Abstract
Sjogren’s syndrome is an autoimmune disease characterized by inflammation and destruction of lacrimal and salivary glands. The development of the inflammation requires the migration of lymphocytes from the blood into these tissues. This migration involves multistep cascades with binding of lacrimal gland endothelial adhesion molecules to their ligands on circulating lymphocytes. We used nonobese diabetic mice, which develop autoimmune-mediated lacrimal gland inflammation, as an experimental model to define the adhesion molecules that control lymphocyte migration into inflamed lacrimal glands. We found that vascular endothelia in inflamed areas of lacrimal gland expressed vascular cell adhesion molecule (VCAM)-1 and the peripheral node addressin (PNAd), but not mucosal addressin cell adhesion molecule-1. Most lymphocytes in the inflamed glands expressed α4 integrin, L-selectin, and lymphocyte function-associated antigen (LFA)-1. In vivo studies revealed that antibodies against VCAM-1, α4 integrin, PNAd, L-selectin, or LFA-1 almost completely blocked lymphocyte migration from blood into inflamed lacrimal glands. There was no inhibition of migration by antibodies against mucosal addressin cell adhesion molecule-1 or α4β7 integrin. These results indicate that endothelial/lymphocyte adhesion cascades involving VCAM-1/α4β1 integrin, PNAd/L-selectin, and LFA-1 control the migration of lymphocytes into inflamed lacrimal gland. These adhesion molecules offer potential therapeutic targets to block the development of lacrimal gland inflammation and destruction.
The development of effective immune responses—whether to foreign organisms such as bacteria, or to endogenous antigens in an autoimmune disease—depends in large part on the ability of lymphocytes to migrate to sites of antigen deposition and presentation. The migration of lymphocytes from blood into tissues is not a random process: it is controlled by highly specific interactions between the lymphocytes and the vascular endothelia of blood vessels at the sites of lymphocyte egress. 1 The sites of lymphocyte egress include secondary lymphoid tissues–such as lymph nodes (LNs) and Peyer’s patches (PPs)—and tertiary lymphoid tissues, like inflamed pancreatic islets and lacrimal glands. 2-4 The endothelial/lymphocyte interactions in these tissues comprise multistep cascades with at least four sequential adhesion and activation steps: 1) an initial transient tethering and rolling; 2) if the lymphocytes encounter appropriate activating molecules, rolling may be followed by a lymphocyte activation step, which then leads to; 3) firm adhesion or sticking mediated by activated integrins, that may be followed by; 4) lymphocyte diapedesis into tissue. 5-8 These cascades can be tissue-specific, with much of the specificity determined by selective expression of adhesion molecules by endothelia in various tissues, and of their ligands by circulating lymphocytes.
Nonobese diabetic (NOD) mice are a well-established animal model for human insulin-dependent diabetes mellitus. These mice develop autoimmune-mediated inflammation of pancreatic islets (insulitis) with destruction of the insulin-producing β cells. 9 Along with islet inflammation, NOD mice develop autoimmune-mediated inflammation and destruction of lacrimal and submandibular salivary glands. 10 Thus, NOD mice also serve as an animal model for human Sjogren’s syndrome, which is an autoimmune disease characterized by lymphocytic inflammation and destruction of salivary glands (sialoadenitis) and lacrimal glands (dacryoadenitis), leading to the development of dry mouth and dry eyes because of insufficient glandular secretions. 11 There are several mouse models of Sjogren’s syndrome; however, the NOD mouse best mimics the histopathology and the loss of secretory function found in humans with Sjogren’s syndrome. 12,13
In this study, we investigated the adhesion molecule phenotype of vascular endothelia and lymphocytes in inflamed lacrimal glands. We found that vascular cell adhesion molecule-1 (VCAM-1) and peripheral node addressin (PNAd), but not mucosal addressin cell adhesion molecule-1 (MAdCAM-1), are expressed by endothelia in inflamed areas of lacrimal glands. Using adhesion-blocking monoclonal antibodies (mAbs) in in vivo assays, we show that VCAM-1/α4β1 integrin, PNAd/L-selectin, and lymphocyte function-associated antigen (LFA)-1 adhesion pathways are involved in migration of lymphocytes from blood into inflamed lacrimal gland.
Materials and Methods
Mice and Rats
NOD mice were bred in our colony from stock originally obtained from Taconic Farms (Germantown, NY). Prediabetic female and male NOD mice were used for histology studies; prediabetic male NOD mice were used for immunohistochemical and immunofluorescence staining and as hosts for the in vivo migration studies. BALB/c mice and Sprague-Dawley rats were obtained from our animal facility.
Antibodies and Other Reagents
mAbs included anti-α4 [mAb PS/2; American Type Culture Collection (ATCC), Manassas, VA], anti-β7 (Fib504; provided by Dr. D. Andrew, Cambridge, MA), anti-α4β7 heterodimer (DATK-32; provided by Dr. D. Andrew), anti-αE (M290; provided by Dr. P. Kilshaw, Cambridge, UK), anti-L-selectin (MEL-14; ATCC), anti-LFA-1 (FD441.8, ATCC), anti-CD3 (145–2C11; BD PharMingen, San Diego, CA), anti-CD4 (GK1.5, ATCC), anti-CD8 (53-6.72, ATCC), anti-CD16/CD32 (Fc Block, BD PharMingen), anti-CD19 (1D3, BD PharMingen), anti-CD45 (M1/9, ATCC), anti-CD45R/B220 (RA3-6B2; provided by Dr. R. Coffman, Palo Alto, CA), anti-MAdCAM-1 (MECA-367, provided by Dr. E. Butcher, Stanford, CA), anti-PNAd (MECA-79, provided by Dr. E. Butcher), anti-VCAM-1 (M/K-2.7, ATCC; and 4B12, provided by Dr. B. Engelhardt, Bad Nauheim, Germany), anti-ICAM-1 (YN1/1.7, ATCC), anti-E-selectin (10E9.6, BD PharMingen), and anti-P-selectin (RB40, ATCC). Negative control mAbs included anti-human CD44 (9B5, provided by Dr. E. Butcher) and anti-cerebellar granular cell antigen (OZ-42; provided by Dr. L. Pickford, Palo Alto, CA). For flow cytometry studies, mAbs were conjugated with fluorescein or allophycocyanin (APC) as described, 14 or purchased from BD PharMingen as Cy-Chrome conjugates.
Biotinylated anti-rat IgM and peroxidase-streptavidin were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Biotinylated anti-rat IgG was obtained from Vector Laboratories (Burlingame, CA), PE-conjugated anti-rat IgG from Biosource International (Camarillo, CA), OCT from Miles (Naperville, IL), and 3,3′-diaminobenzidine tetrahydrochloride from Sigma (St. Louis, MO). Carboxy-fluorescein succinimidyl ester (CFSE) and tetramethylrhodamine isothiocyanate (TRITC) were purchased from Molecular Probes (Eugene, OR).
Histology and Immunohistochemistry
Mice were sacrificed at ages indicated in the text; lacrimal glands and mesenteric LNs (MLNs) were removed and fixed in formalin or snap-frozen in OCT. Hematoxylin and eosin (H&E)-stained sections of the formalin-fixed lacrimal glands were examined by light microscopy. For each gland that showed an inflammatory infiltrate, the areas of the infiltrate and of the gland were measured on three nonconsecutive sections using an image analysis system as described (VAS II; Mideo Systems, Huntington Beach, CA). 15 We then calculated the percentage of the gland involved by inflammation (area of inflammation/area of gland × 100).
