Abstract
A salient feature of normal wound healing is the development and resolution of an acute inflammatory response. Although much is known about the function of inflammatory cells within wounds, little is known about the chemotactic and activation signals that influence this response. As the CC chemokines macrophage inflammatory protein-1α (MIP-1α) and monocyte chemotactic protein-1 (MCP-1) are abundant in acute wounds, wound repair was examined in MIP-1α−/− and MCP-1−/− mice. Surprisingly, wound re-epithelialization, angiogenesis, and collagen synthesis in MIP-1α−/− mice was nearly identical to wild-type controls. In contrast, MCP-1−/− mice displayed significantly delayed wound re-epithelialization, with the greatest delay at day 3 after injury (28 ± 5% versus 79 ± 14% re-epithelialization, P < 0.005). Wound angiogenesis was also delayed in MCP-1−/− mice, with a 48% reduction in capillary density at day 5 after injury. Collagen synthesis was impeded as well, with the wounds of MCP-1−/− mice containing significantly less hydroxyproline than those of control mice (25 ± 3 versus 50 ± 8 μg/wound at day 5, P < 0.0001). No change in the number of wound macrophages was observed in MCP-1−/− mice, suggesting that monocyte recruitment into wounds is independent of this chemokine. The data suggest that MCP-1 plays a critical role in healing wounds, most likely by influencing the effector state of macrophages and other cell types.
Chemokines are small molecular weight proteins that possess chemoattractant properties for a number of immune cells. Induction of inflammatory infiltration by the CC chemokine family is generally monocyte- and lymphocyte-specific. Two well-studied CC chemokines that are strongly chemotactic for macrophages are monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α). MCP-1 mediates the recruitment of monocytes in several inflammation models and diseases. 1,2 The absence of MCP-1 leads to attenuated monocyte influx in thioglycolate-induced peritonitis and in delayed-type hypersensitivity response. 3 Mice lacking the MCP-1 receptor, CCR2, cannot recruit macrophages to the inflamed peritoneum, 4 are not able to clear Listeria monocytogenes infection, 5 or to develop granulomas. 6 MCP-1 is abundant in atherosclerotic lesions, leading to the postulation that MCP-1 and other chemokines, such as MIP-1α, may be involved in monocyte recruitment and the subsequent development of these lesions. 7
Similar to MCP-1, MIP-1α can mediate the recruitment of monocytes in several inflammatory diseases. 8-12 MIP-1α also leads to a local accumulation of neutrophils when it is subcutaneously injected. 13,14 MIP-1α has been shown to play a role in several inflammatory diseases. For example, bleomycin-induced pulmonary fibrosis is decreased when MIP-1α is inhibited, 15 and influenza-induced pneumonitis is dependent on the presence of MIP-1α. 16 These many studies support the notion that both MCP-1 and MIP-1α are key molecules in macrophage trafficking into inflammatory foci.
A number of studies have demonstrated the expression and function of chemokines in healing wounds. 17-20 Overall, the expression pattern of chemokines in wounds has been shown to correlate well with the known sequence of neutrophil, macrophage, and lymphocyte recruitment in wounds. 19 In particular, studies in our own laboratory have demonstrated that both MCP-1 and MIP-1α are expressed in high levels in murine full-thickness dermal wounds at time points preceding and coincident with maximal macrophage infiltration. 21,22 Taken together, these and other studies have established a role for chemokines in wound inflammation.
The generation of the MIP-1α- and MCP-1-deficient mice (MIP-1α−/− and MCP-1−/−) provided an opportunity to test the requirement of these chemokines for wound repair in a genetically defined system. Given the central role of these chemokines in many different inflammatory responses, as well as their established presence in early wound healing, we hypothesized that mice deficient in either MCP-1 or MIP-1α would display altered wound repair. This study characterizes and compares wound healing in MCP-1 and MIP-1α genetically deficient mice.
