Abstract
Dynamic process of apoptosis has not been elucidated in adult rat cardiomyocytes. Soluble Fas ligand (0.1 μg/ml) in the presence of actinomycin D (0.05 μg/ml) induced apoptosis in cultured adult rat cardiomyocytes, as documented by activated caspase-3, DNA fragmentation, and apoptotic ultrastructure. In the present model, we observed 60 adult cardiomyocytes with a normal rod shape under a real-time videomicroscope continuously for 48 hours. Seventeen cells (28%) were unchanged and 7 cells (12%) showed oncosis (so-called necrosis) in which no beating was evident. In the remaining 36 cells (apoptosis, 60%), a slow beating (17 ± 3/min) was initiated 16 ± 1 hours later. Approximately 1 hour later, the rod cells showed long-axial shortening as bone- or club-like, or square-shaped, accompanied with faster beating rates (35 ± 7/min). In 29 cells (type A1 and A2), marked shrinkage occurred; the cellular shape became almost completely round with a smooth surface and the beating ceased 3.0 ± 0.4 hours later. Then, smooth budding appeared 0.6 ± 0.2 hours later. Apoptotic bodies were found in 8 cells 10 ± 4 hours later (type A1, 13%) but not in 21 cells (type A2, 35%). In the other 7 cells (type A3, 12%), the cell surface became rough 8 ± 3 hours later and the beating ceased. Maximal beating rate was greatest in type A1 (72 ± 26/min) and greater in type A2 (29 ± 5/min) than in type A3 (10 ± 2/min). Electron microscopy confirmed apoptotic ultrastructure even in the cardiomyocytes with bone-, club-like, or square shapes, suggesting that type A3 as well as A1 and A2 is also under apoptotic process. A caspase inhibitor, zVAD.fmk, blocked beating, apoptotic morphology, and DNA fragmentation, indicating these depended on caspase activation. In the caspase-dependent apoptotic process of cultured adult cardiomyocytes, beating and the following deformity of the cellular edges were the initial signs and the rate of beating was related with the subsequent three different processes of apoptosis.
The evidence of apoptosis documented by apoptotic ultrastructure, DNA fragmentation, and caspase 3 activation has been reported in the Fas-stimulated neonatal cardiomyocytes in culture. 1 Recently, it was also reported in cultured adult rat cardiomyocytes subjected to hypoxia/reoxygenation. 2
Apoptosis is known to be an energy-requiring process of cell death. 3 A series of apoptotic processes have been observed in various cell types in culture except for cardiomyocytes, using a videomicroscope. 4-7 These studies showed a dynamic process of apoptosis within hours common to various cell types. However, adult cardiac myocytes are terminally differentiated cells and are special in terms of both structure and function. They are provided with well-developed contractile proteins that are sensitive to Ca2+ load and continually contract and relax in the in vivo situations. It is suggested that compared with other cell types, the apoptotic process must be special in adult cardiac myocytes, deserving its specificity, ie, intrinsically provided active movement. Thus, it would be of extreme interest to observe the overall process of adult cardiomyocyte apoptosis in light of its dynamism.
In the present study, we tried to delineate the overall dynamic process of apoptosis using serial videomicroscopic recordings in adult cardiomyocytes in a Fas stimulation model. Fas, one of the representative death receptors, is well known to induce apoptosis in various cells including cardiac myocytes. 1,8 Up-regulation of Fas has been reported at both mRNA and protein levels in various pathological conditions of the heart. Hypoxia up-regulated Fas mRNA in hypoxic cultured cardiomyocytes 9 and Fas protein was augmented in salvaged myocytes after infarction. 10 Fas may participate in cardiomyocyte death during acute myocardial infarction, 11 and hearts affected by autoimmune myocarditis overexpressed both Fas and Fas ligand. 12 Recently, volume overload and adriamycin treatment have been reported to up-regulate Fas in the heart, 13,14 which suggest the possible involvement of the Fas/Fas ligand system in the pathophysiology of various heart diseases. Secondly, the videomicroscopic morphology was compared with electron microscopic findings and DNA fragmentation.
Materials and Methods
Isolation and Culture of Cardiomyocytes
Isolation and culture of adult rat ventricular cardiomyocytes from adult male Sprague-Dawley rats (200 to 250 g) were performed using previously established techniques with slight modifications. 15-17 Rod-shaped cells at concentration of 5.0 × 10 4 cells/ml were plated in laminin-coated dishes or slide glass chambers, and incubated with a serum-free Krebs-Ringer buffer containing 200 μmol/L CaCl2 and 50 μg/ml gentamicin for 3 hours in a CO2 incubator (95% air-5% CO2) before the experiments.
Treatments of Cardiomyocytes
To induce apoptosis, the cardiomyocytes were incubated with 0.1 μg/ml soluble Fas ligand (FasL; Oncogene, Boston, MA) plus 0.05 μg/ml actinomycin D (Wako, Osaka, Japan) up to 48 hours., which were compared with those incubated with the buffer alone (control). The effect of caspase inhibitor was examined on the present apoptosis system by the simultaneous treatment with zVAD.fmk (25 μmol/L; Enzyme Systems Products, Livermore, CA). An oncosis model was prepared by the addition of surfactant, Triton X (Sigma, St. Louis, MO), at the concentration of 0.001% to the cultured cardiomyocytes.
