Abstract
Extensive studies have demonstrated that the Akt/AKT1 pathway is essential for cell survival and inhibition of apoptosis; however, alterations of Akt/AKT1 in human primary tumors have not been well documented. In this report, significantly increased AKT1 kinase activity was detected in primary carcinomas of prostate (16 of 30), breast (19 of 50), and ovary (11 of 28). The results were confirmed by Western blot and immunohistochemical staining analyses with phospho-Ser473 Akt antibody. The majority of AKT1-activated tumors are high grade and stage III/lV (13 of 16 prostate, 15 of 19 breast, and 8 of 11 ovarian carcinomas). Previous studies showed that wild-type AKT1 was unable to transform NIH3T3 cells. To demonstrate the biological significance of AKT1 activation in human cancer, constitutively activated AKT1 (Myr-Akt) was introduced into NIH3T3 cells. Overexpression of Myr-Akt in the stably transfected cells resulted in malignant phenotype, as determined by growth in soft agar and tumor formation in nude mice. These data indicate that AKT1 kinase, which is frequently activated in human cancer, is a determinant in oncogenesis and a potential target for cancer intervention.
Akt, also known as protein kinase B, represents a subfamily of the serine/threonine protein kinases. 1-5 Akt/AKT1/PKBα signaling has been extensively studied throughout the last 6 years. It has been shown that Akt is activated by a variety of stimuli in a phosphoinositide-3-OH kinase (PI 3-kinase)-dependent manner. 6-9 Activation of Akt by growth factors depends on the integrity of the PH domain, which binds to the PI 3-kinase product, PI(3,4,5)P3, and phosphorylation of Thr-308 and Ser-473 by PDK1 and PDK2/ILK, respectively. In addition, growth factor-induced Akt activation is also mediated by Ras, Src, and Gab1. 10-12 In numerous cell types, it has been shown that Akt induces survival and suppresses apoptosis induced by a variety of stimuli, including growth factor withdrawal and loss of cell adhesion. The mechanisms by which Akt promotes cell survival include phosphorylation of the pro-apoptotic proteins BAD, caspase-9, Forkhead transcription factors, and IκB kinase α, resulting in reduced binding of BAD to Bcl-XL, inhibition of caspase-9 protease activity, Fas ligand gene transcription, and activation of the nuclear factor-κB cascade. 13-17 Akt has also been shown to inhibit the Raf-MEK-ERK pathway through phosphorylation of Raf-1 in myotubes and overcome constitutively activated MAPK-induced cell-cycle arrest in MCF7 cells. 18,19
Although Akt/AKT1 is essential for cell survival and anti-apoptosis, alterations of Akt/AKT1 have not been consistently observed in any human malignancy. In fact, amplification of AKT1 has been reported in only a single gastric carcinoma. 20 In this communication, we describe frequent activation of AKT1 in human carcinomas of prostate, breast, and ovary. We also demonstrate the biological significance of AKT1 activation in human cancer by showing that constitutively activated, but not wild-type, Akt is highly oncogenic in NIH3T3 cells.
Materials and Methods
Tumor Specimens, Cell Lines, Transfection, and Transformation Assay
All primary human cancer specimens were obtained from patients who underwent surgery at the H. Lee Moffitt Cancer Center and each sample contained at least 70% tumor cells as was confirmed by histological examination. The tissues were snap-frozen and stored at −70°C. Slides from each case were reviewed for grade and stage following the criteria of the American Joint Committee on Cancer, 1988 edition. NIH3T3 cells were cultured at 37°C in Dulbecco’s modified Eagle’s medium supplemented with 10% calf serum. Transfection was performed with LipofectAMINE PLUS (Life Technologies, Inc., Rockville, MD). Stable clonal cell lines were established after G418 selection. Soft agar suspension and tumorigenesis assays were performed as previously described. 21
Plasmids
Hemagglutin epitope (HA)-tagged wild-type, constitutively active (Myr-Akt and Akt-E40K), and dominant-negative (kinase-inactive mutant Myr-Akt-K179M) Akt were described previously. 22
Immunoprecipitation and Western Blotting Analysis
The frozen tissue was lysed by a tissue tearor in a lysis buffer containing 20 mmol/L Tris-HCl (pH 7.5), 137 mmol/L NaCl, 15% (v/v) glycerol, 1% Nonidet P-40, 2 mmol/L phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin and leupeptin, 2 mmol/L benzamidine, 20 mmol/L NaF, 10 mmol/L NaPPi, 1 mmol/L sodium vanadate, and 25 mmol/L β-glycerophosphate. An equal amount of protein was analyzed for protein expression and enzyme activity. For immunoprecipitation, lysates were precleared with protein A-protein G (2:1) agarose beads at 4°C for 20 minutes. After removal of the beads by centrifugation, lysates were incubated with the indicated antibody in the presence of protein A-protein G (2:1) agarose beads for 2 hours at 4°C. The beads were washed once with 50 mmol/L Tris-HCl (pH 7.5), 0.5 mol/L LiCl, 0.5% Triton X-10, twice with phosphate-buffered saline (PBS), and once with 10 mmol/L Tris-HCl (pH 7.5), 10 mmol/L MgCl2, 10 mmol/L MnCl2, and 1 mmol/L dithiothreitol, all containing 20 mmol/L β-glycerophosphate and 0.1 mmol/L sodium vanadate. Immunoprecipitates were subjected to in vitro kinase assays or Western blotting analysis. Protein expression was determined by probing Western blots of immunoprecipitates with phospho-Ser473 Akt (New England Biolabs, Beverly, MA) or anti-AKT1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibody. Detection of antigen-bound antibody was performed with the ECL Western blotting analysis system (Amersham, Arlington Heights, IL).
