Abstract
Adenomyosis is a fairly frequent disorder in adult women characterized by the haphazard location of endometrial glands and stroma deep within the myometrium of the uterus. This study compared the effects on uterine development of the selective estrogen receptor modulators, tamoxifen, toremifene, and raloxifene with estradiol when given orally to female mice on days 2 to 5 after birth. Uterine adenomyosis was found in all (14 of 14) mice dosed with tamoxifen and most mice (12 of 14) treated with toremifene, but in none of the vehicle-dosed controls, in only one animal treated with raloxifene at 42 and 90 days after dosing and in none of the mice treated with estradiol at 42 days. At 6 days, the uterus in the groups that developed a high incidence of adenomyosis showed histological evidence of disturbed differentiation of the myometrium. Gene-expression XY-scatterplots using Clontech mouse 1.2 Atlas mouse cDNA expression arrays analyzing total uterine RNA showed nerve growth factor-α, preadipocyte factor-1, and insulin-like growth factor-2 were key genes differentially modified by tamoxifen or toremifene treatment, relative to the controls. As these genes may play an important role in regulating differentiation and development of the myometrium, these data suggest that adenomyosis may be caused primarily by defects in the formation of the myometrium.
Adenomyosis is a fairly frequent, often symptomatic disorder characterized by the haphazard location of endometrial glands and stroma deep within the myometrium of the uterus. It is found most commonly in multiparous premenopausal women between 40 and 50 years of age 1 but it has also been reported in postmenopausal breast cancer patients treated with the selective estrogen receptor modulator (SERM) tamoxifen. 2
Although several theories for the development of adenomyosis have been proposed, its precise etiology remains uncertain and the trigger for this aberrant growth remains unexplained. The conventional view is that adenomyosis results from the abnormal down-growth and invagination of the endometrium into the myometrium, because continuity between the basal endometrium and underlying adenomyosis can often be seen in tissue sections. It has been suggested that this occurs because of weakness in the uterine smooth muscle or increased uterine pressure. The focal smooth muscle hypertrophy and hyperplasia, observed in association with zones of adenomyosis, is explained as a response of the myometrium to the presence of ectopic endometrial cells. Traditionally, adenomyosis is seen as separate from endometriosis, the condition in which endometrial tissue is found in extrauterine locations. It is argued that endometrium reaches these extrauterine locations by seeding of endometrial cells via the fallopian tubes.
However, it has been postulated that both adenomyosis and endometriosis arises by a process of metaplasia. Mai and colleagues 3 have proposed that both conditions result from induction of endometrial epithelial cells by the presence of endometrial stroma formed by multipotent pericytes situated in the uterine body and extrauterine locations. More recently, magnetic resonance imaging and vaginal sonography has highlighted anatomical defects in the myometrium in adenomyosis and endometriosis in women. Instead of smooth muscle changes representing a response to invading endometrial tissue, this imaging data has suggested that the primary defect in these conditions is myometrial dysfunction. 4,5
A number of primate and rodent models have been used in the study of adenomyosis. Adenomyosis and endometriosis occurs naturally in non-human primates notably Macaca mulata but useful numbers of cases can only be obtained from large breeding colonies or after surgical implantation of autologous endometrium. 6 Several mouse strains also develop adenomyosis spontaneously, where it can be found in a small percentage of adult animals. 7,8 Adenomyosis has also been induced in mice by hormonal manipulation such as produced by the implantation of a single anterior pituitary gland into the uterine lumen. 9,10 The effects of surgical manipulation and tissue repair as well as diverse local hormonal alterations may confound interpretations of the findings in this latter model.
In this article we report on the development of adenomyosis in mice after the administration of the SERMs, tamoxifen or toremifene, for 5 days during the neonatal period. This model, which produces a high incidence of adenomyosis by 3 months of age, allowed examination of the early events in the myometrium and endometrium that might be responsible for the development of adenomyosis. Histological findings, estrogen receptor (ER) status, and gene expression data after tamoxifen or toremifene compared with raloxifene or estradiol treatment during this neonatal period suggested that the predisposition to adenomyosis may be primarily related to alterations in stromal and myometrial differentiation that alter myometrium structure and function in adulthood.
