Abstract
The lysosome-associated membrane proteins (LAMPs)-1 and -2 are major constituents of the lysosomal membrane. These molecules are known to be among the most glycosylated proteins of several types of cells and cancer cells, and their expression in cancer cells is marked by a distinct difference in the structures of the oligosaccharides as compared to nonmalignant cells. We analyzed by immunohistochemistry the intensity and distribution of LAMP-1 and LAMP-2 in 9 human colorectal cancer cases and in 16 control cases, including inflammatory diseases (diverticulitis, ulcerative colitis, and Crohn’s disease). LAMP proteins were expressed more intensely in the epithelium of colorectal neoplasms than in normal mucosa (P < 0.05), and no significant differences were found between adenoma and cancer cells (P > 0.05) in the same tissue section. Further, in sites of inactive inflammatory diseases and nonneoplastic areas in cancer specimens, no significant increases in epithelial LAMP proteins were observed, even in the proliferative zone of the lower crypt epithelium. Northern blot analysis showed increased expression of LAMP-1 and LAMP-2A in two of three colorectal cancers examined and increased LAMP-2B in all three cancers. Our findings suggest that LAMPs are related to neoplastic progression, but there is no direct association between the expression of LAMP molecules and cell proliferation.
Two lysosome-associated membrane proteins, LAMP-1 and LAMP-2, are type 1 integral membrane proteins localized primarily on the periphery of the lysosome as major constituents of the lysosomal membrane. 1-7 Although the majority of LAMP molecules reside in the lysosome, some (<5%) of LAMP-1 and LAMP-2 are also expressed on the cell surface of a variety of cultured cells. 1,4,7 Both glycoproteins consist of a polypeptide core of ∼40 kd. The molecules are among the most heavily glycosylated of cellular proteins with ∼50% of their mass as carbohydrate. 3,8,9 The LAMP proteins, with their high oligosaccharide content, are suggested to be the major cellular proteins associated with changes in glycosylation that accompany malignant transformation. 9 Although encoded by separate genes, with LAMP-1 located on chromosomes 13q34 and LAMP-2 on Xq24-25, 10 the two proteins are closely similar in their primary structure, with ∼37% sequence homology. 9,11-15 LAMP-2 is also found as splice variant molecules that are encoded by at least two or three transcripts resulting in variant transmembrane and cytoplasmic domains. Three transcripts in chicken 16 and mouse 16,17 and two human transcripts 18,19 have been reported to date. Although the LAMP proteins have assumed importance as markers of lysosomes in a wide variety of biological studies, the function of the molecules is not proved. One speculation is that LAMP-1 is a housekeeping protein 20,21 whereas LAMP-2 may have some additional or other role. 19
In a previous study, we conducted an immunohistochemical and molecular analysis of the relative degree of expression of LAMP-1 as compared to LAMP-2 in various normal human tissues. 19 Although previous immunohistochemical and electron microscopic studies 9 uniformly support the conclusion that LAMP molecules have a predominant steady-state localization in the lysosome, we showed that some human tissue cells have dissociation between expression of LAMP proteins and lysosomal hydrolases. 19 Moreover, the azurophilic granules that are defined as primary lysosomes, ie, a membrane-bound organelle containing acid hydrolases, in neutrophilic leukocytes and their precursors in the bone marrow have little of the LAMP molecules. 22
It has been observed repeatedly that neoplastic transformation is associated with a variety of structural changes in cell surface carbohydrates, most notably increased sialylation and β1-6-linked branching of complex-type asparagine (Asn)-linked oligosaccharides (-GlcNAcβ1-6Manα1-6Manβ1-). 23 Further, malignant transformation of rodent and human cells is associated with an increase in the amount of tetra-antennary and tri-antennary N-glycans. 24-26 The increased amount of these N-glycans is often associated with the increased amount of poly-N-acetyllactosamine. 27 LAMP-1 and LAMP-2 are the major carriers of polylactosaminoglycans in various cells. 28,29 It has also been shown that one of the important factors that enable tumor cells to become invasive is their ability to secrete lysosomal enzymes that can degrade surrounding extracellular matrix. 28-30 Presumably, LAMP-1 and LAMP-2 molecules may be correlated not only with malignant transformation of tumor cells but also their property of invasion. It is possible, therefore, that expression of LAMP-1 and/or LAMP-2 molecules in neoplastic cells is related to neoplastic progression.
In this study, we conducted an examination of the LAMP distributions in human colorectal cancer and compared the findings to inflammatory diseases (diverticulitis, ulcerative colitis, and Crohn’s disease).