Frozen sections of lacrimal glands and MLNs were stained using a three-stage immunoperoxidase system. Briefly, the sections were sequentially incubated with rat primary antibody, biotin-conjugated anti-rat IgG or anti-rat IgM, peroxidase-streptavidin, diaminobenzidine/hydrogen peroxide, and methylene blue counterstain. There were two washes with phosphate-buffered saline (PBS) between each step. Slides were examined by light microscopy. 15 On slides of lacrimal glands from 2- to 3-, 5-, 10-, 20-, 40-, and 60-week-old male NOD mice, the numbers of vessels expressing PNAd and/or VCAM-1 per mm 2 of lacrimal gland area were determined by image analysis as described. 15 In addition, if the section showed an inflammatory infiltrate, the percentage of the gland involved by inflammation was determined as described above.
Immunofluorescence Staining and Flow Cytometry
Male NOD mice (10-, 30-, and 60-weeks old) were sacrificed, and then perfused with 10 ml of fluorescence-activated cell sorting (FACS) buffer (PBS with 2% bovine calf serum and 0.05% azide) through the left ventricle to remove intravascular lymphocytes. Lacrimal glands and MLNs were harvested, minced, teased with needles, and filtered over a nylon mesh. For each experiment, lacrimal gland cells were pooled from 6 to 10 mice of the same age. The cell suspensions were collected in FACS buffer and stained using four-color immunofluorescence protocols as described. 16 Briefly, suspensions were sequentially incubated with anti-adhesion molecule mAb, PE-anti-rat IgG, 10% normal rat serum, Fc Block, and a mix of APC-anti-CD45, Cy-Chrome-anti-CD3, and fluorescein anti-CD19. There were two washes with FACS buffer after each step, except after incubation with normal rat serum, when Fc Block was added to the suspensions without washing. For negative controls, specific mAbs were replaced by species, isotype- and conjugation-matched irrelevant mAbs. Stained cells were analyzed on a FACScaliber flow cytometer (Becton-Dickinson, San Jose, CA) as described. 16 Analysis was performed on 30,000 to 80,000 cells/sample that were within the lymphocyte scatter gate and a CD45+ fluorescence gate. Data are presented as histograms, or as the percentage of cells reacting with a specific mAb minus percentage of cells reacting with the negative control mAb.
In Vivo Lymphocyte Migration Studies
Lymphocytes from spleen, MLNs, and peripheral LNs (PLNs) of 10- to 20-week-old NOD mice were labeled by incubating 2 × 10 7 cells/ml with 0.8 μg/ml of TRITC in labeling buffer (50% RPMI, 48.5% HBSS without calcium and magnesium, 1.5% bovine calf serum, and 10 mmol/L HEPES) at 37°C for 15 minutes. 17 Lymphocytes from rat spleen and MLNs were labeled with CFSE under the same conditions. To remove excess dye, the cells were centrifuged through bovine calf serum, washed, and resuspended in transfer medium (Dulbecco’s modified Eagle’s medium, 1% bovine calf serum, and 10 mmol/L HEPES).
To examine the roles of endothelial adhesion molecules in the migration of lymphocytes from the bloodstream into inflamed lacrimal glands, each host NOD mouse (15- to 20-week-old males) received 500 μg intravenously of anti-endothelial adhesion molecule or negative control mAb, followed 30 minutes later by 5 × 10 7 TRITC-labeled mouse cells and 100 μg of mAb intravenously. Host mice were sacrificed 2 hours after the cell transfer. Flow cytometry was used to determine the percentage of donor cells of the total cells in host MLN, PLN, PP, and erythrocyte-lysed spleen suspensions, and in blood mononuclear cell preparations. The number of donor cells/mm 2 of inflamed lacrimal gland area was determined by confocal microscopy of frozen sections. For each tissue, results for each anti-endothelial adhesion molecule treatment group are expressed as the percentage of migration in the negative control mAb treatment group.
To determine which lymphocyte adhesion molecules are involved in the migration of lymphocytes from the bloodstream into inflamed lacrimal glands, TRITC-labeled mouse cells were treated with 10 μg/ml of anti-lymphocyte adhesion molecule or negative control mAb for 10 minutes on ice. mAb-treated TRITC-labeled mouse cells (5 × 107), 1 × 10 8 CFSE-labeled rat cells, and 250 μg of mAb were transferred intravenously into each host mouse. The rat cells, which do not bind the antibodies against mouse lymphocyte adhesion molecules, serve as an internal standard to control for differences between host mice in blood flow to tissues and in the efficiency of the injection. An aliquot of the labeled mouse and rat cell mixture was saved to obtain the input mouse/internal control rat cell ratio. Host mice were sacrificed 2 hours after transfer. The ratios of donor mouse/internal control rat cells in host MLN, PLNs, PPs, spleen, and blood were determined by flow cytometry. Donor mouse/internal control rat cell ratios for lacrimal gland were calculated from the numbers of donor mouse and rat cells detected by confocal microscopy of frozen sections. The ratios for all tissues were normalized by the input mouse/internal control rat cell ratio. For each tissue, the results for each anti-lymphocyte adhesion molecule mAb treatment group are presented as percentage migration of control mAb treatment group.
Data Analysis
Numerical data are presented as mean ± SD. Student’s t-test was used to evaluate differences between treatment groups in the in vivo migration studies. P < 0.05 is considered to be significant.
Results
Assessment of Inflammation in Lacrimal Glands
Our first step was to define the time course of inflammation development in lacrimal glands of the NOD mice from our colony. Lacrimal glands from 1- to 60-week-old prediabetic male and female mice were harvested and fixed in formalin. H&E-stained sections of these glands were examined by light microscopy and the extent of inflammation was quantitated by morphometric analysis.
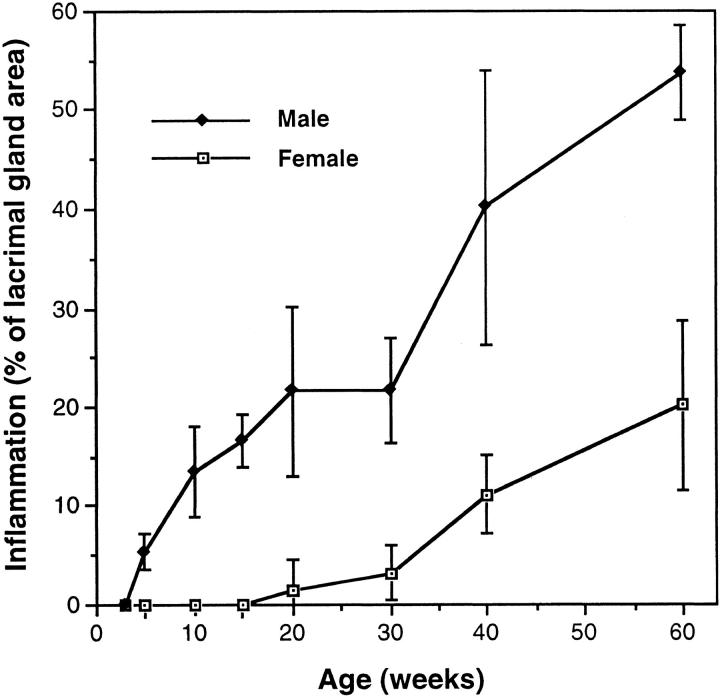
In our NOD colony, as in other colonies, lacrimal gland inflammation develops at a younger age in males than in females (Figure 1) ▶ . 18,19 There was no inflammation in lacrimal glands of our 1- to 3-week-old male mice. However, we saw small aggregates of T and B cells in lacrimal glands from all of the 5-week-old male NOD mice that we examined (n = 6 mice). These aggregates were in a perivascular and periductular distribution, and, on the average, occupied ∼5% of the gland area (Figure 1) ▶ . At 10 to 30 weeks of age, sections of lacrimal glands from most male NOD mice showed several large foci of perivascular and periductular inflammation. These infiltrates had poorly segregated B and T cell areas (Figure 2) ▶ . By 40 weeks of age, infiltrates extended beyond the perivascular/periductular regions to involve the acinar tissue. In a few of the 40- to 60-week-old male mice, an extensive lymphocytic infiltrate combined with tissue destruction made it difficult to identify residual glandular tissue.