Materials and Methods
Animals and Treatments
Pregnant CD-1 mice were from Charles River, Kent, UK. Groups of 10 female neonatal mice were dosed orally via capillary tubing on days 2 to 5 after birth (day of birth is day 1), for 4 days consecutively. Mice were dosed with 5.3 nmol/kg estradiol, 2.7 μmol/kg tamoxifen base, toremifene citrate, or raloxifene hydrochloride suspended in peanut oil/lecithin/condensed milk mixture (2:0.2:3 v/v) at a dose volume of 5 μl/g body weight. Controls received vehicle only. Oral dosing was used in preference to subcutaneous on animal welfare grounds. On day 6, mice were sacrificed by decapitation and uteri were removed, weighed, and fixed in 3.7% neutral-buffered formalin, Carnoy’s medium, or snap-frozen in liquid nitrogen. Four controls and groups of four mice, also dosed on days 2 to 5 after birth, for 4 days consecutively with tamoxifen, toremifene, raloxifene, and estradiol were examined at 42 days. In addition, 10 controls and groups of 10 mice treated with a similar regimen of tamoxifen, toremifene, and raloxifene and controls were examined at 90 days.
For microscopic examination, standard histological sections of the uterus vagina and ovaries were prepared from formalin-fixed material and were stained with hematoxylin and eosin. Oil red O staining for lipid was conducted on unfixed frozen sections. Animal care and procedures were conducted in accordance with the codes and practice of the Animals (Scientific Procedures) Act 1986 of the United Kingdom.
RNA Isolation and Mouse 1.2 Atlas cDNA Microarray Analysis
Total RNA was extracted from the uteri of each group of treated neonatal mice using a Microisolation kit (Stratagene, La Jolla, CA) according to manufacturer’s instructions. For each group, uterine tissues from four to six newborn mice were pooled. DNase-treated total RNA (5 μg) was used to synthesize 32P-radiolabeled cDNA probes. RNA was transcribed by reverse transcriptase with (α-32P) dATP and specific primers for genes represented on the Clontech mouse 1.2 atlas array (Clontech, Palo Alto, CA). Clontech atlas cDNA expression arrays include 1176 mouse cDNA spotted onto a nylon membrane. Plasmid and bacteriophage DNAs are included as negative controls to confirm hybridization specificity along with several housekeeping genes that act as orientation marks and as positive controls for normalizing RNA abundance. Labeled probe (pooled fractions, 2 to 20 × 10 6 cpm) was added to Cot-1 DNA and incubated at 68°C. Prepared probe was hybridized with the array for 18 hours at 68°C with constant agitation. Arrays were processed in duplicate for each treatment group. The arrays were washed according to the manufacturer’s protocol and exposed to a phosphor imaging plate for up to 72 hours. Hybridization signals were quantified with a phosphor imager (Bio-Rad, Hercules, CA) and analyzed using image analysis software (Molecular Dynamics).
Immunocytochemistry Analysis
Formalin-fixed, 5-μm paraffin sections of rodent uterus were de-waxed by immersing in Histoclear, taken to water, and microwaved in citrate buffer, pH 6.0, for 20 minutes at 700 W. Sections were placed in distilled water and endogenous peroxidase activity was blocked with 3% (v/v) H2O2 in water for 20 minutes. ERα was detected using a Novocastra mouse monoclonal antibody (NCL-ER-6F11) raised against a recombinant protein corresponding to the full-length human ERα. The antibody was diluted 1:40. ERβ was detected using an Upstate Biotechnology rabbit polyclonal antibody (06-629) raised against a synthetic peptide representing amino acids 54 to 71 of mouse ERβ. The antibody was diluted 1:10. Primary antibody was detected with DAKO StreptABComplex/HP Duet kit, following the manufacturer’s instructions, and visualized with 3,3′-diaminobenzidine tetrahydrochloride solution. Sections were lightly counterstained with hematoxylin before de-hydration though graded alcohol, clearing, and mounting with DPX. Human breast carcinoma sections were used as a positive control and omission of primary antibody was used as a negative control on parallel sections.
Statistical Analysis
Statistical analysis was performed using Minitab version 10 (Minitab Inc., PA). Difference between groups was tested using analysis of variance with Dunnett’s test for significance at the 5% level.
Results
Animal and Uterine Weights
A comparison of effects on uterine weights of tamoxifen, toremifene, and raloxifene at day 6 after dosing and at day 42 and day 90, relative to vehicle-dosed controls, is shown in Table 1 ▶ . At day 6, all treatments resulted in a significant increase in uterine weights relative to controls. In contrast, after 42 days both tamoxifen and toremifene showed a significant decrease in weight compared to controls and, after 90 days, only the uteri of the raloxifene-treated animals showed an increase in weight, whereas tamoxifen treatment resulted in a significant decrease.
Table 1.
