Abstract
Atherosclerotic lesions are characterized by prominent macrophage and T-cell infiltration and atherosclerosis is widely recognized as an inflammatory disease. The cytokine interleukin-15 (IL-15) has T-cell chemotactic and pro-inflammatory properties and promotes the recruitment of T cells to sites of inflammation. We have therefore examined IL-15 expression in the atherosclerotic ApoE-deficient mouse model as well as in human atherosclerotic lesions. In gene expression arrays, a transcript corresponding to IL-15 mRNA was elevated in atherosclerotic aortas of ApoE-deficient mice fed a Western diet for 10 and 20 weeks, corresponding to lesions of the fatty streak and fibrofatty plaque stage, respectively. Immunostaining for IL-15 localized to aortic smooth muscle cells in nonatherosclerotic C57BL/6 mice, whereas both macrophages and smooth muscle cells stained positive for IL-15 in atherosclerotic lesions of ApoE-deficient mice. Finally, advanced atherosclerotic lesions of human carotid arteries were immunostained to determine whether IL-15 is involved in human disease. IL-15 protein was present also in the human lesions with a distribution primarily overlapping that of macrophages. In conclusion, IL-15 is up-regulated in both human and animal atherosclerotic lesions and may contribute to the recruitment of T cells and their activation during atherogenesis.
Atherosclerosis, the main cause of morbidity and mortality in industrialized societies, is a complex disease with both genetic and environmental causes. 1 Infiltrates of activated macrophages and T cells are prominent both in human 2 and murine atherosclerotic lesions, 3 and a growing body of evidence implies that atherosclerosis can be considered an inflammatory disease. 4,5 Mononuclear cell recruitment mediated by endothelial leukocyte adhesion molecules 6-8 and chemoattractants, 9,10 as well as signal pathways essential for adaptive immunity 11,12 has been demonstrated to be important in the development of atherosclerosis. T cells isolated from human atherosclerotic plaques are predominantly CD4+CD45RO+ memory cells, 13 some of which recognize oxidatively modified low-density lipoproteins (oxLDL) in an MHC class II-dependent manner. 14 Immunization with oxLDL reduces the development of atherosclerosis in mouse and rabbit models. 15 However, the precise role for the adaptive immunity in atherosclerosis is still unclear and other antigens, such as heat shock proteins, 16 β2-glycoprotein, 17 and Chlamydia pneumoniae, 18 may also be involved.
The cytokine interleukin-15 (IL-15) has been detected in different inflammatory conditions. 19 IL-15 is constitutively expressed in a wide variety of cells such as monocytes, skeletal muscle cells, endothelial cells, epithelial cells, and fibroblasts. 20-22 Posttranscriptional regulation of IL-15 is prominent and may occur both at the level of translation and intracellular trafficking. 23 Inflammatory stimuli such as bacterial lipopolysaccharide and interferon-γ (IFN-γ) up-regulate IL-15 mRNA in freshly isolated monocytes 20,24 and nuclear factor-κB and interferon regulatory factor-E motifs are conserved in the 5′-flanking region of the IL-15 gene both in humans and mice. 25,26
IL-15 is a potent pro-inflammatory mediator inducing the expression of tumor necrosis factor-α, IL-1, and interferon-γ in rheumatoid arthritis. 27 IL-15 has been shown to be both a potent growth factor 20 and chemoattractant for T cells. 28 It is involved in the recruitment of activated memory T cells to sites of inflammation by a novel, recently described mechanism. 29,30 IL-15 induces the expression of hyaluronan by endothelial cells, promoting the binding to the hyaluronan receptor CD44 on activated T cells. 31 This mechanism has been shown to operate under shear stress of the kind encountered in arterial flow and is followed by a secondary CD44/VLA-4-mediated adhesion that leads to extravasation of the activated T cells. 32 Antibodies to CD44 and integrin-α4 prevented experimental autoimmune encephalomyelitis induced by the transfer of myelin basic protein-specific T cells in rats. 33
In addition to its role in T-cell recruitment and cytokine secretion, IL-15 has angiogenic activity 34 that may be important in atherosclerosis and other inflammatory diseases. Finally, it promotes antigen-specific activation of T cells by acting in a manner similar to IL-2. 20
IL-15 can promote T cell adhesion and activation, cytokine secretion as well as angiogenesis, and may thus play a role in atherosclerosis. By analyzing gene expression in atherosclerotic ApoE−/− mice with cDNA array technique, we detected the expression of IL-15 mRNA in atherosclerotic aortas of ApoE−/− mice. The expression was confirmed on both mRNA and protein levels. IL-15 was expressed by macrophages and smooth muscle cells in lesions of ApoE−/− mice and could also be detected in human atherosclerotic lesions. These data show that IL-15 expression is up-regulated in atherosclerosis and suggest that this cytokine may be important in its pathogenesis.
