Abstract
Inappropriate neutrophil activation has been implicated in the pathology of several clinically important inflammatory conditions. Although murine models are extensively used in the investigation of such pathological processes, a reliable method by which viable, quiescent neutrophils can be isolated from murine blood has not been developed. Here we describe a novel method based on negative immunomagnetic separation, which yields highly pure populations of murine neutrophils. Blood is incubated with a cocktail of antibodies against specific cell markers on unwanted cells, and then with secondary antibody-coated magnetic beads. After running the preparation through a column within a magnetic field, labeled cells are retained, and a neutrophil-rich effluent is collected. This method yields a >95% pure suspension of >97% viable neutrophils, recovering ∼70% of neutrophils from whole blood. Flow cytometric analysis shows little difference in surface L-selectin and CD18 expression on isolated neutrophils compared with neutrophils in whole blood, indicating that neutrophils are minimally activated bythe isolation process. Stimulation with phorbol 12-myristate 13-acetate (PMA) reduced L-selectin andincreased CD18 expression. Isolated neutrophilsmigrate under agarose in response to fMLP, and fluorescently labeled neutrophils transfused into recipient mice interact with postcapillary venules in a manner comparable to endogenous leukocytes. These findings show that neutrophils isolated using this method can be used for inflammatory studies in vitro and in vivo.
Neutrophils are phagocytic leukocytes involved in host defense against infection, which can also contribute to pathology in inflammatory diseases. An understanding of the role of neutrophils in inflammation is of particular importance in the study of inflammatory diseases such as rheumatoid arthritis, 1 ischemia-reperfusion injury, 2 and acute respiratory distress syndrome. 3 Neutrophils migrate from vessels in response to inflammatory stimuli. This process has many stages and is regulated at each by a range of inflammatory mediators and adhesion molecules. 4-6 Leukocyte migration mechanisms have been studied extensively using isolated human neutrophils and cell lines both in vitro and in vivo. 7-9 In addition, many in vivo models of inflammation have been developed in mice because of the wide availability of genetically modified strains. 10 For example, genetically modified mice have been used to examine the role of adhesion molecules and chemokine receptors in inflammation in vivo. 11-15 Potentially informative studies in which interactions of neutrophils from one genetic strain are studied in vessels of another genetic strain have been limited, however, by the lack of a reliable method for isolation of murine neutrophils from whole blood.
Methods currently available for the isolation of mouse neutrophils rely either on differences in the buoyancy of mouse blood cells for density gradient centrifugation 16,17 or require a neutrophil-specific migratory stimulus to be administered in vivo into a body cavity. 18,19 The former method does not permit reproducibly pure populations of neutrophils to be isolated, as differences in buoyancy between mouse leukocytes are not sufficient for easy separation. Hematological variation between different mouse strains further limits this technique. Using the latter method, neutrophils migrate into a cavity in response to an inflammatory stimulus to be isolated. Although this method elicits highly pure neutrophil populations, migrated neutrophils display a phenotype that is dramatically different compared to quiescent neutrophils, for example with regard to their internal cytoskeletal arrangement and surface expression of adhesion molecules and receptors. 20-27 Results obtained using pre–migrated cells in models of inflammation may not be predictive because the neutrophils have already engaged in an inflammatory response.
In the present work we describe a novel method for the isolation of >95% pure populations of unactivated neutrophils from whole blood using negative immunomagnetic separation. 28 Preliminary data shows that these isolated neutrophils are capable of both migration in response to chemokines in vitro and also rolling in postcapillary venules in vivo.
Materials and Methods
Reagents
Dextran (T500) was purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). Bovine serum albumin, PMA, fMLP, and fluorescein isothiocyanate (FITC)-labeled rabbit anti-rat IgG were purchased from Sigma (Poole, UK). Rat anti-mouse antibodies to CD2 (RM2–5), CD5 (53-7.3) and CD45R (RA3-6B2), phycoerythrin (PE)-labeled rat anti-mouse Ly-6G (RB6-8C5), PE-labeled rat anti-mouse L-selectin (CD62L; MEL-14), CellWash and PharmLyse were purchased from PharMingen (Oxford, UK). Rat anti-mouse ICAM-1 (YN1/1) was a gift from Dr. C. Wegner (Abbott Laboratories, Abbott Park, IL). Rat anti-mouse F4/80 antigen (CI:A3-1), FITC-labeled rat anti-mouse CD18 (C71/16), PE-labeled rat IgG2a isotype-negative control, and FITC-labeled rat IgG2a isotype-negative control were purchased from Serotec (Kidlington, UK). Goat anti-rat IgG microbeads were obtained from Miltenyi Biotech (Bisley, UK). Falcon 3001 tissue culture dishes were purchased from Becton Dickinson (Oxford, UK). Agarose (LE analytical grade) was purchased from Promega (Southampton, UK). RPMI-1640 and Hanks’ balanced salt solution were purchased from Life Technologies Ltd. (Paisley, UK). Carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) was purchased from Molecular Probes (Leiden, Netherlands).
Immunomagnetic Cell Separation
Mice (25 to 30 g) were anesthetized with an intraperitoneal injection of ketamine (Ketaset, 125 mg/kg; Willows Francis Veterinary, Crawley, UK) and acepromazine (2.75 mg/kg; C-Vet Veterinary Products, Lancashire, UK). Heparin (50 U) was also administered intraperitoneally with anesthetic. Blood (1 ml) was collected by cardiac puncture using a heparinized syringe and transferred into dextran (3 ml, 1.25% w/v in saline). Tubes were then filled to a total of 10 ml with dextran solution and inverted. Erythrocytes were allowed to sediment for 30 minutes at room temperature and the leukocyte-rich supernatant collected. Cells were washed with buffer [sterile-filtered phosphate-buffered saline (PBS) without cations containing 0.5% w/v low-endotoxin bovine serum albumin, pH 7.4, 4°C] and a total leukocyte count was performed. A leukocyte differential count for the strain of donor mouse used was also performed. Antibodies to cell surface markers were selected based on published data 29,30 to specifically label non–neutrophil cell types. In murine peripheral blood these are lymphocytes and monocytes. Anti-CD2, anti-CD5, and anti-CD45R were chosen to specifically label lymphocytes, anti-F4/80 antigen to specifically label monocytes, and anti-ICAM-1 as a pan-lymphocyte/monocyte antibody. Flow cytometry was used to determine saturating doses of antibody that specifically bound non–neutrophil cell types (see below). Whole–blood was then incubated at 4°C for 30 minutes with an antibody cocktail at concentrations based on the total number of lymphocytes and monocytes in the sample. Final antibody doses were as follows: anti-CD2 (1.5 μg/10 6 lymphocytes), anti-CD5 (2 μg/10 6 lymphocytes), anti-CD45R (10 μg/10 6 lymphocytes), anti-F4/80 antigen (2 μg/10 6 monocytes), and anti-ICAM-1 (0.6 μg/10 6 leukocytes). After removal of excess antibody by addition of 8 ml of buffer and centrifugation (6 minutes, 300 × g, 4°C), cells were resuspended in PBS (80 μl) and incubated with goat anti-rat IgG MicroBeads (20 μl/10 7 cells) at 4°C for 15 minutes. A chilled BS separation column was connected to a VarioMACS magnet (Miltenyi Biotech, Bisley, UK) and prepared with cold sterile water and buffer according to manufacturer’s instructions. The leukocyte/microbead mixture was then added to the column and the neutrophil-rich effluent collected. The unwanted cells, previously labeled with magnetic beads, were retained within the metallic matrix of the column. The neutrophil-rich effluent was then centrifuged (6 minutes, 300 × g, 4°C), the supernatant discarded and residual erythrocytes removed by hypotonic lysis. Hypotonic lysis was performed by the addition of 7 ml of 0.2% NaCl solution, gently inverted ×10, followed by hypertonic rescue of neutrophils with an equivolume of 1.6% NaCl solution supplemented with 0.1% glucose and inverted once. Neutrophils were washed of erythrocyte debris and resuspended in PBS. Total cell counts were performed using a hemocytometer and differential cell counts were made using cytospins of a sample (100 μl) of the final neutrophil-rich cell suspension stained with Diff-Quick rapid staining set (BDH, Poole, UK) and neutrophils identified by their multilobular nuclei. From these data the yield and purity of the preparation was established.