Materials and Methods
Animals and Wound Model
MIP-1α−/− (B6.Scya3) 16 and MCP-1−/− (Scya2) 3 mice were generated as previously described. The genetic background of the MIP-1α−/− mice is C57BL/6, and the genetic background of MCP-1−/− mice is ∼93% C57BL/6 and ∼7% SJL. 23 The mice were maintained and bred at Loyola University Medical Center animal facilities. Control C57BL/6 mice were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). For wounding studies, mice were anesthetized by inhalation of methoxyflurane (Metofane; Schering-Plough Animal Health Corp., Union, NJ.). Full-thickness excisional wounds of 3 mm in diameter were made on the shaved dorsum of 6- to 7-week-old mice with a standard biopsy punch (Acuderm, Inc., Ft. Lauderdale, FL). At various time points after injury, the mice were euthanized by halothane inhalation (Halocarbon Laboratories, Riveredge, NJ), and the wound with its surrounding tissue were removed. Wounds were either flash-frozen in liquid nitrogen for hydroxyproline analysis or embedded in TBS Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) for histological analysis. All samples were stored at −80°C until the time of analysis. Sex- and age-matched mice were used for all experiments. Animal protocols used in this study were reviewed and approved by the Loyola University Institutional Animal Care and Use Committee.
Histology and Immunohistology
Ten-μm sections were prepared from frozen embedded tissues. To visualize general wound morphology, slides were stained with hematoxylin and eosin (Sigma Chemical Company, St. Louis, MO). For immunohistochemical staining, the sections were fixed in acetone for 15 minutes. All incubations and washes were performed at room temperature. After three 5-minute washes in phosphate-buffered saline (PBS), pH 7.4, sections were treated with 0.3% (v/v) H2O2 in methanol to quench endogenous peroxidase. The slides were washed in PBS, and blocked with a 1:10 dilution of normal mouse serum (Harlan Bioproducts for Science, Inc., Indianapolis, IN) in PBS for 30 minutes. To detect endothelial cells, sections were incubated in 1.0 μg/ml of MEC 13.3 primary antibody (anti-CD31; Pharmingen International, San Diego, CA). For the detection of macrophages, 1.7 μg/ml of rat anti-mouse MOMA-2 antibody (Sertotec Inc., Raleigh, NC) was used. This antibody recognizes an intracellular antigen in monocytes and macrophages. 24 After a 30-minute incubation with the primary antibody, the slides were washed for 5 minutes, three times, in PBS and incubated with 13.0 μg/ml of biotinylated mouse anti-rat IgG antibody (Jackson Laboratories, West Grove, PA) for another 30 minutes. After three 5-minute washes in PBS, slides were incubated with avidin-biotin-horseradish peroxidase complex (ABC-HRP; Vector Laboratories, Burlingame, CA) for 30 minutes. After the final set of washes, slides were incubated in an HRP substrate, 3,3′-diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 10 minutes and the sections were counterstained with Harris hematoxylin (Sigma Chemical Company, St. Louis, MO). Slides were mounted with Cytoseal (Stephens Scientific, Kalamazoo, MI). To quantify macrophages, wound bed areas were outlined and measured using Scion Image beta 3b acquisition and analysis software (Scion Corp., Frederick, MD). MOMA-2-positive cells were counted within the wound bed, and the number of macrophages per mm 2 determined. For each group, two wound sections from each of four individual mice were analyzed. All counting was performed in a blinded manner.
Analysis of Re-Epithelialization
To analyze the degree of re-epithelialization, the central portion of the wound was viewed under ×100 or ×400 power. The width of the wound, and the distance that the epithelium had traversed were measured. The percent re-epithelialization was calculated with the following formula:
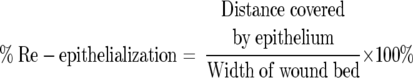 |
Analysis of Angiogenesis
To analyze blood vessel density in wound samples, endothelial cells were visualized by CD31 immunohistochemistry. Images of CD31-immunostained wound sections were captured and analyzed using Scion Image beta 3b acquisition and analysis software (Scion Corp.). The wound bed was outlined using a freehand drawing tool and the area was measured. CD31+ area within the wound was highlighted with false coloring and the intensity and pattern of the captured image was adjusted to match those of the slide. Background signals were eliminated either with a picture-editing function in the program or by setting a higher threshold that includes CD31+ signals while excluding those from the hematoxylin counterstain. The CD31-positive area within the wound bed was measured and the percent vascularization was calculated as:
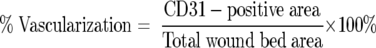 |
Collagen Analysis
Collagen content of wounds was assessed by determining the amount of hydroxyproline. 25 Frozen wound tissue was hydrolyzed in 2.0 ml of 6 N HCl for 3 hours at 130°C or overnight at 110°C. The reaction was neutralized with 2.5 N NaOH and diluted 40-fold with deionized H2O. One ml of a 0.05 mol/L N-chloro-p-toluene-sulfonamide solution was added to 2 ml of the neutralized/diluted solution and incubated for 20 minutes at room temperature. One ml of 3.15 mol/L perchloric acid was added to the solution and was incubated for 5 more minutes at room temperature. One ml of 20% solution of p-dimethylaminobenzaldehyde was then added and the resulting mixture was incubated for 20 minutes at 60°C. The samples were cooled with cold tap water. Absorbance was measured at 557 nm, and the amount of hydroxyproline determined by comparison to a standard curve. All reagents in this assay were purchased from Sigma Chemical Company.