Immunohistochemistry
Formalin-fixed paraffin-embedded sections were deparaffinized in xylene and rehydrated through graded alcohol to distilled water. The sections were subjected to antigen retrieval by boiling in a microwave for 30 minutes in 0.01 mol/L sodium citrate buffer (pH 6.0) and then exposed to 3% hydrogen peroxide diluted in water for 20 minutes at room temperature to block endogenous peroxidase activity. Sections were incubated in a blocking solution (PBS containing blocking serum) for 20 minutes followed by 20 minutes with an avidin/biotin blocking solution (Vector Laboratories, Burlingame, CA). The primary antibody to phospho-S473 Akt (Upstate Biotechnology, Lake Placid, NY) or PTEN (Upstate Biotechnology) was applied and incubated overnight at 4°C. After incubation, the slides were treated with biotinylated secondary antibody, washed, and treated with streptavidin and biotinylated horseradish peroxidase according to the manufacturer’s instruction (Vector Laboratories, Burlingame, CA). After washing, the signal was visualized by diaminobenzidine tetrahydrochloride. A negative control reaction with no primary antibody was always performed alongside the reaction-containing sample.
In Vitro Protein Kinase Assay
In vitro Akt kinase assays were performed as previously described. 11 Briefly, the reaction was performed in the presence of 10 μCi of [γ-32P]ATP (New England Nuclear, Boston, MA) and 3 μmol/L of cold ATP in 30 μl of buffer containing 20 mmol/L Hepes (pH 7.4), 10 mmol/L MgCl2, 10 mmol/L MnCl2, and 1 mmol/L dithiothreitol. Histone H2B was used as the exogenous substrate. After incubation at room temperature for 30 minutes, the reactions were stopped by adding protein-loading buffer, and the products were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Each experiment was repeated three times. The relative amounts of incorporated radioactivity were determined by autoradiography and quantitated with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
PI 3-Kinase Assay
Anti-p85 (Santa Cruz) antibody was used to immunoprecipitate p110 catalytic subunits of PI 3-kinase from the tumor lysate. The immunoprecipitates were washed once with cold PBS, twice with 0.5 mol/L LiCl, 0.1 mol/L Tris (pH 7.4), and finally with 10 mmol/L Tris (pH 7.5), 100 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid. The presence of PI 3-kinase activity in immunoprecipitates was determined by incubating the beads with reaction buffer containing 10 mmol/L HEPES (pH 7.4), 10 mmol/L MgCl2, 50 μmol/L ATP, 20 μCi [γ-32P]ATP, and 10 μg l-α-phosphatidylinositol-4,5-bis phosphate (PI-4,5-P2; Biomol, Plymouth Meeting, PA) or l-α-phosphatidylinositol-4-phosphate (Sigma Chemical Co., St. Louis, MO) for 20 minutes at 25°C. The reactions were stopped by adding 100 μl of 1 mol/L HCl. Phospholipids were extracted with 200 μl of CHCl3CH3/MeOH. Phosphorylated products were separated by thin-layer chromatography as previously described. 23 The conversion of PI-4,5-P2 to PI-3,4,5-P3 and PI-4-P1 to PI-3,4-P2 was determined by autoradiography and quantitated by using a PhosphorImager.