Materials and Methods
Tissues
Formaldehyde-fixed and paraffin-embedded specimens were provided by the Department of Pathology of The Johns Hopkins University School of Medicine, Baltimore, MD. Nine cases of human primary colorectal cancer were selected to include adenocarcinoma, adenoma, and mucosa in one set of slides. As controls, two cases of abdominal gun-shot wound penetrating the colon and five cases of diverticulitis were selected. Four cases of Crohn’s disease and five cases of ulcerative colitis were also selected. The histopathological diagnosis for each case was confirmed in hematoxylin and eosin-stained slides.
Immunohistochemistry
The anti-human LAMP-1 mouse monoclonal antibody H4A3 7 was used at 1:150 dilution and anti-human LAMP-2 mouse monoclonal antibody H4B4 7 at 1:100 dilution. Anti-human CD44 mouse monoclonal antibody U9M2 (a gift from Dr. James Hildreth of The Johns Hopkins University, Baltimore, MD) 31 at 5 μg/ml was used as positive control antibody as a substitute for the primary antibody of different specificity but of the same origin. Anti-human p53 mouse monoclonal antibody DO7 (Signet, Dedham, MA) at 1:50 dilution was used as negative control antibody.
Five-μm serial sections were treated with xylene to remove paraffin, rehydrated, treated with 3% hydrogen peroxide in methanol to eliminate endogenous peroxidase activity, and treated for 15 minutes in a microwave oven 32 with 0.05 mol/L glycine-HCl, pH 3.5, 33 for antigen retrieval. The sections were then incubated with 5% normal horse serum with 0.01% Triton X in phosphate-buffered saline (PBS) at pH 7.4 for 20 minutes to eliminate nonspecific background immunostaining. After these procedures, sections were incubated with the H4A3, H4B4, U9M2, or DO7 antibody diluted in PBS containing 5% normal horse serum with 0.01% Triton X for 24 hours at room temperature. The sections were next treated for 1 hour at room temperature with affinity-purified horse biotinylated anti-mouse IgG (BA-2000; Vector Laboratories, Burlingame, CA) diluted 1:200, followed by incubation in avidin-biotin peroxidase complex 34 (Vectastatin Elite avidin-biotin-peroxidase complex ABC reagent, Vectastatin ABC kit standard, PK6100; Vector Laboratories) for 1 hour at room temperature. ABC was visualized by incubating with immunopure metal-enhanced diaminobenzidine substrate kit (Pierce, Rockford, IL). A brown reaction product appeared, and then the reaction was terminated by transferring the sections to water. After counterstaining with methyl green (Sigma, St. Louis, MO), sections were dehydrated, and a coverslip was attached with Permount (Fisher Scientific, Pittsburgh, PA).
Evaluation of Expression by Image Analysis
Areas of tissue represented in slides from each of the nine cases of human primary colorectal cancer were categorized as normal mucosa, adenoma, or cancer based on their histopathological features. Control slides from gun-shot wound specimens were categorized as “gun-shot inflamed mucosa,” or “gun-shot normal mucosa.” Inflammatory disease control tissues with diverticulitis, Crohn’s disease, and ulcerative colitis were termed “active” or “inactive” based on acute inflammation. In each category, three high-power view fields (×200 magnification) were selected randomly and captured by a charge-coupled device camera (SPOT; Diagnostic Instruments, Sterling Heights, MI) on a light microscope for computerized image analysis.
Image analysis was performed on a Macintosh computer using the public domain NIH Image program (developed at the National Institutes of Health and available from the Internet by anonymous FTP from zippy.nimh. nih.gov or on floppy disk from the National Technical Information Service, Springfield, VA, part number PB95–500195GEI). In each captured field, area (A) and mean intensity (I) of positive staining were measured. The value of (A) × (I) in each field was used for comparison of immunohistochemical positivity (Table 1) ▶ .
Table 1.
Evaluation of Immunohistochemistry
| LAMP-1 | LAMP-2 | |||
|---|---|---|---|---|
| (A) × (I) | SD | (A) × (I) | SD | |
| Normal | 5.9*† | 3.1 | 1.7‡§ | 1.8 |
| Adenoma | 38.3* | 27.4 | 11.7‡ | 10.4 |
| Cancer | 53.5† | 49.9 | 10.2§ | 10.0 |
| Gun-shot inflamed | 7.3 | 3.9 | 1.6 | 0.6 |
| Diverticulitis | 25.8 | 24.1 | 10.4 | 15.1 |
| Crohn’s disease | 46.4 | 34.5 | 8.7 | 12.6 |
| Ulcerative colitis | 11.2 | 9.6 | 0.2 | 0.3 |
A, area of positive staining; I, intensity of positive staining; SD, standard deviation.