Effects on Animal and Uterine Weights of Treatment with SERMs
| Age of mouse (days) | Controls | Tamoxifen | Toremifene | Raloxifene |
|---|---|---|---|---|
| 6 | ||||
| Body wt (g) | 4.24 ± 0.13 | 4.47 ± 0.24 | 3.56 ± 0.24 | 4.20 ± 0.09 |
| Uterine wt* | 0.24 ± 0.03 | 0.48 ± 0.05† | 0.45 ± 0.04† | 0.36 ± 0.03† |
| 42 | ||||
| Body wt (g) | 28.4 ± 0.80 | 27.5 ± 0.38 | 27.1 ± 0.48 | 28.3 ± 2.47 |
| Uterine wt* | 0.63 ± 0.06 | 0.39 ± 0.04† | 0.43 ± 0.05† | 0.69 ± 0.11 |
| 90 | ||||
| Body wt (g) | 32.2 ± 0.60 | 29.1 ± 0.72 | 35.3 ± 2.14 | 30.0 ± 0.86 |
| Uterine wt* | 0.62 ± 0.05 | 0.38 ± 0.05† | 0.50 ± 0.03 | 0.77 ± 0.07† |
*Mean uterine weight expressed as percentage of body weight.
†Statistically significant at the 5% level.
Histopathological Findings
Adult Animals
At the 90-day time point, all 10 mice treated with tamoxifen and 9 of 10 treated with toremifene in the neonatal period showed adenomyosis, only 1 of 10 animals treated with raloxifene, and none of the three groups of 10 untreated controls. In affected animals adenomyosis was characterized histologically by the presence of simple endometrial glands, associated with variable but small amounts of endometrial stroma that infiltrated all layers of the myometrium, reaching but not penetrating the uterine peritoneal surface (Figure 1) ▶ . The loose mesometrial aspects of the myometrium were preferentially involved. Another feature associated with affected animals was the disruption to the concentric and longitudinal smooth muscle bands. Instead of regular concentric layers of smooth muscle cells seen in control mice at 90 days (Figure 1) ▶ , there were interwoven, thickened bands of smooth muscle cells interspersed with prominent layers of collagen and prominent blood vessels. In many of these altered zones there was a scattering of polymorphonuclear cells, particularly eosinophils that was not evident among controls. These stromal changes were frequently, but not always, associated with the presence of ectopic endometrial glands. The endometrial glands and the vagina epithelium were unaltered in treated groups and both control and treated mice showed typical cyclical alterations. Likewise, ovaries were similar in treated mice and controls. At the 42-day time point adenomyosis was also observed in all mice treated with tamoxifen, three of four treated with toremifene, but none of controls or mice treated with raloxifene or estradiol. In treated animals the vagina, oviducts, and ovaries showed no significant histological differences from controls.
Figure 1.
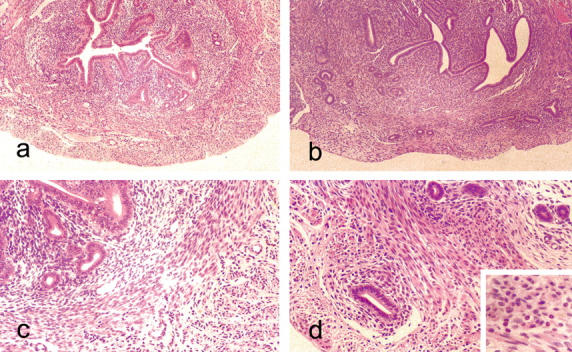
Light microscopic appearances of the mouse uterus at 90 days. a: Untreated control showing endometrial glands and stroma surrounded by well-defined, concentric layers of smooth muscle cells. H&E: original magnification, ×55. b: Uterus from anti-estrogen (tamoxifen)-treated mouse showing adenomyosis with endometrial glands penetrating deeply into a myometrium that is devoid of the concentric bands of smooth muscle seen in the controls. H&E: original magnification, ×55. c: Higher power view of section seen in a showing well-defined layer of smooth muscle that sharply demarcates the endometrium. H&E: original magnification, ×140. d: Same case as in b showing glands penetrating the myometrium. The epithelium lining the penetrating glands shows little or no evidence of hyperplasia. H&E: original magnification, ×140. Inset shows the polymorphonuclear cells within the myometrium. H&E: original magnification, ×220.
Neonatal Animals
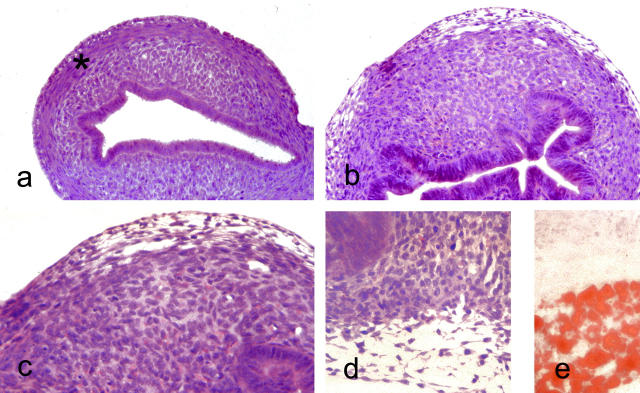
At 6 days, in mice treated with raloxifene or estradiol the uterus showed similar histological appearances to controls. The endometrial lumen was a simple slit lined by hyperchromatic epithelial cells. The lumen was surrounded by a layer of cellular stromal tissue in which occasional mitoses were evident. A uniform layer of young concentric smooth muscle fibers also containing mitotic figures surrounded the endometrial tissue. A thin external layer of longitudinal smooth muscle cells was also present (Figure 2) ▶ .