Analysis of the Dynamic Process of Adult Cardiomyocyte Apoptosis by Serial Videomicroscopic Recording
A plastic incubation chamber in which a mixed gas of 95% air and 5% CO2 was constantly infused and the temperature controlled at 37°C (Olympus, Tokyo, Japan) was placed on the stage of an Olympus IMT-2 inverted microscope, and a dish with cultured cells was transferred to the chamber. For videomicroscopy, a charge-coupled device camera was interfaced between the microscope and the tape recorder. Images at a magnification of ×400 were recorded at normal time for 48 hours. Sixty adult cardiomyocytes with a normal rod shape were recorded just after the treatment with 0.1 μg/ml FasL plus 0.05 μg/ml actinomycin D. To check caspase involvement in the present model, 30 normal rod-shaped cells simultaneously treated with 25 μmol/L of zVAD.fmk, in addition to FasL plus actinomycin D, were recorded similarly. In separate experiments, 35 normal rod-shaped cells incubated with the Krebs-Ringer buffer alone were similarly recorded for 48 hours as a normal control group, as were 20 other normal rod-shaped cells incubated with the buffer containing 0.001% Triton X as an oncosis model.
Viability, DNA Fragmentation, and Caspase-3 Activity of Adult Cardiomyocyte Apoptosis In Vitro
Assessment of Cell Viability: After 0-, 6-, 24-, and 48-hour culture, cells were exposed to 0.1% trypan blue for 5 minutes, and the number of stained and unstained cells in the dishes was counted. Experiments were done in triplicate.
DNA Extraction and Electrophoresis: DNA released from 4 × 10 5 cardiomyocytes were extracted after 0-, 6-, and 24-hour culture and separated by electrophoresis in agarose gels according to Arends and colleagues. 18
DNA in Situ Nick End-Labeling (TUNEL): After a 0-, 6-, and 24-hour incubation, the cells cultured on slide chambers were fixed with 10% buffered formalin solution for 6 hours at room temperature. TUNEL was performed on them using an ApopTag in situ apoptosis detection kit (Intergen, Purchase, NY). Slides were then counterstained using hematoxylin. The positive control was prostate tissue from a rabbit castrated 2 days before.
Caspase-3 Activity: Activation of caspase-3 was detected after 0-, 6-, 12-, 24-, and 48-hour culture by the caspase-3 colorimetric protease assay kit (MBL, Nagoya, Japan). This assay is based on spectrophotometric detection of the chromophore p-nitroanilide (pNa) after cleavage from the labeled caspase substrate.
Transmission and Scanning Electron Microscopy
After 0-, 24-, and 48-hour culture, the cells on dishes and those on slide chambers were fixed with phosphate-buffered 2.5% glutaraldehyde (pH 7.4) for 4 hours followed by postfixation with 1% osmium tetroxide for 1 hour and prepared for transmission and scanning electron microscopy in conventional manners. For transmission electron microscopy, cells were dehydrated, embedded in Epon medium, and cut into ultrathin sections (80 nm). They were stained with uranyl acetate and lead citrate, and observed with an H-800 Hitachi transmission electron microscope (Hitachi, Tokyo, Japan). For scanning electron microscopy, cells were dehydrated, critical-point dried, gold coated, and examined with an S-450 Hitachi scanning electron microscope.
Statistical Analysis
Values were expressed as the mean ± SEM. Statistical comparisons were performed by analysis of variance followed by Newman-Keul’s multiple comparisons test. A P value <0.05 was considered significant.