Figure 1.
Lacrimal gland inflammation in NOD mice. H&E-stained slides were prepared from formalin-fixed, paraffin-embedded lacrimal glands. Morphometric analysis was used to determine the percentage of gland area in each mouse that contained an inflammatory infiltrate. Each value is mean ± SD from four to six mice.
Figure 2.
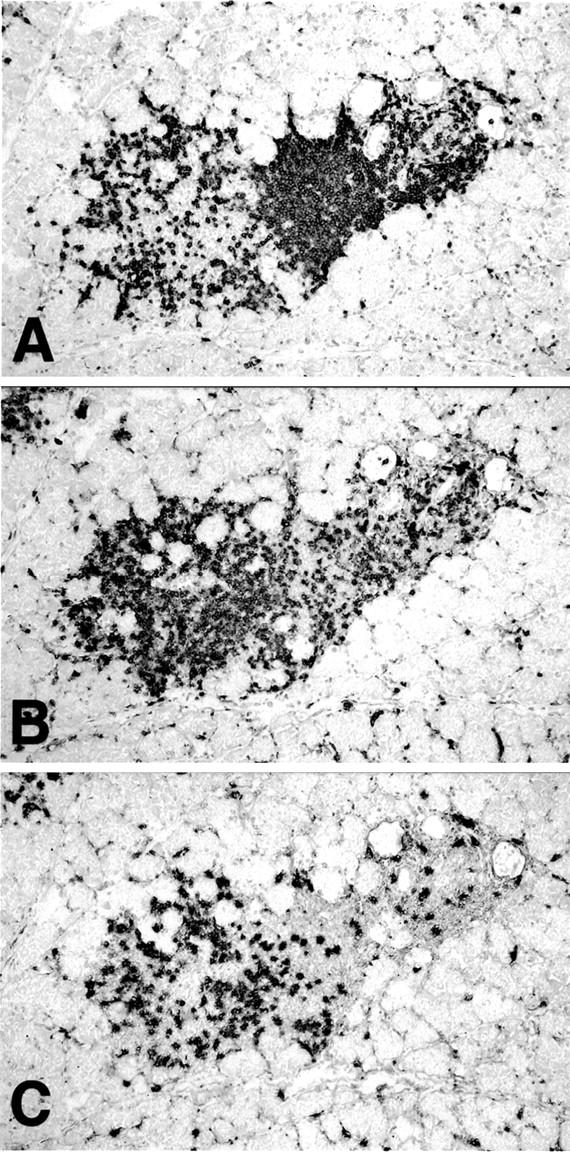
B and T lymphocytes in inflamed lacrimal gland. Serial frozen sections of lacrimal gland from a 20-week-old male NOD mouse were stained with antibodies to detect: B cells (mAb RA3-6B2) (A); CD4+ T cells (mAb GK1.5) (B); and CD8+ T cells (mAb 53-6.72) (C). Immunoperoxidase stain with methylene blue counterstain. Original magnifications, ×40.
Although lacrimal glands from female NOD mice contained inflammatory infiltrates similar to those seen in male mice, the development of the infiltrates was delayed by many weeks (Figure 1) ▶ . Small foci of inflammation were first seen in most female mice at 20 weeks of age, and large foci of perivascular/periductular inflammation were found in lacrimal glands of 40- to 60-week-old females. We did not see lymphocytic infiltrates in lacrimal glands from age-matched BALB/c mice of either gender.
Expression of Endothelial and Lymphocyte Adhesion Molecules in Lacrimal Glands
Our first step in defining the adhesion mechanisms that control the migration of lymphocytes from the blood into inflamed lacrimal glands was to determine which endothelial and lymphocyte adhesion molecules are expressed in inflamed glands. We used immunohistochemical staining of frozen sections of inflamed lacrimal glands from NOD mice, and, for comparison, uninflamed lacrimal glands from BALB/c and young NOD mice, to study endothelial adhesion molecule expression (Figures 3 and 4) ▶ ▶ . Suspension immunofluorescence staining and flow cytometric analysis were used to define the expression of adhesion molecules on lymphocytes from NOD-inflamed lacrimal glands (Figure 5) ▶ . We focused on endothelial adhesion molecules and their lymphocyte ligands that are involved in lymphocyte migration into tertiary sites of chronic inflammation. These include VCAM-1, an Ig family member that is expressed by vascular endothelia in a wide variety of chronically inflamed tissues, and its lymphocyte ligands α4β1 and α4β7 integrin; 18,20-23 endothelial PNAd and lymphocyte L-selectin, which play a major role in lymphocyte migration into PLNs and a minor role in migration into PPs, and may be involved in lymphocyte migration to some tertiary lymphoid tissues; 4,5,7,24 endothelial MAdCAM-1 and lymphocyte α4β7 integrin, which mediate lymphocyte migration into intestinal secondary lymphoid tissues such as PPs, and to a few inflamed tissues such as pancreatic islets and gut; 4,5,25,26 endothelial ICAM-1 and lymphocyte LFA-1, which are involved in lymphocyte migration into most secondary and tertiary lymphoid tissues; 27,28 and endothelial E-selectin and P-selectin, which mediate lymphocyte migration to some tertiary lymphoid tissues, such as inflamed skin. 29
Figure 3.