Materials and Methods
Animals and Tissue Preparation
Female ApoE−/− mice 35 on the B6 background (strain C57BL/6H-ApoETM1UNC129) were obtained from M&B Breeding and Research Center (M&B A/S, Ry, Denmark) and normal C57BL/6 from Charles River Sverige AB (Uppsala, Sweden) at 6 to 8 weeks of ages. The ApoE−/− mice were fed Western diet containing 0.15% cholesterol for 10 or 20 weeks. 3 The mice were sacrificed by exsanguination under carbon dioxide anesthesia. After perfusion with ice-cold phosphate-buffered saline (PBS), the hearts and aortas were dissected out and placed in ice-cold PBS. The samples were further rinsed with PBS under dissection microscope before pooling for freezing. Nonatherosclerotic aortic tissue was obtained in a similar manner from C57BL/6 mice on chow diet.
Gene Array Analysis
The aortas of five mice were pooled, frozen, and stored at −80°C until RNA preparation. The frozen samples were homogenized in a dismembrator (B. Braun, Melsungen AG, Germany). Lysis buffer (Dynal, NY) was added to the homogenate, mRNA isolated on oligo-dT-conjugated magnetic beads (Dynabeads, Dynal AS, Oslo, Norway). The mRNA quantity was estimated using DNA dip stick (Invitrogen, Carlsbad, CA). The pooled mRNA from 3 × 5 mice (0.6 μg total) from each group was precipitated with Na-acetate. cDNA was labeled with [α-33P]-dATP and hybridized to the mouse gene expression array (Clontech Laboratories Inc., Palo Alto, CA) following the instructions of the manufacturer. The membranes for the two groups of each time were exposed on the same phosphor plate (Fuji BAS 2040; Fujifilm, Tokyo, Japan) for 4 to 14 hours and were quantified on a BAS 2500 Bio-Imaging Analyzer (Fujifilm).
The image was imported into the Image Gauge version 3.0 computer program (Fujifilm). The pixel/light intensity was measured for one gene at a turn, applying exactly equal areas on both groups. To normalize the individual genes, the average gene-expression intensities of for each group were divided by the average of gene-expression intensity of the C57BL/6 mice on 10 weeks of diet. The expression intensities of the individual genes of the different groups were thereafter divided by the respective normalization factors.
Real-Time Polymerase Chain Reaction
Twenty ng of mRNA from each sample was reverse-transcribed using Superscript II according to the manufacturers manual (Life Technologies, Rockville, MD). cDNA (1.5 μl) was amplified by real-time polymerase chain reaction (PCR) with 1× TaqMan buffer, 5 mmol/L MgCl2, 200 μmol/L of each dNTP, 200 μmol/L of each primer, 1.25 pmol/L of probe, 0.25 U Amp-Erase uracil N-glycosylase 1.25 U AmpliTaq gold (PE Biosystems, Foster City, CA). For the amplification of the IL-15 gene, 36 the primers IL-15 FW: 5-CAT CCA TCT CGT GCT ACT TGT GTT-3; and IL-15 RW: 5-CAT CTA TCC AGT TGG CCT CTG TTT-3 (Life Technologies, Inc., Grand Island, NY); and the probe IL-15 TM: 5-AGG GAG ACC TAC ACT GAC ACA GCC CAA AA-3 (PE Biosystems) were used. For normalization of RNA loading control samples were run using β-actin, 36 applying the primers β-actin FW: 5-AGA GGG AAA TCG TGC GTG AC-3, and β-actin RW: 5-CAA TAG TGA TGA CCT GGC CGT-3 (Life Technologies, Inc.) and the probe β-actin TM: 5-CAC TGC CGC ATC CTC TTC CTC CC-3 (PE Biosystems). Each sample was analyzed in duplicate (2 minutes at 50°C, 10 minutes at 95°C, 0.15 minute at 95°C, and 1 minute at 60°C) using ABI Prism 7700 Sequence Detector (PE Biosystems). The PCR amplification was estimated by correlation to a standard curve. The reactions were performed in MicroAmp optical 96-well reaction plates (PE Biosystems).