Results
Videomicroscopic Findings of the Serial Dynamic Process of Apoptosis Specific for Adult Cardiomyocytes
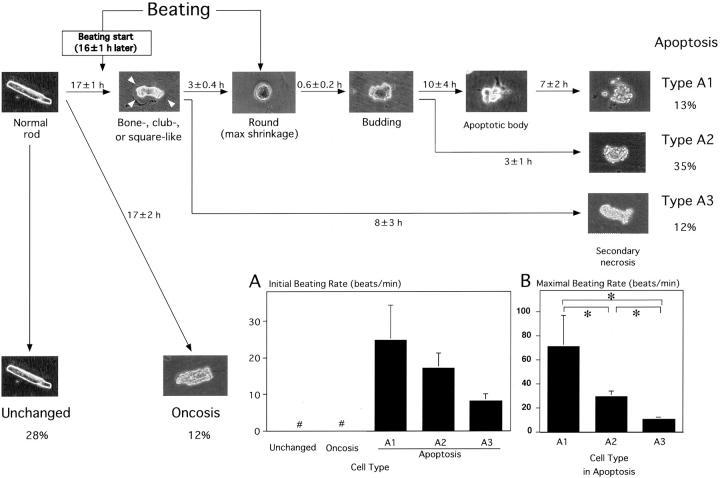
The overall process of structural and functional alterations of the 60 Fas-stimulated adult cardiomyocytes is summarized in Figure 1 ▶ . All 60 cardiomyocytes showed normal rod shapes and no beating at the start of the experiment. Of the 60 normal cardiomyocytes, 17 cells (28%) showed no morphological change and no beating within 48 hours after Fas stimulation. They preserved their smooth-surface rod shape throughout the time period. In the other 36 rod-shaped cells (apoptosis, 60%), a slow and intermittent beating (17 ± 3 beats/min) was initiated at 16 ± 1.4 hours after the treatment. Approximately 1 hour later, the cellular long-axial diameter was shortened as either a bone-like shape with rounded edges, a club-like shape with rounding of one-side edge, or square shaped without deformity of the cellular edges. These cell surfaces were smooth. In 29 cells of apoptosis (type A1 and A2), cellular shrinkage progressed and was accompanied by a faster beating compared with the initial slow beating. When they attained the maximal shrinkage to become almost completely round cells with a smooth surface 3.0 ± 0.4 hours later, the beating disappeared. Then, after 0.6 ± 0.2 hours, multiple budding formation occurred on the cells, of which the surface was smooth. Eight cells (type A1, 13%) showed further formation of apoptotic bodies 10 ± 4 hours later, whereas 21 cells (type A2, 35%) did not. Cell surfaces of apoptotic bodies were preserved smooth. However, most cell surfaces of types A1 and A2 were gradually rough to become secondary postapoptotic necrosis (7 ± 2 hours later in type A1 and 3 ± 0.7 hours in type A2). In seven other cells of apoptosis (type A3, 12%), the cell surface of club- or bone-like-shaped cardiomyocytes became rough (secondary postapoptotic necrosis) 8 ± 3 hours later, and the beating ceased. There was no budding or apoptotic body formation in type A3. In the remaining seven cells (oncosis, 12%), the cell surface gradually became rough at 17 ± 2 hours, accompanied with no beating and neither bone-, club-, or square-like deformation, completely rounded, budding or apoptotic body formation (oncotic necrosis). The cell type population is summarized in Table 1 ▶ .
Figure 1.
Schematic presentation of the overall process of serial morphological and functional changes in 60 adult cardiomyocytes treated with FasL plus actinomycin D based on 48-hour videomicroscopic observation. Inset includes bar graphs comparing the initial beating rate (A) and the maximal beating rate (B) among types A1, A2, and A3 (apoptosis), oncosis, and the unchanged. *, A significant difference at P < 0.05.
Table 1.
Percentage of Types of Cardiomyocytes (Apoptosis, Oncosis, and Unchanged) after 48 Hours Incubation in the Control Group, Triton X-Treated Group (Oncosis Model), FA-Treated Group, and FA and zVAD.fmk-Treated Group
| Group | n | Apoptosis, % | Type A1, % | Type A2, % | Type A3, % | Oncosis, % | Unchanged, % |
|---|---|---|---|---|---|---|---|
| Control | 35 | 15 | 0 | 11 | 3 | 9 | 77 |
| Triton X | 20 | 0 | 0 | 0 | 0 | 100 | 0 |
| FA | 60 | 60 | 13 | 35 | 12 | 12 | 28 |
| FA+zVAD.fmk | 30 | 30 | 0 | 17 | 13 | 7 | 63 |
FA, soluble Fas ligand (0.1 μg/ml) plus actinomycin D (0.05 μg/ml).
Beating was specific for types A1, A2, and A3 cells, but was not seen in oncosis and in the unchanged cells. The initial slow beating (type A1, 24 ± 9 beats/min; type A2, 16 ± 4 beats/min; type A3, 8 ± 2 beats/min) was continually replaced by the faster beating as the morphological alteration proceeded. The maximal beating rate was 71 ± 26 beats/min in type A1, 29 ± 5 beats/min in type A2, and 10 ± 2 beats/min in type A3; there were significant differences among them (Figure 1) ▶ .
Beating cells were decreased in the group by the additional treatment with the caspase inhibitor, zVAD.fmk (Table 1) ▶ . Of 30 adult cardiomyocytes, only 9 cells (30%) showed beating and type A2 (17%) or type A3 (13%) change. In addition, 2 cells (7%) were oncotic and 19 cells (63%) were unchanged.
In 35 adult cardiomyocytes incubated with medium alone (control), 27 cells (77%) were unchanged and 3 cells (9%) underwent oncotic processes. The remaining five cells (14%) behaved in a similar manner to apoptosis as seen in the Fas-stimulated group; four cells (11%) were type A2, and one cell was type A3.
In the surfactant-induced oncosis model, the cells showed a rigorous contracture after the cell surface became severely rough. All finally became fluffy and oval- to round-shaped and finished the alteration within 1 hour after the treatment with 0.001% Triton X. They never showed spontaneous beating, bone-, club-, or square-like deformation, complete rounding, budding, or apoptotic body formation during the course.
Effect of Fas-Stimulation on Cell Viability, DNA Fragmentation, and Caspase-3 Activity
The viability of the adult rat cardiomyocytes incubated with FasL plus actinomycin D was significantly decreased to 73.2 ± 5.0% after 24 hours and to 65.2 ± 6.3% of the cardiomyocytes after 48 hours, when compared with the control values at the corresponding times (Figure 2A) ▶ .
