Abstract
Methionine aminopeptidase-2 (MetAP2) is the molecular target of the angiogenesis inhibitors, fumagillin and ovalacin. Fumagillin can also inhibit cancer cell proliferation, implying that MetAP2 may play a quite complex role in tumor progression. Here, we examined the expression and function of MetAP2 in an in vitro model of human mesothelioma. We found that mesothelioma cells expressed higher MetAP2 mRNA levels than primary normal mesothelial cells. Consistently, fumagillin induced apoptosis, owing to early mitochondrial damage, in malignant, but not in normal mesothelial cells. Transfection of mesothelioma cells with a MetAP2 anti-sense oligonucleotide determined a time-dependent inhibition of cell survival and induced nucleosome formation. Interestingly, mRNA and protein levels of the anti-apoptotic gene bcl-2 as well as telomerase activity were selectively reduced after MetAP2 inhibition in mesothelioma cells, whereas bcl-2 overexpression counteracted the effect of MetAP2 inhibition on telomerase activity and apoptosis. MetAP2 inhibition also increased caspase activity and the caspase inhibitor, zVAD-fmk, prevented fumagillin-induced apoptosis, but it did not alter telomerase activity. These results indicate that MetAP2 is a main regulator of proliferative and apoptotic pathways in mesothelioma cells and suggest that MetAP2 inhibition may represent a potential target for therapeutic intervention in human mesothelioma.
Angiogenesis is considered a relevant pathogenetic event in cancer development. 1 In fact, formation of new blood vessels is essential to provide an adequate supply of nutrients to cancer cells and sustain their growth and invasiveness. 2 As a consequence, the use of anti-angiogenic molecules may represent a novel approach to cancer treatment. A number of angiostatic compounds have been identified and their efficacy as anti-neoplastic agents is currently under scrutiny. 3,4
Methionine aminopeptidases (MetAPs) are enzymes involved in the removal of the N-terminal methionine from peptides and proteins. Two main MetAP isoforms have been identified so far, precisely MetAP1 and MetAP2. 5 The most significant structural difference between the two is a large helical domain insertion on the surface of the type 2 isozymes. 6 MetAP2, previously identified as an eukaryotic initiation factor-2-associated 67-kd protein, p67, 7 regulates protein synthesis by protecting the α subunit of eukaryotic initiation factor-2 from phosphorylation. 8 MetAP2 expression correlates with cell growth and nondividing cells do not show immunodetectable levels of this enzyme. 9 Moreover, MetAP2 is greatly induced by phorbol myristate acetate. 8 Recently, MetAP2 has been identified as the molecular target of the angiostatic agents fumagillin and ovalicin. These compounds selectively and covalently bind MetAP2 and block its aminopeptidase activity. 9,10 Therefore, MetAP2 might play a relevant role in cancer angiogenesis as well as in the regulation of the proliferative activity of cells expressing this enzyme.
Mesothelioma is an asbestos-related tumor that has been recently associated with the presence of SV40 DNA sequences. 11 Prognosis is poor because of its marked resistance to conventional treatments, including surgical resection, chemotherapy, and radiotherapy, and the median survival of patients with this disease remains between 8 to 18 months. Mesothelioma cell growth is greatly influenced by the capability of these cells to release a number of growth factors including the pro-angiogenic fibroblast growth factor type 2, vascular endothelial growth factor (VEGF), and hepatocyte growth factor/scatter factor. 12,13 In particular, it has been shown that mesothelioma cells express high VEGF, VEGF-C, and VEGF receptor mRNA levels 14 and that elevated VEGF levels can be measured in pleural effusions of patients with mesothelioma. 15 Moreover, we have also observed that VEGF potently stimulates mesothelioma cell proliferation by phosphorylation of the VEGF receptors, KDR and Flt-1, and that anti-VEGF antibodies inhibit the growth of these cells. 16 Thus, angiogenic factors may promote mesothelioma growth not only by inducing tumor angiogenesis, but also by direct stimulation of cell proliferation. As a consequence, angiostatic molecules, capable of antagonizing growth factor actions, might have a considerable impact on mesothelioma cell survival.