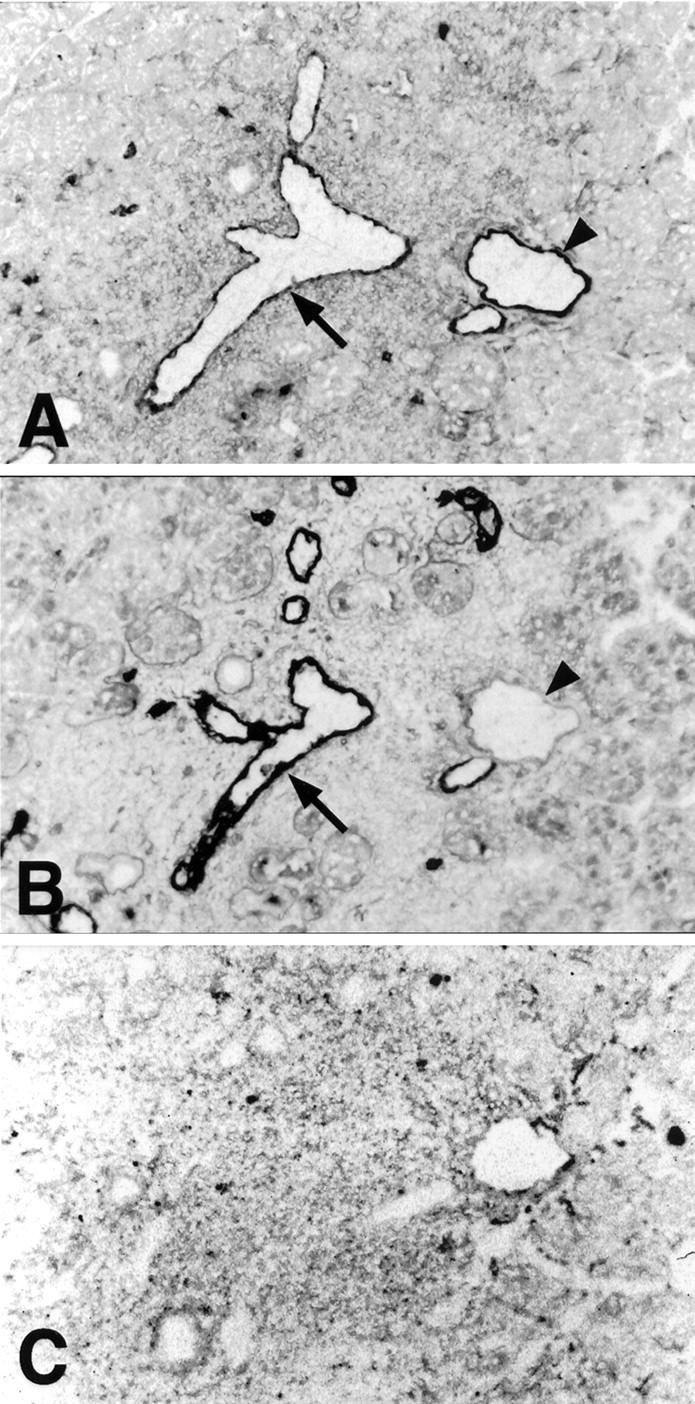
VCAM-1 and PNAd, but not MAdCAM-1, are expressed by vascular endothelia in inflamed lacrimal gland. Semi-serial frozen sections of lacrimal gland from a 20-week-old male NOD mouse were stained with anti-VCAM-1 (mAb M/K-2.7) (A), anti-PNAd (mAb MECA-79) (B), and anti-MAdCAM-1 (mAb MECA-367) (C) antibodies. Many vessels in inflamed foci co-expressed VCAM-1 (A, arrow) and PNAd (B, arrow). A few vessels were VCAM-1+ (A, arrowhead) and PNAd− (B, arrowhead), or VCAM-1− PNAd+ (not shown). Immunoperoxidase stain with methylene blue counterstain. Original magnifications, ×40.
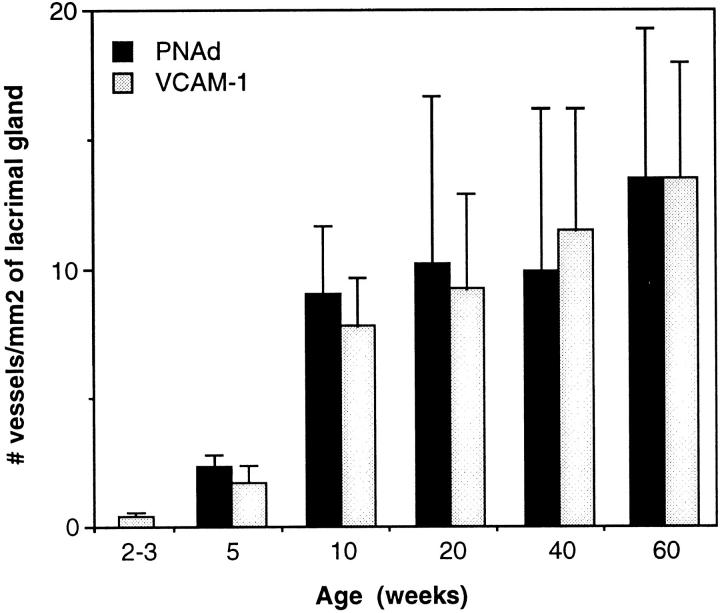
Figure 4.
Vascular expression of PNAd and VCAM-1 in inflamed lacrimal glands. Frozen sections of lacrimal glands from male NOD mice were stained with monoclonal antibodies against PNAd and VCAM-1 using an immunoperoxidase technique. The numbers of vessels expressing each adhesion molecule per mm 2 of lacrimal gland area were determined by light microscopic examination and morphometric analysis. Each bar is mean ± SD from four to five mice. PNAd was not detected in lacrimal glands from the 2- to 3-week-old mice. Mean percentage of lacrimal gland area involved by inflammation was 0% (2 to 3 weeks), 5.2% (5 weeks), 16.6% (10 weeks), 15.0% (20 weeks), 26.9% (40 weeks), and 42.0% (60 weeks).
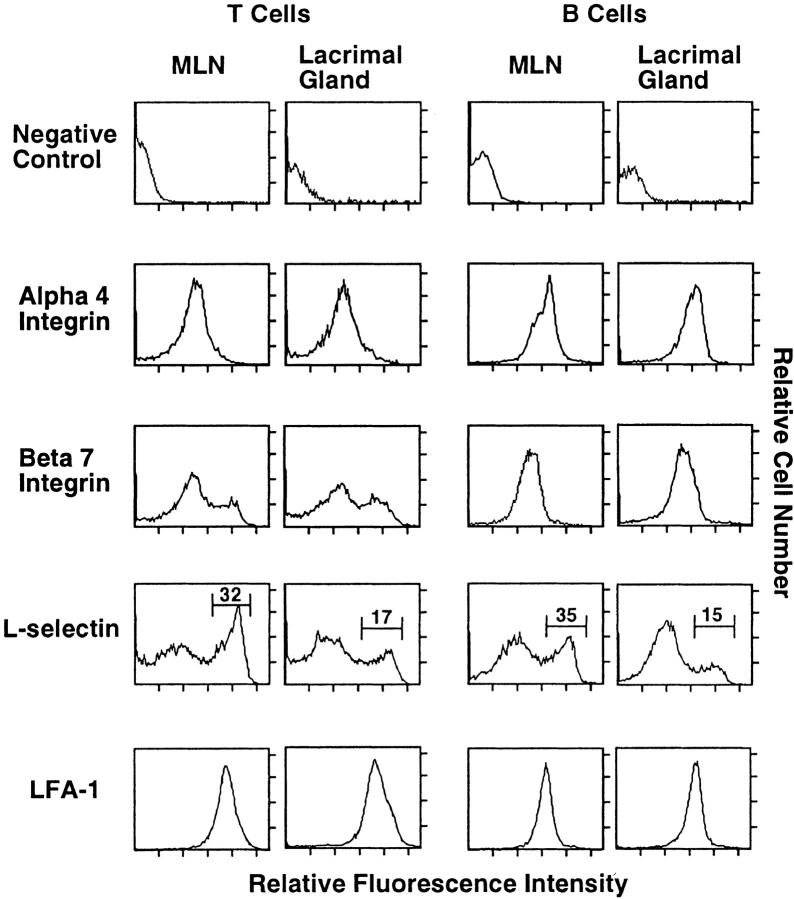
Figure 5.
Adhesion molecule expression on T and B cells from inflamed lacrimal glands. Cell suspensions from NOD lacrimal gland and, for comparison, from mesenteric lymph nodes (MLNs), were stained using a four-color immunofluorescence technique and analyzed by flow cytometry as described in the text. The histograms show the expression of adhesion molecules by T cells (left two columns) and B cells (right two columns) from lacrimal gland and MLNs from 30-week-old male NOD mice; the markers and numbers on the L-selectin histograms indicate the populations that express high levels of L-selectin. Similar adhesion molecule profiles were seen on lacrimal gland cells from 10- and 60-week-old male NOD mice.