Results
Frequent AKT1 Activation in Human Cancer
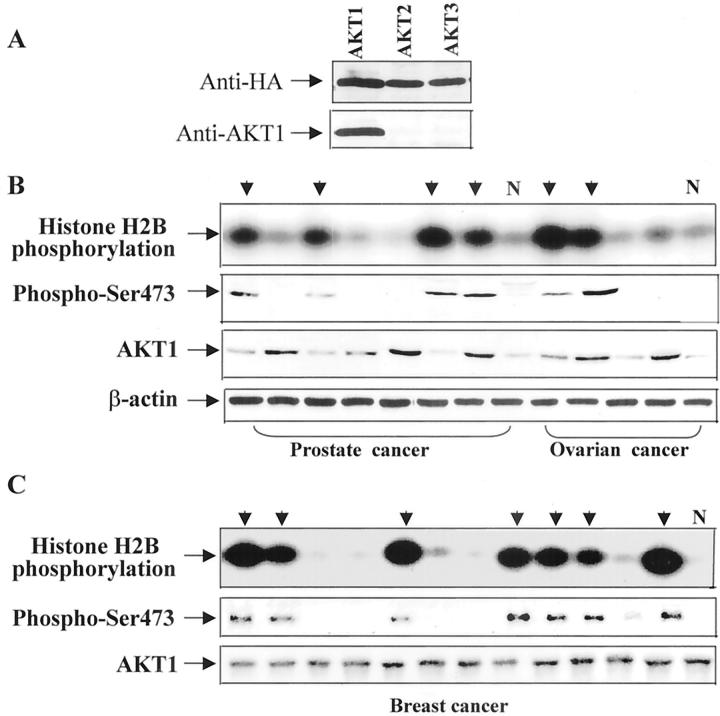
Despite the fact that the PI 3-kinase/Akt/AKT1 pathway is essential for cell survival and anti-apoptosis, consistent alterations of AKT1 in human primary tumors have not been well documented. We have previously examined AKT1 and AKT2 alterations at the DNA and/or mRNA levels in more than 100 cancer cell lines (including NCI 60 cancer cell lines screen) and more than 300 primary tumors from various organs. Amplification and/or overexpression of AKT2 were observed in 15 to 25% of ovarian and pancreatic tumors examined, 2,24,25 whereas no AKT1 alteration at the DNA or mRNA level was detected (J. Q. Cheng and J.R. Testa, unpublished data). The essential role of AKT1 kinase in cell survival prompted us to examine if AKT1 kinase activity is elevated in human cancer. We first examined whether anti-AKT1 antibody (D-17, Santa Cruz) specifically recognizes AKT1. HEK293 cells were transiently transfected with HA-AKT1, HA-AKT2, or HA-AKT3, lysed, and immunoprecipitated with monoclonal anti-HA antibody. The immunoprecipitates were separated and detected with AKT1 antibody. Figure 1A ▶ showed that anti-AKT1 antibody only reacted with HA-AKT1. We next immunoprecipitated AKT1 from lysates prepared from frozen tumors of prostate, breast, and ovary with anti-AKT1 antibody. The AKT1 immunoprecipitates were subjected to in vitro kinase assays. Significantly increased AKT1 kinase activity was observed in 16 of 30 prostate adenocarcinomas, 19 of 42 ductal breast cancers, and 11 of 23 ovarian serous adenocarcinomas. No elevated AKT1 activity was detected in eight lobular breast carcinomas, three endometrioid, and two borderline ovarian cancers examined, implying that alteration of AKT1 kinase level primarily involves ductal breast and serous ovarian carcinomas. Moreover, we observed that the majority of AKT1 activated-tumors are high grade and stage III/lV (Table 1) ▶ , suggesting that activation of AKT1 plays an important role in tumor progression rather than initiation.
Figure 1.
Activation of AKT1 in human cancers. A: Western blot. HEK293 cells were transiently transfected with HA-AKT1, HA-AKT2, or HA-AKT3 expression construct, lysed, immunoprecipitated with anti-HA antibody, and detected with anti-HA (top) or anti-AKT1 antibody (bottom). The top panels of B and C are in vitro kinase assays of AKT1 immunoprecipitates from frozen tumor specimens of prostate and ovary (B), and breast (C). AKT1 kinase levels were highly elevated in cases indicated by arrows. Normal tissue lysates (N) from prostate, ovary, and breast were used as controls. The second panels of B and C are Western blot analyses of AKT1 immunoprecipitates probed with anti-phospho-Ser473 Akt antibody. The cases exhibiting elevated AKT1 kinase activity displayed phosphorylation bands. The third and fourth panels of B and bottom panel of C are Western blot analyses with anti-AKT1 and anti-β-actin antibodies.
Table 1.
Frequencies of AKT1 Activation and Tumor Stage and Grade
| n | Kinase activity | P value | ||
|---|---|---|---|---|
| Normal | High | |||
| Stage | ||||
| Prostate cancer | ||||
| T1–T2 | 12 | 9 | 3 | 0.013§ |
| T3–T4 | 18 | 5 | 13 | |
| Breast cancer | ||||
| I–II | 19 | 15 | 4 | 0.038§ |
| III–IV | 31 | 16 | 15 | |
| Ovarian cancer | ||||
| I–II | 9 | 7 | 2 | 0.155 |
| III–IV | 19 | 10 | 9 | |
| Grade | ||||
| Prostate cancer | ||||
| <7* | 16 | 10 | 6 | 0.055 |
| >7 | 14 | 4 | 10 | |
| Breast cancer | ||||
| 1–2† | 16 | 14 | 2 | 0.009§ |
| 3‡ | 34 | 17 | 17 | |
| Ovarian cancer | ||||
| 1–2† | 11 | 8 | 3 | 0.186 |
| 3‡ | 17 | 9 | 8 | |
*Gleason score.
†Well (1) or moderately (2) differentiated.
‡Poorly differentiated.
§P < 0.05.