Determination of Neutrophil Purity
We have used two methods to determine neutrophil purity. First, we used the traditional method of differential counts of cytospin as described above and, in addition, we have performed flow cytometric analysis of murine leukocytes before and after negative immunomagnetic separation. For flow cytometry, whole-blood leukocytes were identified on a dot plot by forward- and side-scatter characteristics. The neutrophil subpopulation was separately identified using PE-conjugated granulocyte-specific antibody RB6-8CS as previously described. 13 Statistical analysis of the resulting neutrophil gate revealed the number of neutrophils as a percentage of the acquired leukocyte events. Using the same acquisition parameters, the above protocol was repeated using cell suspensions after negative immunomagnetic separation to determine the increase in purity of neutrophil isolates.
Flow Cytometry for Determination of Antibody Doses
Animals were anesthetized as described above and whole-blood was collected into ethylenediaminetetraacetic acid-coated tubes (Becton Dickinson, Oxford, UK). To determine leukocyte concentration, 10 μl blood samples were diluted 1:10 with 0.9% acetic acid and counted on a hemocytometer. Total lymphocyte/monocyte number per blood sample was then determined using differential counts of whole-blood smears. Blood samples (100 μl) were dispensed into flow cytometry tubes followed by different doses (μg/10 6 cells) of each rat anti-mouse primary antibody directed against CD2, CD5, CD45R, F4/80 antigen, or ICAM-1 to appropriate tubes. All tubes were incubated at 4°C for 30 minutes. Volumes were then expanded with 2 ml of CellWash followed by centrifugation at 300 × g for 6 minutes at 4°C. FITC-labeled secondary antibody (anti-rat IgG) was added to primary antibody-labeled cells. Secondary antibody was also added to unlabeled blood samples as a control of nonspecific binding. PE-labeled antibody against mouse Ly6G was added to whole-blood samples to specifically identify the granulocyte population. All tubes were incubated at 4°C for 30 minutes. To remove erythrocytes, 2 ml of PharmLyse was added to each tube and incubated for 15 minutes at room temperature, protected from light. All tubes were centrifuged as before, cell pellets were resuspended in 2 ml of CellWash, and centrifugation repeated. Cell pellets were finally resuspended in 500 μl of PBS for flow cytometric analysis using a FacScan cytometer (Becton Dickinson, Oxford, UK). Autofluorescence and secondary antibody control fluorescence were recorded on fluorescence intensity histograms. FITC fluorescence of the entire blood sample was obtained. Incremental shifts of the fluorescence intensity peak to the right indicated greater secondary antibody binding, and therefore greater primary antibody binding. To assess if neutrophils were labeled with primary antibody, the anti-Ly6G gate was used to determine any significant change in fluorescence intensity of the neutrophil population.
Viability and Activation Status of Isolated Neutrophils
Viability of isolated neutrophils was assessed by the exclusion of 0.1% trypan blue dye. 31 The activation status of isolated neutrophils was determined by flow cytometric analysis of cell surface expression of L-selectin and CD18. Levels of these activation markers on isolated neutrophils were compared with levels on neutrophils in whole-blood, and on PMA-stimulated isolated neutrophils. Whole-blood neutrophils and isolated neutrophils were counted as above; isolated neutrophils were resuspended in PBS at a density of 1 × 10 6 cells/ml. Samples (100 μl) of whole-blood or isolated neutrophils were dispensed into flow cytometry tubes. As a positive control, a series of tubes containing isolated neutrophils were preincubated for 10 minutes at 37°C in the presence of PMA (10−7 mol/L). PE-conjugated anti-L-selectin, FITC-conjugated anti-CD18 or isotype control antibodies were then administered to appropriate tubes and incubated at 4°C for 30 minutes. To remove erythrocytes from whole-blood, 2 ml of PharmLyse was added to each tube and incubated for 15 minutes at room temperature and protected from light. All tubes were then centrifuged at 300 × g for 6 minutes at 4°C and supernatant removed. Cell pellets were resuspended in 2 ml of CellWash and centrifugation repeated. Cells were finally resuspended in 500 μl of PBS and analyzed using a FacScan cytometer.
In Vitro Neutrophil Chemotaxis
To determine whether isolated neutrophils could respond to inflammatory stimulation in vitro we observed the chemotactic response of isolated neutrophils in a previously described under-agarose assay. 32 Briefly, 3 ml of agarose solution (50:50 Hanks’ balanced salt solution/RPMI-1640, 0.6% agarose) was poured into Falcon 3001 tissue culture dishes. Various concentrations of agarose were assessed to select conditions for optimal murine neutrophil migration. After the gels had solidified, two wells (diameter, 3 mm) were cut 2.2 mm apart on each plate. Isolated neutrophils (1 × 10 5 in 10 μl) were placed in the cell well and either fMLP (3 to 300 pmol in 10 μl of RPMI-1640 and 0.5% w/v bovine serum albumin) or control medium placed in the chemoattractant well. All plates were incubated for 2.5 hours in a 5% CO2 incubator at 37°C during which time neutrophil migration occurred. Cells were fixed to plates (4 ml methanol for 30 minutes followed by 4 ml 37% formaldehyde for 30 minutes) and, after removal of gels, stained with methylene blue. Images of plates were digitized [Nikon Eclipse E600 microscope (Nikon UK Ltd., Surrey, UK), RGB color digital TV camera (Basler Vision Technologies, Ahrensberg, Germany)] and distances of cell migration measured using image analysis software (Lucia DI; Nikon UK Ltd., Surrey, UK). Maximum migration distance (average of 20 furthest cells) from the edge of the cell well in the opposite direction to the chemoattractant source was subtracted from maximum migration distance in the direction of the chemoattractant to give a measure of directed migration.