All the values are relative ones which derived from image analysis. Statistically significant relationships were observed among *(P = 0.00), †(P = 0.00), ‡(P = 0.00), and §(P = 0.00).
Statistics
Each measurement was composed of subsamples. Testing group mean difference was evaluated using F-ratio of group effect and nested effect by SAS procedure GLM. 35
Northern Analysis
To confirm the immunohistochemistry data, we examined the LAMP RNA expression in human colorectal cancer specimens. Because fresh specimens were not available from the same patients examined in the immunohistochemical analysis, three cases of human colorectal cancer tissues were obtained from surgical specimens at Kyushu University Hospital after operation. Samples were prepared and processed as described previously. 19 In brief, total cellular RNA from human colorectal cancer tissues was isolated using the standard guanidium-isothiocyanate method. 36 RNA (10 μg/lane) was electrophoresed through a 1.5% gel containing 0.4 mol/L formaldehyde and transferred to positively charged nylon membranes (Boehringer-Mannheim, Indianapolis, IN) by capillary action. The RNA blots were probed with DNA fragments prepared by polymerase chain reaction amplification using a PCR DIG probe synthesis kit (Boehringer-Mannheim). The mRNA of LAMP-1 (2455 bp; forward primer: ttc tca aca tca acc cca aca; reverse primer: cac agt cgg caa ttc cta caa) was analyzed by using the 249-bp probe; mRNA of LAMP-2A (standard form, 1868 bp; forward primer: cac aag gaa agt att cta cag; reverse primer: cac cat cat gct gga tat gag) was evaluated by the 166-bp probe; and LAMP-2B (variant form, 4006 bp; forward primer: cac aag gaa agt att cta cag; reverse primer: gga tat cag act ctg taa cac) by the 154-bp probe. mRNA of 293 cells (human embryonal kidney) was used for positive control and NRK cells (normal rat kidney) for negative control (data not shown).
Each RNA sample was quantitated by UV absorption for equal loading. To minimize the effect of possible degradation of RNA in the tissue, RNA samples were further semiquantitated with ethidium bromide staining of 18S ribosomal bands by using a densitometry computer program (Kodak Digital Science 1D; Kodak, New Haven, CT) after electrophoresis.
Results
Immunoreactivity with the monoclonal antibodies to LAMP-1 and LAMP-2 was seen in each of the cancer cases (Figure 1, A–F) ▶ and control cases. The cellular staining pattern in all tissues was chiefly granular and cytoplasmic, in keeping with the lysosomal localization of the proteins. No distinct cell surface staining was observed, although cell boundary staining was seen: it was difficult to distinguish by immunohistochemistry technique whether the staining was inside or outside the cells. Intracytoplasmic mucin was not stained by these two antibodies in any of the normal, adenoma, or cancer areas, but extracellular necrotic or degenerated tissues inside the glandular areas in adenoma, or cancer showed staining (Figure 1, C–F) ▶ . In most of the cases the relative degree of staining for LAMP-1 and LAMP-2 varied greatly among the different areas. No morphological explanation of this heterogeneity was found. The staining with LAMP-1 was more intense than with LAMP-2, but there were no distinct differences in staining location for LAMP-1 and LAMP-2.
Figure 1.