Statistical Analysis
Data were analyzed using GraphPad Prism, version 2.01 (GraphPad Software Inc, San Diego, CA). The means and SEM were calculated for each data set. For the comparison of two groups, an unpaired t-test was used. For data from time-course experiments, a two-way analysis of variance was used. This method determines if there are significant variations in the healing response as a result of genetic strain and/or time. For both tests, significance was taken as P < 0.05.
Results
Comparison of Wound Closure in MCP-1−/− and MIP-1α−/− Mice
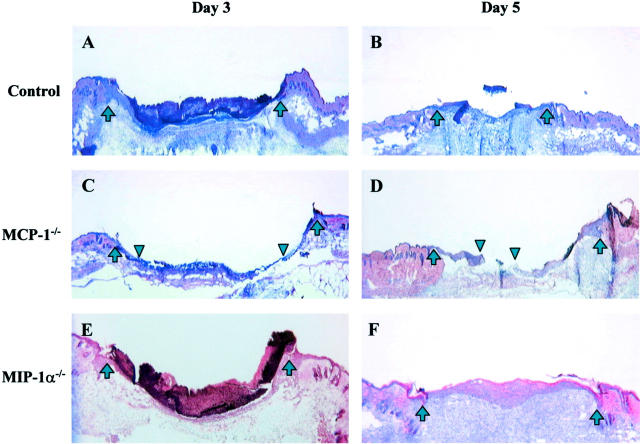
We first examined the degree of re-epithelialization in wounds from MCP-1−/−, MIP-1α−/− and control mice at 3 days after injury. This time point was previously characterized as the mid-point of re-epithelialization in this model. 26 At 3 days after injury, wounds from MCP-1−/− mice showed a significant delay in re-epithelialization (28 ± 6% re-epithelialization versus 79 ± 14% in controls, P < 0.005; Table 1 ▶ and Figure 1 ▶ ). Although day 5 wounds from control mice were primarily re-epithelialized, those from MCP-1−/− mice were still incomplete (Figure 1) ▶ . In contrast to the delay in wound closure seen in the MCP-1−/− mice, re-epithelialization of wounds in MIP-1α−/− mice was similar to wild-type control (70 ± 27%, MIP-1α−/− versus 80 ± 2%, control, on day 3 after injury, not significant; Table 1 ▶ and Figure 1 ▶ ).
Table 1.
Effects of MIP-1α or MCP-1 Deficiency on Day 3 Wound Re-Epithelialization
| Strain | n | Percent re-epithelialization |
|---|---|---|
| MCP-1−/− | 6 | 28 ± 6* |
| Control | 6 | 79 ± 14 |
| MIP-1α−/− | 4 | 70 ± 27 |
| Control | 4 | 80 ± 2 |
*P < 0.005 versus controls.
Figure 1.
Histological comparison of wounds from C57BL/6 controls (A and B), MCP-1−/− (C and D), and MIP-1α−/− (E and F) mice on day 3 (A, C, and E) and day 5 (B, D, and F) after injury. H&E-stained sections were photographed at ×25 power. The wound margins are indicated by upward arrows. In C and D, the margins of the advancing epithelial layer are indicted by downward arrowheads. By 3 days after injury, most wounds from control and MIP-1α−/− mice showed complete re-epithelialization (A and E). In contrast, day 3 wounds from MCP-1−/− mice showed delayed re-epithelialization (C). At day 5, wounds from MCP-1−/− mice still exhibited incomplete re-epithelialization (D).