In Vivo Neutrophil Rolling
The rolling of isolated neutrophils in vivo was analyzed by observation of the murine cremaster microcirculationusing intravital microscopy. Mice (25 to 30 g) were anesthetized with an intraperitoneal injection of ketaminehydrochloride (100 mg/kg) after a premedication of sodium pentobarbitone (Sagatal, 30 mg/kg; Rhône Mérieux Ltd., Essex, UK) and atropine sulfate (0.1 mg/kg; Pheonix Pharmaceuticals, Gloucester, UK). The trachea and carotid artery were cannulated with polythene tubing to aid respiration and permit administration of fluorescent-isolated neutrophils, respectively. The testis was exposed by a small scrotal incision and the cremaster muscle exteriorized and pinned over a glass coverslip. The cremaster tissue was superfused with thermocontrolled (37°C) bicarbonate-buffered saline equilibrated with CO2 in N2. Intravital microscopy was performed as previously described 33 using a Nikon E600-FN microscope fitted with an epifluorescence attachment and immersion objectives. Isolated neutrophils were fluorescently labeled by incubation with CFDA-SE for 15 minutes at room temperature as previously described. 34 Fluorescent neutrophils were injected (0.5 × 10 6 cells/200 μl) via the carotid artery and leukocyte-endothelial cell interactions in postcapillary venules within the cremaster were captured using a charge-coupled device camera (DC-330; DAGE MTI Inc., Michigan City, IN) and recorded onto sVHS video cassettes (AG-4700; Panasonic, Brackneu, UK). Image analysis was performed using NIH-Image program (available from http://www.ccp14.ac.uk/ccp/web-mirrors/nih-image/nih-image/download.html) as previously described. 33
Results
Flow Cytometry for Determination of Antibody Doses
Figure 1, a and b ▶ , shows the determination of an optimal primary antibody dose using anti-ICAM-1 as an example. As described above, primary antibody binding was assessed in the whole leukocyte population as well as to the neutrophil subpopulation, the latter separately identified with Ly-6G staining. The neutrophil subpopulation was assessed to monitor antibody binding to low-expressed surface molecules or nonspecific binding at high antibody doses. As can be seen from Figure 1a ▶ , binding of anti-ICAM to whole-blood leukocytes increased with primary antibody dose. Figure 1b ▶ shows, however, that anti-ICAM-1 binding to neutrophils also occurred at the higher dose (1.8 μg of ICAM-1/10 6 cells). Consequently, as labeling of neutrophils would lead to their retention within the magnetic column, doses of primary antibodies that showed an absence of, or very little binding to, neutrophils were selected.
Figure 1.
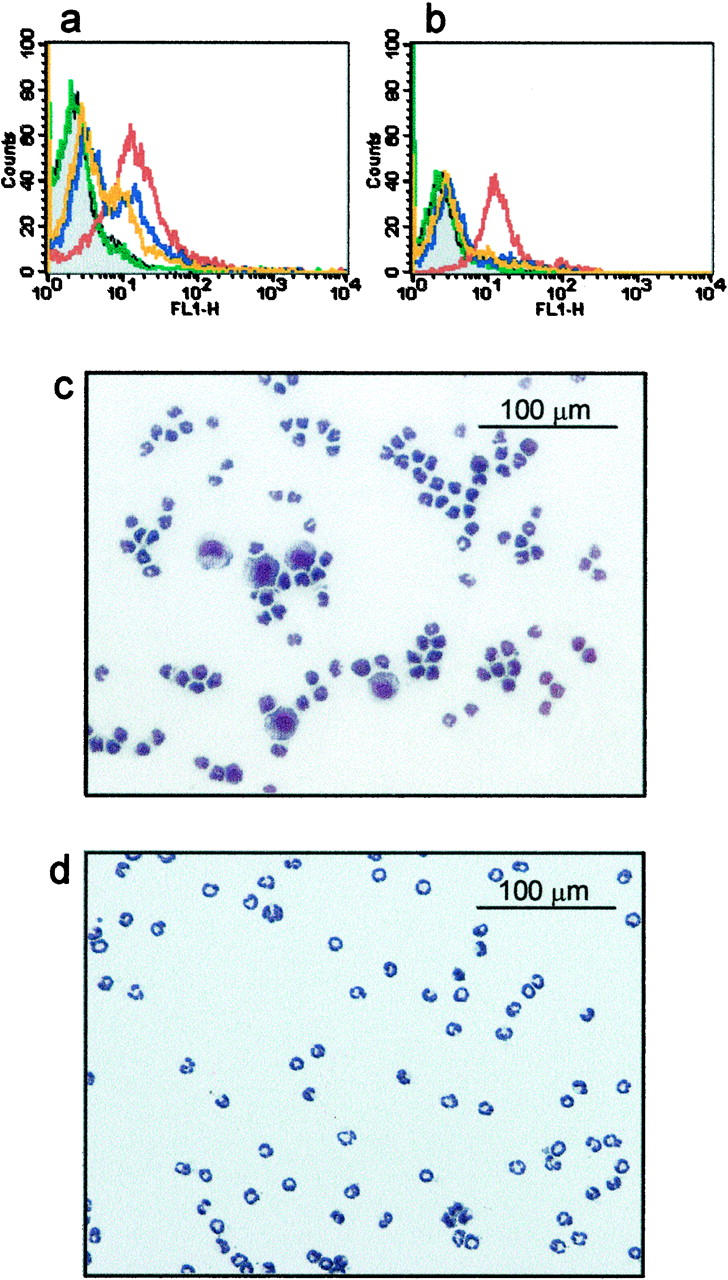
Flow cytometric analysis of primary antibody binding and typical cytospins with and without the use of anti-ICAM-1. Doses of each primary antibody (for example anti-ICAM-1 shown here) were determined by measuring fluorescence intensities of a fluorescent secondary antibody (anti-rat IgG). a: Whole-blood leukocytes incubated with higher doses of anti-ICAM-1 demonstrated increased binding of secondary antibody (gray, autofluorescence; green, isotype control; orange, 0.2 μg of anti-ICAM-1/10 6 cells; blue, 0.6 μg of anti-ICAM-1/10 6 cells; red, 1.8 μg of anti-ICAM-1/10 6 cells). b: The neutrophil subpopulation (separately identified by Ly-6G staining), however, also showed anti-ICAM-1 binding at the highest dose (1.8 μg of anti-ICAM-1/10 6 cells). Consequently, primary antibody doses were selected that gave optimal neutrophil purity and yield. c and d: Cytospins of isolated neutrophils stained with Diff-Quick after negative immunomagnetic separation. The efficacy of ICAM-1 to deplete monocytes is evident from cytospins of cell isolates where anti-ICAM-1 (0.6 μg/10 6 cells) has been excluded or included in the primary antibody cocktail (c and d, respectively). Original magnifications, ×200.