It has been shown that phosphorylation of threonine-308 and serine-473 is required for activation of AKT1 and that phospho-Ser473 Akt antibody recognizes only the phosphorylated/active form of Akt. 9 To confirm the results obtained from in vitro kinase assays, we performed Western blotting analysis with this antibody. To avoid the possibility of cross-reaction of the phospho-Ser473 antibody with other isoforms of Akt/PKB family, the tissue lysates were first immunoprecipitated with the specific anti-AKT1 antibody. The AKT1 immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-phospho-Ser473 antibody. Phosphorylated AKT1 was detected only in the tumors with elevated AKT1 kinase activity (second panels of Figure 1, B and C ▶ ).
To further demonstrate AKT1 activation and determine whether activated AKT1 is derived from tumor cells or stromal tissues in the tumor specimens with elevated AKT1 activity identified by in vitro kinase assay, immunohistochemical staining of tumor paraffin sections was performed with phospho-Ser473 Akt antibody. We first demonstrated that the phospho-Ser473 Akt antibody is capable of recognizing phosphorylated AKT1 by immunostaining paraffin sections prepared from serum-starved and serum-starved/EGF-stimulated MCF7 cells (Figure 2 ▶ , panels 1 and 2). Phosphorylation status of AKT1 in these cells was confirmed by Western blot analysis with phospho-S473 Akt antibody (data not shown). The tumor paraffin sections from the 16 prostate, 19 breast, and 11 ovarian tumor specimens with elevated AKT1 kinase activity strongly immunoreacted with phospho-Ser473 Akt antibody (Figure 2 ▶ , panels 3 to 5), whereas no immunostaining was observed in normal tissues and the tumor samples without increased AKT1 activity (Figure 2 ▶ , panel 6). Interestingly, phosphorylated AKT1 was located in the tumor cell membrane and cytoplasm but not the nucleus, which is in conflict with the previously reported observation that activated Akt could translocate to the nucleus in ectopically Akt-overexpressing cells. 26,27 Our data also showed that overexpression of GFP-tagged AKT1 translocates to the nucleus in NIH3T3 cells after IGF-1 stimulation (data not shown), indicating that activated Akt in the primary tumor cells could have a different subcellular localization from the cells overexpressing exogenous Akt.
Figure 2.
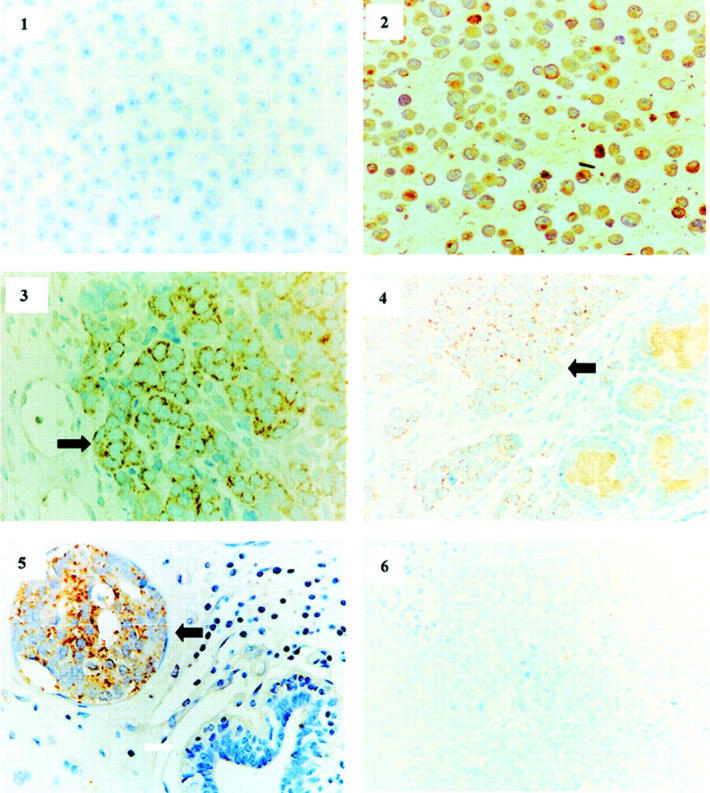
Phospho-AKT1 was detected in tumor cells and located only in cytoplasm. Immunochemical staining of the paraffin sections prepared from serum-starved (1), EGF-stimulated MCF7 cells (2), and adenocarcinomas of ovary (3), prostate (4), and breast (5 and 6) with phospho-S473 Akt antibody. Strong staining was observed in EGF-stimulated MCF7 cells and ovarian, breast, and prostate tumor cells, indicated by black arrows. No immunoreaction was detected in unstimulated MCF7 cells (1) normal prostate gland and ductal epithelial cells (4 and 5, white arrow), and the breast tumor without elevated AKT1 activity (6).