Figure 2.
Light microscopic appearances of uterus of 6-day-old mice. a: Untreated mouse uterus showing slit-like endometrial lumen lined by columnar cells with well-defined layers of endometrial stroma and smooth muscle. H&E: original magnification, ×140. b: Anti-estrogen (toremifene)-treated mouse at 6 days showing slightly hyperplastic endometrial glands and complete disruption of the stroma that is composed of stroma-like tissue with prominent small blood vessel. H&E: original magnification, ×140. c: Higher power view of stroma from b. At the periphery of the uterus, a layer of clear cells is evident. H&E: original magnification, ×220. d: The vacuolated nature of the clear cells, mature forms of which contained lipid (e: Oil red O; original magnification, ×220).
By contrast, at 6 days, in all mice treated with tamoxifen and toremifene, this regular arrangement of smooth muscle and stroma was lost. Almost the entire body of the uterus comprised stromal tissue and was devoid of cells showing overt smooth muscle differentiation (Figure 2) ▶ . Within this stroma small blood vessels were prominent. Another striking feature of the outer aspect of the altered myometrium was the presence of vacuolated cells that special stains showed were lipocytes (Figure 2) ▶ . The endometrial lumen retained its slit-like character with some infolding but it was lined by glandular tissue that was moderately hyperplastic with enlarged cells containing pseudostratified nuclei compared with controls or mice given raloxifene or estradiol.
Immunocytochemistry
ERα and ERβ labeling of control neonatal uterine tissue was most dense in the cells of the endometrial stroma, with little or no staining in smooth muscle or endometrial glandular cells. In tamoxifen-treated mice, labeling of the disordered stroma was similar to that of endometrial stroma in controls. Labeling of nuclei from the hyperplastic glandular tissue was also more prominent than in controls (Figure 3) ▶ .
Figure 3.
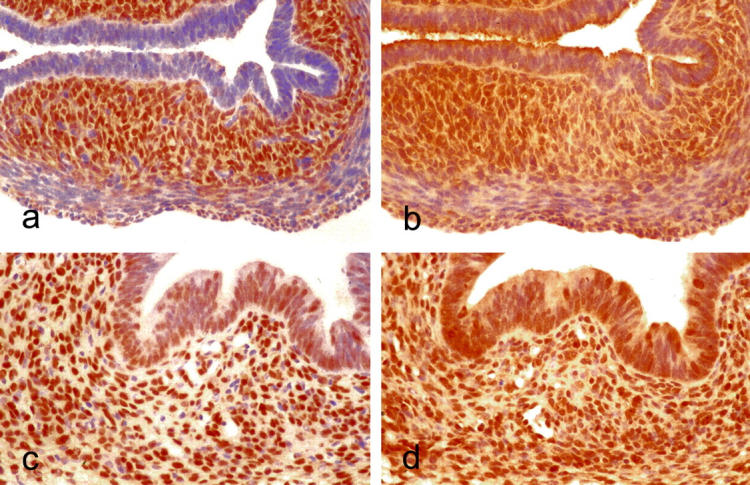
Expression of ERs in the uterus from control and treated mice, immunoperoxidase, hematoxylin counterstain. a: Control, ERα. Original magnification, ×220. b: Control, ERβ. Original magnification, ×220. c: Tamoxifen-treated, ERα. Original magnification, ×220. d: Tamoxifen-treated, ERβ. Original magnification, ×220.
Gene Microarray Analysis
Changes in gene expression in uterine tissues of neonatal mice were assessed 6 days after birth. Figure 4 ▶ shows log-log scatterplots of the cDNA microarray data. Comparisons were made between cDNA hybridizations of pooled uterine samples of controls and either tamoxifen (Figure 4a) ▶ , toremifene (Figure 4b) ▶ , raloxifene (Figure 4c) ▶ , or estradiol (Figure 4d) ▶ . Each point on the scatterplots represents 1 of the 1176 genes on the Atlas array. The density of each point has been normalized to the sum of the density of all of the genes. There was good reproducibility between duplicate assays performed on the pooled RNA extracts. Changes in gene expression resulting from tamoxifen or toremifene treatment were broadly similar, whereas raloxifene had a much less marked effect. Comparing expression between tamoxifen and control: 22 genes were up-regulated and 22 were down-regulated greater than twofold. Toremifene treatment resulted in the up-regulation of 45 genes and the down-regulation of 31 genes greater than twofold when compared with control. Comparison of estradiol to control showed a greater than twofold up-regulation of 19 genes and the down-regulation of 47 genes. In contrast, treatment with raloxifene resulted in a greater than twofold up-regulation of just four genes and down-regulation of eight genes.