Determination of Neutrophil Purity
Figure 1c ▶ shows a cytospin of neutrophils isolated when anti-ICAM-1 was absent from the primary antibody cocktail. The effect of including anti-ICAM-1 in the antibody cocktail to remove contaminating monocytes is clearly illustrated in Figure 1d ▶ . The isolation protocol routinely yielded ∼0.5 × 10 6 cells/ml whole blood representing a recovery of ∼70% of circulating neutrophils. The final cell suspension was typically >95% neutrophils.
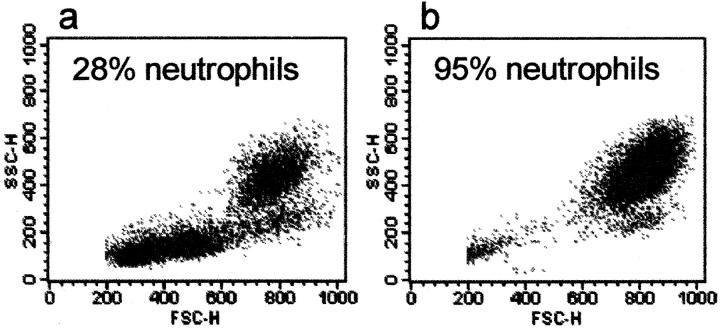
We used flow cytometry to confirm the proportion of neutrophils before and after negative immunomagnetic separation (Figure 2) ▶ . Statistical analysis of the neutrophil gate revealed that neutrophils represented ∼28% of whole-blood murine leukocytes (Figure 2a) ▶ . After negative immunomagnetic separation, the same protocol and acquisition parameters were used for the final cell suspensions and these were shown to be >95% neutrophils. Residual contaminating cells in these suspensions displayed characteristic forward and side-scatter properties of monocytes (Figure 2b) ▶ .
Figure 2.
Flow cytometric analysis of BALB/c murine leukocytes before and after negative immunomagnetic separation. a: Identification of the neutrophil subpopulation using Ly-6G staining revealed that neutrophils represented ∼28% of the donor’s whole-blood leukocytes. b: After negative immunomagnetic separation of the same whole-blood sample as above, cell suspensions were typically >95% pure neutrophils. Ten thousand events were acquired in each analysis.
Viability and Activation Status of Isolated Neutrophils
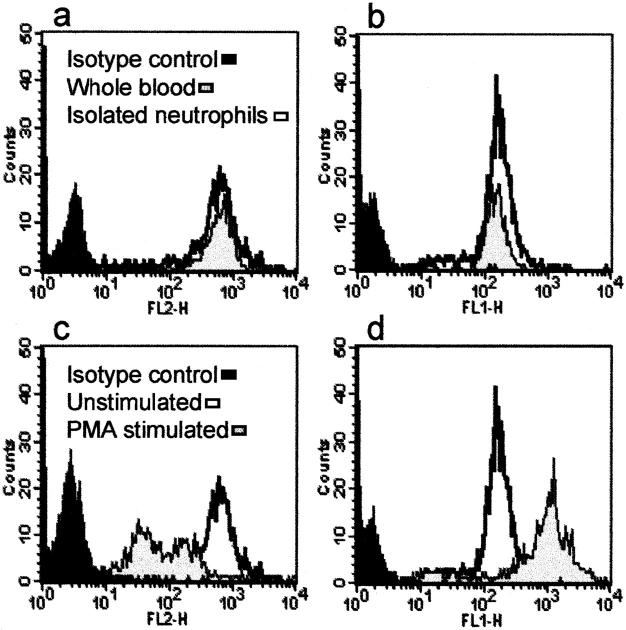
Exclusion of trypan blue dye revealed that isolated neutrophils were >97% viable. Flow cytometric analysis of isolated neutrophils showed little change in surface L-selectin or CD18 expression compared with neutrophils gated with anti-Ly-6G in whole blood (Figure 3, a and b ▶ , respectively). However, stimulation of isolated neutrophils with PMA (10−7 mol/L) resulted in a reduction in surface L-selectin and increase in CD18 expression (Figure 3, c and d) ▶ demonstrating that although neutrophils were not activated by the isolation procedure, they were still susceptible to activation by applied stimuli. The PMA-stimulated increase of CD18 expression reflects a similar up-regulation of CD11b on the cell surface (mean fluorescence intensity: unstimulated whole-blood neutrophils = 109.49; PMA-stimulated whole blood neutrophils = 584.37; unstimulated isolated neutrophils = 84.61; PMA-stimulated isolated neutrophils = 737.17). These changes are characteristic of neutrophil activation. 35-38
Figure 3.
Flow cytometric analysis of neutrophil surface expression of L-selectin and CD18. Levels of PE-labeled L-selectin (a) and FITC-labeled CD18 (b) on isolated neutrophils were compared with those on whole-blood neutrophils. The effect of PMA (10−7 mol/L) stimulation on expression of L-selectin (c) and CD18 (d) on isolated neutrophils is also shown.
In Vitro Neutrophil Chemotaxis
Exposure of isolated neutrophils to a chemoattractant in an under-agarose assay caused a characteristic chemotactic migratory response toward fMLP that was dependent on the dose of fMLP used (Figure 4, A and B) ▶ . Doses of 10 to 100 pmol (10 μl of 10−6 to 10−5 mol/L) induced significant (P < 0.05) chemotaxis compared to control. Optimal chemotaxis was induced by 30 pmol of fMLP, whereas higher doses (300 pmol) reduced directed migration. These results are consistent with the accepted hypothesis that high doses of chemoattractant can desensitize leukocytes and result in an inhibition of neutrophil orientation and chemotaxis. 39,40 Other chemoattractants have also been used in our laboratory to assess the ability of isolated neutrophils to migrate under agarose. In our hands, studies incorporating LTB4 have shown a maximal distance of directed migration of ∼120 μm toward a source of 30 pmol (10 μl of 3 × 10−7 mol/L).
Figure 4.