Wound Healing in MIP-1α−/− Mice
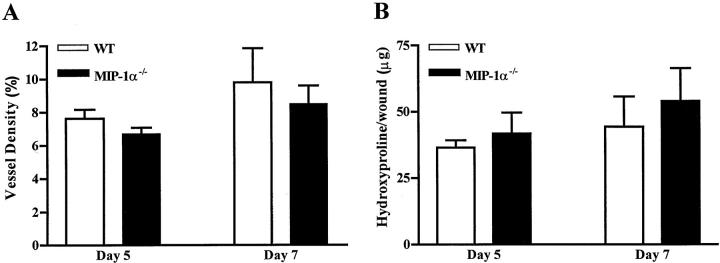
To confirm that MIP-1α−/− mice heal normally, we examined two additional aspects of wound healing in this strain. Wound angiogenesis, assessed as vascular density, was comparable for MIP-1α−/− and control wild-type mice at all time points examined (Figure 2) ▶ . Wound collagen content, as measured by the level of hydroxyproline, was nearly identical in MIP-1α−/− and control mice (Figure 2) ▶ . Analysis of healing in larger excisional wounds (8-mm diameter) unveiled no significant differences in healing between MIP-1α−/− and control mice (data not shown). Taken together, the data reinforce the concept that wound healing proceeds normally in MIP-1α−/− mice.
Figure 2.
Effects of MIP-1α deficiency on dermal wound repair. A: Determination of wound angiogenesis in MIP-1α−/− mice. Vessel density was measured in wounds at 5 and 7 days after injury. CD31-stained tissues were analyzed by image analysis and CD31+ areas quantified and compared to total wound area (100%). Data are expressed as mean ± SEM (n = 4). B: Determination of wound hydroxyproline content as an indicator of collagen levels. Hydroxyproline content per wound (±SEM) was measured at days 5 and 7 after injury. Day 5, n = 4 for C57BL/6 and n = 6 for MIP-1α−/−. Day 7, n = 4 for both strains.
Wound Healing in MCP-1−/− Mice
The delay in wound healing that had been detected in the MCP-1−/− mice was examined in further detail. Time course analysis of re-epithelialization revealed a profound delay in epithelial regrowth in the MCP-1−/− strain. When compared to wild-type, MCP-1−/− mice displayed a significant delay in wound re-epithelialization on days 1, 3, and 5 after injury (Figure 3) ▶ . The wounds of MCP-1−/− mice ultimately reached closure at day 10, taking approximately twice as long as those of control animals to achieve complete re-epithelialization. A time course analysis of angiogenesis established that the vascular density of wounds from