We stained frozen sections of lacrimal glands from 2- to 60-week-old male NOD and BALB/c mice with antibodies against endothelial adhesion molecules. VCAM-1 was expressed on a few vessels in uninflamed lacrimal glands from 2- to 3-week-old NOD mice (Figure 4) ▶ and 2- to 60-week-old BALB/c mice (data not shown). We saw a marked increase in endothelial VCAM-1 expression at the histological onset of inflammation (5 weeks of age) and during the early stages of inflammation (from 5 to 10 weeks of age) (Figure 4) ▶ . This was followed by a gradual increase in VCAM-1 expression. Specifically, the density of VCAM-1-expressing vessels increased ∼18-fold between 2 to 3 and 10 weeks of age (from a mean of 0.4 VCAM-1+ vessels/mm 2 of lacrimal gland area to 7.4 vessels/mm2) and approximately twofold between 10 and 60 weeks of age (from a mean of 7.4 vessels/mm 2 to 13.1 vessels/mm2) (Figure 4) ▶ . Most VCAM-1-expressing vessels (>90% in 5-week-old NOD mice and >95% in 10- to 60-week-old NOD mice) were located in areas of inflammation.
There was no expression of PNAd in uninflamed lacrimal glands from 2- to 3-week-old NOD mice (Figure 4) ▶ and any BALB/c mice (data not shown). PNAd was first seen on vessels in NOD lacrimal glands at the onset of inflammation (5 weeks of age) (Figure 4) ▶ . The numbers of PNAd-expressing vessels increased markedly between 5 and 10 weeks of age, and more gradually thereafter. PNAd expression was confined to vessels in the areas of inflammation; most of these vessels also expressed VCAM-1 (Figure 3) ▶ .
There was no expression of MAdCAM-1 by vascular endothelia in the NOD lacrimal glands, even in the areas of severe inflammation (Figure 3) ▶ (n = 28 mice; three or more nonconsecutive sections from each mouse). MLNs, used as a positive control tissue in each staining experiment, showed strong expression of MAdCAM-1 by the HEV. ICAM-1 and ICAM-2 were expressed by all vessels in the inflamed foci. We did not find vascular E-selectin or P-selectin in inflamed areas of any lacrimal gland.
We used four-color immunofluorescence staining and flow cytometric analysis to define the expression of adhesion molecules by lacrimal gland T and B lymphocytes. We studied male NOD mice of three different ages (10, 30, and 60 weeks) to determine whether there are differences in lymphocyte adhesion molecule expression between the early, middle, and late stages of the inflammatory process. We found that adhesion molecule expression by lacrimal gland lymphocytes did not change appreciably with age (data not shown). Moreover, adhesion molecule profiles of the lacrimal gland lymphocytes were similar to those of MLN lymphocytes (Figure 5) ▶ . Specifically, in both tissues, all lymphocytes expressed LFA-1 and α4 integrin. B cells in lacrimal gland and MLNs showed moderate expression of β7 integrin. T cells showed a bimodal pattern, with most T cells in both tissues expressing low levels of β7, and a smaller population expressing high levels of β7. Fewer than 20% of lacrimal gland and MLN T cells expressed αE integrin (which can pair with β7 to form αEβ7) (data not shown). There was no expression of αE integrin by B cells in these tissues (data not shown). L-selectin was expressed by most lymphocytes in both tissues; however, there was a difference in expression levels between cells in lacrimal gland and MLNs. In MLNs, 30 to 40% of T and B cells expressed high levels of L-selectin. In contrast, 15 to 20% of lacrimal gland lymphocytes were L-selectinhi (Figure 5) ▶ .
VCAM-1/α4β1 Integrin, PNAd/L-Selectin, and LFA-1 Adhesion Pathways Are Involved in Lymphocyte Migration to Inflamed Lacrimal Glands
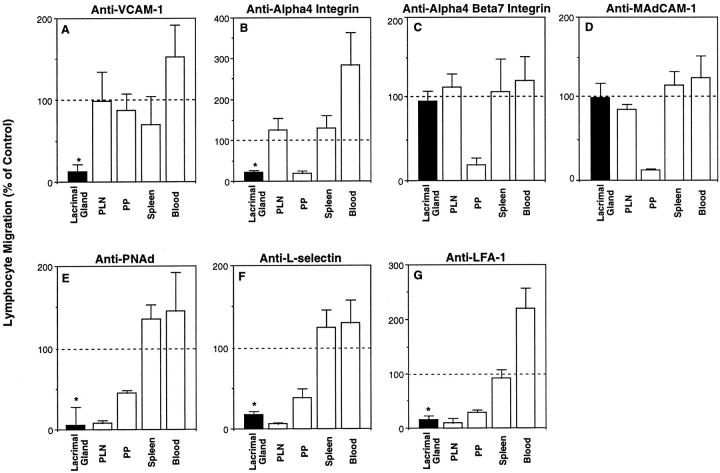
Endothelial VCAM-1 and its lymphocyte ligands α4β1 and α4β7 are thought to be involved in lymphocyte migration into many tertiary lymphoid tissues. 20-23 We found that VCAM-1 is expressed by vascular endothelia in inflamed foci in NOD lacrimal glands (Figures 3 and 4) ▶ ▶ , and that the α4 integrin chain is expressed by the infiltrating lymphocytes (Figure 5) ▶ . These results suggest that lymphocytes may migrate into inflamed lacrimal glands using VCAM-1/α4β1 and/or VCAM-1/α4β7 interactions. To evaluate these possibilities, we used short-term in vivo lymphocyte migration assays to examine the ability of blocking mAbs against VCAM-1 (mAb 4B12), α4 integrin (PS/2), and α4β7 integrin heterodimer (DATK-32) to inhibit the migration of lymphocytes from the bloodstream into inflamed lacrimal glands of 15- to 20-week-old male NOD mice. As shown in Figure 6A ▶ , anti-VCAM-1 mAb blocked most of the lymphocyte migration to inflamed lacrimal gland without significantly affecting the migration to secondary lymphoid tissues. In a similar manner, anti-α4 mAb blocked lymphocyte migration into inflamed lacrimal glands by ∼78% (Figure 6B) ▶ . There was no blocking of lymphocyte migration into lacrimal gland by an antibody (DATK-32) directed against a combinatorial epitope on the α4β7 integrin (Figure 6C) ▶ , or by an antibody against MAdCAM-1 (which is also a ligand for α4β7) (Figure 6D) ▶ . As expected, anti-α4β7 and anti-MAdCAM-1 mAbs inhibited lymphocyte migration into MLNs (not shown) and PPs (Figure 6, C and D) ▶ . 5 These results indicate that VCAM-1 and α4β1 integrin are involved in lymphocyte migration from blood into inflamed lacrimal glands.
Figure 6.
VCAM-1/α4β1, PNAd/L-selectin, and LFA-1 adhesion pathways are involved in the migration of lymphocytes to inflamed lacrimal gland. In vivo migration assays were used to determine the involvement of specific endothelial and lymphocyte adhesion molecules in the migration of lymphocytes from the bloodstream into inflamed lacrimal glands of 15- to 20-week-old male NOD mice. A, D, and E: NOD host mice were given anti-endothelial adhesion molecule or negative control antibody (500 μg iv) followed by TRITC-labeled mouse lymphocytes (iv). Host mice were sacrificed 2 hours later, and the numbers of donor mouse cells in host tissues were determined by confocal microscopy of lacrimal glands and flow cytometric analysis of peripheral lymph nodes (PLN), Peyer’s patches (PP), spleen, and blood. B, C, F, and G: TRITC-labeled mouse lymphocytes were treated with anti-lymphocyte adhesion molecule or negative control antibody, and injected intravenously with CFSE-labeled internal control rat lymphocytes into NOD host mice. Host mice were sacrificed 2 hours later and the ratio of donor mouse to rat cells in each tissue determined by confocal microscopy or flow cytometric analysis. The ratios for all tissues were then normalized by the donor mouse to rat cell ratio used for the transfer. Each graph is from one representative experiment of at least two experiments (A–G). Specific mAbs are anti-VCAM-1 (mAb M/K-2.7) (A), anti-α4 integrin (mAb PS/2) (B), anti-α4β7 integrin (mAb DATK-32) (C), anti-MAdCAM-1 (mAb MECA-367) (D), anti-PNAd (mAb MECA-79) (E), anti-L-selectin (mAb MEL-14) (F), and anti-LFA-1 (mAb FD441.8) (G). Bars indicate mean ± SD of lymphocyte migration in specific mAb treatment group (n = 3 mice), when migration in negative control mAb treatment group (n = 3 mice) is defined as 100% (horizontal line). *, P < 0.05, Student’s t-test, for lacrimal gland, specific antibody treatment versus control antibody treatment.