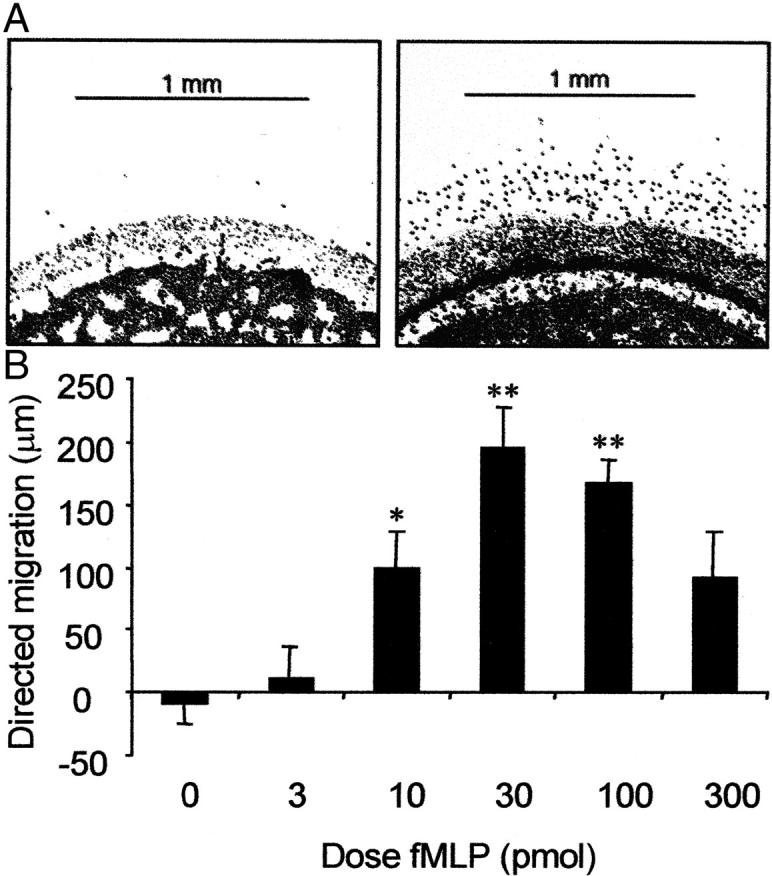
A: Photomicrographs of stained neutrophils showing in vitro migration of isolated neutrophils toward fMLP. In each frame cell wells are perpendicular to chemoattractant wells (top; not shown). Left: Cells in control plates were not exposed to a fMLP gradient and displayed minimal migration. Right: An optimal dose of fMLP (30 pmol) induced a distinct migratory response from isolated neutrophils toward the chemoattractant source. B: Distance of directed migration under agarose by isolated neutrophils in response to increasing concentrations of fMLP after incubation at 37°C for 2.5 hours. To determine directed migration, spontaneous migration (chemokinesis) was subtracted from the distance migrated toward the chemoattractant source. Data are presented as mean ± SEM (n = 4). Data were analyzed for statistical significance using a two-way analysis of variance followed by Dunnett’s t-test. *, P < 0.05; **, P < 0.01 compared with control.
In Vivo Neutrophil Rolling
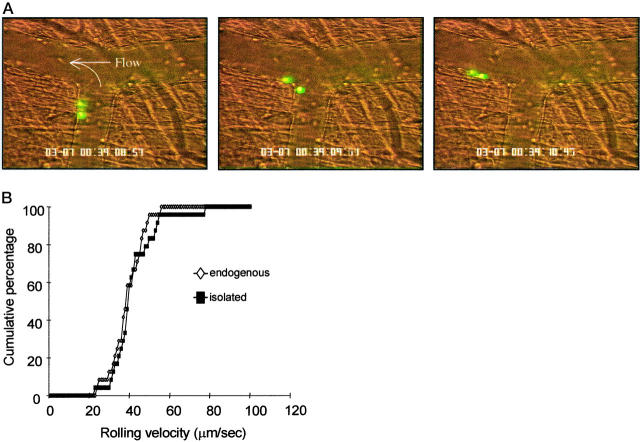
Exteriorization of the cremaster muscle requires a small incision in the scrotum through which the testis, epididymis, and the cremaster that encloses them are gently extracted. A lateral incision from the tip of the cremaster toward the point of exit from the scrotum allows the cremaster muscle to be pinned across a glass coverslip while superfused with a thermocontrolled bicarbonate buffer. This short procedure rapidly induces leukocyte rolling that has been shown to be predominantly P-selectin-dependent at 30 minutes. 41 Histological examination of surgically stimulated vessels reveals that >90% of the rolling population are neutrophils. 42 Observation of isolated neutrophils in vivo was recorded at ∼30 minutes after surgical preparation. Fluorescent-isolated neutrophils were observed to circulate for at least 30 minutes after transfusion into recipient mice. Figure 5A ▶ shows consecutive frames of two isolated, fluorescently labeled neutrophils rolling in vivo. QuickTime movies of these rolling cells are available on the website (http://www.amjpathol.org). These images illustrate the ability of isolated neutrophils to interact with the postcapillary vascular endothelium.
Figure 5.
A: Rolling of isolated neutrophils in vivo. Sequential video frames (left to right) show isolated neutrophils rolling along the vascular endothelium. Cells were fluorescently labeled with CFDA-SE and intravital microscopy performed on the murine cremaster microcirculation ∼30 minutes after surgery. QuickTime movies of these rolling cells are available on the website. B: Rolling velocities of isolated neutrophils and endogenous leukocytes in vivo. Images of rolling cells were recorded onto videotape immediately after injection of isolated neutrophils, ∼30 minutes after surgical preparation of the recipient mice. Velocities were determined by measuring distance traveled over successive video frames for a period of at least 2 seconds per cell. Data are presented as cumulative velocity histograms for at least 20 cells per histogram.
Rolling of fluorescent (isolated and re-injected) neutrophils did not differ overtly from that of endogenous leukocytes. Approximately 33% of isolated neutrophils passing through observed vessels were rolling, and frame-by-frame analysis of these interactions revealed that the rolling velocities of the isolated neutrophils were indistinguishable from those of endogenous leukocytes rolling in the same vessels (Figure 5B) ▶ .
Discussion
Many in vivo models are used to investigate the contribution of neutrophils to inflammatory processes. Before the advent of methods for production of genetically modified mice, investigations were driven by the availability of pharmacological tools such as antibodies and antagonists to study the involvement of specific molecules in inflammation. The current widespread availability of knockout and transgenic mice has had a tremendous influence on the study of all biological processes including inflammation, resulting in an increased dependence on mouse models. 43-46 The ability to isolate murine neutrophils is, therefore, of great potential use for research into the inflammatory processes in these models. Until now, efficient and reproducible isolation of unactivated murine neutrophils has not been possible.