Because MetAP2 is the intracellular target of anti-angiogenic agents and mesothelioma cells may be particularly sensitive to exposure to angiostatic molecules, we examined MetAP2 expression in mesothelioma cells, as well as the impact of this enzyme on cell survival and on some of the intracellular pathways regulating apoptosis. Here, we report that mesothelioma cells express higher MetAP2 mRNA levels compared to normal mesothelial cells and that MetAP2 inhibition induces mesothelioma cell apoptosis associated with a reduction in telomerase activity and bcl-2 down-regulation.
Materials and Methods
Cell Lines and Culture Conditions
Human primary mesothelial cells were established from patients and identified morphologically and by extensive phenotypic analysis as previously described. 17 We used three different primary normal mesothelial cells, NM-2 and NM-3 (isolated from the peritoneal fluid of two distinct patients with nonneoplastic ascites) and NM-1 (obtained by pooling pleural effusion cells from four patients with congestive heart failure or liver disease). After 2 weeks in culture, 100% of the normal mesothelial cells stained positive for calretinin. They were expanded thereafter and used for the experiments between the third and seventh passage. Malignant mesothelial cell lines were established as previously described. 18 Three distinct cell lines with different phenotype, biphasic (MM-B1), fibrosarcomatous (MM-F1), and epithelioid (HM) were used for the experiments between the eighth and twelfth passage. All mesothelial cells were maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 1% l-glutamine, 1% penicillin-streptomycin (all from HyClone, Rome, Italy) at 37°C and 5% CO2.
Human umbilical vein endothelial cells (HUVECs) were obtained by Clonetics (San Diego, CA) and maintained in Opti-MEM medium (Life Technologies Inc., Milan, Italy) supplemented with 20% fetal calf serum. These cells were used between the second and fifth passage.
Proliferation Assay
Approximately 5 × 10 4 cells were exposed to oligonucleotides as described. 19 Briefly, cells were washed three times with prewarmed (37°C), serum-free RPMI and incubated with either a MetAP2 anti-sense or a scrambled oligonucleotide previously mixed with 10 μg/ml of lipofectin (Life Technologies, Inc.) in serum-free RPMI. Cells were incubated for 4 hours at 37°C, 5% CO2. The medium was then removed and replaced with complete medium. Cells were collected after 1, 2, 3, and 4 days of incubation, stained with a 0.4% trypan blue solution (Sigma Chemical Co., Milan, Italy) and counted with a hemocytometer. All cell counts were done in triplicate. For analysis of DNA synthesis, 4 × 10 4 cells/ml were cultured for 24 hours in complete RPMI 1640 medium. Cells were then maintained in serum-free medium for 24 hours. This was replaced by complete medium containing the test agents and incubated for varying periods of time. At the end of the incubation, [3H]-thymidine (0.5 μCi/ml) was added for an additional 4 hours. Cells were then washed with phosphate-buffered saline (PBS)2+, incubated with ice-cold 5% trichloroacetic acid for 10 minutes, washed again with ethanol/ether (2:1) before being lysed with 0.5 ml of a solution containing PBS2+, 0.5% Triton, 200 mmol/L NH4OH, and 0.1% bovine serum albumin. Lysates were added to 3 ml of scintillation fluid and radioactivity was determined in a β-scintillation analyzer (model Tri-Carb 2100TR; Packard, Milan, Italy) and counted for 1 minute.
Apoptosis
Apoptosis was assessed by DNA laddering and nucleosome formation. For DNA-laddering detection, DNA was extracted with phenol and subsequently precipitated with ethanol from cells treated from 1 to 4 days with either a MetAP2 anti-sense oligonucleotide or fumagillin in complete medium. Low-molecular weight DNA fragments were visualized by 2% agarose gel electrophoresis and ethidium bromide staining. Nucleosome formation was analyzed using a cell-death detection enzyme-linked immunosorbent assay (ELISA) kit (Roche Molecular Biochemicals, Milan, Italy). In experiments with the caspase inhibitor, benzyloxycarbonyl-Val-Ala-Asp(OMe)-CH2 (zVAD-fmk), the time of exposure to the MetAP2 anti-sense oligonucleotide was extended to 24 hours to obtain a reduction of the MetAP2 protein level before the addition of the caspase inhibitor.