Figure 4.
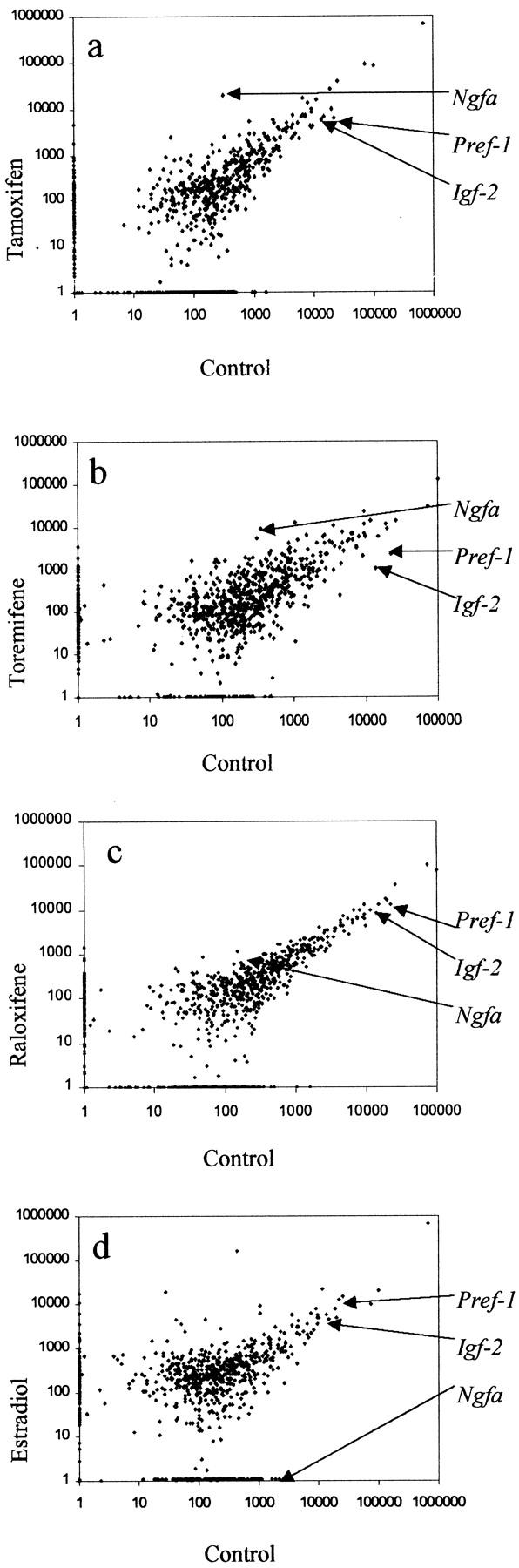
Differential expression of genes by tamoxifen, toremifene, raloxifene, and estradiol compared to control, in the 6-day neonatal mouse uterus using cDNA microarray analysis. The levels of gene expression were determined in pooled uteri as described at 6 days after administration of tamoxifen (a), toremifene (b), raloxifene (c), and estradiol (d). Each point on the scattergrams represents the mean expression of a target gene.
A total of three genes, and their putative functions, which were up- or down-regulated compared with control by tamoxifen, toremifene but not raloxifene are listed in Table 2 ▶ . The 7S nerve growth factor-α subunit (Ngfa) was strongly up-regulated by tamoxifen and toremifene treatment but in contrast, was strongly down-regulated by estradiol (Table 2) ▶ . The paternally imprinted insulin-like growth factor 2 (Igf-2) and preadipocyte factor-1 (Pref-1) were down-regulated by tamoxifen, toremifene, raloxifene, and estradiol.
Table 2.
Changes in Gene Expression Determined by cDNA Arrays
| Gene | Fold change* | Putative function | |||
|---|---|---|---|---|---|
| Tamoxifen | Toremifene | Raloxifene | Estradiol | ||
| Igf-2 precursor | −2.5 (−1.1) | −13 (ND) | −1.5 (−1.2) | −2.5 (−1.2) | Insulin-like growth factors stimulate mitogenic activity in human endometrial stromal cells. IGF-2 is involved in the growth of uterine smooth muscle tumours. IGF mediate and modulate steroid hormone actions in the endometrium. IGF-2 is an embryonic growth promoter and cell survival factor. |
| Pref-1 | −3.5 (−1.3) | −9.5 (ND) | −1.5 (−1.4) | −1.5 (−1.4) | Pref-1 inhibits the differentiation of preadipocytes into adipocytes and is down-regulated during their differentiation. |
| Ngfa | +65 (+4.3) | +16.5 (ND) | +2.5 (+1.1) | −307 (−7.4) | The receptor p75NTR is highly expressed in the smooth muscle cells of the mouse uterus. Further studies in a mouse muscle-derived cell line (C2C12) demonstrate down-regulation of nerve growth factor and p75NTR during myogenic differentiation. |
*Fold induction (positive numbers) and reduction (negative numbers) compared with expression in controls.