The negative immunomagnetic separation technique described above has several distinct advantages over previously described neutrophil isolation methods. The method of isolation we describe is based on negative selection. The advantage of negative immunomagnetic separation compared to a positive selection technique is that neutrophils are not labeled with antibodies directed against their cell surface markers, therefore reducing exposure to potential activating agents. In addition, other workers have previously observed a high activation status of human neutrophils isolated by density gradient centrifugation compared to those isolated by immunomagnetic separation. 38,47 Density-gradient centrifugation, which is the method of choice for isolation of neutrophils from humans and other species, has inherent difficulties when it comes to isolation of murine neutrophils. Variable levels of neutrophils and other leukocytes in the peripheral circulation of mice, 44,48 and insignificant buoyancy differences between mouse neutrophils and lymphocytes hinder the identification of distinct bands after density centrifugation. Different cell types are therefore difficult to separate, leading to a compromise between yield and purity (ie, retaining discrete parts of the neutrophil band ensures purity but decreases potential yield). In a recent study by Kruger and colleagues, 49 isolation of murine neutrophils from peripheral blood was abandoned as the numbers of neutrophils yielded from density-gradient centrifugation was insufficient. Kruger and colleagues, 49 and also other investigators, 50 isolated neutrophils from bone marrow but the maximal purity of the populations was lower than, or only as great as, the minimal purity we achieve with immunomagnetic separation. Also, the densities of gradients used for isolation of bone marrow neutrophils from different strains of mice were not the same. Our method overcomes these problems because total and differential cell counts are made before isolation. Doses of each antibody are then adjusted accordingly, maximizing the yield and purity of neutrophils given by each preparation. We have specifically developed this technique so that the amount of each antibody used is adjusted for the number of cells it is directed against. This allows the method to be used for any strain of mouse regardless of total leukocyte counts and differentials. This is of practical importance when studying genetically modified mice because deletion of certain molecules can have profound effects on circulating neutrophils. 51 Indeed, we have used the method to successfully isolate neutrophils from C57BL/6 (>95% pure, yield ∼0.5 × 10 6 cells/ml whole blood), BALB/c (>97% pure, ∼0.5 × 10 6 cells/ml) and interleukin-1β-converting enzyme knockout (ICE−/−) mice (>94% pure, ∼0.5 × 10 6 cells/ml).
To deplete monocytes from whole-blood leukocyte suspensions, F4/80 antigen was used as a monocyte-specific marker. As F4/80 antigen predominantly labels mature monocytes, anti-ICAM-1 was selected as a pan-lymphocyte/monocyte antibody to remove residual monocytes. ICAM-1 has been previously demonstrated to be expressed on circulating unstimulated rodent monocytes with negligible amounts expressed on neutrophils. 30 We therefore postulated that careful administration of low doses of anti-ICAM-1 to the primary antibody cocktail would increase the purity of the neutrophil isolate without severely compromising yield. We found this to be a highly successful approach because addition of anti-ICAM-1 to the antibody cocktail increased the purity of the isolated neutrophil populations from 85 to >95%.
Although we have taken every possible measure to maximize yield, our method gives ∼0.5 × 10 6 neutrophils per ml of blood. Typically, ∼3 × 10 6 total leukocytes per 1 ml whole blood was obtained. Based on blood smears from the mouse strains used here, neutrophils represented ∼20 to 25% of total leukocytes in the peripheral circulation. Therefore, our method is able to retrieve ∼70 to 80% of neutrophils from whole blood. Other workers have previously administered neutrophil-specific inflammatory stimuli, such as thioglycollate 17-19 to the peritoneal cavity to obtain peritoneal lavage containing >95% pure populations of neutrophils. This technique is elegantly simple, but is limited by the fact that exposure of the neutrophils to an inflammatory agent and migration into the peritoneum may alter the activity of neutrophils in subsequent assays. It is now well established, for example, that expression of surface molecules such as CD29 (β1-integrin) and CD18 (β2-integrin) are significantly increased after neutrophil migration 20-22 and that L-selectin is shed. 23,24
Our preliminary investigations indicate that neutrophils isolated using negative immunomagnetic separation are viable for subsequent studies in vitro and in vivo. We have used the under-agarose migration assay to demonstrate the ability of isolated murine neutrophils to respond to chemotactic stimuli including fMLP and LTB4. Other studies that have used a variety of chemoattractants to investigate the chemotactic ability of murine leukocytes have shown fMLP to be a highly effective activating agent for murine neutrophils. 49 It was observed, however, that mouse neutrophils migrated a smaller distance under agarose when compared to human neutrophils under similar experimental conditions. The migration of human neutrophils to fMLP under agarose in our hands is consistent with published data. 32,40 In contrast, the average directed migration distance of murine neutrophils to an optimal dose of fMLP was ∼10% of that of human neutrophils. Furthermore, the optimal dose of fMLP that induced maximal migration of murine neutrophils under agarose (30 pmol) is ∼30 times higher than that previously observed, by ourselves and others, for human neutrophils. 40 Other workers have also reported data supporting differential migratory behavior between human and murine cells, 19,40,52 suggesting that this phenomenon is because of species difference. Nevertheless, isolated murine neutrophils are clearly responsive and migrate under agarose toward a chemotactic stimulus.
Our data obtained from in vivo experiments using isolated fluorescently labeled neutrophils show no significant difference in rolling behavior compared to endogenous cells. In addition, the rolling fraction of isolated neutrophils (33%) was similar to that of endogenous leukocytes routinely observed in vivo in our laboratory. Loading of isolated neutrophils with CFDA-SE is not believed to activate these cells as other workers have previously used this dye in studies of leukocyte activation 53 and leukocyte rolling. 34 Other workers have also compared the effects of various fluorescent dyes on neutrophil-endothelial cell interactions using rolling velocity as a sensitive parameter of neutrophil activation. 54 That the rolling velocities of isolated neutrophils closely resemble endogenous neutrophils in vivo support the flow cytometry data suggesting that neutrophils have not been unduly activated by the isolation or the fluorescent labeling procedure. This conclusion is supported by additional flow cytometric observations in our laboratory of CFDA-SE-labeled cells that do not differ in forward-scatter characteristics compared to unstimulated neutrophils (data not shown). Shape change characteristics have been used in a number of studies investigating cell activation. 55-57 Furthermore, fluorescent-labeled isolated neutrophils continued to circulate for at least 30 minutes after transfusion into the recipient mouse. This observation suggests that these cells are not activated by the isolation or labeling procedures.
Studying responses of genetically modified mice to inflammatory stimulation has already generated a wealth of information regarding precise molecular mechanisms of inflammatory responses. 58-62 The ability to isolate neutrophils from one mouse and study their interactions in another mouse offers the potential to study genetic combinations. This can also be achieved by bone marrow transplantation although considerably more time is required. As mentioned, some strains of genetically modified mice, such as the CD18−/− and ICAM-1−/− mutants, have circulating neutrophil numbers significantly greater than those found in wild-type mice. 44,48,51 Interpretation of data obtained using these mice with regard to neutrophil migration and inflammation is therefore made difficult. Isolation and labeling of neutrophils from these mutants, and injection into wild-type mice or vice versa would allow similar studies to be performed without such complications.