PNAd is expressed by PLN and PP HEV and by vascular endothelia in many sites of chronic inflammation. 4,24,30,31 Although PNAd is not expressed by endothelia in uninflamed lacrimal gland, we saw prominent expression by vessels in areas of inflammation (Figures 3 and 4) ▶ ▶ . This led us to hypothesize that PNAd and its lymphocyte ligand L-selectin are involved in the migration of lymphocytes into inflamed lacrimal gland. To test this hypothesis, we examined the ability of anti-PNAd and anti-L-selectin mAbs to block the migration of lymphocytes into NOD lacrimal glands.
Treatment of NOD host mice with anti-PNAd mAb blocked 93% of the lymphocyte migration into inflamed lacrimal glands (Figure 6E) ▶ . In a similar manner, incubation of donor lymphocytes with a blocking anti-L-selectin mAb inhibited 82% of the migration to inflamed lacrimal glands (Figure 6F ▶ and Figure 7 ▶ ). As expected, blocking of PNAd or L-selectin almost completely inhibited lymphocyte migration to PLNs, and partially inhibited lymphocyte migration into PPs in host mice (Figures 6, E and F) ▶ . 5,7,31 These results support the hypothesis that PNAd and L-selectin target the migration of lymphocytes to inflamed lacrimal gland.
Figure 7.

L-selectin plays a significant role in lymphocyte migration into inflamed lacrimal gland. TRITC-labeled mouse lymphocytes, treated with negative control (A and B) or anti-L-selectin mAb (C and D), were transferred intravenously with CFSE-labeled internal control rat lymphocytes into 15- to 20-week-old male NOD mice. Host mice were sacrificed 2 hours after transfer. Lymphocytes that migrated from the bloodstream into inflamed lacrimal glands were observed and counted by confocal microscopy. A and B: Serial frozen sections of lacrimal gland from host mouse that received negative control mAb-treated mouse cells. H&E stain (A) demonstrates an area of inflammation. Two-color confocal micrograph (B) shows that almost equal numbers of donor mouse (red) cells and rat (green) cells have migrated into the inflamed area. C and D: Serial frozen sections of lacrimal gland from host mouse that received anti-L-selectin mAb-treated mouse cells. H&E stain (C) shows an area of inflammation. In the two-color confocal micrograph (D), many rat cells (green), but only one anti-L-selectin-treated mouse cell (red cell highlighted by arrow), are seen in the inflamed area, indicating that anti-L-selectin blocked most of the migration of mouse cells into the gland. A and C: H&E. B and D: Two-color confocal micrograph. Original magnifications, ×40.
LFA-1 is involved in lymphocyte migration to most secondary and tertiary lymphoid tissues. 27,28 To determine whether LFA-1 is involved in lymphocyte migration to inflamed lacrimal glands, we incubated TRITC-labeled mouse lymphocytes with an anti-LFA-1 mAb, and transferred them intravenously along with CFSE-labeled rat internal control cells into male NOD mice. Anti-LFA-1 inhibited 84% of the migration of the mouse lymphocytes from blood into inflamed lacrimal glands (Figure 6G) ▶ . As expected, anti-LFA-1 mAb treatment also blocked much of the lymphocyte migration to PLNs and PPs. 5,7,27,28
Discussion
Lymphocyte Migration Pathways in Tissue-Specific Autoimmune Diseases
Most lymphocytes migrate continuously throughout the body. The major route of recirculation for naive T and B lymphocytes is from blood through secondary lymphoid tissues such as LNs and PPs, into the draining lymphatics and back to blood. 2 Antigen stimulation of naive lymphocytes generates memory and effector lymphocytes that migrate effectively from blood into tertiary lymphoid tissues. 3 These memory/effector populations show striking tissue selectivity in their abilities to migrate to inflamed sites, such as lung, skin, and gut. 23,32,33
Studies of local immune responses, including those in autoimmune diseases such as insulin-dependent diabetes mellitus and thyroiditis, suggest the following migration pathways are involved in the initiation and maintenance of lacrimal gland inflammation. 34-36 Dendritic cells capture and process autoantigens in the lacrimal glands, and then migrate through the lymphatics to the regional LNs. The initial T cell priming is thought to occur in these regional LNs where the dendritic cells present autoantigen to recirculating naive T cells. 3 This generates T memory/effector cells that migrate effectively from the blood into the lacrimal gland, where they meet their cognate antigens. 3,23,32,33,37 The local inflammatory response is initiated, with production of activating and chemoattractant molecules including cytokines and chemokines, and up-regulation of endothelial adhesion molecules that support the migration of cells from the bloodstream into the tissue. The subsequent influx of antigen-specific and effector lymphocytes and other leukocytes into the lacrimal glands causes tissue destruction and clinical symptoms such as dry eyes. 11
In this study, we used tissue-section immunohistochemistry and suspension immunofluorescence staining to determine the patterns of expression of endothelial and lymphocyte adhesion molecules in lacrimal glands from NOD mice. The physiological importance of these molecules in lymphocyte migration from blood into inflamed lacrimal glands was determined using short-term in vivo migration studies. Our results indicate that VCAM-1/α4β1, PNAd/L-selectin, and LFA-1 adhesion pathways play important roles in this migration.
Endothelial/Lymphocyte Adhesion Pathways Involved in Lymphocyte Migration into Inflamed Lacrimal Gland
VCAM-1 and α4β1 Integrin
Endothelial VCAM-1 and lymphocyte α4β1 integrin mediate the migration of lymphocytes to tertiary lymphoid tissues, including lung, brain, and skin. 21-23 Although α4β7 can also bind to VCAM-1, α4β1 is thought to be the major physiological ligand for VCAM-1. 20
In NOD lacrimal gland, we found that: 1) VCAM-1-expressing vessels are located mainly in areas of inflammation (Figure 3) ▶ ; 2) the numbers of VCAM-1+ vessels increase with increasing inflammation (Figure 4) ▶ ; 3) essentially all T and B cells in inflamed lacrimal gland express the α4 integrin chain (Figure 5) ▶ ; and 4) antibodies against VCAM-1 and α4 integrin, but not against the α4β7 heterodimer, block lymphocyte migration into inflamed lacrimal gland (we do not have an antibody that reacts specifically with β1 chain or the α4β1 heterodimer) (Figure 6; A, B, and C ▶ ). These results indicate that α4β1 and VCAM-1 participate in lymphocyte migration to inflamed lacrimal glands.