Values in parentheses represent fold change determined by reverse transcriptase-polymerase chain reaction.
ND, not determined.
The results of the cDNA microarray analysis were confirmed by semiquantitative reverse transcriptase-polymerase chain reaction using cDNA synthesized from the total RNA samples used with the microarray. Products were identified by DNA sequencing and corresponded to expected gene sequences. Similar changes in expression were observed for Ngfa, Igf-2, and Pref-1 using reverse transcriptase-polymerase chain reaction however, the fold change by this different technique was lower (Table 2) ▶ .
Discussion
The data from this study clearly shows that short-term treatment of neonatal mice with the SERMs tamoxifen and toremifene, but not raloxifene, leads to adenomyosis in a high proportion of animals by 3 months of age. Although several strains of mice develop adenomyosis spontaneously in adulthood, adenomyosis is uncommon in this strain at 3 months of age usually making its appearance after 6 months of age.
Why tamoxifen and toremifene but not raloxifene should have this effect remains unclear, although different SERMs are known to possess diverse effects in various tissues and organs.
The histological features of the adenomyosis in this present study using this 5-day treatment regimen of SERMs possessed the histological features described previously both in animal models and in humans. 1,11,12 Moreover, this was produced without the confounding effects of surgical trauma of the mouse pituitary implantation model. Nodules of endometrial glands and stroma were present deep within the myometrium, sometimes extending to, but not penetrating, the serosa. Notable was the disordered arrangement of the smooth muscle of the myometrium. In contrast to controls where smooth muscle fibers were arranged in uniform concentric layers around the endometrium, in tamoxifen- and toremifene-treated mice, this zone comprised irregular smooth muscle bundles interspersed with collagen and often penetrated by prominent blood vessels. The smooth muscle changes were more extensive than the zones of abnormal glands. This suggests that the penetration of endometrial glands might follow the changes to myometrium rather than precede them. These areas of altered smooth muscle organization were associated with a scattering of polymorphonuclear leukocytes, notably eosinophils. The presence of eosinophils has been reported in both human endometriosis and carcinoma where it has been postulated that they are involved in general tissue-remodeling. 13 It is of note that there was neither evidence of vaginal adenosis nor the extension of the oviduct epithelium through the muscularis described in stilbestrol-treated CD-1 mice by Newbold and colleagues 14 in the treated mice.
The histological alterations and principle genetic changes, which occurred in the neonatal uterus immediately after treatment with tamoxifen and toremifene, provide additional support for the concept that the primary derangement of the uterine mesenchymal tissue differentiation is the basis for the development of adenomyosis. At 6 days the uterine body in mice treated with tamoxifen and toremifene, but not raloxifene or estradiol, was devoid of the normal developing layers of smooth muscle but retained histological appearances and ER status of endometrial stroma. In addition, some unusual patterns of differentiation were noted, particularly the presence of lipocytes in the outermost parts of the uterus, features resembling adipose tissue of the developing mesenteric tissue in the surrounding mesenchyme.