In summary, the isolation method described here provides the ability to study murine neutrophils within murine models, both in vitro and in vivo, thus eliminating the inherent questions of species differences, and providing more accurate models of disease. The data presented show negative immunomagnetic separation of murine blood can yield unactivated, viable, and responsive neutrophils.
Footnotes
Address reprint requests to Dr. V. Ridger, Cardiovascular Research Group, Clinical Sciences Centre, Northern General Hospital, Herries Rd., Sheffield, S5 7AU, UK. E-mail: v.c.ridger@sheffield.ac.uk.
Supported by the British Heart Foundation (FS/99040), the Northern General Hospital Research Committee (RG004090), and the Wellcome Trust (057108, 042592, 043571).
References
- 1.Kitsis E, Weissmann G: The role of the neutrophil in rheumatoid arthritis. Clin Orthop 1991, 265:63-72 [PubMed] [Google Scholar]
- 2.Entman ML, Smith CW: Postreperfusion inflammation: a model for reaction to injury in cardiovascular disease. Cardiovasc Res 1994, 28:1301-1311 [DOI] [PubMed] [Google Scholar]
- 3.Demling RH: The modern version of adult respiratory distress syndrome. Annu Rev Med 1995, 46:193-202 [DOI] [PubMed] [Google Scholar]
- 4.Springer TA: Adhesion receptors of the immune system. Nature 1990, 346:425-434 [DOI] [PubMed] [Google Scholar]
- 5.Ward PA, Lentsch AB: The acute inflammatory response and its regulation. Arch Surg 1999, 134:666-669 [DOI] [PubMed] [Google Scholar]
- 6.White M: Mediators of inflammation and the inflammatory process. J Allergy Clin Immunol 1999, 103:S378-S381 [DOI] [PubMed] [Google Scholar]
- 7.Russell KJ, McRedmond J, Mukherji N, Costello C, Keatings V, Linnane S, Henry M, Fitzgerald MX, O’Connor CM: Neutrophil adhesion molecule surface expression and responsiveness in cystic fibrosis. Am J Respir Crit Care Med 1998, 157:756-761 [DOI] [PubMed] [Google Scholar]
- 8.Reinhardt PH, Elliott JF, Kubes P: Neutrophils can adhere via alpha4beta1-integrin under flow conditions. Blood 1997, 89:3837-3846 [PubMed] [Google Scholar]
- 9.von Andrian UH, Berger EM, Ramezani L, Chambers JD, Ochs HD, Harlan JM, Paulson JC, Etzioni A, Arfors KE: In vivo behavior of neutrophils from two patients with distinct inherited leukocyte adhesion deficiency syndromes. J Clin Invest 1993, 91:2893-2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes RO, Wagner DD: Genetic manipulation of vascular adhesion molecules in mice. J Clin Invest 1996, 98:2193-2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homeister JW, Zhang M, Frenette PS, Hynes RO, Wagner DD, Lowe JB, Marks RM: Overlapping functions of E- and P-selectin in neutrophil recruitment during acute inflammation. Blood 1998, 92:2345-2352 [PubMed] [Google Scholar]
- 12.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD: The combined role of P- and E-selectins in atherosclerosis. J Clin Invest 1998, 102:145-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizgerd JP, Horwitz BH, Quillen HC, Scott ML, Doerschuk CM: Effects of CD18 deficiency on the emigration of murine neutrophils during pneumonia. J Immunol 1999, 163:995-999 [PubMed] [Google Scholar]
- 14.Balish E, Wagner RD, Vazquez-Torres A, Jones-Carson J, Pierson C, Warner T: Mucosal and systemic candidiasis in IL-8Rh−/− BALB/c mice. J Leukoc Biol 1999, 66:144-150 [DOI] [PubMed] [Google Scholar]
- 15.Terkeltaub R, Baird S, Sears P, Santiago R, Boisvert W: The murine homolog of the interleukin-8 receptor CXCR-2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum 1998, 41:900-909 [DOI] [PubMed] [Google Scholar]
- 16.Coxon A, Tang T, Mayadas TN: Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor. J Exp Med 1999, 190:923-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN: A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 1996, 5:653-666 [DOI] [PubMed] [Google Scholar]
- 18.Gao JL, Lee EJ, Murphy PM: Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med 1999, 189:657-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartt JK, Barish G, Murphy PM, Gao JL: N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med 1999, 190:741-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussel E, Gingras MC: Transendothelial migration induces rapid expression on neutrophils of granule-release VLA6 used for tissue infiltration. J Leukoc Biol 1997, 62:356-362 [DOI] [PubMed] [Google Scholar]
- 21.Kubes P, Niu XF, Smith CW, Kehrli ME, Jr, Reinhardt PH, Woodman RC: A novel beta 1-dependent adhesion pathway on neutrophils: a mechanism invoked by dihydrocytochalasin B or endothelial transmigration. FASEB J 1995, 9:1103-1111 [PubMed] [Google Scholar]
- 22.Poon BY, Ward CA, Giles WR, Kubes P: Emigrated neutrophils regulate ventricular contractility via alpha4 integrin. Circ Res 1999, 84:1245-1251 [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn DM, Bargatze RF, Butcher EC: Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol 1987, 138:4313-4321 [PubMed] [Google Scholar]
- 24.Jutila MA, Rott L, Berg EL, Butcher EC: Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol 1989, 143:3318-3324 [PubMed] [Google Scholar]
- 25.Wagner JG, Roth RA: Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 2000, 52:349-374 [PubMed] [Google Scholar]
- 26.al-Mokdad M, Shibata F, Nakagawa H: Effects of cytokine-induced neutrophil chemoattractants (CINCs) on shape change, adhesiveness and phagocytosis of rat neutrophils. Biol Pharm Bull 1997, 20:920-923 [DOI] [PubMed] [Google Scholar]
- 27.Becker EL: The short and happy life of neutrophil activation. J Leukoc Biol 1990, 47:378-389 [DOI] [PubMed] [Google Scholar]
- 28.Miltenyi S, Muller W, Weichel W, Radbruch A: High gradient magnetic cell separation with MACS. Cytometry 1990, 11:231-238 [DOI] [PubMed] [Google Scholar]
- 29.Lai L, Alaverdi N, Maltais L, Morse HC, III: Mouse cell surface antigens: nomenclature and immunophenotyping. J Immunol 1998, 160:3861-3868 [PubMed] [Google Scholar]
- 30.Tailor A, Das AM, Getting SJ, Flower RJ, Perretti M: Subacute treatment of rats with dexamethasone reduces ICAM-1 levels on circulating monocytes. Biochem Biophys Res Commun 1997, 231:675-678 [DOI] [PubMed] [Google Scholar]
- 31.Boiadjieva S, Hallberg C, Hogstrom M, Busch C: Methods in laboratory investigation. Exclusion of trypan blue from microcarriers by endothelial cells: an in vitro barrier function test. Lab Invest 1984, 50:239-246 [PubMed] [Google Scholar]