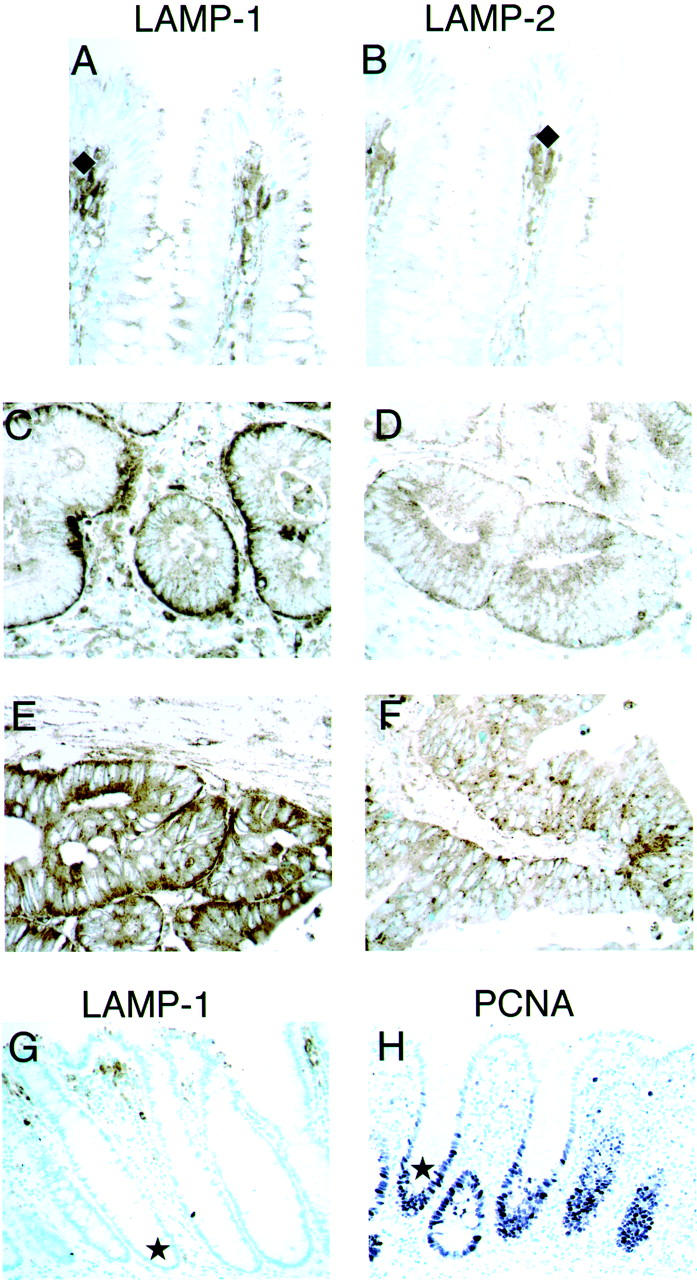
Immunohistochemical expression of LAMPs and PCNA in nonneoplastic and neoplastic human colorectal tissues. Staining of LAMP-1 is shown in the left column (A, C, E, and G), LAMP-2 in the right column (B, D, and F), and PCNA is shown in H. Counterstained with methyl green. Original magnifications, ×400 (A–F), ×200 (G and H). Nonneoplastic tissues: A, LAMP-1 (normal); and B, LAMP-2 (normal mucosa). In the areas categorized as normal in human colorectal cancer tissues, although LAMP proteins were expressed in epithelial cells, immunoreactivity was significantly less than the neoplastic adenoma and cancer tissues. Positively stained macrophages are present in the upper lamina propria of normal areas (♦ in A and B). Neoplastic tissues: C, LAMP-1 (adenoma); E, LAMP-1 (cancer); D, LAMP-2 (adenoma); and F, LAMP-2 (cancer). The epithelial cells of adenoma and cancer were strongly stained. The stromal areas of the neoplasm, including inflammatory cells, macrophages, and fibroblasts, also showed intense positive staining. The localization of staining of the neoplastic tissues was similar with the two antibodies, with greater intensity of LAMP-1 staining. Nonneoplastic tissues: G, LAMP-1; and H, PCNA. Positive PCNA staining (nuclear stain by PC10 anti-PCNA monoclonal antibody) occurs in the lower crypt epithelium (★ in H) but there is no staining of proliferative cells in normal tissue by LAMP antibodies (★ in G).
LAMP proteins were expressed in normal colorectal epithelial cells to some degree. By contrast immunoreactivity was significantly greater in adenomas and cancers (Figure 1, A–F ▶ ; Table 1 ▶ ) (P < 0.05). In some tumors single or clustered cancer cells showed intense staining. Differences in intensity of LAMP staining were not related to the differences in the histopathological typing of the tumors: malignant cells of well or poorly differentiated cancer cases showed similar positive staining (data not shown), and the localization and intensity of staining of adenomas and cancers were similar with both of the LAMP antibodies (Figure 1, C–F ▶ ; Table 1 ▶ ) (P > 0.05).
Normal mucosa of cancer specimens was not significantly stained in the proliferative zone of the lower crypt epithelium (Figure 1G) ▶ as demonstrated by proliferating cell nuclear antigen (PCNA) proliferation marker staining (Figure 1H) ▶ . As we previously reported, 19 there was LAMP staining in macrophages (Figure 1; A, B, and G ▶ ) in the upper lamina propria of normal mucosa. In stromal areas of neoplastic tissue, there was intense staining of inflammatory cells, macrophages, and fibroblasts.