MAdCAM-1 and α4β7 Integrin
Endothelial MAdCAM-1 and its lymphocyte ligand α4β7 integrin direct the migration of predominantly naive lymphocytes to intestinal secondary lymphoid tissues (PPs and MLNs). 5,25,38 MAdCAM-1 is also expressed by venules in lamina propria in normal and inflamed intestine, and by HEV in inflamed islets in NOD mice, and is thought to be involved in lymphocyte recruitment to these sites. 4,25,26,30,39,40 MAdCAM-1 does not serve as an adhesion molecule in all mucosal lymphoid tissues, however. For example, HEV in several mucosal sites—including tonsil, bronchus-associated lymphoid tissues (BALT) (Xu, Butcher, and Michie, unpublished observation), nasal-associated lymphoid tissues, inflamed oral mucosa, and inflamed conjunctiva—fail to express functionally significant levels of MAdCAM-1. 40-42
Although lacrimal glands are mucosal tissues, we did not find expression of MAdCAM-1 in normal or inflamed glands (Figure 3) ▶ . Moreover, antibodies against the α4β7 integrin and MAdCAM-1 did not block lymphocyte migration into inflamed lacrimal gland (Figure 6, C and D) ▶ . Thus, MAdCAM-1/α4β7 interactions do not direct the migration of lymphocytes from the bloodstream into inflamed lacrimal glands.
PNAd and L-Selectin
PNAd, which was originally identified on PLN HEV by reactivity with mAb MECA-79, consists of carbohydrate epitopes that bind L-selectin. 31,43 PNAd/L-selectin interactions are important in the migration of naive lymphocytes to PLNs, and play a role in lymphocyte migration to PPs. 5,7,31,44 MECA-79 reactive venules are also found in some sites of chronic inflammation, and may be involved in lymphocyte migration to these sites. 4,24,30
In the current study, we did not see vascular expression of PNAd in uninflamed lacrimal glands from BALB/c or very young NOD mice. However, in NOD lacrimal glands, PNAd expression was seen at the onset of inflammation. Moreover, the numbers of PNAd+ vessels increased along with the inflammation (Figure 4) ▶ . Our in vivo assays show that inhibition of either PNAd or L-selectin blocks >80% of lymphocyte migration to inflamed lacrimal glands (Figure 6, E and F) ▶ . Our suspension staining and flow cytometry studies indicate that L-selectin is expressed by almost all T and B cells in inflamed lacrimal gland (Figure 5) ▶ . However, most of the lymphocytes express low to medium levels of L-selectin. Lymphocyte activation is known to induce shedding of surface L-selectin. 45 Thus, blood-borne lymphocytes may have used L-selectin to interact with the lacrimal gland endothelium, and then down-regulated the receptor after migration into the gland parenchyma. Alternatively, inflamed lacrimal glands may selectively recruit or retain lymphocyte subsets with low to medium levels of L-selectin.
LFA-1
LFA-1 is involved in lymphocyte migration to many secondary and tertiary lymphoid tissues. 27,28 In situ microscopy studies show that LFA-1 is essential for activation-dependent sticking (step 3) of lymphocytes to HEV in LNs and PPs. 5,7 Members of the ICAM family of adhesion molecules are thought to serve as endothelial ligands for LFA-1. Using short-term in vivo assays, we found that treatment of lymphocytes with an antibody against LFA-1 blocks almost all migration of lymphocytes from blood into inflamed lacrimal glands (Figure 6G) ▶ .
Multistep Cascades for Lymphocyte Migration into Inflamed Lacrimal Gland
An important feature in the regulation of lymphocyte recirculation is the ability of lymphocytes to recognize and bind to the surface of blood vessel endothelial cells before migrating through the vessel wall into surrounding tissue. Recent studies have shown that adhesion interactions of vascular endothelia with lymphocytes under flow or shear consist of multistep cascades with at least four steps: 1) an initial transient tethering and rolling: most lymphocyte adhesion molecules, such as L-selectin, that are involved in rolling are found on the tips of the lymphocytes’ microvilli, where they can easily contact the endothelium; 2) if the lymphocytes encounter appropriate activating factors such as chemokines in the local environment, rolling may be followed by a lymphocyte activation step mediated primarily through G protein-linked chemoattractant receptors, which then leads to; 3) firm adhesion or sticking mediated by activated integrins interacting with endothelial immunoglobulin family members, that may be followed by; 4) lymphocyte diapedesis through the endothelium into tissue, probably also directed by chemokines. 5-8
Our studies, in conjunction with known roles for adhesion molecules in the cascades in LNs and PPs, suggest that lymphocyte migration from blood into inflamed lacrimal glands uses a multistep cascade as follows: lymphocytes tether and roll (step 1) using VCAM-1/α4β1 and PNAd/L-selectin; after activation (step 2) by as yet undefined cytokines or chemokines, there is firm adhesion (step 3) mediated by lymphocyte LFA-1 binding to endothelial ICAMs, and lymphocyte α4β1 binding to endothelial VCAM-1; diapedesis (step 4) through the endothelium into lacrimal gland parenchyma is mediated by unknown adhesion molecules, and by activating factors that may include the chemokines RANTES and IP-10. 46
Summary
In conclusion, we found that inflamed lacrimal glands show prominent vascular expression of endothelial adhesion molecules VCAM-1 and PNAd. Moreover, we show that lymphocyte migration from the blood into inflamed lacrimal glands requires an endothelial/lymphocyte adhesion cascade involving VCAM-1/α4β1 integrin, PNAd/L-selectin, and LFA-1. Inhibition of these lymphocyte and endothelial adhesion molecules may permit selective modulation of lacrimal gland inflammation, and thus prevention of lacrimal gland destruction, in Sjogren’s syndrome.
Footnotes
Address reprint requests to Sara Michie, M.D., SUMC-Pathology-L235, Stanford, CA 94305-5324. E-mail: smichie@stanford.edu.
Supported by the National Institutes of Health (AI 47574) (to S. A. M.) and by a Merit Review Award from the Department of Veterans Affairs (to S. A. M.).
Present address of A. Mikulowska-Mennis: Arcturus Engineering, Inc., 400 Logue Ave., Mountain View, CA, 94043-4019.
