Abstract
Penicillium aurantiogriseum Dierckx was cultivated on six agar substrates (barley meal agar, oat meal agar, wheat meal agar, malt extract agar, Czapek agar, and Norkrans agar) and on oat grain for 5 days in cultivation vessels provided with an inlet and an outlet for air. Volatile metabolites produced by the cultures were collected on a porous polymer adsorbent by passing an airstream through the vessel. Volatile metabolites were collected between days 2 and 5 after inoculation. CO2 production was simultaneously measured, and after the cultivation period ergosterol contents and the numbers of CFU of the cultures were determined. Alcohols of low molecular weight and sesquiterpenes were the dominant compounds found. During growth on oat grain the production of 8-carbon alcohols and 3-methyl-1-butanol was higher and the production of terpenes was lower than during growth on agar substrates. The compositions of the volatile metabolites from oat grain were more similar to those from wheat grain, which was used as a substrate in a previous investigation, than to those produced on any of the agar substrates. Regarding the agar substrates, the production of terpenes was most pronounced on the artificial substrates (Czapek agar and Norkrans agar) whereas alcohol production was highest on substrates based on cereals. The production of volatile metabolites was highly correlated with the production of CO2 and moderately correlated with ergosterol contents, whereas no correlation with the numbers of CFU was found. Thus, the volatile metabolites formed and the ergosterol contents of fungal cultures should be good indicators of present and past fungal activity.
Full text
PDF
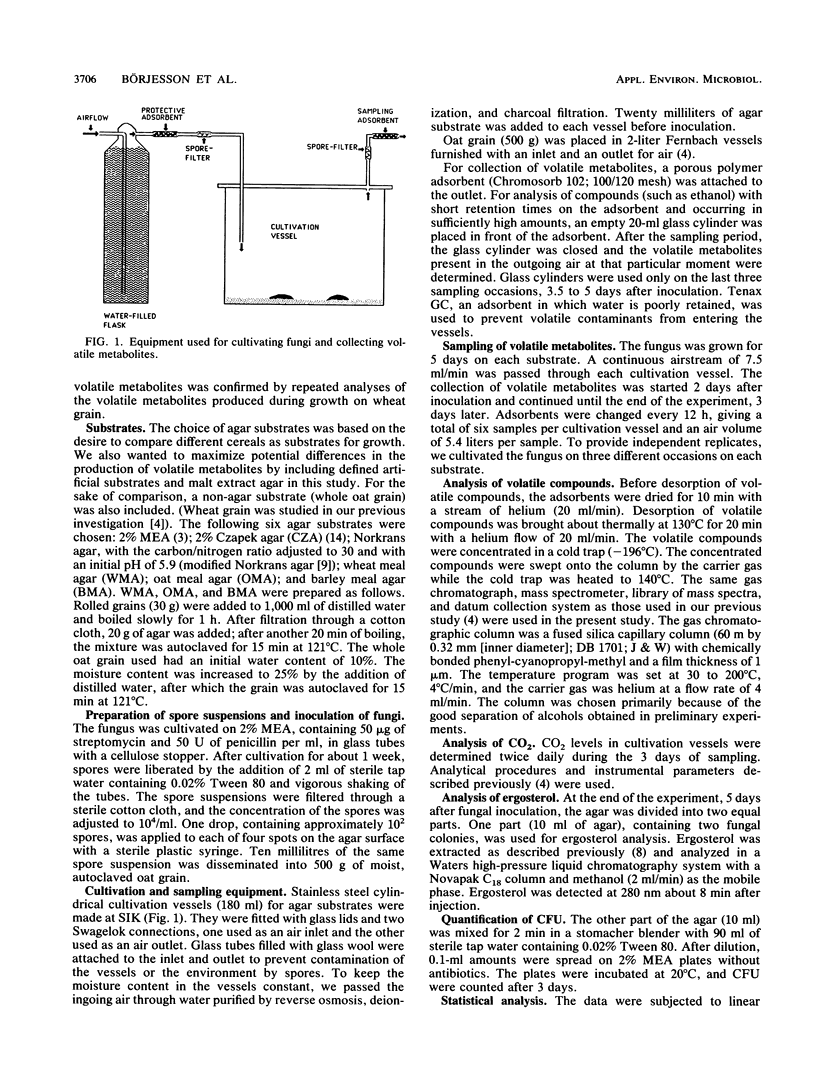
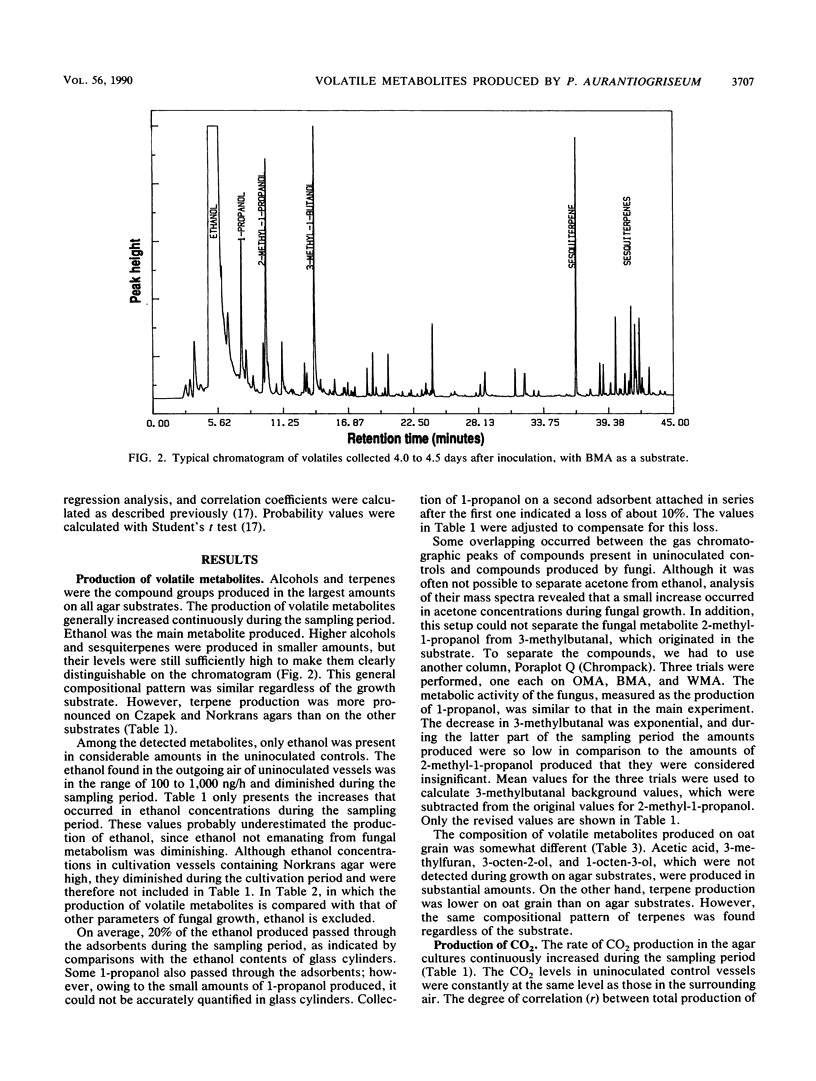
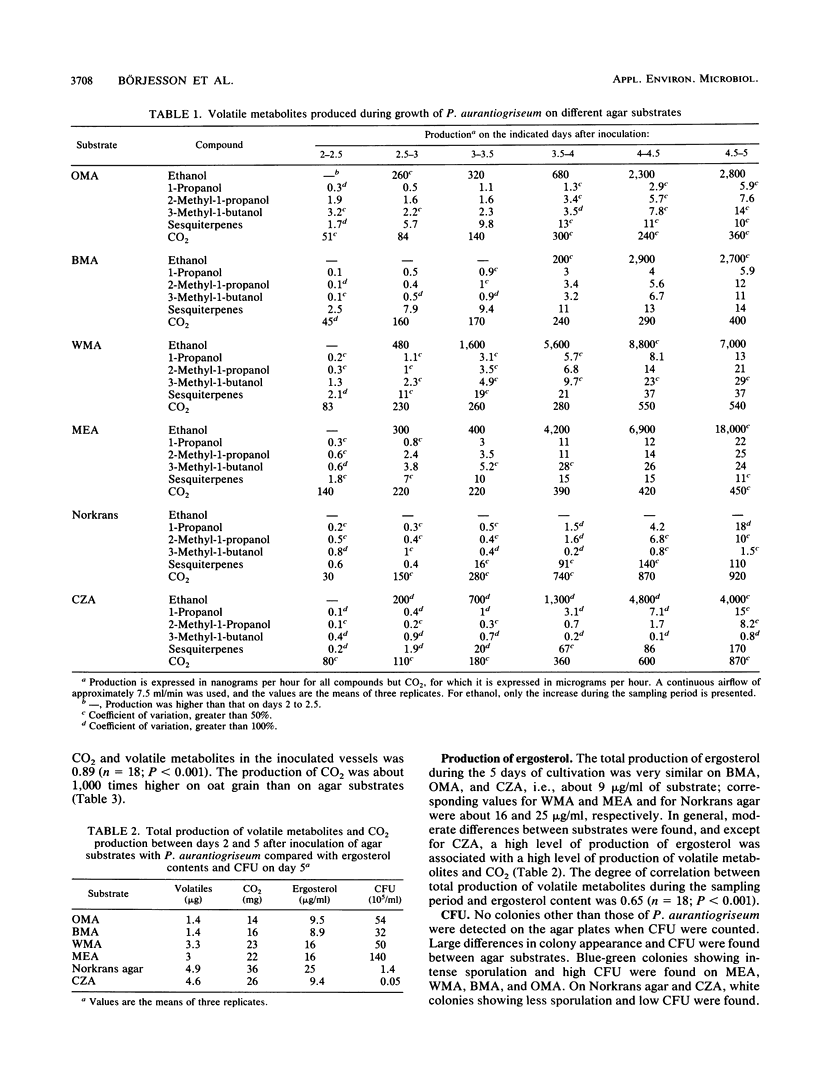


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kaminski E., Stawicki S., Wasowicz E. Volatile Flavor Compounds Produced by Molds of Aspergillus, Penicillium, and Fungi imperfecti. Appl Microbiol. 1974 Jun;27(6):1001–1004. doi: 10.1128/am.27.6.1001-1004.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y., Arsuffi T. L., Fallon R. D. Fundamental procedures for determining ergosterol content of decaying plant material by liquid chromatography. Appl Environ Microbiol. 1988 Jul;54(7):1876–1879. doi: 10.1128/aem.54.7.1876-1879.