In the actively inflamed areas of the nonneoplastic control cases (gun-shot wound, diverticulitis, Crohn’s disease, and ulcerative colitis), there was strong staining of inflammatory cells and macrophages (data not shown) as compared to the staining of normal epithelium, including normal mucosa in cancer specimens. There were no significant differences in staining intensity by image analysis between neoplastic tissues and the active sites of the inflammatory control cases (P > 0.05) (Table 1) ▶ . The positive cells of neoplasia were mostly the adenoma or cancer cells, whereas in control cases the positive areas were mostly composed of inflammatory cells and macrophages. Thus, both of the LAMP antibodies recognized not only neoplastic cells but also inflammatory cells. The anti-CD44 and anti-p53 control antibodies showed appropriate patterns of reactivity that differed from those of the LAMP patterns.
Northern Analysis
Northern analysis of mRNA extracted from human colorectal cancer tissues was evaluated for LAMP-1, LAMP-2A, and LAMP-2B expression in tumor and mucosa. In two of three cases, LAMP-1 mRNA from tumors showed greater increase than normal counterparts (Figure 2A ▶ and Table 2 ▶ ). LAMP-2A, except in case 3, and LAMP 2B mRNA also showed greater expression in tumor (Figure 2B ▶ and Table 2 ▶ ), with a 73 to 126% increase as compared to normal mucosa. The 293 control RNAs showed positive bands with each LAMP probe and the NRK control RNA was negative (data not shown).
Figure 2.
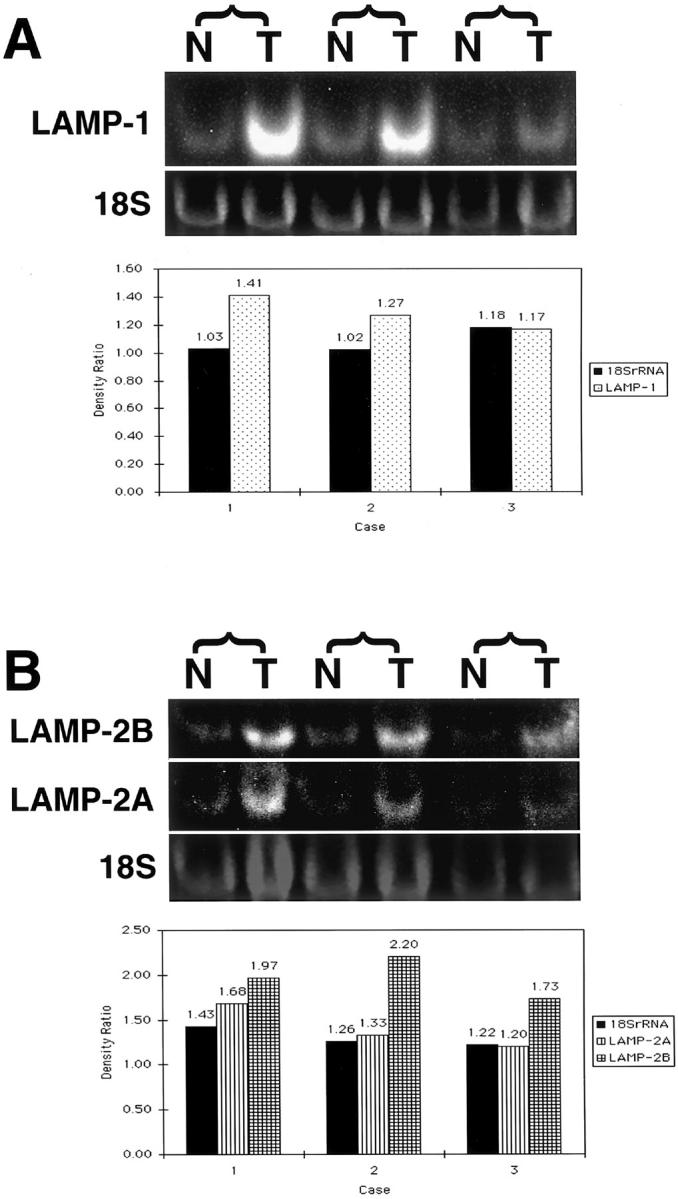
Northern blot analysis of total RNA prepared from three cases of human colorectal cancer. The mRNAs of LAMP-1 (2455 bp) were analyzed by using the 249-bp probe, mRNA of LAMP-2A (standard form, 1868 bp) by the 166-bp probe, and LAMP-2B (variant form, 4006 bp) by the 154-bp probe. RNA samples were semi-quantitated with ethidium bromide staining of 18S ribosomal bands by using a densitometry computer program. Top: Higher expression of LAMP-1 transcripts in the tumor tissue (T) as compared with its normal counterpart (N) (A and B). Bottom: The density ratios (T/N) of bands derived from tumor (T) and normal (N) samples between 18S rRNA, LAMP-1 (A), and LAMP-2A and -2B (B) (Table 2) ▶ . mRNA from tumors showed greater increase in their density compared with normal counterparts.