MCP-1−/− mice was also significantly decreased from control (Figure 3) ▶ . This decrease in vascularity was most pronounced on day 5 after injury and differences from wild-type disappeared by day 10 after injury. The influence of MCP-1 deficiency on the level of wound hydroxyproline, an indirect measure of collagen, was also determined (Figure 3) ▶ . When compared to wounds from control mice, a significant retardation in collagen production was observed in wounds of MCP-1−/− mice. The greatest delay occurred on day 5 after injury. By day 10, the hydroxyproline level in MCP-1−/− mice reached a level similar to that of wild-type controls.
Figure 3.
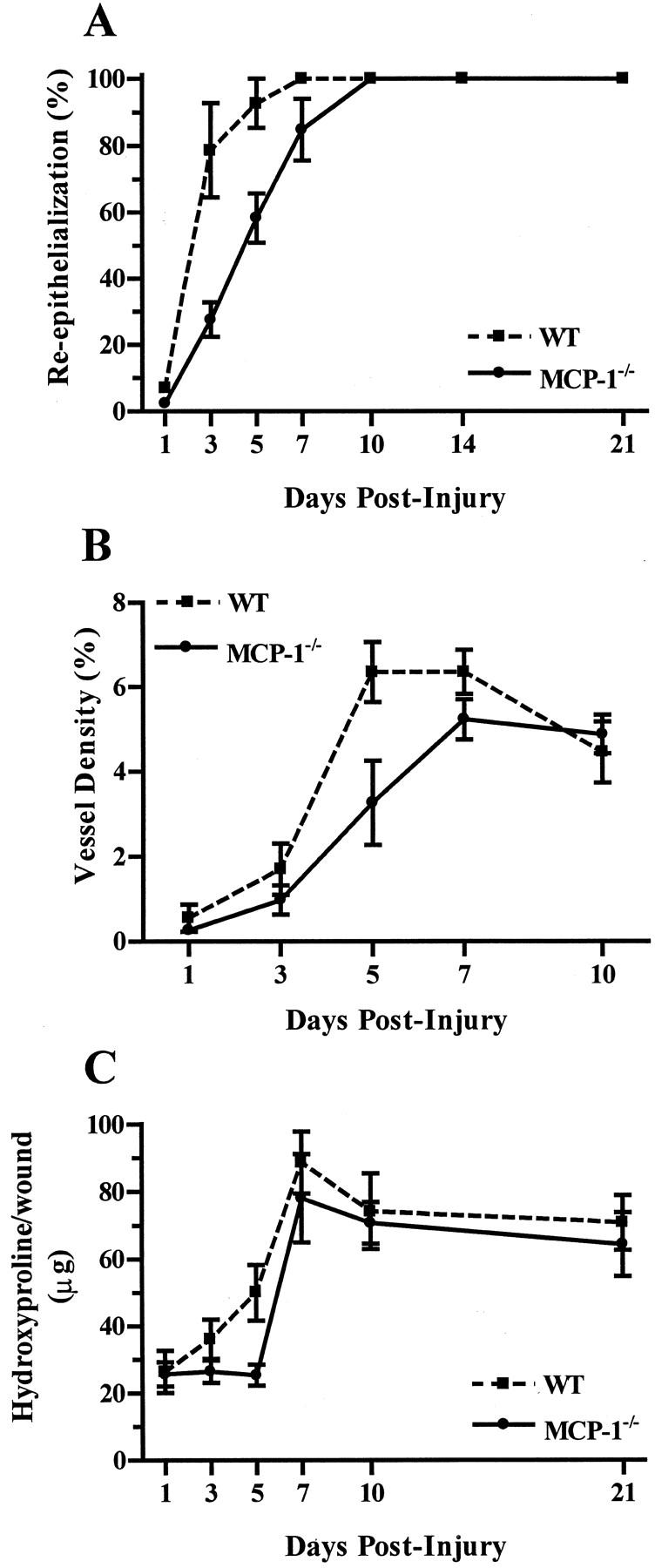
Effects of MCP-1 deficiency on dermal wound repair. A: Time course of re-epithelialization in MCP-1−/− mice compared to wild-type controls. Percent re-epithelialization (±SEM) is shown [n = 6 (days 1 to 14), n = 4 (day 21)]. P < 0.0001 between groups by two-way analysis of variance. B: Time course of wound angiogenesis in MCP-1−/− mice. Blood vessel density (±SEM) is shown (n = 6). P < 0.02 between groups by two-way analysis of variance. C: Collagen content of wounds from MCP-1−/− mice. As an indicator of collagen amount, hydroxyproline content (±SEM) of wounds was determined. [n = 6 (days 1 to 10), n = 4 (day 21)]. P < 0.05 between groups by two-way analysis of variance.
Macrophage Content in Wounds of MCP-1−/− and MIP-1α−/− Mice
The number of macrophages in the wound increases during healing because of their local recruitment from peripheral blood. Both MCP-1 and MIP-1α chemokines may participate in this process as chemotactic factors. The number of macrophages in day 3 after injury wounds was not significantly different between MCP-1−/− mice, MIP-1α−/− mice, and wild-type controls (Table 2) ▶ . Enumeration of wound macrophages with an antibody specific for mature macrophages, F4/80, 27 yielded similar results to those obtained using the MOMA-2 antibody (data not shown).
Table 2.