Multiple Mechanisms Resulted in AKT1 Activation
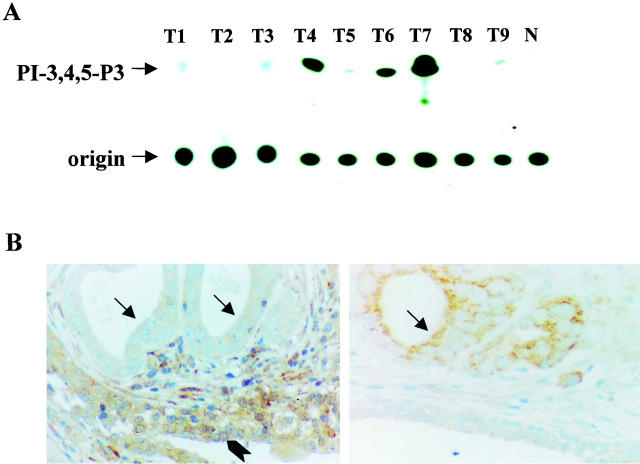
Because AKT1 kinase activity is regulated positively by PI 3-kinase and negatively by PTEN/MMAC1, 28 we examined PI 3-kinase activity and PTEN expression in the tumors exhibiting AKT1 activation (Figure 3) ▶ . Elevated PI 3-kinase activity was observed in 7 of the 19 breast and 5 of the 11 ovarian carcinomas, but in none of the prostate tumors that exhibit AKT1 activation. Immunohistochemical staining revealed no PTEN expression in 10 of 16 prostate and 2 of 11 ovarian cancer specimens with elevated AKT1 activity, whereas all breast carcinomas that showed AKT1 activation expressed PTEN. Absence of PTEN is well correlated with positive staining of phosphorylated AKT1 on the tumor tissue sections (Figure 3 ▶ and data not shown). In addition, we performed single-strand conformational polymorphism/sequencing analyses in AKT1-activated tumor specimens that have neither PI 3-kinase nor PTEN alteration. No AKT1 mutation was detected, implying that there are other mechanisms leading to AKT1 activation in the specimens without alterations of either PI 3-kinase or PTEN, which needs further investigation.
Figure 3.
Activation of PI 3-kinase or down-regulation of PTEN in human tumors. A: In vitro PI 3-kinase assay of the anti-p85 immunoprecipitates from nine tumor specimens that exhibit elevated AKT1 kinase activity. Elevated levels of PI 3-kinase activity were detected in cases 4, 6, and 7. B: Immunostaining of paraffin sections of prostate adenocarcinomas with anti-PTEN (left) and anti-phospho-Ser473 Akt (right) antibodies. PTEN is negative in tumor cells (black arrows), but positive in hyperplastic glands (arrowhead). However, phosphorylation of Akt was only detected in tumor cells (black arrow).
Constitutively Active Forms of Akt/AKT1 Are Highly Oncogenic in NIH3T3 Cells
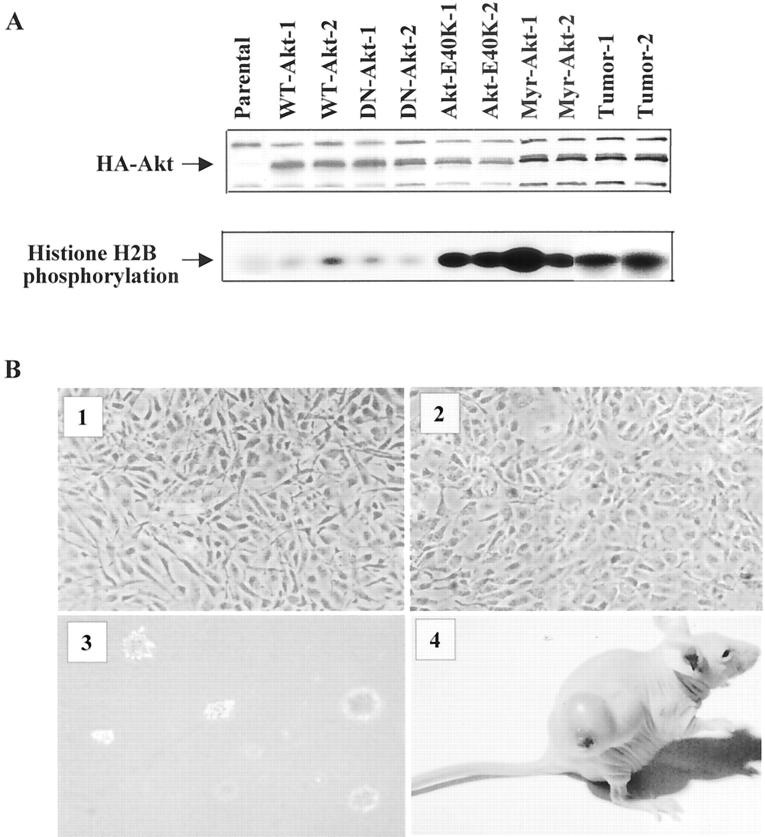
We and others previously demonstrated that overexpression of wild-type of Akt (WT-Akt) is unable to transform NIH3T3 cells. 21,29 To determine whether activation of AKT1 in human tumors has biological implication, we have introduced HA-tagged constitutively active forms of Akt (Myr-Akt and Akt-E40K), wild-type, and myristoylated kinase-inactive mutant (Myr-Akt-K179M) Akt into NIH3T3 cells individually. After G418 selection, five stable clonal cell lines from each transfection were obtained. Western blot and in vitro kinase analyses revealed that all of the clonal cell lines express Akt protein (Figure 4A) ▶ . High levels of kinase activity were detected in constitutively active Akt (Myr-Akt and Akt-E40K)-transfected clonal cell lines. Figure 4A ▶ shows Akt expression and kinase activity in two clonal cell lines from each transfection. Cells transfected with constitutively active forms of Akt, but not Myr-Akt-K179M and WT-Akt were morphologically transformed, grew in medium with low serum (0.1%), formed colonies in soft agar suspension, and were highly tumorigenic in nude mice (Figure 4B) ▶ . Tumors were observed 1 to 3 weeks after