Table 2.
Relative Density Values of Bands; Tumor Compared to Mucosa
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| LAMP-1 | 1.41 | 1.27 | 1.17 |
| 18S rRNA | 1.03 | 1.02 | 1.18 |
| LAMP-2A | 1.68 | 1.33 | 1.20 |
| LAMP-2B | 1.97 | 2.20 | 1.73 |
| 18S rRNA | 1.43 | 1.26 | 1.22 |
All the values are relative and stand for the density of bands derived from tumor compared to mucosa samples. In each case and category, the density of normal samples was set at 1.00. Thus, the relative density value equals the density ratio (T/N). For instance, in case 1, although 18S rRNA from tumor showed 3% increase in density compared with its normal counterpart, LAMP-1 mRNA from tumor showed 41% increase in density compared with mRNA from normal. All the data are based on the results in Figure 2 ▶ .
Discussion
The most significant message of this study is that LAMP molecules were highly expressed in colorectal neoplastic tissues, either adenoma or cancer, compared to nonneoplastic counterparts. Numerous studies indicate that neoplastic transformation is associated with a variety of structural changes in cell surface carbohydrates, most notably increased sialylation and β1-6-linked branching of complex type asparagine (Asn)-linked oligosaccharides. 23 Saitoh and colleagues 27 have shown previously that LAMPs are the major carriers of polylactosaminoglycans in various cells. The ability of LAMP to bind to extracellular matrix components was inversely related to the degree of its glycosylation, and it was suggested that the increased β1-6 branching of the molecules contributed to the increased metastatic potential by decreasing adhesiveness. 37 Although increased glycosylation may play a role by decreasing adhesiveness, it is also possible that LAMP-1 and LAMP-2 carry oligosaccharide ligands that are recognized by adhesion molecules. 38 It has been demonstrated that tumor cells bind to endothelial cells through E-selectin-mediated adhesion. 39-41 Moreover, highly metastatic tumor cells were found to adhere more efficiently to endothelial cells, compared to low metastatic tumor cells. 42 Although there are no clear explanations regarding the opposite roles of glycosylation, the differences between positive and negative roles in adhesion are likely because of the differences of milieus such as inside the stroma or the venules, where glycosylation has its function.
Further, this study indicated that in sites of inactive inflammatory diseases and nonneoplastic areas in cancer specimens, no significant increases in epithelial LAMP proteins were observed, even in the proliferative zone of the lower crypt epithelium. These findings suggest that there is no direct association between the expression of LAMP molecules and cell proliferation. In other words, these LAMP molecules are related to neoplastic progression by a mechanism other than cell proliferation. A recent study offers potential clues to the functions of LAMPs. Lichter-Koneckie and colleagues 17 studied the developmental expression patterns of murine LAMP-2 transcripts and reported that the expression pattern was tissue type- and cell type-specific as differentiation progressed. Also, they suggested that two different mechanisms at the transcriptional and posttranslational level generated a variety of LAMP-2 proteins that probably served different developmental functions. Additional investigations are needed of the expression patterns of LAMPs, either mRNAs or proteins, in a quantitative manner by using various cancer tissues, inflammatory tissues, and also embryonic tissues of developmentally different stages.
Acknowledgments
We thank Mr. Jeffrey J. Floyd, Ms. Rahj Robinson, and Ms. Mihoko Oginosawa for technical assistance; and Drs. S. Shimajiri, A. Tanimoto, and Y. Sasaguri for providing help.
Footnotes
Address reprint requests to Koh Furata, Department of Clinical Chemistry and Laboratory Medicine, National Cancer Center Hospital, 5-1-1, Tsukiji, Tokyo Japan 104-0045. E-mail: kfuruta@ncc.go.jp.
Supported in part by The Foundation for the Advancement of Clinical Medicine and Grant-in-Aid for Scientific Research (C) 11670200 from the Japan Society for the Promotion of Science.
Current address of Stanley R. Hamilton: Division of Pathology and Laboratory Medicine, University of Texas MD Anderson Cancer Center, Box 85, Room G1.3754, 1515 Holcombe Blvd., Houston, TX 77030-4095.