Figure 2.
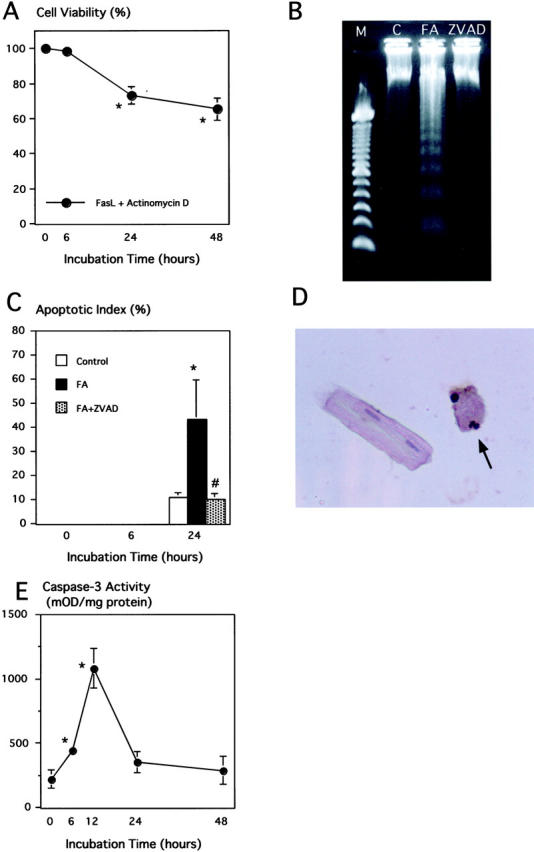
Effect of Fas stimulation on viability, DNA fragmentation, and caspase-3 actiivty of cultured adult cardiomyocytes. A: The percentage of the dye-excluding cells were calculated in proportion to the vehicle control at the corresponding time. *, P < 0.05 compared with the control. B: Gel electrophoresis of DNA extracted from cultured cardiomyocytes incubated for 24 hours with normal buffer (C), FasL plus actinomycin D (FA), or FA plus zVAD.fmk (ZVAD). Lane M indicates 100-bp marker ladder. Lane FA shows a clear ladder pattern. C: Apoptotic index of Fas-stimulated cardiomyocytes at 0, 6, and 24 hours after incubation. Note that there was no evidence of TUNEL-positive cells at 0 and 6 hours. FA, FasL plus actinomycin D; ZVAD, FA plus zVAD.fmk. *, P < 005 versus control; #, P < 0.05 versus FA. D: A TUNEL-positive (closed arrow) and negative adult cardiomyocytes (open arrows) after 24 hours incubation with FasL plus actinomycin D. Original magnification, ×400. E: Quantitative analysis of caspase-3 activity in cultured adult cardiomyocytes treated with FasL plus actinomycin D. Caspase-3 activity was measured using DEVD-pNa; n = 5. *, P < 0.05 compared with control.
DNA ladder and TUNEL positivity were not observed 0 and 6 hours after Fas stimulation. However, only in the Fas-stimulated group without the caspase inhibitor, the DNA ladder appeared at 24 hours after stimulation (Figure 2B) ▶ . The apoptotic index based on TUNEL at 24 hours was 10.7 ± 1.9% in the control and 43.8 ± 16.3% in the Fas-stimulated group (P < 0.05). Treatment with zVAD.fmk concealed the DNA ladder pattern and significantly reduced the apoptotic index (9.8 ± 2.6%, P < 0.05) (Figure 2, B and C) ▶ . TUNEL positivity was never observed in rod-shaped cardiomyocytes (Figure 2D) ▶ .
There was a significant increase in caspase-3 activity after 6 hours of Fas stimulation that peaked at 12 hours (Figure 2E) ▶ . The activity was: millioptical density/mg protein, 445 ± 32 at 6 hours and 1083 ± 156 at 12 hours whereas the baseline value at 0 hour was 221 ± 74.
Ultrastructural Features
At 24 and 48 hours after Fas stimulation, cardiomyocytes with various shapes described in videomicroscopic findings were observed (Figure 3) ▶ . That is, the rod-shaped cardiomyocytes showed almost normal ultrastructure (Figure 3A) ▶ . The cells with shortening of the long-axis, such as the bone-like, club-like or square-shaped cells already had glossy condensation of chromatin as an apoptotic nuclear change in the periphery of the nucleus (Figure 3B) ▶ . In the cytoplasm, myofibrillar derangement with disappearance of the Z-band was partially evident especially in the edges of the bone- or club-like shaped cells (Figure 3B) ▶ . The smooth-surface round cells with maximal shrinkage showed an extensive condensation of nuclear chromatin, fragmented nuclei, and shriveled cytoplasm containing diffusely deranged myofibrils (Figure 3C) ▶ . Their plasma membrane was intact in most cardiomyocytes. The cells with multiple budding were seen (Figure 3D) ▶ ; these buddings were relatively big and appeared smooth. Smooth budding contained fragmented nuclei containing condensed chromatin, a clump of mitochondria, and deranged myofibrils. Notably, although nuclear changes were indeed typically apoptotic with respect to the sharp delineation, and the condensed chromatin mostly shaped doughnut-like but not half moon- or horseshoe-like (Figure 3; B, C, and D ▶ ). Moreover, mitochondria appeared highly condensed and contained wrinkled bodies inside (Figure 3; C, D, and E ▶ ). Typical apoptotic bodies were also demonstrated (Figure 3E) ▶ . Secondary postapoptotic necrosis, defined as degeneration of subcellular organelles and rupture of the plasma membrane despite the presence of apoptotic condensation of chromatin, was observed. The number of round cardiomyocytes with secondary postapoptotic necrosis was increased at 48 hours after Fas stimulation. A part of the round cells showed the features of oncotic necrosis such as oncotic nuclear change, disrupted plasma membrane, degeneration of cytoplasmic organelles, swollen mitochondria with amorphous dense bodies, and myofibril supercontraction surrounded by edematous cytoplasm (Figure 3F) ▶ .