Macrophage Content of Day 3 Wounds from MIP-1α−/− and MCP-1−/− Mice
| Strain | n | Macrophage/mm2 |
|---|---|---|
| MIP-1α−/− | 4 | 166 ± 20 |
| MCP-1−/− | 4 | 141 ± 37 |
| Control | 4 | 183 ± 36 |
Discussion
Among the ever-growing list of chemokines, two CC family members, MIP-1α and MCP-1 are found in high levels in the early phase of wound repair. 18-22 The presence of these two chemokines precedes and coincides with maximal macrophage infiltration, suggesting that they may mediate the recruitment of these inflammatory cells. The availability of genetically deficient mice allowed for an assessment of the role of MIP-1α and MCP-1 on wound healing in a defined system. In MIP-1α−/− mice, examination of the degree of epithelial growth, wound angiogenesis, and collagen synthesis, demonstrated no significant delay in repair when compared to control. Given that MIP-1α is actively transcribed in early wounds, and that MIP-1α protein is abundant during the inflammatory phase of wound repair, the lack of an observable phenotype in the MIP-1α genetically deficient strain is a most surprising finding. 22 The data seem to suggest that MIP-1α production is inconsequential in normal wounds. A second possibility is that compensatory activity is generated in the MIP-1α−/− mice. In these mice, the chemokine system may have accommodated to MIP-1α deficiency during development. The large degree of permissive binding between chemokines and their receptors suggests that chemokine compensation might easily occur. MIP-1α is known to be a ligand for CCR1, CCR5, and CCR9, 28,29 yet these three receptors bind to several chemokines other than MIP-1α. For example, CCR1 also binds to RANTES and MCP-3, and the ligands for CCR5 include RANTES and MIP-1β. CCR9 is a receptor for MCP-1, -2, and -4; MIP-1β; RANTES; eotaxin; and HCC-1. At least two pieces of evidence support the concept that MIP-1α−/− mice have developed a compensatory mechanism that overcomes the loss of this single chemokine. First, although normal healing is observed in MIP-1α−/− mice, treatment of normal mice with neutralizing anti-MIP-1α antiserum results in significantly delayed wound healing. 22 Neutralization of MIP-1α by antiserum seems likely to represent the abrogation of MIP-1α activity in a situation in which chemokine compensation has not developed. A second and comparable example of possible compensation for MIP-1α has been demonstrated in a murine experimental autoimmune encephalomyelitis model. 30 Whereas treatment of normal mice with neutralizing anti-MIP-1α antiserum inhibits EAE development, 31 MIP-1α−/− mice are fully susceptible to the disease. Both of these examples suggest that MIP-1α−/− mice produce other factors, perhaps other chemokines, which fully compensate in some inflammatory circumstances.
In contrast to the normal healing observed in MIP-1α−/− mice, MCP-1−/− mice did not display overt compensation, and exhibited an observable delay in multiple parameters of healing. In these mice, re-epithelialization, angiogenesis, and collagen production were remarkably decreased. Although delays were apparent and often dramatic in the early phases of repair, by day 10 the differences were no longer significant, and the values of the wounds from MCP-1−/− mice were similar to those of control animals. Thus, although there is a delay during the healing process, the ultimate endpoints are similar in wounds from both knockout and control animals. The transient but significant nature of the delay is very similar to those observed in wounds from mice deficient in basic fibroblast growth factor 32 and in wounds of aged mice. 26
Although there is a delay in the repair process, no reduction in macrophages, as identified by MOMA-2 antibody, was seen in wounds of the MCP-1−/− mouse. Because MOMA-2 detects nearly all monocytes and macrophages, without distinction as to activation status, the possibility exists that specific subsets of macrophages, such as activated populations, are decreased in the MCP-1−/− mice. The finding of normal levels of macrophages in MCP-1−/− mice, in the face of altered healing, is consistent with the notion that it is not only the presence, but also the appropriate activity of these cells that is important for proper progression of wound healing. 33-35 As macrophages are a rich source of growth factors, the absence of MCP-1 may have affected the ability of these cells to produce or secrete the necessary factors. Additional studies to investigate shifts in wound macrophage activity in the MCP-1−/− mice are needed to substantiate this notion.
Our observation of normal inflammatory cell infiltration in the dermal healing model is somewhat different from those from other inflammatory models. MCP-1−/− 3 and CCR2−/− 4-6 mice had impaired peritoneal inflammation elicited by thioglycolate because of a lack of monocytes/macrophages in the peritoneum. In the autoimmune-prone MRL-faslpr mice that lack MCP-1, the disease was not manifested, because there was a reduction of autoimmune cell invasion. 36 The MCP-1−/− leukocytes did not exhibit any delay in proliferation when stimulated in vitro, suggesting that alterations in chemotaxis itself, rather than intrinsic cellular defects were responsible. In mouse models of atherosclerosis, 23,37,38 the lack of MCP-1 led to a reduction in macrophage infiltration, and consequently, a decrease in plaque formation. These varied observations suggest a differential requirement for MCP-1 in each particular inflammatory disease environment. MCP-1−/− mice exhibited differential deficits in the various parameters of wound repair, suggesting specific roles for this chemokine. First, our results support the hypothesis that the role of MCP-1 in wounds involves macrophage activation but not recruitment. Second, in regard to the proliferative aspects of wound healing, MCP-1 seems to have greater role in the promotion of epithelial and vascular growth than in the induction of collagen synthesis. Interestingly, among these three proliferative parameters, collagen synthesis showed the least amount of delay in the MCP-1−/− mice. Therefore, within wounds, the role of MCP-1 seems compartmentalized to specific regenerative processes.