References
- 1.Hughes EN, August JT: Characterization of plasma membrane proteins identified by monoclonal antibodies. J Biol Chem 1981, 256:664-671 [PubMed] [Google Scholar]
- 2.Chen JW, Murphy TL, Willingham MC, Pastan I, August JT: Identification of two lysosomal membrane glycoproteins. J Cell Biol 1985, 101:85-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JW, Chen GL, D’Souza MP, Murphy TL, August JT: Lysosomal membrane glycoproteins: properties of LAMP-1 and LAMP-2. Biochem Soc Symp 1986, 51:97-112 [PubMed] [Google Scholar]
- 4.Lippincott-Schwartz J, Fambrough DM: Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell 1987, 49:669-677 [DOI] [PubMed] [Google Scholar]
- 5.Fukuda M, Viitala J, Matteson J, Carlsson SR: Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-LAMP-1 and h-LAMP-2. Comparison of their deduced amino acid sequences. J Biol Chem 1988, 263:18920-18928 [PubMed] [Google Scholar]
- 6.Carlsson SR, Roth J, Piller F, Fukuda M: Isolation and characterization of human lysosomal membrane glycoproteins, h-LAMP-1 and h-LAMP-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem 1988, 263:18911-18919 [PubMed] [Google Scholar]
- 7.Mane SM, Marzella L, Bainton DF, Holt VK, Cha Y, Hildreth JEK, August JT: Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys 1989, 268:360-378 [DOI] [PubMed] [Google Scholar]
- 8.Hughes EN, August JT: Coprecipitation of heat shock proteins with a cell surface glycoprotein. Proc Natl Acad Sci USA 1982, 79:2305-2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda M: Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem 1991, 266:21327-21330 [PubMed] [Google Scholar]
- 10.Mattei MG, Matterson J, Chen JW, Williams MA, Fukuda M: Two human lysosomal membrane glycoproteins, h-LAMP-1 and h-LAMP-2, are encoded by genes localized to chromosome 13q34 and chromosome Xq24-25, respectively. J Biol Chem 1990, 265:7548-7551 [PubMed] [Google Scholar]
- 11.Fambrough DM, Takeyasu K, Lippincott-Schwarz J, Siegel NR: Structure of LEP100, a glycoprotein that shuttles between lysosomes and the plasma membrane, deduced from the nucleotide sequence of the encoding cDNA. J Cell Biol 1988, 106:61-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JW, Cha Y, Yuksel KU, Gracy RW, August JT: Isolation and sequencing of a cDNA clone encoding lysosomal membrane glycoprotein mouse LAMP-1. Sequence similarity to proteins bearing onco-differentiation antigens. J Biol Chem 1988, 263:8754-8758 [PubMed] [Google Scholar]
- 13.Viitala J, Carlsson SR, Siebert PD, Fukuda M: Molecular cloning of cDNAs encoding LAMP A, a human lysosomal membrane glycoprotein with apparent Mr ≈ 120,000. Proc Natl Acad Sci USA 1988, 85:3743-3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha Y, Holland SM, August JT: The cDNA sequence of mouse LAMP-2. Evidence for two classes of lysosomal membrane glycoproteins. J Biol Chem 1990, 265:5008-5013 [PubMed] [Google Scholar]
- 15.Howe CL, Granger BL, Hull M, Green SA, Gabel CA, Helenius A, Mellman I: Derived protein sequence, oligosaccharides, and membrane insertion of the 120-kDa lysosomal membrane glycoprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci USA 1988, 85:7577-7581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gough NR, Hatem CL, Fambrough DM: The family of LAMP-2 proteins arises by alternative splicing from a single gene—characterization of the avian LAMP-2 gene and identification of mammalian homologs of LAMP-2b and LAMP-2c. DNA Cell Biol 1995, 14:863-867 [DOI] [PubMed] [Google Scholar]
- 17.Lichter-Konecki U, Moter SE, Krawisz BR, Schlotter M, Hipke C, Konecki DS: Expression patterns of murine lysosome-associated membrane protein 2 (LAMP-2) transcripts during morphogenesis. Differentiation 1999, 65:43-58 [DOI] [PubMed] [Google Scholar]
- 18.Konecki DS, Foetisch K, Zimmer KP, Schlotter M, Lichter-Konecki U: An alternatively spliced form of the human lysosome-associated membrane protein-2 gene is expressed in a tissue-specific manner. Biochem Biophys Res Comm 1995, 215:757-767 [DOI] [PubMed] [Google Scholar]
- 19.Furuta K, Yang X-L, Chen J-S, Hamilton SR, August JT: Differential expression of the lysosome-associated membrane proteins in normal human tissues. Arch Biochem Biophys 1999, 365:75-82 [DOI] [PubMed] [Google Scholar]