- 32.Nelson RD, Quie PG, Simmons RL: Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol 1975, 115:1650-1656 [PubMed] [Google Scholar]
- 33.Norman KE, Anderson GP, Kolb HC, Ley K, Ernst B: Sialyl Lewis(x) (sLe(x)) and an sLe(x) mimetic, CGP69669A, disrupt E-selectin-dependent leukocyte rolling in vivo. Blood 1998, 91:475-483 [PubMed] [Google Scholar]
- 34.Norman KE, Moore KL, McEver RP, Ley K: Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood 1995, 86:4417-4421 [PubMed] [Google Scholar]
- 35.Skubitz KM, Butterfield J, Ma K, Skubitz AP: Changes in neutrophil surface phenotype during hemodialysis. Inflammation 1998, 22:559-572 [DOI] [PubMed] [Google Scholar]
- 36.Torsteinsdottir I, Arvidson NG, Hallgren R, Hakansson L: Enhanced expression of integrins and CD66b on peripheral blood neutrophils and eosinophils in patients with rheumatoid arthritis, and the effects of glucocorticoids. Scand J Immunol 1999, 50:433-439 [DOI] [PubMed] [Google Scholar]
- 37.Bless NM, Warner RL, Padgaonkar VA, Lentsch AB, Czermak BJ, Schmal H, Friedl HP, Ward PA: Roles for C-X-C chemokines and C5a in lung injury after hindlimb ischemia-reperfusion. Am J Physiol 1999, 276:L57-L63 [DOI] [PubMed] [Google Scholar]
- 38.Kuijpers TW, Tool ATJ, van der Schoot CE, Ginsel LA, Onderwater JJM, Roos D, Verhoeven AJ: Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood 1991, 78:1105-1111 [PubMed] [Google Scholar]
- 39.Devreotes PN, Zigmond SH: Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol 1988, 4:649-686 [DOI] [PubMed] [Google Scholar]
- 40.Foxman EF, Campbell JJ, Butcher EC: Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol 1997, 139:1349-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL: Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med 1995, 181:669-675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunkel EJ, Ley K: Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res 1996, 79:1196-1204 [DOI] [PubMed] [Google Scholar]
- 43.Doyle NA, Bhagwan SD, Meek BB, Kutkoski GJ, Steeber DA, Tedder TF, Doerschuk CM: Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J Clin Invest 1997, 99:526-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, Doerschuk CM: Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med 1997, 186:1357-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gloor B, Todd KE, Lane JS, Rigberg DA, Reber HA: Mechanism of increased lung injury after acute pancreatitis in IL-10 knockout mice. J Surg Res 1998, 80:110-114 [DOI] [PubMed] [Google Scholar]
- 46.Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL: Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. J Exp Med 1996, 183:2329-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahler S, Kowalski C, Brosig A, Kupatt C, Becker BF, Gerlach E: The function of neutrophils isolated by a magnetic antibody cell separation technique is not altered in comparison to a density gradient centrifugation method. J Immunol Methods 1997, 200:173-179 [DOI] [PubMed] [Google Scholar]
- 48.Qin L, Quinlan WM, Doyle NA, Graham L, Sligh JE, Takei F, Beaudet AL, Doerschuk CM: The roles of CD11/CD18 and ICAM-1 in acute Pseudomonas aeruginosa-induced pneumonia in mice. J Immunol 1996, 157:5016-5021 [PubMed] [Google Scholar]
- 49.Kruger J, Butler JR, Cherapanov V, Dong Q, Ginzberg H, Govindarajan A, Grinstein S, Siminovitch KA, Downey GP: Deficiency of Src homology 2-containing phosphatase 1 results in abnormalities in murine neutrophil function: studies in motheaten mice. J Immunol 2000, 165:5847-5859 [DOI] [PubMed] [Google Scholar]
- 50.Zöllner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes HG, Vestweber D: L-selectin from human, but not from mouse neutrophils binds directly to E-selectin. J Cell Biol 1997, 136:707-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Wayne Smith C, Montgomery CA, Rich S, Beaudet AL: Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med 1998, 188:119-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iizawa O, Akamatsu H, Niwa Y: Neutrophil chemotaxis, phagocytosis, and generation of reaction oxygen species show a hierarchy of responsiveness to increasing concentrations of N-formyl-Met-Leu-Phe. Biol Signals 1995, 4:14-18 [DOI] [PubMed] [Google Scholar]
- 53.Hodgkin PD, Lee JH, Lyons AB: B cell differentiation and isotype switching is related to division cycle number. J Exp Med 1996, 184:277-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbitt KB, Rainger GE, Nash GB: Effects of fluorescent dyes on selectin and integrin-mediated stages of adhesion and migration of flowing leukocytes. J Immunol Methods 2000, 239:109-119 [DOI] [PubMed] [Google Scholar]
- 55.Sklar LA, Oades ZG, Finney DA: Neutrophil degranulation detected by right angle light scattering: spectroscopic methods suitable for simultaneous analysis of degranulation or shape change, elastase release, and cell aggregation. J Immunol 1984, 133:1483-1487 [PubMed] [Google Scholar]
- 56.Sabroe I, Hartnell A, Jopling LA, Bell S, Ponath PD, Pease JE, Collins PD, Williams TJ: Differential regulation of eosinophil chemokine signalling via CCR3 and non-CCR3 pathways. J Immunol 1999, 162:2946-2955 [PubMed] [Google Scholar]
- 57.Donabedian H, Sawyer T, Senitzer D: Inhibition of neutrophil shape change by an inhibitor of chemotaxis. J Leukoc Biol 1987, 42:510-518 [DOI] [PubMed] [Google Scholar]
- 58.Hopken UE, Lu B, Gerard NP, Gerard C: Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med 1997, 186:749-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunkel EJ, Chomas JE, Ley K: Role of primary and secondary capture for leukocyte accumulation in vivo. Circ Res 1998, 82:30-38 [DOI] [PubMed] [Google Scholar]
- 60.Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K: Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest 1998, 102:1526-1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizgerd JP, Bullard DC, Hicks MJ, Beaudet AL, Doerschuk CM: Chronic inflammatory disease alters adhesion molecule requirements for acute neutrophil emigration in mouse skin. J Immunol 1999, 162:5444-5448 [PubMed] [Google Scholar]
- 62.Kunkel EJ, Dunne JL, Ley K: Leukocyte arrest during cytokine-dependent inflammation in vivo. J Immunol 2000, 164:3301-3308 [DOI] [PubMed] [Google Scholar]