15.Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, Verhoef G, Menestrina F, Todeschini G, Paulli M, Lazzarino M, Giardini R, Aiello A, Foss H-D, Araujo I, Fizzotti M, Pelicci P-G, Flenghi L, Martelli MF, Santucci A: ALK+ lymphoma: clinico-pathological findings and outcome. Blood 1999, 93:2697-2706 [PubMed] [Google Scholar]
- 16.Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, Mori N, Fujimoto J, Miyauchi J, Mikata A: Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995, 86:1954-1960 [PubMed] [Google Scholar]
- 17.Gascoyne RD, Aoun P, Wu D, Chhanabhai M, Skinnider BF, Greiner TC, Morris SW, Connors JM, Vose JM, Viswanatha DS, Coldman A, Weisenburger DD: Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 1999, 93:3913-3921 [PubMed] [Google Scholar]
- 18.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM: Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984, 226:1097-1099 [DOI] [PubMed] [Google Scholar]
- 19.Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ: Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell 1985, 41:899-906 [DOI] [PubMed] [Google Scholar]
- 20.McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ: bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 1989, 57:79-88 [DOI] [PubMed] [Google Scholar]
- 21.McDonnell TJ, Korsmeyer SJ: Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18). Nature 1991, 349:254-256 [DOI] [PubMed] [Google Scholar]
- 22.Korsmeyer SJ: BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1999, 59:1693S-1700S [PubMed] [Google Scholar]
- 23.Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74:609-619 [DOI] [PubMed] [Google Scholar]
- 24.Minn AJ, Boise LH, Thompson CB: Bcl-x(S) antagonizes the protective effects of Bcl-x(L). J Biol Chem 1996, 271:6306-6312 [DOI] [PubMed] [Google Scholar]
- 25.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB: bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74:597-608 [DOI] [PubMed] [Google Scholar]
- 26.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ: Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med 1995, 182:821-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science 1998, 281:1322-1326 [DOI] [PubMed] [Google Scholar]
- 28.Tang SC, Visser L, Hepperle B, Hanson J, Poppema S: Clinical significance of bcl-2-MBR gene rearrangement and protein expression in diffuse large-cell non-Hodgkin’s lymphoma: an analysis of 83 cases. J Clin Oncol 1994, 12:149-154 [DOI] [PubMed] [Google Scholar]
- 29.Hermine O, Haioun C, Lepage E, d’Agay MF, Briere J, Lavignac C, Fillet G, Salles G, Marolleau JP, Diebold J, Reyas F, Gaulard P: Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin’s lymphoma. Groupe d’Etude des Lymphomes de l’Adulte (GELA). Blood 1996, 87:265-272 [PubMed] [Google Scholar]
- 30.Kramer MH, Hermans J, Parker J, Krol AD, Kluin-Nelemans JC, Haak HL, van Groningen K, van Krieken JH, de Jong D, Kluin PM: Clinical significance of bcl2 and p53 protein expression in diffuse large B-cell lymphoma: a population-based study. J Clin Oncol 1996, 14:2131-2138 [DOI] [PubMed] [Google Scholar]
- 31.Gascoyne RD, Adomat SA, Krajewski S, Krajewska M, Horsman DE, Tolcher AW, O’Reilly SE, Hoskins P, Coldman AJ, Reed JC, Connors JM: Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997, 90:244-251 [PubMed] [Google Scholar]
- 32.Gascoyne RD, Krajewska M, Krajewski S, Connors JM, Reed JC: Prognostic significance of Bax protein expression in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997, 90:3173-3178 [PubMed] [Google Scholar]
- 33.Bairey O, Zimra Y, Shaklai M, Okon E, Rabizadeh E: Bcl-2, Bcl-X, Bax, and Bak expression in short- and long-lived patients with diffuse large B-cell lymphomas. Clin Cancer Res 1999, 5:2860-2866 [PubMed] [Google Scholar]