In contrast to the major changes in mesenchymal differentiation, alterations to histological and receptor status in endometrial glandular tissue, produced by treatment, were modest. This was reflected by the lack of major alteration in the ER gene in endometrial glandular tissues. Not only did endometrial glands show no major differences in ER status, the endometrium itself continued to show regular cyclical alterations in treated groups similar to those in controls. These data suggest that the functional status of the endometrial glandular tissue is not greatly altered in adenomyosis. In the present study, no cystic ovaries were observed and corpora lutea were seen in all of the treatment groups. This contrasts to previous findings at 14 to 17 months after dosing where corpora lutea were absent when tamoxifen was given subcutaneously on days 1 to 5 to newborn CD-1 mice. 15
Although the conventional view is that adenomyosis represents down-growth of endometrial glands and stroma into the myometrium with secondary local tissue response, 1 other recent evidence also underlines the importance of mesenchymal tissue in the development of adenomyosis. It has been postulated, based on histopathological and immunocytochemical analysis of the human uterus, that the abnormal smooth muscle in adenomyosis results from a similar metaplastic process proposed for endometriosis where smooth muscle has been found associated with endometrium in extrauterine locations. It has been suggested that aberrant endometrial stroma may be produced by metaplasia from primitive pericytes. It is postulated that this is capable of inducing the formation of endometrial glands through local autocrine or paracrine mechanisms controlled by genetic, hormonal, and immunological factors. 3,16 It has also been suggested that excessive myocyte proliferation may be the cause of adenomyosis rather than its consequence based on T2-weighted magnetic resonance imaging and vaginal sonography in women with adenomyosis. These techniques show more extensive derangement of the myometrium compared with the patchy and focal nature of adenomyosis. 4,5
The pathological changes in mice were mirrored by alterations seen at 6 days in genes that are believed to be important in the developmental regulation of mesenchymal cells. The up-regulation of Ngfa by tamoxifen and toremifene (Figure 4 ▶ , Table 2 ▶ ) is in marked contrast to its down-regulation by estradiol treatment. Nerve growth factor and its low-affinity receptor p75NTR are believed to have not only a role in synchronizing the developing visceral nervous system but also on myogenic differentiation. The receptor p75NTR is highly expressed in the smooth muscle cells of the mouse uterus. 17 Further studies in a mouse muscle-derived cell line (C2C12) demonstrate down-regulation of nerve growth factor and p75NTR during myogenic differentiation. 18 We suggest that the up-regulation of Ngfa observed in the tamoxifen- and toremifene-treated uteri may contribute to the repression of myometrial differentiation in these tissues.
Pref-1 is abundant in preadipocytes and is down-regulated during adipocyte differentiation; it is also known that the expression of Pref-1 inhibits differentiation of mouse 3T3-L1 preadipocytes. 19 The microarray data presented here demonstrate the down-regulation of Pref-1 in tamoxifen- and toremifene-treated neonatal uteri. We suggest that the down-regulation of Pref-1 contributes to the appearance of adipocytes observed in the abnormally differentiated neonate tissue.
The paternally imprinted Igf-2 precursor is repressed by all of the SERMs tested, although the effect was most marked with toremifene treatment. In contrast, the maternally imprinted gene for the mannose 6-phosphate/insulin-like growth factor-2 receptor showed little treatment-related change in expression (not shown). Insulin-like growth factors are believed to mediate and modulate steroid hormone actions in the endometrium, 20 IGF-2 expression is also involved in endometrial differentiation. 21 A previous study of patients with endometriosis showed a reduced level of staining for IGF-2 in the eutopic endometria of affected women. 22 Elevated expression of IGF-2 is often found in tumors, and loss of imprinting is one mechanism by which its expression is deregulated. 23 However, we speculate that the observed reduction in Igf-2 expression may contribute to the altered growth and differentiation of the uterus.
With the present technology, we could only examine changes in gene expression taking RNA from the whole uterus. Clearly, the response of the different endometrial, stromal, and myometrial components will differ. If the molecules are expressed in different cell types the changes in their levels may be larger than when analyzed with the present methods. Techniques such as laser capture microdissection will be needed to examine cell-specific responses to these drugs.
In summary, these experimental data from this study support the hypothesis that adenomyosis represents a condition of the uterine body in which the stromal cells have a primary pathogenetic role although some contribution of accelerated epithelial downgrowth cannot be entirely excluded. Disruption of the mesenchymal layers surrounding the endometrium in the neonatal period can give rise to disordered development of uterine stroma, smooth muscle, blood vessels, and possibly its innervation. This alteration to the normal functional fibromuscular anatomy of the uterine body provides the framework for the abnormal and aberrant growth of endometrial tissue. Most importantly, these data suggest that discrete hormonal derangements, that produce defects in the formation of the myometrium early in neonatal life in humans, may explain the predisposition to adenomyosis in adulthood.
Acknowledgments
We thank Mr. Richard Edwards for performing the immunohistochemistry; Barbara Dorman and Nihal Razvi for excellent scientific support; and Dr. Terry Orton, AstraZeneca for the Clontech arrays.
Footnotes
Address reprint requests to Emma Parrott, MRC Toxicology Unit, Hodgkin Building, Lancaster Rd., Leicester LE1 9HN, United Kingdom. E-mail elp8@le.ac.uk.