Morphology
Cells suspensions were fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.2, for 30 minutes, rinsed three times with the same buffer, postfixed with 1% OsO4, dehydrated with increasing concentrations of ethanol and embedded in the Epon 812 resin. Semi-thin sections were prepared using an appropriate microtome, stained with 1% toluidine blue at 40°C and examined by light microscopy. Ultra-thin sections, collected on 200-mesh copper grids, were stained with uranyl acetate and lead citrate and examined with a Zeiss 109 electron microscope at 80 kV.
Northern Blotting
Total RNA was extracted from subconfluent cells using the Trizol reagent (Life Technologies). RNA (15 μg) was separated on a 1% agarose/formaldehyde gel, transferred to a Hybond N+ nylon membrane overnight, and UV-cross-linked (Stratalinker 2400; Stratagene, Milan, Italy). The MetAP1, MetAP2, bcl-2, and bax probes were synthesized from full-length human cDNA by random primed 32P-labeling. Blots were hybridized at 42°C overnight in 5× standard saline citrate. Filters were washed twice at 65°C in 2× standard saline citrate containing 0.1% sodium dodecyl sulfate and exposed to X-ray films for 2 days at −70°C. All Northern blots were reprobed with random primed 32P-labeled human β-actin cDNA to confirm equivalent loading of RNA samples.
MetAP2 Anti-Sense Oligonucleotides
The MetAP2 phosphorothioate anti-sense oligonucleotide (5′-AGTATTTACTTTCTCCCAAG-3′) and its relative scrambled sequence (5′-CTTGGGAGAAAGTAAATACT-3′) were synthesized by Sigma-Genosys Ltd. The anti-sense start position on MetAP2 mRNA coding region is 1284. This region corresponds to the large helical domain insertion on the surface of the type 2 isozyme that differentiates it from the type 1 isoform. 5,6
Bcl-2 Stable Transfectants
Cells seeded at the density of 2 × 106/100-mm diameter plate were transfected by the calcium-phosphate precipitation method with a plasmid DNA containing the full-length human bcl-2 cDNA and as a selectable marker, a neomycin phosphotransferase gene. Bcl-2 expression in individually isolated clones was determined by immunoblotting with an anti-Bcl-2 antibody (N-19; Santa Cruz Biotechnology, Milan, Italy). Once a stable cell line was obtained from each clone, neomycin was removed from the culture medium. The clones were maintained in drug-free medium and Lcl-2 expression was periodically determined. As a control, cells were transfected with either an unrelated, neomycin-resistant PKR-plasmid DNA or with a plasmid DNA containing a neomycin phosphotransferase gene.
Immunoblotting and ELISA
Forty μg of protein lysates were resolved using a 10 to 12% sodium dodecyl sulfate-polyacrylamide gel and proteins transferred to polyvinylidene difluoride membranes. These were incubated for a minimum of 2 hours with either a monoclonal antibody against Bcl-2 (Santa Cruz Biotechnology) or with a rabbit polyclonal anti-MetAP2 antibody (CM33) (Zymed Inc., Histo-Line Laboratories, Milan, Italy) and subsequently exposed to a horseradish peroxidase-conjugated secondary antibody. Bands were visualized using the ECL Plus detection kit (Amersham Corp., Milan, Italy). As an internal control the upper portion of the blot was cut and probed with an antibody recognizing an unrelated protein (β-actin). Quantitation of Bcl-2 protein was performed using an ELISA immunoassay kit (Oncogene Science, Cambridge, MA) following the manufacture’s instructions.
Telomeric Repeat Amplification Protocol (TRAP) Assay
Either a nonamplified conventional standard or a polymerase chain reaction-ELISA-based assay (Roche Molecular Biochemicals) was used to measure telomerase activity. 20,21 Cell equivalents (1 × 10 3 to 5 × 103) were used to visualize the DNA ladder with the standard protocol, whereas for polymerase chain reaction-ELISA, 2 × 10 3 cell equivalents were used. The polymerase chain reaction-ELISA protocol was provided by the assay kit manufacturer (Roche Molecular Biochemicals). Each set of TRAP assays included control reaction tubes without any extract or with RNase A (200 μg/ml)-treated extracts.