A growing body of literature supports the concept that interconnections exist among the chemokine, cytokine, and growth factor networks. MCP-1 is known to influence macrophage activity, and can also stimulate both fibroblasts 39,40 and endothelial cells. 41 Each of these three cell types is known to produce growth factors and other cytokines within wounds. 42 These studies suggest the possibility that impaired wound healing, such as that observed in aged or diabetic patients, may involve perturbations in the chemokine network. An argument for such chemokine dysregulation is supported by a recent study of patients with chronic nonhealing venous leg ulcers. Compared to healing wounds, chronic wounds from these patients exhibited altered chemokine and inflammatory cytokine profiles. 43 In a more specific example of cytokine interaction in wounds, interleukin-10 has been shown to exert a regulatory role via inhibition of the expression of MIP-1α and MCP-1, leading to a diminished wound inflammatory response. 44 Additional investigations will be needed to assess the importance of chemokine, cytokine, and growth factor alterations to the problem of impaired wound healing.
Acknowledgments
We thank Aime L. Burns-Harring for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Luisa A. DiPietro, Burn and Shock Trauma Institute, Loyola University Medical Center, 2160 S. First Ave., Maywood, IL 60153. E-mail: ldipiet@luc.edu.
Supported by National Institutes of Health grants AI 44861–01 (to D. G. Q.), GM 55344 and AG 16067 (to E. J. K.), GM 55238 and GM 50875 (to L. A. D.).
References
- 1.Furie MB, Randolph GJ: Chemokines and tissue injury. Am J Pathol 1995, 146:1287-1301 [PMC free article] [PubMed] [Google Scholar]
- 2.Strieter RM, Standiford J, Huffnagle GB, Colletti LM, Lukacs NW, Kunkel SL: “The good, the bad, and the ugly”: the role of chemokines in models of human disease. J Immunol 1996, 156:3583-3686 [PubMed] [Google Scholar]
- 3.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ: Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 1998, 187:601-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF: Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997, 100:2552-2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurihara T, Warr G, Loy J, Bravo R: Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997, 186:1757-1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N: Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA 1997, 94:12053-12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reape TJ, Groot PH: Chemokines and atherosclerosis. Atherosclerosis 1999, 147:213-225 [DOI] [PubMed] [Google Scholar]
- 8.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Strieter RM: Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest 1994, 93:921-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driscoll KE: Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res 1994, 20:473-490 [DOI] [PubMed] [Google Scholar]
- 10.Lahrtz F, Piali L, Spanaus KS, Seebach J, Fontana A: Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol 1998, 85:33-43 [DOI] [PubMed] [Google Scholar]
- 11.Balashov KE, Rottman JB, Weiner HL, Hancock WW: CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA 1999, 96:6873-6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wada T, Furuichi K, Segawa-Takaeda C, Shimizu M, Sakai N, Takeda SI, Takasawa K, Kida H, Kobayashi KI, Mukaida N, Ohmoto Y, Matsushima K, Yokoyama H: MIP-1alpha and MCP-1 contribute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int 1999, 56:995-1003 [DOI] [PubMed] [Google Scholar]
- 13.Wolpe SD, Davatelis G, Sherry B, Beutler B, Hesse DG, Nguyen HT, Moldawer LL, Nathan CF, Lowry SF, Cerami A: Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med 1998, 167:570-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SC, Brummet ME, Shahabuddin S, Woodworth TG, Georas SN, Leiferman KM, Gilman SC, Stellato C, Gladue RP, Schleimer RP, Beck LA: Cutaneous injection of human subjects with macrophage inflammatory protein-1 alpha induces significant recruitment of neutrophils and monocytes. J Immunol 2000, 164:3392-3401 [DOI] [PubMed] [Google Scholar]
- 15.Smith RE, Strieter RM, Phan SH, Lukacs NW, Huffnagle GB, Wilke CA, Burdick MD, Lincoln P, Evanoff H, Kunkel SL: Production and function of murine macrophage inflammatory protein-1 alpha in bleomycin-induced lung injury. J Immunol 1994, 153:4704-4712 [PubMed] [Google Scholar]
- 16.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O: Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 1995, 269:1583-1585 [DOI] [PubMed] [Google Scholar]