Figure 3.
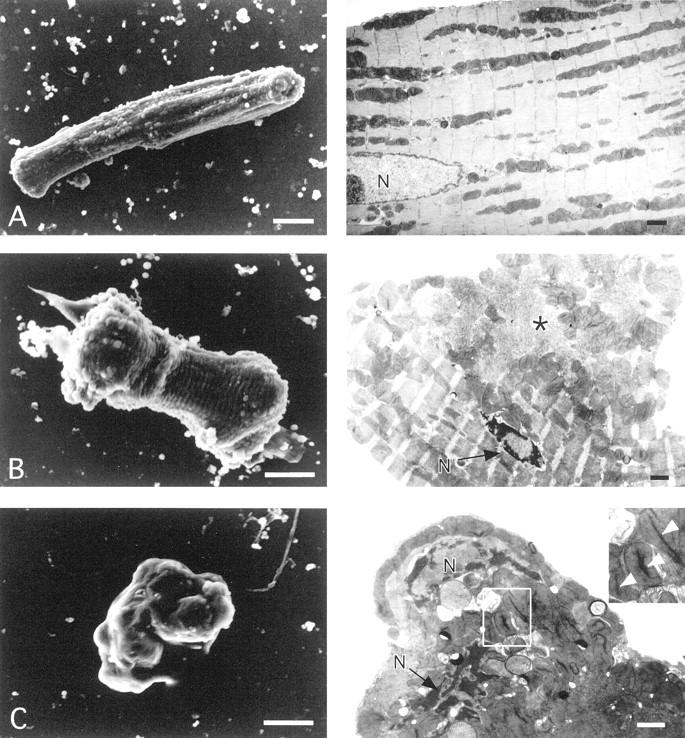
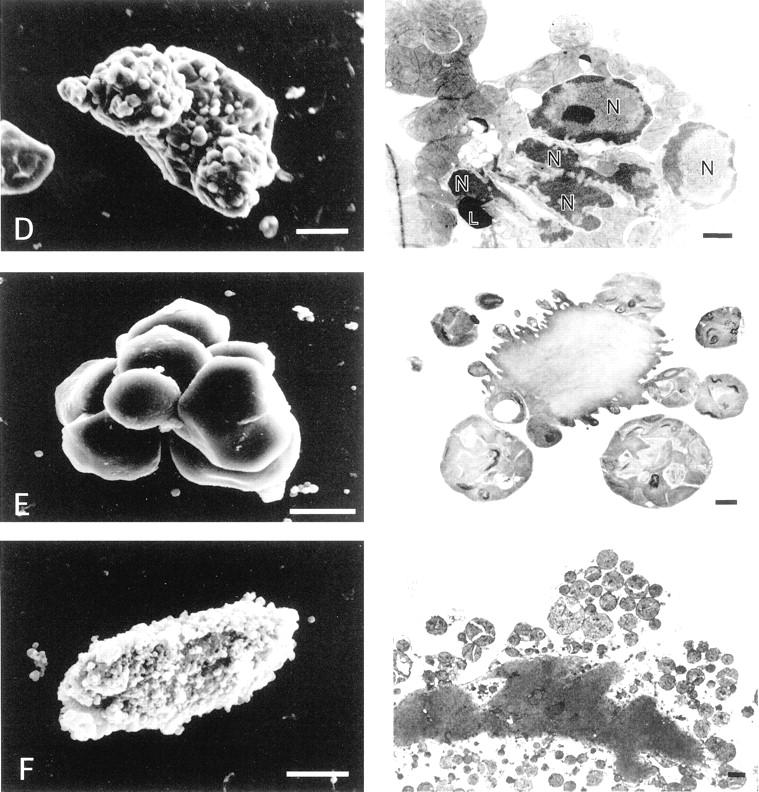
Scanning (left) and transmission (right) electron micrographs of cultured adult cardiomyocytes treated with FasL plus actinomycin D. Scale bars, 10 μm (left); and 1 μm (right). A: A normal rod-shaped cell. The nucleus (N), myofibrils (Mf), and mitochondria (Mt) appear intact. B: A bone-like-shaped cell. At this stage, well-defined, condensed chromatin was distributed to the periphery of the nucleus (N), and myofibrils were partially deranged, losing the Z-band (an asterisk indicates the position). C: A round cell with maximal shrinkage. The cell surface is smooth. Fragmented nuclei (N) containing condensed chromatin with a doughnut-like shape, condensed mitochondria, and a lipid-like structure (L) are observed. Myofibril rows (Mf) are completely deranged. Inset: The square-surrounded portion with higher magnification, showing details of mitochondrial changes. Arrowheads indicate wrinkled bodies in the condensed mitochondria. D: A cell with extensive buddings. Membrane rupture is not evident. This budding contained extensively fragmented nuclei with condensed chromatin (N) and a lipid-like structure (L). E: A cardiomyocyte forming apoptotic bodies (cellular fragmentation). The surface of each apoptotic body is smooth. Each apoptotic body contains subcellular organelles such as condensed mitochondria with wrinkled bodies and deranged myofibrils. F: An oncotic cell with extensive rupture of the plasma membrane and release of cellular contents. Mitochondrial changes of oncosis, such as swelling, disruption of cristae, and amorphous dense bodies inside, are very distinct from those of apoptosis.