Immunohistochemistry
Human atherosclerotic tissue specimens were obtained from patients undergoing carotid endarterectomy surgery. The mouse hearts and the human tissue samples were snap-frozen in n-heptane chilled with liquid nitrogen. Frozen cryostat sections were dried and fixed with 100% acetone at room temperature for 10 minutes.
The following antibodies were used: polyclonal goat-anti-mouse/human IL-15 (clone L-20; Santa Cruz Biotechnology, Santa Cruz, CA); monoclonal rat anti-mouse CD4 (clone A15.1.17), biotin-labeled monoclonal rat anti-mouse Mac-1 (CD11b, clone M1/70), biotin-labeled monoclonal rat anti-mouse CD44 (clone IM7) (all from PharMingen, San Diego, CA); monoclonal mouse anti-human anti-CD68 (clone PG-M1; DAKO A/S, Glostrup, Denmark); alkaline phosphatase-conjugated monoclonal α-smooth muscle actin (clone 1A4; Sigma Chemical Co., Saint Louis, MO); the secondary horse anti-goat and horse anti-mouse antibodies (Vector Laboratories, Burlingame, CA).
For IL-15 staining, the antibody was diluted in a Saponin buffer containing 0.1% (w/v) saponin (Sigma), Earl’s buffered salt solution and 1% (v/v) Hepes buffer and kept in the saponin buffer throughout the staining procedure until the development when saponin was omitted. The sections were incubated for 1 hour at room temperature (dilution, 1:200) with the primary antibody. After incubation with a biotin-labeled horse anti-goat secondary antibody (dilution, 1:200) the sections were incubated with avidin-biotin-peroxidase and developed with a diaminobenzidine/H2O2 substrate solution as described. 3 For blocking experiments, the primary antibody was preincubated overnight with or without a 5× excess of the antigenic IL-15 peptide (sc-1296P; Santa Cruz Biotechnology) according to the manufacturer’s recommendation before staining the sections. All surface markers were used at optimal concentrations as determined by staining of spleen sections. For detection of anti-mouse α-smooth muscle actin, sections were incubated with primary antibody conjugated with alkaline phosphatase for 1 hour at room temperature and developed with Fast Red (Vector Laboratories). Finally, all sections were counterstained with Harris’ hematoxylin.
Statistical Analysis
The results of the quantitative mRNA analysis are expressed as means ± SEM. Differences between the groups were considered significant at P < 0.05, using two-tailed Students’ t-test.
Results
IL-15 mRNA Expression in Mouse Aortas Detected by Gene Expression Array and Quantitative Real-Time PCR
To identify differences in gene expression between normal arterial walls and atherosclerotic lesions, we used a membrane-based mouse gene expression array. Aortas from atherosclerotic ApoE−/− mice fed a Western diet for 10 and 20 weeks were compared with aortas from age-matched C57BL/6 mice. By gene array analysis IL-15 expression was 1.66-fold increased at 10 weeks and 2.22-fold increased at 20 weeks of diet (Figure 1A) ▶ . For comparison, the expression of markers for plaque-infiltrating macrophages (Mac-1) and T cells (CD4) are shown.