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrman J. A gas chromatographic investigation of the influence of different carbon sources on the production of volatile compounds by Dipodascus aggregatus. Arch Mikrobiol. 1971;75(2):145–162. doi: 10.1007/BF00408002. [DOI] [PubMed] [Google Scholar]
- Norrman J. The influence of different nitrogen sources on the production of volatile compounds by Dipodascus aggregatus. Arch Mikrobiol. 1971;80(4):338–350. doi: 10.1007/BF00406221. [DOI] [PubMed] [Google Scholar]
- Sprecher E., Hanssen H. P. Influence of strain specificity and culture conditions on terpene production by fungi. Planta Med. 1982 Jan;44(1):41–43. doi: 10.1055/s-2007-971398. [DOI] [PubMed] [Google Scholar]
- Tuma D., Sinha R. N., Muir W. E., Abramson D. Odor volatiles associated with microflora in damp ventilated and non-ventilated bin-stored bulk wheat. Int J Food Microbiol. 1989 May;8(2):103–119. doi: 10.1016/0168-1605(89)90065-2. [DOI] [PubMed] [Google Scholar]
- Wilkins C. K., Scholl S. Volatile metabolites of some barley storage molds. Int J Food Microbiol. 1989 Feb;8(1):11–17. doi: 10.1016/0168-1605(89)90075-5. [DOI] [PubMed] [Google Scholar]