- 34.Xerri L, Devilard E, Bouabdallah R, Hassoun J, Chaperot L, Birg F, Plumas J: Quantitative analysis detects ubiquitous expression of apoptotic regulators in B cell non-Hodgkin’s lymphomas. Leukemia 1999, 13:1548-1553 [DOI] [PubMed] [Google Scholar]
- 35.Schlaifer D, Krajewski S, Galoin S, Rigal-Huguet F, Laurent G, Massip P, Pris J, Delsol G, Reed JC, Brousset P: Immunodetection of apoptosis-regulating proteins in lymphomas from patients with and without human immunodeficiency virus infection. Am J Pathol 1996, 149:177-185 [PMC free article] [PubMed] [Google Scholar]
- 36.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD: The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol 1999, 10:1419-1432 [DOI] [PubMed] [Google Scholar]
- 37.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984, 133:1710-1715 [PubMed] [Google Scholar]
- 38.Shi S, Key M, Kalra K: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991, 39:741-748 [DOI] [PubMed] [Google Scholar]
- 39.Bruckheimer EM, Cho S, Brisbay S, Johnson DJ, Gingrich JR, Greenberg N, McDonnell TJ: The impact of bcl-2 expression and bax deficiency on prostate homeostasis in vivo. Oncogene 2000, 19:2404-2412 [DOI] [PubMed] [Google Scholar]
- 40.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, Yamamoto T: Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci USA 1996, 93:4181-4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai RY, Ouyang T, Miething C, Morris SW, Peschel C, Duyster J: Nucleophosmin–anaplastic lymphoma kinase associated with anaplastic large–cell lymphoma activates the phosphatidylinositol 3–kinase/Akt antiapoptotic signaling pathway. Blood 2000, 96:4319-4327 [PubMed] [Google Scholar]
- 43.Ladanyi M: Aberrant ALK tyrosine kinase signaling. Different cellular lineages, common oncogenic mechanisms. Am J Pathol 2000, 157:341-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghia P, Boussiotis VA, Schultze JL, Cardoso AA, Dorfman DM, Gribben JG, Freedman AS, Nadler LM: Unbalanced expression of bcl-2 family proteins in follicular lymphoma: contribution of CD40 signaling in promoting survival. Blood 1998, 91:244-251 [PubMed] [Google Scholar]
- 45.Dirks W, Schone S, Uphoff C, Quentmeier H, Pradella S, Drexler HG: Expression and function of CD95 (FAS/APO-1) in leukaemia-lymphoma tumour lines. Br J Haematol 1997, 96:584-593 [DOI] [PubMed] [Google Scholar]
- 46.Wellmann A, Thieblemont C, Pittaluga S, Sakai A, Jaffe ES, Siebert P, Raffeld M: Detection of differentially expressed genes in lymphomas using cDNA arrays: identification of clusterin as a new diagnostic marker for anaplastic large-cell lymphomas. Blood 2000, 96:398-404 [PubMed] [Google Scholar]
- 47.Collard MW, Griswold MD: Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry 1987, 26:3297-3303 [DOI] [PubMed] [Google Scholar]
- 48.Yang CR, Leskov K, Hosley-Eberlein K, Criswell T, Pink JJ, Kinsella TJ, Boothman DA: Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc Natl Acad Sci USA 2000, 97:5907-5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoneda-Kato N, Fukuhara S, Kato J: Apoptosis induced by the myelodysplastic syndrome-associated NPM-MLF1 chimeric protein. Oncogene 1999, 18:3716-3724 [DOI] [PubMed] [Google Scholar]
- 50.Haralambieva E, Pulford KA, Lamant L, Pileri S, Roncador G, Gatter KC, Delsol G, Mason DY: Anaplastic large-cell lymphomas of B-cell phenotype are anaplastic lymphoma kinase (ALK) negative and belong to the spectrum of diffuse large B-cell lymphomas. Br J Haematol 2000, 109:584-591 [DOI] [PubMed] [Google Scholar]