Figure 1.
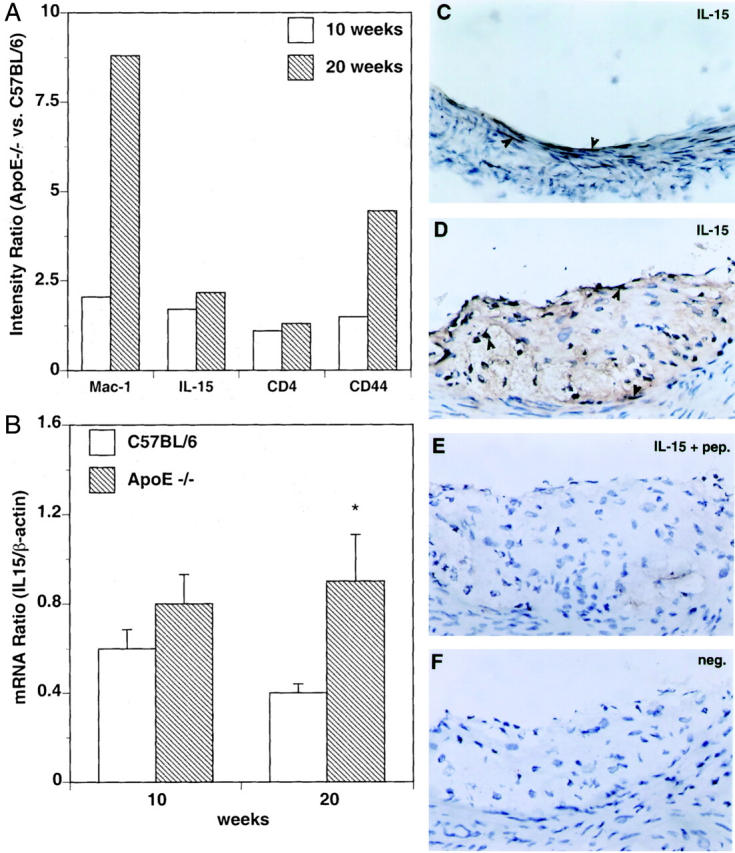
A: Gene expression analysis of aortas of ApoE−/− mice with the Clontech mouse gene array detected increased expression levels of Mac-1, IL-15, CD4, and CD44 mRNAs compared with aortas of C57BL/6 mice. The bar graph shows intensity ratios of gene expression in ApoE−/− mice divided by the gene expression in C57BL/6 mice at 10 (white bars) and 20 weeks (gray bars) of diet. B: Quantitative real-time PCR analysis of IL-15 mRNA of the aortas of both ApoE−/− (gray bars) and C57BL/6 (white bars) mice after 10 and 20 weeks of diet showed a similar pattern to the gene array. The y axis indicates the mRNA ratio of IL-15 divided by the β-actin. Mean ± SEM of three pooled samples each containing five mice for each time point. At 20 weeks, the IL-15 mRNA levels were significantly increased in the ApoE−/− mice compared to nonatherogenic C57BL/6 mice. C–F: Immunohistochemical analysis of IL-15 in sections of C57BL/6 mice (C) and ApoE−/− mice (D–F) after 10 weeks of diet. To evaluate the specificity of the immunostaining, the primary antibody was either preincubated with the antigenic IL-15 peptide to block the binding (E) or omitted (F). Antibody binding was visualized by the avidin-biotin-peroxidase detection system. Original magnification, ×400. The arrowheads indicate positive labeling for IL-15.
To confirm the up-regulation of IL-15 mRNA expression, quantitative real-time PCR was used. IL-15 expression in aortas of ApoE−/− mice was increased 1.35-fold when compared to C57BL/6 at 10 weeks of diet, whereas the difference was 2.02-fold at 20 weeks of diet (Figure 1B) ▶ .
Immunohistochemical Detection of IL-15 Protein in Murine and Human Arteries
To examine if mRNA expression resulted in production of IL-15 protein, sections from the root of the aorta of ApoE−/− mice and C57BL/6 mice were analyzed by immunohistochemistry (Figure 1 ▶ ; C–F, and Figure 2, C–F ▶ ). IL-15 was expressed in smooth muscle and endothelial cells of normal, nonatherosclerotic aortas of C57BL/6 mice (Figure 1C) ▶ . In addition, IL-15 immunostaining could be detected in cardiomyocytes (data not shown). In consecutive sections of atherosclerotic lesions of ApoE−/− mice, both Mac-1-positive macrophage dense areas and α-smooth muscle actin-positive layers stained positively for IL-15 (Figure 2, A–F) ▶ . CD4+-positive cells were also localized to IL-15-rich subendothelial areas (Figure 2, G and H) ▶ , whereas CD44 stained the majority of cells present in the lesion (Figure 2, I and J) ▶ .
Figure 2.