Caspase 3 Activity
Caspase 3 activity was measured using the Caspase 3 colorimetric assay kit from Clontech (Palo Alto, CA). Briefly, equal amounts of proteins from cellular lysates were added to 5 × 10−5 M substrate (Asp-Glu-Val-Asp-p-nitroanilide) in assay buffer. Samples were incubated at room temperature and analyzed using a spectrophotometer (ELISA microplate reader, Bio-Rad, Richmond, CA).
Statistical Analyses
Results are expressed as means ± SD. All statistical comparisons were made with two-sided Student’s t-test. A P value of less than 0.05 was considered statistically significant.
Results
Fumagillin Reduces Proliferation and Induces Apoptosis in Mesothelioma Cells Because of Early Mitochondrial Damage
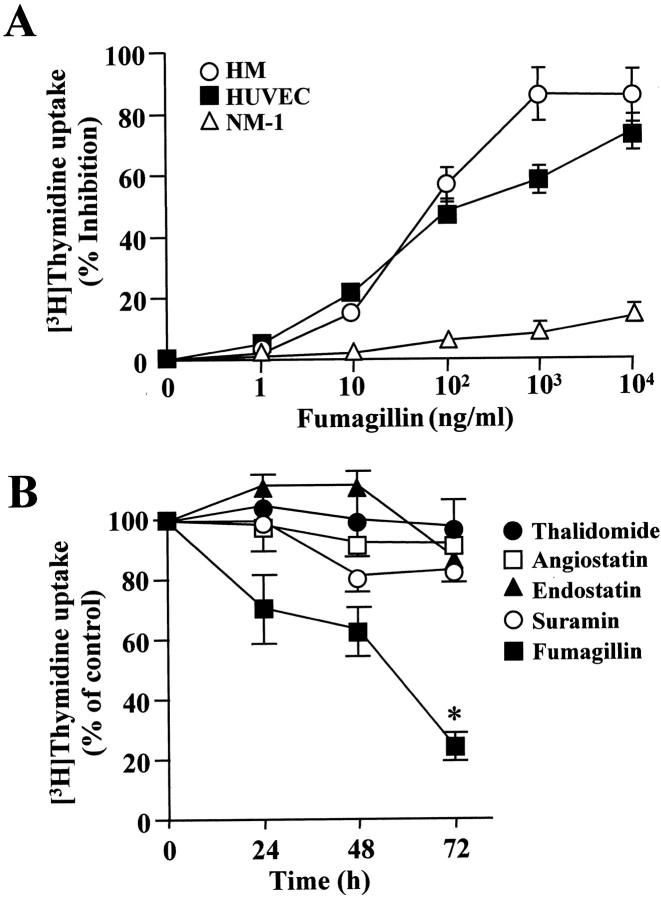
Angiostatic agents may have a considerable impact on tumor progression. To determine whether mesothelioma cell proliferation and survival were affected by angiostatic molecules, we exposed mesothelioma cells to varying concentrations of the known angiostatic molecule, fumagillin. We observed that fumagillin potently inhibited [3H]-thymidine uptake by mesothelioma cells, with an apparent IC50 of ∼0.1 μg/ml. Fumagillin also significantly reduced DNA synthesis in HUVECs, but it did not alter [3H]-thymidine uptake by normal mesothelial cells (Figure 1A) ▶ . To assess selectivity of fumagillin, mesothelioma cells were exposed for different times to a panel of well-characterized anti-angiogenic compounds including thalidomide (10 μg/ml), angiostatin (5 μg/ml), endostatin (10 μg/ml), and suramin (10 μg/ml). The activity of these compounds was preliminary tested using a HUVEC proliferation assay (results not shown). It was observed that fumagillin time-dependently inhibited DNA synthesis in all of the mesothelioma cell lines examined. In contrast, thalidomide, angiostatin, endostatin, and suramin did not have any appreciable effect (Figure 1B) ▶ . In particular, [3H]-thymidine uptake by HM cells was reduced by 72 ± 8% after 96 hours of exposure to 0.5 μg/ml of fumagillin (P ≤ 0.05, fumagillin-treated versus untreated cells).