References
- 1.Ferenczy A: Pathophysiology of adenomyosis. Hum Reprod Update 1998, 4:312-322 [DOI] [PubMed] [Google Scholar]
- 2.Cohen I, Beyth Y, Shapira J, Tepper R, Fishman A, Cordoba M, Bernheim J, Yigael D, Altaras MM: High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol Obstet Invest 1997, 44:200-205 [DOI] [PubMed] [Google Scholar]
- 3.Mai KT, Yazdi HM, Perkins DG, Parks W: Pathogenetic role of the stromal cells in endometriosis and adenomyosis. Histopathology 1997, 30:430-442 [DOI] [PubMed] [Google Scholar]
- 4.Brosens JJ, Barker FG, de Souza NM: Myometrial zonal differentiation and uterine junctional zone hyperplasia in the non-pregnant uterus. Hum Reprod Update 1998, 4:496-502 [DOI] [PubMed] [Google Scholar]
- 5.Kunz G, Beil D, Huppert P, Leyendecker G: Structural abnormalities of the uterine wall in women with endometriosis and infertility visualized by vaginal sonography and magnetic resonance imaging. Hum Reprod 2000, 15:76-82 [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie WF, Casey HW: Animal model of human disease. Endometriosis. Animal model: endometriosis in rhesus monkeys. Am J Pathol 1975, 80:341-344 [PMC free article] [PubMed] [Google Scholar]
- 7.Guttner J: Adenomyosis in mice. Z Versuchstierkd 1980, 22:249-251 [PubMed] [Google Scholar]
- 8.Faccini JM, Abbott DP, Paulus GJJ: Uterus. Mouse Histopathology: A Glossary for Use in Toxicity and Carcinogenicity. 1990, Elsevier, Amsterdam
- 9.Mori T, Kyokuwa M, Nagasawa H: Animal model of uterine adenomyosis: induction of the lesion in rats by ectopic pituitary isografting. Lab Anim Sci 1998, 48:64-68 [PubMed] [Google Scholar]
- 10.Koujyo T, Hatakeyama S, Yamada H, Iwabuchi K, Kajino K, Ogasawara K, Onoe K, Fujimoto S: Induction of endometriosis and adenomyosis by transvaginal pituitary transplantation in mice with and without natural killer cell activity. Am J Reprod Immunol 1998, 40:441-446 [DOI] [PubMed] [Google Scholar]
- 11.Ota H, Igarashi S, Tanaka T: Morphometric evaluation of stromal vascularization in the endometrium in adenomyosis. Hum Reprod 1998, 13:715-719 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Matsuda M, Sakamoto S, Mori T: Changes in uterine microvessels as a possible pathogenic factor in the development of adenomyosis induced by pituitary grafting in mice. Acta Histochem Cytochem 1999, 32:387-391 [Google Scholar]
- 13.Blumenthal RD, Samoszuk M, Taylor AP, Brown G, Alisauskas R, Goldenberg DM: Degranulating eosinophils in human endometriosis. Am J Pathol 2000, 156:1581-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newbold RR, Bullock BC, McLachlan J: Diverticulosis and salpingitis isthmica nodosa (SIN) of the fallopian tubes. Am J Pathol 1984, 117:333-335 [PMC free article] [PubMed] [Google Scholar]
- 15.Newbold RR, Jefferson WN, Padilla-Burgos E, Bullock BC: Uterine carcinoma in mice treated neonatally with tamoxifen. Carcinogenesis 1997, 18:2293-2298 [DOI] [PubMed] [Google Scholar]
- 16.Anaf V, Simon P, Fayt I, Noel J: Smooth muscles are frequent components of endometriotic lesions. Hum Reprod 2000, 15:767-771 [DOI] [PubMed] [Google Scholar]
- 17.Seidl K, Erck C, Buchberger A: Evidence for the participation of nerve growth factor and its low-affinity receptor (p75NTR) in the regulation of the myogenic program. J Cell Physiol 1998, 176:10-21 [DOI] [PubMed] [Google Scholar]
- 18.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H: Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived neurotrophic functions. Am J Pathol 1999, 155:1183-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garces C, Ruiz-Hidalgo MJ, Bonvini E, Goldstein J, Laborda J: Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation 1999, 64:103-114 [DOI] [PubMed] [Google Scholar]
- 20.Rutanen EM, Salmi A, Nyman T: mRNA expression of insulin-like growth factor-I (IGF-I) is suppressed and those of IGF-II and IGF-binding protein-1 are constantly expressed in the endometrium during use of an intrauterine levonorgestrel system. Mol Hum Reprod 1997, 3:749-754 [DOI] [PubMed] [Google Scholar]
- 21.Rutanen EM: Insulin-like growth factors in endometrial function. Gynecol Endocrinol 1998, 12:399-406 [DOI] [PubMed] [Google Scholar]
- 22.Sbracia M, Zupi E, Alo P, Manna C, Marconi D, Scarpellini F, Grasso JA, Di Tondo U, Romanini C: Differential expression of IGF-I and IGF-II in eutopic and ectopic endometria of women with endometriosis and in women without endometriosis. Am J Reprod Immunol 1997, 37:326-329 [DOI] [PubMed] [Google Scholar]
- 23.De Souza AT, Yamada T, Mills JJ, Jirtle RL: Imprinted genes in liver carcinogenesis. FASEB J 1997, 11:60-67 [DOI] [PubMed] [Google Scholar]