References
- 1.Carlos TM, Harlan JM: Leukocyte-endothelial adhesion molecules. Blood 1994, 84:2068-2101 [PubMed] [Google Scholar]
- 2.Gowans JL, Knight EJ: The route of recirculation of lymphocytes in the rat. Proc R Soc Lond [Biol] 1964, 159:257-269 [DOI] [PubMed] [Google Scholar]
- 3.Mackay CR, Marston WL, Dudler L: Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med 1990, 171:801-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanninen A, Taylor C, Streeter PR, Stark LS, Sarte JM, Shizuru JA, Simell O, Michie SA: Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest 1993, 92:2509-2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargatze RF, Jutila MA, Butcher EC: Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity 1995, 3:99-108 [DOI] [PubMed] [Google Scholar]
- 6.Stein JV, Rot A, Luo Y, Marasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH: The CC chemokine TCA-4 triggers LFA-1-mediated arrest of rolling lymphocytes in peripheral lymph node high endothelial venules. J Exp Med 2000, 191:61-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnock RA, Askari S, Butcher EC, von Andrian UH: Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med 1998, 187:205-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warnock RA, Campbell JJ, Dorf ME, Matsuzawa A, McEvoy LM, Butcher EC: The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer’s patch high endothelial venules. J Exp Med 2000, 191:77-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castano L, Eisenbarth GS: Type-I diabetes: a chronic autoimmune disease of human, mouse and rat. Annu Rev Immunol 1990, 8:647-679 [DOI] [PubMed] [Google Scholar]
- 10.Asamoto H, Akazawa Y, Tashiro S, Oishi M, Azuma T, Koide S, Sudo K, Yokota H, Tochino Y: Infiltration of lymphocytes in various organs of the NOD (non-obese diabetic) mouse. J Jpn Diabetic Soc 1984, 27:775-781 [Google Scholar]
- 11.Fox RI, Maruyama T: Pathogenesis and treatment of Sjogren’s syndrome. Curr Opin Rheumatol 1997, 9:393-399 [DOI] [PubMed] [Google Scholar]
- 12.Humphreys-Beher MG, Hu Y, Nakagawa Y, Wang PL, Purushotham KR: Utilization of the non-obese diabetic mouse as an animal model for the study of secondary Sjogren’s syndrome. Adv Exp Med Biol 1994, 350:631-636 [DOI] [PubMed] [Google Scholar]
- 13.Moore PA, Bounous DI, Kaswan RL, Humphreys-Beher MG: Histologic examination of the NOD-mouse lacrimal glands, a potential model for idiopathic autoimmune dacryoadenitis in Sjogren’s syndrome. Lab Anim Sci 1996, 46:125-128 [PubMed] [Google Scholar]
- 14.Jung TM, Dailey MO: A novel and inexpensive source of allophycocyanin for multicolor flow cytometry. J Immunol Methods 1989, 121:9-18 [DOI] [PubMed] [Google Scholar]
- 15.Johnson GG, Mikulowska A, Butcher EC, McEvoy LM, Michie SA: Anti-CD43 monoclonal antibody L11 blocks migration of T cells to inflamed pancreatic islets and prevents the development of diabetes in NOD mice. J Immunol 1999, 163:5678-5685 [PubMed] [Google Scholar]
- 16.Engelhardt B, Martin-Simonet MTG, Rott LS, Butcher EC, Michie SA: Adhesion molecule phenotype of T lymphocytes in inflamed CNS. J Neuroimmunol 1998, 84:92-104 [DOI] [PubMed] [Google Scholar]
- 17.Butcher EC, Weissman IL: Direct fluorescent labeling of cells with fluorescein or rhodamine isothiocyanate. I. Technical aspects. J Immunol Methods 1980, 37:97-108 [DOI] [PubMed] [Google Scholar]
- 18.Hunger RE, Muller S, Laissue JA, Hess MW, Carnaud C, Garcia I, Mueller C: Inhibition of submandibular and lacrimal gland infiltration in nonobese diabetic mice by transgenic expression of soluble TNF-receptor p55. J Clin Invest 1996, 98:954-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA, Sullivan DA: Impact of gender on exocrine gland inflammation in mouse models of Sjogren’s syndrome. Exp Eye Res 1999, 69:355-366 [DOI] [PubMed] [Google Scholar]
- 20.Chan BM, Elices MJ, Murphy E, Hemler ME: Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of α4β1 (VLA-4) and α4β7 on the human B cell line JY. J Biol Chem 1992, 267:8366-8370 [PubMed] [Google Scholar]
- 21.Engelhardt B, Laschinger M, Schulz M, Samulowitz U, Vestweber D, Hoch G: The development of experimental autoimmune encephalomyelitis in the mouse requires α4-integrin but not α4β7-integrin. J Clin Invest 1998, 102:2096-2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin JE, Hatfield CA, Winterrowd GE, Brashler JR, Vonderfecht SL, Fidler SF, Griffin RL, Kolbasa KP, Krzesicki RF, Sly LM, Staite ND, Richards IM: Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol 1997, 272:L219-L229 [DOI] [PubMed] [Google Scholar]
- 23.Picker LJ, Martin RJ, Trumble A, Newman LS, Collins PA, Bergstresser PR, Leung DY: Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites. Eur J Immunol 1994, 24:1269-1277 [DOI] [PubMed] [Google Scholar]
- 24.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ: The human peripheral lymph node vascular addressin—an inducible endothelial antigen involved in lymphocyte homing. Am J Pathol 1993, 143:1688-1698 [PMC free article] [PubMed] [Google Scholar]
- 25.Streeter PR, Berg EL, Rouse BTN, Bargatze F, Butcher C: A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 1988, 331:41-46 [DOI] [PubMed] [Google Scholar]
- 26.Yang X-D, Sytwu H-K, McDevitt HO, Michie SA: Involvement of beta7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in non-obese diabetic mice. Diabetes 1997, 46:1542-1547 [DOI] [PubMed] [Google Scholar]
- 27.Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM: Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur J Immunol 1998, 28:1959-1969 [DOI] [PubMed] [Google Scholar]
- 28.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N: Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med 1999, 189:1467-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalina MD, Estess P, Siegelman MH: Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P-selectin but not L-selectin. Blood 1999, 93:580-589 [PubMed] [Google Scholar]
- 30.Faveeuw C, Gagnerault MC, Lepault F: Expression of homing and adhesion molecules in infiltrated islets of Langerhans and salivary glands of nonobese diabetic mice. J Immunol 1994, 152:5969-5978 [PubMed] [Google Scholar]
- 31.Streeter PR, Rouse BTN, Butcher EC: Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol 1988, 107:1853-1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay CR, Andrew DP, Briskin M, Ringler DJ, Butcher EC: Phenotype and migration properties of three major subsets of tissue homing T cells in sheep. Eur J Immunol 1996, 26:2433-2439 [DOI] [PubMed] [Google Scholar]
- 33.Abitorabi MA, Mackay C, Jerome EH, Osorio O, Butcher EC, Erle DJ: Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J Immunol 1996, 156:3111-3117 [PubMed] [Google Scholar]
- 34.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 1998, 392:245-252 [DOI] [PubMed] [Google Scholar]
- 35.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D: Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 1999, 189:331-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voorbij HA, Kabel PJ, de Haan M, Jeucken PH, van der Gaag RD, de Baets MH, Drexhage HA: Dendritic cells and class II MHC expression on thymocytes during the autoimmune thyroid disease of the BB rat. Clin Immunol Immunopathol 1990, 55:9-22 [DOI] [PubMed] [Google Scholar]
- 37.Paronen J, Klemetti P, Kantele JM, Savilahti E, Perheetupa J, Akerblom HK, Vaarala O: Glutamate decarboxylase-reactive peripheral blood lymphocytes from patients with IDDM express gut-specific homing receptor α4β7-integrin. Diabetes 1997, 46:583-588 [DOI] [PubMed] [Google Scholar]
- 38.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC: α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 1993, 74:185-195 [DOI] [PubMed] [Google Scholar]
- 39.Hanninen A, Salmi M, Simell O, Jalkanen S: Mucosa-associated (β7high) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes 1996, 45:1173-1180 [DOI] [PubMed] [Google Scholar]
- 40.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ: Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol 1997, 151:97-110 [PMC free article] [PubMed] [Google Scholar]
- 41.Csencsits KL, Jutila MA, Pascual DW: Nasal-associated lymphoid tissue: phenotypic and functional evidence for the primary role of peripheral node addressin in naive lymphocyte adhesion to high endothelial venules in a mucosal site. J Immunol 1999, 163:1382-1389 [PubMed] [Google Scholar]
- 42.Haynes RJ, Tighe PJ, Scott RA, Singh Dua H: Human conjunctiva contain high endothelial venules that express lymphocyte homing receptors. Exp Eye Res 1999, 69:397-403 [DOI] [PubMed] [Google Scholar]
- 43.Berg EL, Robinson MK, Warnock RA, Butcher EC: The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol 1991, 114:343-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley LM, Watson SR, Swain SL: Entry of naive CD4 T cells into peripheral lymph node requires L-selectin. J Exp Med 1994, 180:2401-2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung TM, Gallatin WM, Weissman IL, Dailey MO: Down-regulation of homing receptors after T cell activation. J Immunol 1988, 141:4110-4117 [PubMed] [Google Scholar]
- 46.Tornwall J, Lane TE, Fox RI, Fox HS: T cell attractant chemokine expression initiates lacrimal gland destruction in nonobese diabetic mice. Lab Invest 1999, 79:1719-1726 [PubMed] [Google Scholar]