Discussion
Using a videomicroscope, the present study revealed an overall series of dynamic processes of classic apoptosis in cultured adult cardiomyocytes. The dynamic process of apoptosis was previously studied using a videomicroscope in various cell types except for cardiomyocytes. As beating was not present in those cells, the apoptotic process was examined by the use of a time-lapse videomicroscope that consisted of intermittent recording. 4-7 In contrast, the present study needed a serial recording of real time to reveal specific processes of cardiomyocyte apoptosis being accompanied by beating.
Apoptosis Is Induced in Adult Cardiomyocytes by Fas Stimulation
The present study showed the appearance of apoptotic ultrastructure in adult cardiomyocytes 24 and 48 hours after Fas stimulation, in addition to caspase-3 activation and DNA fragmentation indicated by positive TUNEL and DNA ladder. These were significantly inhibited by a caspase inhibitor.
The apoptotic ultrastructure in adult cardiomyocytes was basically similar to that seen in other cell types: cytoplasmic shrinkage, nuclear chromatin condensation, nuclear fragmentation, budding or blebbing on the cell surface, cellular fragmentation (apoptotic body formation), and final degeneration (secondary apoptotic necrosis). 4-7,19 In addition to these common features, lipid-like structures reported in neonatal cardiomyocyte apoptosis were also observed. 1 Another distinct feature was the mitochondrial change in apoptotic cells; a close observation revealed the increased electron density of mitochondria and wrinkled cristae in some of these mitochondria. This was in contrast to the mitochondrial changes in oncotic cardiomyocytes, ie, swollen mitochondria with decreased electron density that contained disintegrated cristae and amorphous dense bodies. 20 Details of apoptotic chromatin condensation in adult cardiomyocytes were slightly distinct from those seen in neonatal cardiomyocytes or other cells; condensed chromatin mostly doughnut-like-shaped, but not half moon- or horseshoe-like-shaped.
Under videomicroscopy, type A1 showed adult cardiomyocytes changing from long axial shortening with bone-, club-, or square-like shapes, rounding and budding formation in the midway, into apoptotic bodies and secondary necrosis; type A2 showed those from long axial shortening with bone-, club-, or square-like shapes, rounding, budding formation, and then directly into secondary necrosis; and type A3 showed long-axial shortening with bone-, club-, or square-like shapes directly into secondary necrosis. According to the present electron microscopic analysis, adult cardiomyocytes with long-axial shortening (bone-, club-, or square-like shapes) showed apoptotic ultrastructure, like those with smooth-surface round shapes, budding, apoptotic body, and secondary postapoptotic necrosis. Thus, types A2 and A3 as well as type A1 were considered to be under apoptotic processes.
Caspase-3 was activated in the present model of cardiomyocyte apoptosis. Moreover, additional treatment with a caspase inhibitor, zVAD.fmk, decreased the apoptotic cells, indicating the apoptotic process in the present system was caspase-dependent.
Role of Beating in Apoptosis of Adult Cardiomyocytes
Normally, spontaneous beating is rare in adult cardiomyocytes when cultured in a serum-free medium (rapid attachment model, a popular culture model of adult cardiomyocytes). 21 Under the videomicroscope, the cells that preserved the rod shape for 48 hours after the treatment and oncotic cells did not show beating. In contrast, apoptotic cardiomyocytes first initiated beating when they were still normal rod shapes with neither apoptotic morphology nor DNA fragmentation based on TUNEL, and continued beating until the cells maximally shriveled to be round-shaped. These findings indicated that beating is an initial sign of the apoptotic process.