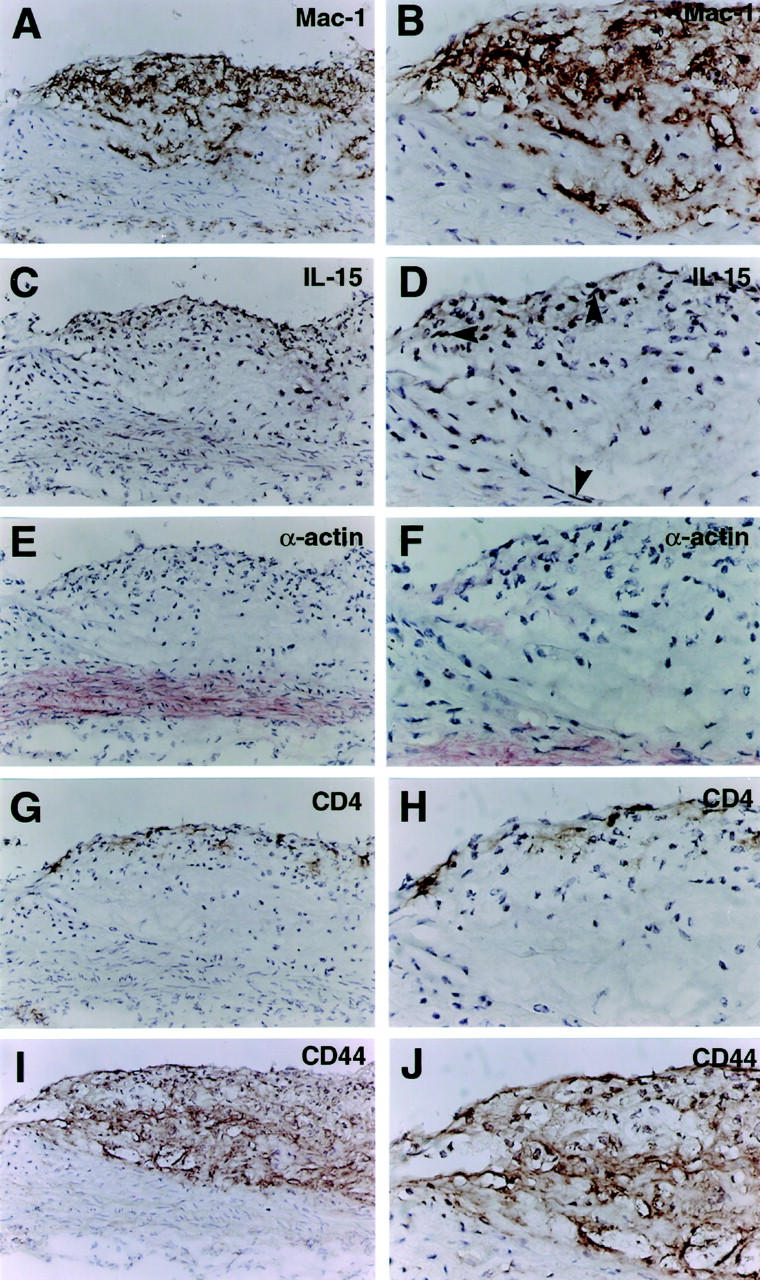
Immunohistochemical analysis of mouse atherosclerotic plaques in serial sections of ApoE−/− mice fed with Western diet for 20 weeks. Antibody binding was visualized by the avidin-biotin-peroxidase detection system (A–D, G–J) and by alkaline phosphatase developed with Fast Red (E and F). Original magnifications, ×200 (left column) and ×400 (right column). A and B: Mac-1 staining for macrophages. C and D: IL-15 staining shows positive labeling of subendothelial areas, rich in macrophages and T cells, and to the smooth muscle layer (C). The arrowheads show positive labeling for IL-15 (D). E and F: Staining for α-smooth muscle actin visualizes smooth muscle cells mainly in the media under the lesion. G and H: CD4 staining for T lymphocytes, which are frequent in subendothelial areas. I and J: Staining for CD44 is abundantly positive throughout the lesion, both in Mac-1-positive and -negative areas.
Finally, IL-15 expression was analyzed by immunohistochemistry in human carotid artery atherosclerosis. Many IL-15-positive cells were observed, particularly in the core region. The staining of IL-15 colocalized with CD68-rich areas in serial sections (Figure 3) ▶ .
Figure 3.