- 17.Gibran NS, Ferguson M, Heimbach DM, Isik FF: Monocyte chemoattractant protein-1 mRNA expression in the human burn wound. J Surg Res 1997, 70:1-6 [DOI] [PubMed] [Google Scholar]
- 18.Fahey TJ, Sherry B, Tracey KJ, van Deventer S, Jones WG, Minei JP, Morgello S, Shires GT, Cerami A: Cytokine production in a model of wound healing: the appearance of MIP-1, MIP-2, cachectin/TNF and IL-1. Cytokine 1990, 2:92-99 [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R: Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol 1998, 153:1849-1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackman SH, Yoak MB, Keerthy S, Beaver BL: Differential expression of chemokines in a mouse model of wound healing. Ann Clin Lab Sci 2000, 30:201-207 [PubMed] [Google Scholar]
- 21.DiPietro LA, Polverini PJ, Rahbe SM, Kovacs EJ: Modulation of JE/MCP-1 expression in dermal wound repair. Am J Pathol 1995, 146:868-875 [PMC free article] [PubMed] [Google Scholar]
- 22.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM: MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 1998, 101:1693-1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF: MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest 1999, 103:773-778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraal G, Rep M, Janse M: Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol 1987, 26:653-661 [DOI] [PubMed] [Google Scholar]
- 25.Woessner JF: The determination of hydroxyproline in tissue and protein samples containing small proportions of the imino acid. Arch Biochem Biophys 1961, 93:440-447 [DOI] [PubMed] [Google Scholar]
- 26.Swift ME, Kleinman HK, DiPietro LA: Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 1999, 79:1479-1487 [PubMed] [Google Scholar]
- 27.Austyn JM, Gordon S: F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981, 11:805-815 [DOI] [PubMed] [Google Scholar]
- 28.Premack BA, Schall TJ: Chemokine receptors: gateways to inflammation and infection. Nat Med 1996, 2:1174-1178 [DOI] [PubMed] [Google Scholar]
- 29.Kunkel SL: Through the looking glass: the diverse in vivo activities of chemokines. J Clin Invest 1999, 104:1333-1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran EH, Kuziel WA, Owens T: Induction of experimental autoimmune encephalomyelitis in C57BL/6 mice deficient in either the chemokine macrophage inflammatory protein-1alpha or its CCR5 receptor. Eur J Immunol 2000, 30:1410-1415 [DOI] [PubMed] [Google Scholar]
- 31.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD: An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 1995, 155:5003-5010 [PubMed] [Google Scholar]
- 32.Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C: Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA 1998, 95:5672-5677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibovich SJ, Ross R: The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975, 78:71-100 [PMC free article] [PubMed] [Google Scholar]
- 34.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C: Macrophages and angiogenesis. J Leukoc Biol 1994, 55:410-422 [DOI] [PubMed] [Google Scholar]
- 35.Martin P: Wound healing—aiming for perfect skin regeneration. Science 1997, 276:75-81 [DOI] [PubMed] [Google Scholar]
- 36.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR: Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med 1999, 190:1813-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boring L, Gosling J, Cleary M, Charo IF: Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998, 394:894-897 [DOI] [PubMed] [Google Scholar]
- 38.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ: Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell 1998, 2:275-281 [DOI] [PubMed] [Google Scholar]
- 39.Gharaee-Kermani M, Denholm EM, Phan SH: Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996, 271:17779-17784 [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T: Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol 2000, 164:6174-6179 [DOI] [PubMed] [Google Scholar]
- 41.Weber KS, Nelson PJ, Grone HJ, Weber C: Expression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol 1999, 19:2085-2093 [DOI] [PubMed] [Google Scholar]
- 42.Whitby DJ, Ferguson MW: Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol 1991, 147:207-215 [DOI] [PubMed] [Google Scholar]
- 43.Fivenson DP, Faria DT, Nickoloff BJ, Polverini PJ, Kunkel S, Burdick M, Strieter RM: Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Rep Regul 1997, 5:310-322 [DOI] [PubMed] [Google Scholar]
- 44.Sato Y, Ohshima T, Kondo T: Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun 1999, 265:194-199 [DOI] [PubMed] [Google Scholar]