It was reported that apoptotic stimulation induces elevation of intracellular Ca2+ level in lymphocytes, 22 and that this elevation might result from changes in sarcoplasmic reticulum Ca2+ regulatory proteins, eg, up-regulation of the inositol triphosphate receptor (IP3R), that occurred during the apoptotic process. 23 Felzen and colleagues 24 recently found electrophysiological alterations such as reduction in the resting potential and action potential amplitudes, increase in action potential duration, elevated diastolic intracellular Ca2+, and increased arrhythmogenic activity in isolated adult cardiomyocytes when they were treated with the agonistic Fas antibody. Importantly, these changes were observed during the very early phase of incubation with the Fas agonist (3 hours) when the apoptotic morphology was not yet apparent. They also reported an association of IP3R up-regulation with the electrophysiological alterations. Cardiomyocyte beating (repeated contraction and relaxation) is regulated by changes in the intracellular Ca2+ level. 25 Therefore, it is possible that in adult cardiomyocytes, both the electrophysiological phenomena described by Felzen and colleagues 24 and the beating in the present study are on the same line: early signs of apoptosis resulting from intracellular apoptotic events occurring before apoptosis, ie, morphological change and DNA fragmentation. Because Fas up-regulation has been reported in myocardial infarction, 10,11 it is interesting to hypothesize that Fas may have a certain role in postmyocardial infarction arrhythmia.
Distinct apoptotic morphologies were identified in the present study, but it is not precisely clear what these morphologies mean. However, we noted a significant difference in the maximal beating rate between types A1, A2, and A3. Thus, the maximal beating rates are apparently related to the subsequent apoptotic process. Because both apoptosis and beating are energy-dependent, rapid or slow beating may reflect the original energy state of the isolated cardiomyocytes. The membrane potential necessary to maintain membrane integrity is also energy-dependent. Therefore, it is suggested that a cell with a high energy level can present a rapid beating rate and complete the apoptotic process from long-axial shortening into apoptotic bodies (type A1). Meanwhile, another with low energy level may show only a slow beating rate and may not be able to form apoptotic bodies (type A2) or budding (type A3) because of membrane damage during the early stage of apoptosis. However, because these speculations are supported by only indirect data, further experiments will be necessary in the future to confirm the hypotheses.
Study Limitation
Two culture models of isolated adult mammalian cardiomyocytes are known, the redifferentiation model and the rapid attachment model, as Jacobson and Piper 21 described. Cardiomyocytes of the rapid attachment model have a merit in maintaining the characteristic morphological features of intact cardiomyocytes. However, spontaneous beating is rare. In contrast, the redifferentiated model in which serum is supplemented in the culture media has a problem in its validity as a model of terminally differentiated cells in vivo because of loss of the specific myocyte structure. 26-28 The detection of apoptotic morphology using this method is not easy because of the cellular deformity, although these cells contract spontaneously. Thus, we used the rapid attachment model in the present study, although a study using the redifferentiation model would also be interesting.
While observing the attached cardiomyocytes under the videomicroscope, a considerable number of the cells floated away and were eliminated from the study. Cell floating occurred not only in the Fas-stimulated group but also in the untreated group. Evaluation of these cells remains unknown.
The Fas/Fas ligand system is one of several well-investigated systems inducing apoptosis. 8 Recently, it was reported that soluble FasL or anti-Fas antibody alone had difficulties inducing apoptosis in neonatal cardiac myocytes, although in the presence of a nontoxic amount of actinomycin D, cycloheximide, or doxorubicin, these cells easily commit to apoptosis by Fas stimulation. 1,29 However, the precise mechanism of this phenomenon remains presently undetermined.
Conclusion
In the Fas-induced, caspase-dependent apoptosis of cultured adult cardiomyocytes, beating and the subsequent deformity of the cellular edges are the initial signs, and the beating rate is associated with the three distinct patterns of the apoptotic processes.
Acknowledgments
We thank Toshie Ohtsubo and Akiko Tsujimoto for technical assistance. We are also indebted to Dr. Yoshihiro Uno (Gifu University School of Medicine) for helpful discussion.
Footnotes
Address reprint requests to Hisayoshi Fujiwara, MD, PhD, Second Department of Internal Medicine, Gifu University School of Medicine, 40 Tsukasa-Machi, Gifu 500-8705, Japan. E-mail: gifuim-gif@umin.ac.jp.