the injection of constitutively activated Akt-transfected cells in all mice, except clone 2 of Akt-E40K cells (Figure 4 ▶ and Table 2 ▶ ). Although vector alone and WT-Akt-transfected NIH3T3 cells developed tumors in 1 of 25 and 2 of 25 mice, respectively, all of these tumors were detected after 38 days (Table 2) ▶ . In addition, high levels of Myr-AKT1 protein and kinase activity were observed in dissected tumors from Myr-Akt nude mice but not in the tumors from vector or WT-Akt mice (Figure 4A) ▶ . These data suggest that kinase activity of Akt/AKT1 is essential for oncogenic transformation in NIH3T3 cells.
Figure 4.
Constitutively activated AKT1 transforms NIH3T3 cells. A: Western blot (top) and in vitro kinase assay (bottom) analyses of Akt expression and kinase activity in stably transfected clones and a tumor sample from nude mice. For Western blot analysis, the lysates were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to membrane, and detected with anti-HA antibody. For assay of kinase activity, immunoprecipitation was performed with anti-HA antibody and the HA-Akt immunoprecipitates were subjected to in vitro kinase assay using histone H2B as substrate. B: The morphology of constitutively active Akt-transfected NIH3T3 cells (2) is more rounded and larger than WT-Akt-transfected cells (1). Constitutively activated Akt-transfected cells grew on soft agar (3) and formed tumors in nude mice (4).
Table 2.
Tumorigenicity of Akt and Control Transfectant NIH3T3 Cell Lines
| Clonal cell lines | Soft agar growth* | Tumorigenicity in nude mice† | Latency (days) |
|---|---|---|---|
| Akt-E40K 1 | +++ | 5 /5 | 14–18 |
| Akt-E40K 2 | ++ | 4 /5 | 17–22 |
| Akt-E40K 3 | +++ | 5 /5 | 15–21 |
| Akt-E40K 4 | +++ | 5 /5 | 13–18 |
| Akt-E40K 5 | +++ | 5 /5 | 13–18 |
| Myr-Akt 1 | +++ | 5 /5 | 11–14 |
| Myr-Akt 2 | +++ | 5 /5 | 8–10 |
| Myr-Akt 3 | +++ | 5 /5 | 10–11 |
| Myr-Akt 4 | +++ | 5 /5 | 7–10 |
| Myr-Akt 5 | +++ | 5 /5 | 8–11 |
| Myr-Akt-K179M (1–5) | − | 0 /25 | |
| Vector control (1–5) | − | 1 /25 | 40 |
| WT-Akt (1–5) | − | 2 /25 | 38–42 |
*Number of colony/60 mm plate: 1–10, +; 11–30, ++; >30, +++.
†The number of injected cells: 1 × 106/mouse.
Discussion
Activation of oncogenic signaling proteins, such as Stat3, mitogen-activated protein kinase, and p110α, has been demonstrated in a number of different tumors. 30-32 Constitutively activated Stat3 and PI 3-kinase are able to induce malignant transformation. 33,34 Recent studies showed that among the most critical tumor-cell survival pathways are those mediated by the Akt/AKT1 kinase. 6-9 In this report, we demonstrate frequently elevated AKT1 kinase activity and phosphorylation of AKT1 in human carcinomas of breast, prostate, and ovary. Moreover, we have also shown that constitutively activated, but not wild-type, AKT1 is highly tumorigenic in NIH3T3 cells. Furthermore, the majority of AKT1-activated tumors are high grade and stage III/lV. These results indicate that activation of AKT1 is a common occurrence in human cancer, especially in more advanced tumors.