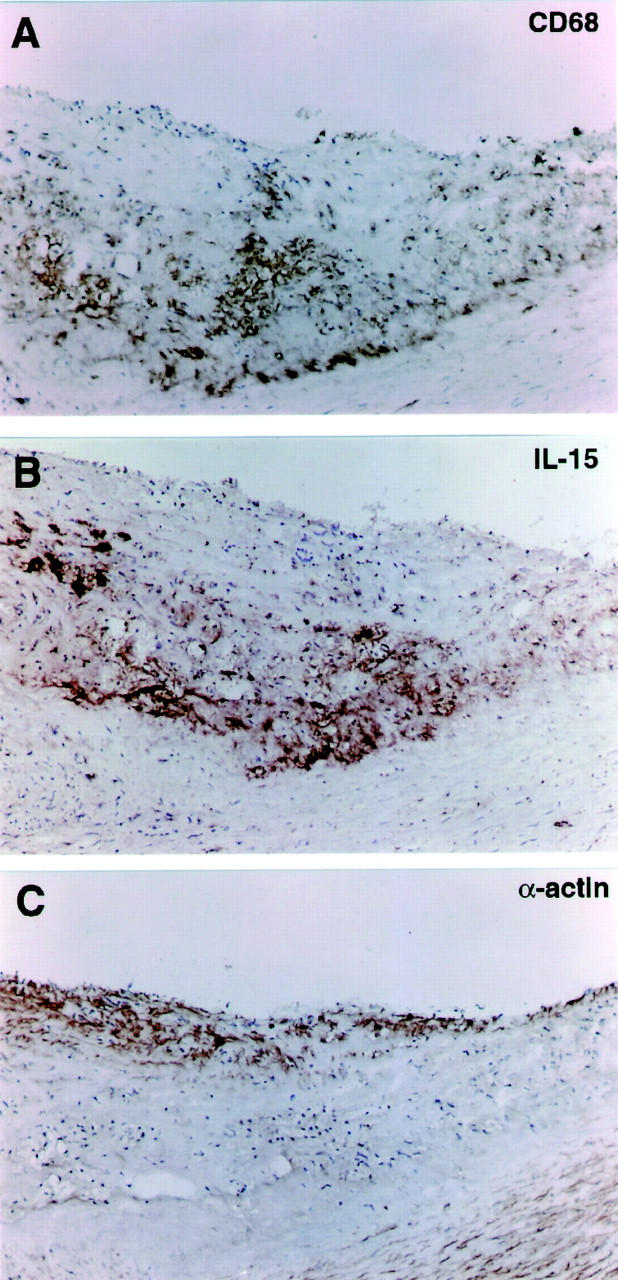
Immunohistochemical analysis of IL-15 in serial sections of human atherosclerotic lesions. Antibody binding was visualized by the avidin-biotin-peroxidase detection system. Original magnifications, ×100. A: CD68 staining for macrophages is most abundant in the core of the lesion. B: IL-15 staining co-localized with the macrophage-rich area in the core of the lesion. C: α-Smooth muscle actin staining depicts smooth muscle cells both in the media and in the cap of the atherosclerotic lesion.
Discussion
IL-15 is a potent immune cytokine that has been implicated in autoimmune disease. We now show that it is expressed, at the mRNA and protein levels, in atherosclerotic lesions of ApoE−/− mice and in advanced human lesions. Its expression co-distributed with that of macrophage markers and because macrophages are known to secrete IL-15, it is likely that they are a major source of this cytokine in the atherosclerotic artery. Interestingly, IL-15 was also expressed, albeit at lower levels, in the aorta of normal C57BL/6 mice, where it was found in smooth muscle cells. It could also be detected in occasional endothelial cells and in cardiomyocytes of surrounding cardiac tissue.
IL-15 production has been found in many cell types 20-22 and this study confirms the production of IL-15 in macrophages, endothelial cells, and cardiomyocytes (not shown). IL-15 has potent pro-inflammatory properties and its expression is tightly regulated at several levels. 23 Transcriptional regulation is mediated via binding sites for nuclear factor-κB and interferon regulatory factor-E in the promotor region of the IL-15 gene. 25,26 Additionally, protein expression is also controlled by regulation of mRNA degradation and translation. Several studies have reported discrepancies between mRNA and protein expression and it was therefore important to establish that IL-15 protein and not only mRNA is present in atherosclerotic lesions. The present immunohistochemical data demonstrate that IL-15 protein is indeed present in lesions, in the human as well as in the ApoE−/− mouse model.
OxLDL has been shown to activate nuclear factor-κB and it seems likely that oxLDL, and other pro-inflammatory components of the forming lesion, could induce IL-15 secretion. CD40 ligation may also promote IL-15 secretion 37 and this could be particularly important in atherosclerotic lesions, where CD40 as well as CD40L are abundantly expressed on vascular as well as immune cells. 38 IL-15 induced through either of these mechanisms could in turn exacerbate local inflammation by activating pro-inflammatory CD4+CD45RO+ memory T cells. 39
Recent studies have shown that IL-15 is a potent immunoregulatory cytokine that can substitute for IL-2 in the activation of antigen-specific T cells. 20 In addition, it promotes NK cell activation, 40 prevents Fas-mediated apoptosis, 41,42 and induces activation of neutrophil granulocytes, 43 B cells, 44 and mast cells. 45 The latter cell type has been identified in human atherosclerotic lesions at sites of plaque rupture. 46 Local IL-15 may induce proliferation of mast cells 45 and up-regulate the production of IL-4 in these cells via Stat6. 47
IL-15 also has been shown to increase hyaluronic acid secretion by endothelial cells. This has been shown to promote the recruitment of inflammatory cells, ie, macrophages and T cells, via a hyaluronan-CD44 interaction. 29,30 CD44 was found to be expressed abundantly in atherosclerotic lesions (this report) and is known to be present on memory T cells, which is the dominating T cell type in human plaques. 48 These findings support the notion that an IL-15-mediated hyaluronan-CD44 ligation could be involved in the recruitment of immune cells to the forming lesion. In addition, CD44 may bind interferon-γ, 49 which might increase the local immune-activating effect of IL-15. Finally, IL-15 has been shown to induce angiogenesis, 34 that could be of importance for the growth of advanced atherosclerotic lesions. 50
In conclusion, IL-15 is expressed in both human and mouse atherosclerotic lesions and may by its pro-inflammatory activities be an important mediator in the progression of the disease.