- 20.Himeno M, Noguchi Y, Sasaki H, Tanaka Y, Furuno K, Kono A, Sakaki Y, Kato K: Isolation and sequencing of a cDNA clone encoding 107 kDa sialoglycoprotein in rat liver lysosomal membranes. FEBS Lett 1989, 244:351-356 [DOI] [PubMed] [Google Scholar]
- 21.Zot AS, Fambrough DM: Structure of a gene for a lysosomal membrane glycoprotein (LEP100). Housekeeping gene with unexpected exon organization. J Biol Chem 1990, 265:20988-20995 [PubMed] [Google Scholar]
- 22.Cieutat AM, Lobel P, August JT, Kjeldsen L, Sengelov H, Borregaard N, Bainton DF: Azurophilic granules of human neutrophilic leukocytes are deficient in lysosome-associated membrane proteins but retain the mannose 6-phosphate recognition marker. Blood 1998, 91:1044-1058 [PubMed] [Google Scholar]
- 23.Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS: β1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 1987, 236:582-585 [DOI] [PubMed] [Google Scholar]
- 24.Yamashita K, Ohkura T, Tachibana Y, Takasaki S, Kobata A: Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem 1984, 259:10834-10840 [PubMed] [Google Scholar]
- 25.Pierce M, Arango J: Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-β(1,6)Man-α(1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem 1986, 261:10772-10777 [PubMed] [Google Scholar]
- 26.Hubbard SC: Differential effects of oncogenic transformation on N-linked oligosaccharide processing at individual glycosylation sites of viral glycoproteins. J Biol Chem 1987, 262:16403-16411 [PubMed] [Google Scholar]
- 27.Saitoh O, Wang WC, Lotan R, Fukuda M: Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 1992, 267:5700-5711 [PubMed] [Google Scholar]
- 28.Nakajima M, Irimura T, Di Ferrante N, Nicolson GL: Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem 1984, 259:2283-2290 [PubMed] [Google Scholar]
- 29.Sloane BF, Rozhin J, Johnson K, Taylor H, Crissman JD, Honn KV: Cathepsin B: association with plasma membrane in metastatic tumors. Proc Natl Acad Sci USA 1986, 83:2483-2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oosta GM, Favreau LV, Beeler DL, Rosenberg RD: Purification and properties of human platelet heparitinase. J Biol Chem 1982, 257:11249-11255 [PubMed] [Google Scholar]
- 31.Guo MML, Hildreth JEK: HIV-induced loss of CD44 expression in monocytic cell lines. J Immunol 1993, 151:2225-2236 [PubMed] [Google Scholar]
- 32.Shi SR, Key ME, Kalra KL: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991, 39:741-748 [DOI] [PubMed] [Google Scholar]
- 33.Taylor CR, Shi SR, Chaiwun B, Young L, Imam SA, Cote RJ: Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections—androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum Pathol 1994, 25:263-270 [DOI] [PubMed] [Google Scholar]
- 34.Hsu S-M, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 35.: SAS Institue Inc: SAS/STAT Software. 1966:p 317 SAS Institute, Inc., Cary, NC
- 36.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 37.Laferte S, Dennis JW: Glycosylation-dependent collagen-binding activities of two membrane glycoproteins in MDAY-D2 tumor cells. Cancer Res 1988, 48:4743-4748 [PubMed] [Google Scholar]
- 38.Bierhuizen MF, Maemura K, Fukuda M: Expression of a differentiation antigen and poly-N-acetyllactosaminyl O-glycans directed by a cloned core 2 beta-1,6-N-acetylglucosaminyltransferase. J Biol Chem 1994, 269:4473-4479 [PubMed] [Google Scholar]
- 39.Rice GE, Bevilacqua MP: An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science 1989, 246:1303-1306 [DOI] [PubMed] [Google Scholar]
- 40.Hession C, Osborn L, Goff D, Chi-Rosso G, Vassallo C, Pasek M, Pittack C, Tizard R, Goelz S, McCarthy K, Hopple S, Lobb R: Endothelial leukocyte adhesion molecule 1: direct expression cloning and functional interactions. Proc Natl Acad Sci USA 1990, 87:1673-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawada R, Lowe JB, Fukuda M: E-selectin-dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem 1993, 268:12675-12681 [PubMed] [Google Scholar]
- 42.Sawada R, Tsuboi S, Fukuda M: Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem 1994, 269:1425-1431 [PubMed] [Google Scholar]