We previously demonstrated that overexpression of wild-type AKT2, but not Akt/AKT1, in NIH3T3 cells resulted in malignant transformation. 21 Ahmed and colleagues 29 also showed that Akt is not tumorigenic when overexpressed in the nontumorigenic rat T-cell lymphoma cell line 5675. In contrast, v-akt-expressing 5675 cells were highly tumorigenic. Because v-akt arose by an in-frame fusion of the viral Gag and Akt, the oncogenic difference between v-akt and wild-type Akt/AKT1 may be because of myristoylation of the amino-terminus of v-akt. 29,35 Several lines of evidence show that attachment of a membrane-targeting sequence (myristoylation/palmitoylation) to the amino-terminus of AKT1/Akt is sufficient to induce its maximal activation and that the PH domain of Akt is required for its membrane translocation and activation. 9 We demonstrated, in this study, that overexpression of constitutively active forms of Akt (Myr-Akt and Akt-E40K) in NIH3T3 cell leads to oncogenic transformation, which supports the results obtained from chicken embryo fibroblasts and Rat1 cells. 36,37 Taken collectively, these data suggest that the kinase activity of Akt contributes to the control of cell malignant transformation and that elevated AKT1 kinase activity plays an important role in development and/or progression of a subset of human cancers.
Previous studies have demonstrated that all of the tumor-associated PTEN mutants that have been biochemically characterized result in activation of AKT1. 28 Recently, elevated PI 3-kinase activity has been observed in human ovarian cancer. 32,38 As discussed above, Ras, Src, and Gab1 mediate growth factor signals to activate the PI 3-kinase/Akt pathway. 10-12 Therefore, activation of AKT1 in human cancer could result from Ras mutation, overexpression/active mutation of growth factor receptor(s), AKT1 mutation, and Src activation. In the present report, we showed that the majority of cases with AKT1 activation had either PTEN down-regulation or PI 3-kinase activation, dependent on the tumor type. Activation of PI 3-kinase was frequently detected in breast and ovarian, but not prostate, carcinomas, whereas the absence of PTEN protein was observed in some prostate and ovarian carcinomas. However, missense mutations of PTEN, although uncommon, cannot be ruled out by immunohistochemistry, because they result in formation of full-length PTEN proteins that may be immunostained by anti-PTEN antibody. Nevertheless, no AKT1 mutation was found in the tumors examined, indicating that elevated AKT1 activity in human cancer results from alterations of upstream regulators of AKT1.
Amplification and/or overexpression of AKT2, but not AKT1, have been detected in a subset of human ovarian, pancreatic, and breast cancers, 2,24,25 suggesting that AKT2 may play a more important role in human malignancy. In the present study, frequent activation of AKT1 kinase in human cancers and malignant transformation resulting from expression of constitutively activated AKT1 provide the first evidence that AKT1 could have a role similar to that of AKT2 in human cancer. Comparison of the AKT and AKT2 protein and/or kinase levels in the same tumor will provide valuable information to better understanding of the importance of AKT1 and AKT2 in human malignancy. Expression of AKT1 and AKT2 protein and alterations of AKT2 at the kinase level in these series of specimens are currently under investigation.
Subcellular localization of activated AKT1 is controversial. 9 Early studies on the subcellular localization of Akt/AKT1 revealed that, whereas c-akt is primarily cytosolic, v-akt is distributed equally between the cell membrane, the cytoplasm, and the nucleus in NIH3T3 cells. 29 Recent studies showed that nuclear translocation of AKT1 and AKT2 in HEK293 and HeLa cells follows in short succession the insulin-induced translocation of AKT1 and AKT2 proteins to the cell membrane. 26,27 However, all of these studies were performed in AKT1- or AKT2-transfected cells. In this report, we demonstrated that activated AKT1 in human primary tumors is distributed only in the plasma membrane and the cytosol, suggesting that activated endogenous AKT1 may not translocate to nucleus. In addition, we noted that immunoreaction to the phospho-Ser-473 antibody in tumor specimens is less strong, which could be because of either weak epitope of single phosphopeptide or epitope masking in paraffin section.
In summary, the data presented in this report showed that AKT1 kinase is frequently activated in human prostate, breast, and ovarian carcinomas. Elevated AKT1 kinase is an essential requirement for its oncogenic activity. These results provide the basis for understanding how the Akt pathway contributes to human oncogenesis. Further studies are required to determine the clinicopathological significance of AKT1 activation and to examine if overexpression of constitutively activated AKT1 develops prostate, breast, and ovarian tumors in transgenic mouse models using tissue-specific promoters.
Acknowledgments
We thank the Tissue Procurement, DNA Sequence, and Pathology Core Facilities at H. Lee Moffitt Cancer Center for providing cancer specimens, sequencing, and immunohistochemical staining; Chen Jiang and Kun Jiang for technical support; and Wen-Ching Lee for critical reading and comments.
Footnotes
Address reprint requests to Jin Q. Cheng, M.D., Ph.D. and Santo V. Nicosia, M.D., Department of Pathology and Laboratory Medicine, University of South Florida College of Medicine, 12901 Bruce B. Downs Blvd., MDC Box 11, Tampa, FL 33612. E-mail: jcheng@hsc.usf.edu.
Supported by grants from the National Institutes of Health (CA77935) and the Department of Defense (OC990075).
M.S. and G.W. contributed equally to this work.