Acknowledgments
We thank Inger Bodin for excellent technical assistance.
Footnotes
Address reprint requests to Sten Stemme, Cardiovascular Research Unit, Center for Molecular Medicine, L8:03, Karolinska Hospital, S-171 76 Stockholm, Sweden. E-mail: sten.stemme@cmm.ki.se.
Supported by grants from the Swedish Medical Research Council (12660, 2042, and 6816), the Swedish Heart-Lung Foundation, the Torsten and Ragnar Söderberg Foundation, the Åke Wiberg Foundation, the Magnus Bergvall Foundation, the Foundation for Old Servants, and the Professor Nanna Svartz Foundation.
References
- 1.Braunwald E: Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med 1997, 337:1360-1369 [DOI] [PubMed] [Google Scholar]
- 2.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK: Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986, 6:131-138 [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Stemme S, Hansson GK: Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am J Pathol 1996, 149:359-366 [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Hansson GK: Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest 1991, 64:5-15 [PubMed] [Google Scholar]
- 5.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 1999, 340:115-126 [DOI] [PubMed] [Google Scholar]
- 6.Cybulsky MI, Gimbrone MJ: Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991, 251:788-791 [DOI] [PubMed] [Google Scholar]
- 7.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD: The combined role of P- and E-selectins in atherosclerosis. J Clin Invest 1998, 102:145-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R: Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol 1998, 18:842-851 [DOI] [PubMed] [Google Scholar]
- 9.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ: Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 1998, 2:275-281 [DOI] [PubMed] [Google Scholar]
- 10.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF: Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997, 100:2552-2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Pablo AM, Jiang X-C, Wang N, Tall AR, Schindler C: IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 1997, 99:2752-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P: Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 1998, 394:200-203 [DOI] [PubMed] [Google Scholar]
- 13.Stemme S, Holm J, Hansson GK: T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb 1992, 12:206-211 [DOI] [PubMed] [Google Scholar]
- 14.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK: T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 1995, 92:3893-3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freigang S, Horkko S, Miller E, Witztum JL, Palinski W: Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler Thromb Vasc Biol 1998, 18:1972-1982 [DOI] [PubMed] [Google Scholar]
- 16.Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G: Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol 1993, 142:1927-1937 [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Harats D, Gilburd B, Afek A, Shaish A, Kopolovic J, Shoenfeld Y: Adoptive transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation 2000, 102:1822-1827 [DOI] [PubMed] [Google Scholar]
- 18.Mosorin M, Surcel HM, Laurila A, Lehtinen M, Karttunen R, Juvonen J, Paavonen J, Morrison RP, Saikku P, Juvonen T: Detection of Chlamydia pneumoniae-reactive T lymphocytes in human atherosclerotic plaques of carotid artery. Arterioscler Thromb Vasc Biol 2000, 20:1061-1067 [DOI] [PubMed] [Google Scholar]
- 19.McInnes IB, Liew FY: Interleukin 15: a proinflammatory role in rheumatoid arthritis synovitis. Immunol Today 1998, 19:75-79 [DOI] [PubMed] [Google Scholar]
- 20.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al: Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994, 264:965-968 [DOI] [PubMed] [Google Scholar]
- 21.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE: Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse-human rheumatoid arthritis model in vivo. J Clin Invest 1998, 101:1261-1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn LS, Haugk KL, Grabstein KH: Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology 1995, 136:3669-3672 [DOI] [PubMed] [Google Scholar]
- 23.Waldmann TA, Tagaya Y: The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 1999, 17:19-49 [DOI] [PubMed] [Google Scholar]
- 24.Doherty TM, Seder RA, Sher A: Induction and regulation of IL-15 expression in murine macrophages. J Immunol 1996, 156:735-741 [PubMed] [Google Scholar]
- 25.McDonald PP, Russo MP, Ferrini S, Cassatella MA: Interleukin-15 (IL-15) induces NF-kappaB activation and IL-8 production in human neutrophils. Blood 1998, 92:4828-4235 [PubMed] [Google Scholar]