Figure 1.
Effect of fumagillin on cell proliferation in mesothelial cells. A: HMs, HUVECs, or normal mesothelial cells (NM-1) were treated with varying concentrations of fumagillin for 24 hours. [3H]-thymidine uptake was then determined as described in Materials and Methods. Results are means ± SD from n = 2 with triplicate measurements. B: HM cells (4 × 104/ml) were incubated for different times with either 0.5 μg/ml of fumagillin, 10 μg/ml of thalidomide, 5 μg/ml of angiostatin, 10 μg/ml of endostatin, or 10 μg/ml of suramin. At the end of the incubation, DNA synthesis was assessed by [3H]-thymidine uptake. Data points depict mean values ± SD from two experiments with quadruplicate determinations (*, P = 0.02).
HM cells treated with fumagillin (0.5 μg/ml) for 96 hours also displayed a DNA fragmentation pattern typical of apoptosis, whereas NM-1 cells, as well as HUVECs, did not show any low-molecular weight DNA fragments (Figure 2A) ▶ . Consistently, a significant increase in nucleosome formation was detected in fumagillin-treated HM cells (Figure 2B) ▶ .
Figure 2.
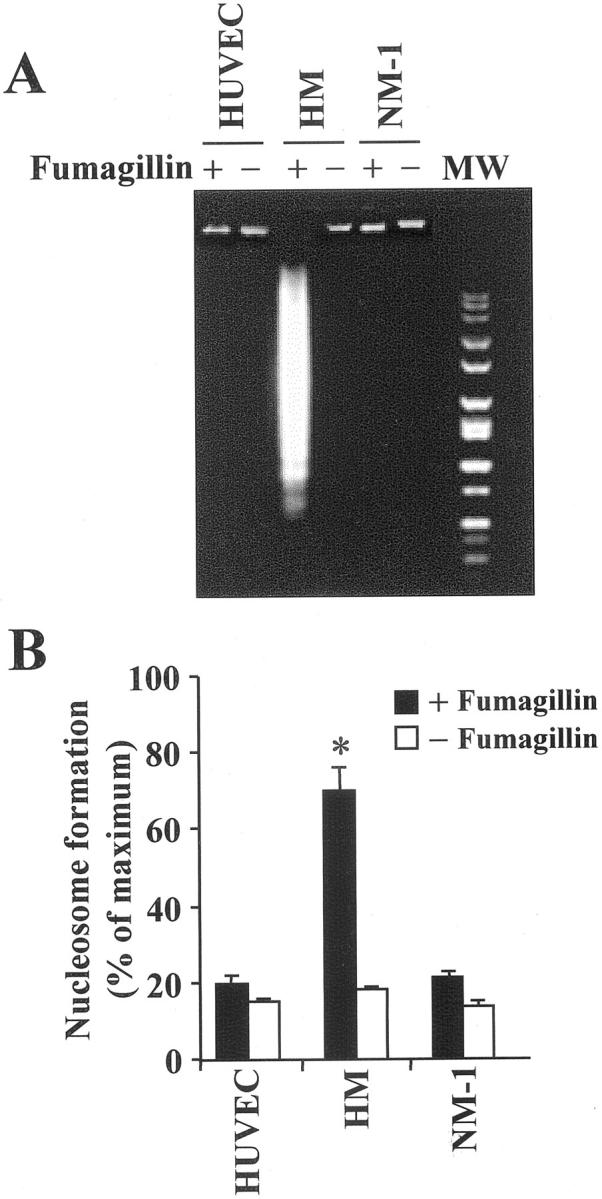
Fumagillin induces apoptosis in mesothelioma cells. A: DNA was extracted from either HUVECs, malignant (HM), or normal (NM-1) mesothelial cells that were exposed for 96 hours to vehicle or fumagillin (0.5 μg/ml). Low-molecular weight DNA was visualized by 2% agarose gel electrophoresis and ethidium bromide staining. MW = 100-bp molecular weight marker. B: Cells were treated with fumagillin (0.5 μg/ml) for 96 hours in complete medium. Nucleosome formation was assessed as indicated in Materials and Methods. One hundred percent corresponded to an optical density reading of 1.278 at 405 nm. Results are mean ± SD from n = 2 done in triplicate.