References
- 1.Bellacosa A, Testa JR, Staal SP, Tsichlis PN: A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 1991, 254:274-277 [DOI] [PubMed] [Google Scholar]
- 2.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR: AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA 1992, 89:9267-9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA: Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA 1991, 88:4171-4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones PF, Jakubowicz T, Hemmings BA: Molecular cloning of a second form of rac protein kinase. Cell Regul 1991, 2:1001-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA: Identification of a human AKT3 (protein kinase Bγ) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Comm 1999, 257:906-910 [DOI] [PubMed] [Google Scholar]
- 6.Franke TF, Yang SL, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN: The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995, 81:727-736 [DOI] [PubMed] [Google Scholar]
- 7.Burgering BMT, Coffer PJ: Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376:599-602 [DOI] [PubMed] [Google Scholar]
- 8.Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA: Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J Biol Chem 1997, 272:30491-30497 [DOI] [PubMed] [Google Scholar]
- 9.Chan TO, Rittenhouse SE, Tsichlis PN: AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem 1999, 68:965-1014 [DOI] [PubMed] [Google Scholar]
- 10.Datta K, Bellacosa A, Chan TO, Tsichlis PN: Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J Biol Chem 1996, 271:30835-30839 [DOI] [PubMed] [Google Scholar]
- 11.Liu A-X, Testa JR, Hamilton TC, Jove R, Nicosia SV, Cheng JQ: AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res 1998, 58:2973-2977 [PubMed] [Google Scholar]
- 12.Holgado-Madruga M, Moscatello DK, Emlet DR, Dieterich R, Wong AJ: Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci USA 1997, 94:12419-12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91:231-241 [DOI] [PubMed] [Google Scholar]
- 14.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G: Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278:687-689 [DOI] [PubMed] [Google Scholar]
- 15.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC: Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282:1318-1321 [DOI] [PubMed] [Google Scholar]
- 16.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB: NFκB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 1999, 401:82-85 [DOI] [PubMed] [Google Scholar]
- 17.Romashkova JA, Makarov SS: NFκB is a target of AKT in anti-apoptotic PDGF signalling. Nature 1999, 401:86-90 [DOI] [PubMed] [Google Scholar]
- 18.Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ: Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science 1999, 286:1738-1741 [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann S, Moelling K: Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 1999, 286:1741-1744 [DOI] [PubMed] [Google Scholar]
- 20.Staal SP: Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA 1987, 84:5034-5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng JQ, Altomare DA, Klein MA, Lee W.-C, Mysliwiec T, Lissy NA, Testa JR: Transforming activity and cell cycle-dependent expression of the AKT2 oncogene: evidence for a link between cell cycle regulation and oncogenesis. Oncogene 1997, 14:2793-2801 [DOI] [PubMed] [Google Scholar]
- 22.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P: Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 1998, 17:313-325 [DOI] [PubMed] [Google Scholar]
- 23.Jiang K, Coppola D, Crespo NC, Nicosia SV, Hamilton AD, Sebti SM, Cheng JQ: The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol 2000, 20:139-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR: Amplification of AKT2 in human pancreatic cancer cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA 1996, 93:3636-3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellacosa A, DeFeo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Bennedetti-Panici P, Mancuso S, Neri G, Testa JR: Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer 1995, 64:280-285 [DOI] [PubMed] [Google Scholar]
- 26.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA: Role of translocation in the activation and function of protein kinase B. J Biol Chem 1997, 272:31515-31524 [DOI] [PubMed] [Google Scholar]
- 27.Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA: Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J Biol Chem 1997, 272:30491-30497 [DOI] [PubMed] [Google Scholar]
- 28.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW: Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998, 95:29-39 [DOI] [PubMed] [Google Scholar]
- 29.Ahmed NN, Franke TF, Bellacosa A, Datta K, Gonzalez-Portal ME, Taguchi T, Testa JR, Tsichlis PN: The proteins encoded by c-akt and v-akt differ in post-translational modification, subcellular localization and oncogenic potential. Oncogene 1993, 8:1957-1963 [PubMed] [Google Scholar]
- 30.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R: Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999, 10:105-115 [DOI] [PubMed] [Google Scholar]
- 31.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ: Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 1999, 59:279-284 [PubMed] [Google Scholar]
- 32.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW: PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet 1999, 21:99-102 [DOI] [PubMed] [Google Scholar]
- 33.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell R, Albanese C, Darnell JE, Jr: Stat3 as an oncogene. Cell 1999, 98:295-303 [DOI] [PubMed] [Google Scholar]
- 34.Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK: Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 1997, 276:1848-1850 [DOI] [PubMed] [Google Scholar]
- 35.Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, Gardner J, Cheng JQ, Testa JR, Tsichlis PN: Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene 1993, 8:745-754 [PubMed] [Google Scholar]
- 36.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK: The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA 1998, 95:14950-14955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirza AM, Kohn AD, Roth RA, McMahon M: Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ 2000, 11:279-292 [PubMed] [Google Scholar]
- 38.Yuan Z, Sun M, Feldman RI, Wang G, Ma X, Coppola D, Nicosia SV, Cheng JQ: Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene 2000, 19:2324-2330 [DOI] [PubMed] [Google Scholar]