- 26.Washizu J, Nishimura H, Nakamura N, Nimura Y, Yoshikai Y: The NF-kappaB binding site is essential for transcriptional activation of the IL-15 gene. Immunogenetics 1998, 48:1-7 [DOI] [PubMed] [Google Scholar]
- 27.McInnes IB, al-Mughales MJ, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY: The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med 1996, 2:175-182 [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson PC, Liew FY: Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med 1995, 181:1255-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE: Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells In vitro and in the SCID mouse—human rheumatoid arthritis model in vivo. J Clin Invest 1998, 101:1261-1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estess P, Nandi A, Mohamadzadeh M, Siegelman MH: Interleukin 15 induces endothelial hyaluronan expression in vitro and promotes activated T cell extravasation through a CD44-dependent pathway in vivo. J Exp Med 1999, 190:9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeGrendele HC, Estess P, Siegelman MH: Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997, 278:672-675 [DOI] [PubMed] [Google Scholar]
- 32.Siegelman MH, Stanescu D, Estess P: The CD44-initiated pathway of T-cell extravasation uses VLA-4 but not LFA-1 for firm adhesion. J Clin Invest 2000, 105:683-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T: Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA 1999, 96:6896-6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angiolillo AL, Kanegane H, Sgadari C, Reaman GH, Tosato G: Interleukin-15 promotes angiogenesis in vivo. Biochem Biophys Res Commun 1997, 233:231-237 [DOI] [PubMed] [Google Scholar]
- 35.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N: Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA 1992, 89:4471-4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overbergh L, Valckx D, Waer M, Mathieu C: Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 1999, 11:305-312 [DOI] [PubMed] [Google Scholar]
- 37.Sugiura T, Kawaguchi Y, Harigai M, Takagi K, Ohta S, Fukusawa C, Hara M, Kamatani N: Increased CD40 expression on muscle cells of polymyositis and dermatomyositis: role of CD40-CD40 ligand interaction in IL-6, IL-8, IL-15, and monocyte chemoattractant protein-1 production. J Immunol 2000, 164:6593-6600 [DOI] [PubMed] [Google Scholar]
- 38.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P: Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA 1997, 94:1931-1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanegane H, Tosato G: Activation of naive and memory T cells by interleukin-15. Blood 1996, 88:230-235 [PubMed] [Google Scholar]
- 40.Carson WE, Giri JG, Lindenmann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA: Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 1994, 180:1395-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruckert R, Assadullah K, Seifert M, Budagian VM, Arnold R, Trombotto C, Paus R, Bulfone-Paus P: Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol 2000, 165:2240-2250 [DOI] [PubMed] [Google Scholar]
- 42.Akbar AN, Salmon M: Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today 1997, 18:72-76 [DOI] [PubMed] [Google Scholar]
- 43.Girard D, Paquet ME, Paquin R, Beaulieu AD: Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood 1996, 88:3176-3184 [PubMed] [Google Scholar]
- 44.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH: IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol 1995, 154:483-490 [PubMed] [Google Scholar]
- 45.Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA: Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J 1996, 15:4928-4939 [PMC free article] [PubMed] [Google Scholar]
- 46.Kovanen PT, Kaartinen M, Paavonen T: Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 1995, 92:1084-1088 [DOI] [PubMed] [Google Scholar]
- 47.Masuda A, Matsuguchi T, Yamaki K, Hayakawa Y, Kubo M, LaRochelle WJ, Yoshikai Y: Interleukin-15 induces rapid tyrosine phosphorylation of STAT6 and the expression of interleukin-4 in mouse mast cells. J Biol Chem 2000, 275:29331-29337 [DOI] [PubMed] [Google Scholar]
- 48.Curry AJ, Portig I, Goodall JC, Kirkpatrick PJ, Gaston JS: T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol 2000, 121:261-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurt-Camejo E, Rosengren B, Sartipy P, Elfsberg K, Camejo G, Svensson L: CD44, a cell surface chondroitin sulfate proteoglycan, mediates binding of interferon-gamma and some of its biological effects on human vascular smooth muscle cells. J Biol Chem 1999, 274:18957-18964 [DOI] [PubMed] [Google Scholar]
- 50.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J: Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 1999, 99:1726-1732 [DOI] [PubMed] [Google Scholar]