A light microscopy analysis on semi-thin sections of HM cells exposed to 0.5 μg/ml of fumagillin for 24 hours, showed membrane blebbing and cytoplasmic vacuolization that was not evident in cultures of either untreated HM or fumagillin-treated NM cells (Figure 3, A and B) ▶ . Moreover, electron microscopy observation of ultra-thin sections of fumagillin-treated HM cells showed a significant alteration of the mitochondrion integrity. In particular, the presence of round osmiophilic structures, the so-called “mitochondrial bodies,” diagnostic of mitochondrial disruption, 22 was observed (Figure 3C) ▶ .
Figure 3.
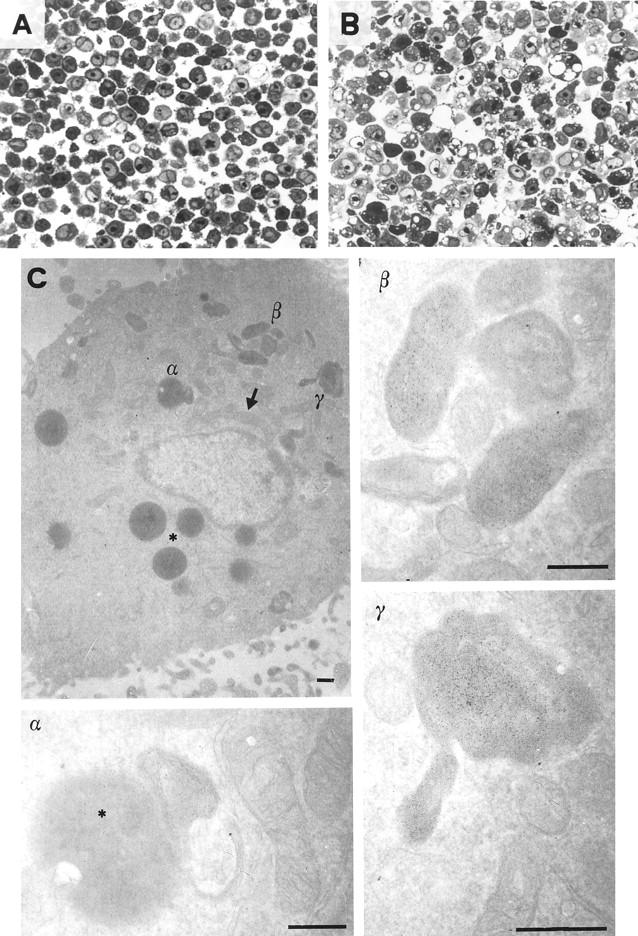
Fumagillin induced morphology changes and mitochondrial disruption in mesothelioma cells. Staining with toluidine blue of semi-thin sections (original magnification, ×400) of mesothelioma cells (HM) exposed for 24 hours to either vehicle (A) or fumagillin (0.5 μg/ml) (B). C: Electron micrographs of ultra-thin sections of HM cells treated with fumagillin for 24 hours. Elongation (arrow), crest disorganization, and round osmiophilic body (asterisks) are indicated. Higher original magnifications ×30,000 (α and β), ×46,000 (γ). Scale bar, 0.5 μm.