Supported, in part, by Grants-in-Aid for Scientific Research (no.11670668 and no.12670704) from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1.Takemura G, Kato S, Aoyama T, Hayakawa Y, Kanoh M, Maruyama R, Arai M, Nishigaki K, Minatoguchi S, Fukuda K, Fujiwara T, Fujiwara H: Characterization of ultrastructure and its relation with DNA fragmentation in Fas-induced apoptosis of cultured cardiac myocytes. J Pathol 2001, 193:546-556 [DOI] [PubMed] [Google Scholar]
- 2.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S: Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 2000, 87:118-125 [DOI] [PubMed] [Google Scholar]
- 3.Richter C, Schweizer M, Cossarizza A, Franceschi C: Control of apoptosis by the cellular ATP level. FEBS Lett 1996, 378:107-110 [DOI] [PubMed] [Google Scholar]
- 4.Evan GI, Wyllie A, Gilbert C, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC: Induction of apoptosis in fibroblasts by c-myc protein. Cell 1992, 69:119-125 [DOI] [PubMed] [Google Scholar]
- 5.Mills JC, Wang S, Erecinska M, Pittman RN: Use of cultured neurons and neuronal cell lines to study morphological, biochemical, and molecular changes occurring in cell death. Methods Cell Biol 1995, 46:217-242 [DOI] [PubMed] [Google Scholar]
- 6.Morris VL, Schmidt EE, MacDonald IC, Groom AC, Chambers AF: Sequential steps in hematogenous metastasis of cancer cells studied by vivo videomicroscopy. Invasion Metastasis 1997, 17:281-296 [PubMed] [Google Scholar]
- 7.Chang SH, Phelps PC, Berezesky IK, Ebersberger ML, Trump BF: Studies on the mechanisms and kinetics of apoptosis induced by microinjection of cytochrome c in rat kidney tubule epithelial cells. Am J Pathol 2000, 156:637-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M: Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res 1994, 75:426-433 [DOI] [PubMed] [Google Scholar]
- 10.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P: Apoptosis and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 1996, 74:86-107 [PubMed] [Google Scholar]
- 11.Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, Debatin KM: Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation 2000, 102:915-920 [DOI] [PubMed] [Google Scholar]
- 12.Ishiyama S, Hiroe M, Nishikawa T, Shimojo T, Abe S, Fujisaki H, Ito H, Yamakawa K, Kobayashi N, Kasajima T, Marumo F: The Fas/Fas ligand system is involved in the pathogenesis of autoimmune myocarditis in rats. J Immunol 1998, 161:4695-4701 [PubMed] [Google Scholar]
- 13.Wollert KC, Heineke J, Westermann J, Ludde M, Fiedler B, Zierhut W, Laurent D, Bauer MK, Schulze-Osthoff K, Drexler H: The cardiac Fas (APO-1/CD95) receptor/Fas ligand system. Relation to diastolic wall stress in volume-overload hypertrophy in vivo and activation of the transcription factor AP-1 in cardiac myocytes. Circulation 2000, 101:1172-1178 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Ueda Y, Juan Y, Ktsuda S, Takahashi H, Koh E: Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats. In vivo study. Circulation 2000, 102:572-578 [DOI] [PubMed] [Google Scholar]
- 15.Piper HM, Probst I, Schwartz P, Hutter FJ, Spieckermann PG: Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol 1982, 14:397-412 [DOI] [PubMed] [Google Scholar]
- 16.Farmer BB, Mancina M, Williams ES, Watanabe AM: Isolation of calcium tolerant myocytes from adult rat heart: review of the literature and description of a method. Life Sci 1983, 33:1-18 [DOI] [PubMed] [Google Scholar]
- 17.Khalid MA, Ashraf M: Direct detection of endogenous hydroxyl radical production in cultured adult cardiomyocytes during anoxia and reoxygenation. Is hydroxyl radical really the most damaging radical species? Circ Res 1993, 72:725-736 [DOI] [PubMed] [Google Scholar]
- 18.Arends MJ, Morris RG, Wyllie AH: Apoptosis. The role of the endonuclease. Am J Pathol 1990, 136:593-608 [PMC free article] [PubMed] [Google Scholar]
- 19.Majno G, Joris I: Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol 1995, 146:3-15 [PMC free article] [PubMed] [Google Scholar]
- 20.Ganote CE, Vander Heide RS: Irreversible injury of isolated adult rat myocytes. Osmotic fragility during metabolic inhibition. Am J Pathol 1988, 132:212-222 [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson SL, Piper HM: Cell cultures of adult cardiomyocytes as models of the myocardium. J Mol Cell Cardiol 1986, 18:661-678 [DOI] [PubMed] [Google Scholar]
- 22.Oshimi Y, Miyazaki S: Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated Ca2+ level. J Immunol 1995, 154:599-609 [PubMed] [Google Scholar]
- 23.Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH: Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-triphosphate receptor. Science 1996, 273:503-507 [DOI] [PubMed] [Google Scholar]
- 24.Felzen B, Shilkrut M, Less H, Sarapov I, Maor G, Coleman R, Robinson RB, Berke G, Binah O: Fas (CD95/Apo-1)-mediated damage to ventricular myocytes induced by cytotoxic T lymphocytes from perforin-deficient mice. A major role for inositol 1,4,5-triphosphate. Circ Res 1998, 82:438-450 [DOI] [PubMed] [Google Scholar]
- 25.Thandroyen FT, Morris AC, Hagler HK, Ziman B, Pai L, Willerson JT, Buja LM: Intracellular calcium transients and arrhythmia in isolated heart cells. Circ Res 1991, 69:810-819 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson SL: Culture of spontaneously contracting myocardial cells from adult rats. Cell Structure Function 1977, 2:1-9 [Google Scholar]
- 27.Claycomb WC, Palazzo MC: Culture of the terminally differentiated adult cardiac muscle cell. A light and scanning electron microscope study. Dev Biol 1980, 80:466-482 [DOI] [PubMed] [Google Scholar]
- 28.Nag AC, Cheng M: Adult mammalian cardiac muscle cells in culture. Tissue Cell 1981, 13:515-523 [DOI] [PubMed] [Google Scholar]
- 29.Yamaoka M, Yamaguchi S, Suzuki T, Okuyama M, Nitobe J, Nakamura N, Mitsui Y, Tomoike H: Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J Mol Cell Cardiol 2000, 32:881-889 [DOI] [PubMed] [Google Scholar]