MetAPs Expression in Malignant and Normal Mesothelial Cells
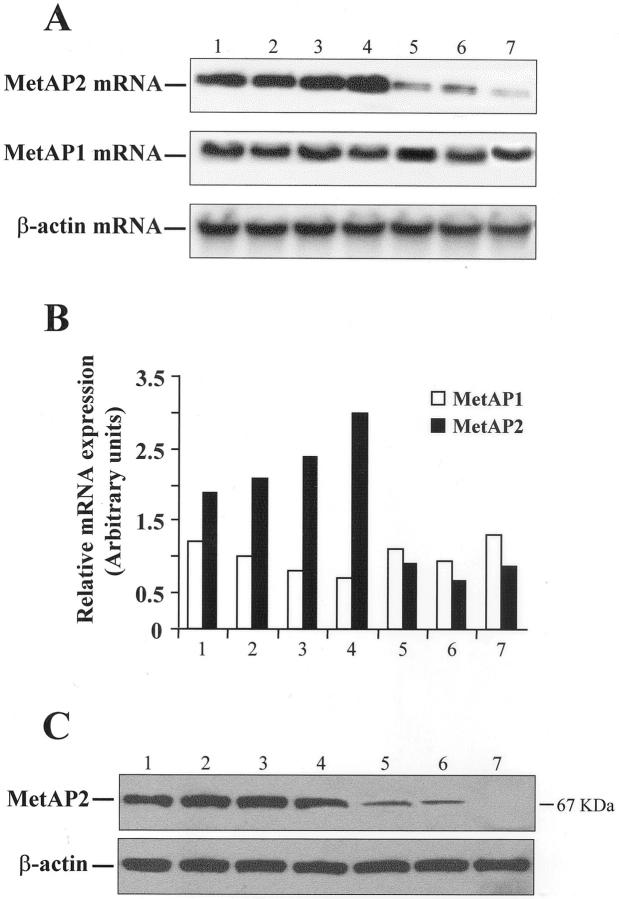
MetAP2 has been identified as the molecular target of fumagillin. 9,10 Therefore, we asked whether the bioactions of fumagillin on mesothelioma cells could be related to MetAP2 expression. To this end, total RNA was extracted from malignant (MM-B1, MM-F1, HM) and primary normal (NM-1, NM-2, NM-3) mesothelial cells and subjected to Northern blot with specific probes for MetAP1 or MetAP2. As shown in Figure 4A ▶ , MM cells expressed high MetAP2 mRNA levels, whereas lower levels were found in NM cells. In contrast, MetAP1 was equally expressed in all cell lines. A densitometric analysis showed that the MetAP2/β-actin ratio was significantly higher in malignant compared to normal mesothelial cells (7.2 ± 1.4 versus 1.4 ± 0.62 arbitrary units; P = 0.002) (Figure 4B) ▶ . Consistently, MetAP2 protein levels were significantly higher in malignant mesothelial cells compared to normal cells (Figure 4C) ▶ .
Figure 4.
MetAP expression in normal and malignant mesothelial cells. A: Total RNA, extracted from HUVEC (lane 1), three mesothelioma cell lines (MM-B1, MM-F1, and HM) (lanes 2 to 4), and three primary normal mesothelial cells (NM-1, NM-2, and NM-3) (lanes 5 to 7), was subjected to Northern blotting using specific probes for MetAP1 and MetAP2. β-actin was used as an internal control. B: Densitometric analysis of results in A. C: Forty μg of lysates from cells listed in A were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted using a specific MetAP2 polyclonal antibody. The MetAP-2 protein was visualized as a band of 67 kd. β-Actin was used as a control for equal protein loading.
A MetAP2 Anti-Sense Oligonucleotide Decreases Mesothelioma Cell Viability and Induces Nucleosome Formation
To obtain a direct evidence of the relationship between MetAP2 expression and mesothelioma cell survival, a sequence-specific phosphorothioate anti-sense oligonucleotide targeted to the coding region of the MetAP2 gene was designed. This oligonucleotide, but not a mismatched oligonucleotide, concentration-dependently decreased MetAP2 mRNA and protein levels in HM cells with an IC50 of ∼50 nmol/L (Figure 5A) ▶ . In contrast, it did not alter MetAP1 expression (results not shown). Maximal inhibition was observed after 48 hours of treatment (results not shown). When added to HM cells, the MetAP2 anti-sense oligonucleotide time-dependently reduced cell viability with a maximum effect after 96 hours (P = 0.015, MetAP2 anti-sense versus scrambled oligo) (Figure 5B) ▶ , whereas it did not alter NM-1 cell viability (result not shown). Further, the MetAP2 anti-sense oligonucleotide induced a significant nucleosome formation in HM, but not in NM-1 cells (0.7 ± 0.052 versus 0.25 ± 0.07, optical density at 405 nm; P = 0.018) (Figure 5C) ▶ .
Figure 5.
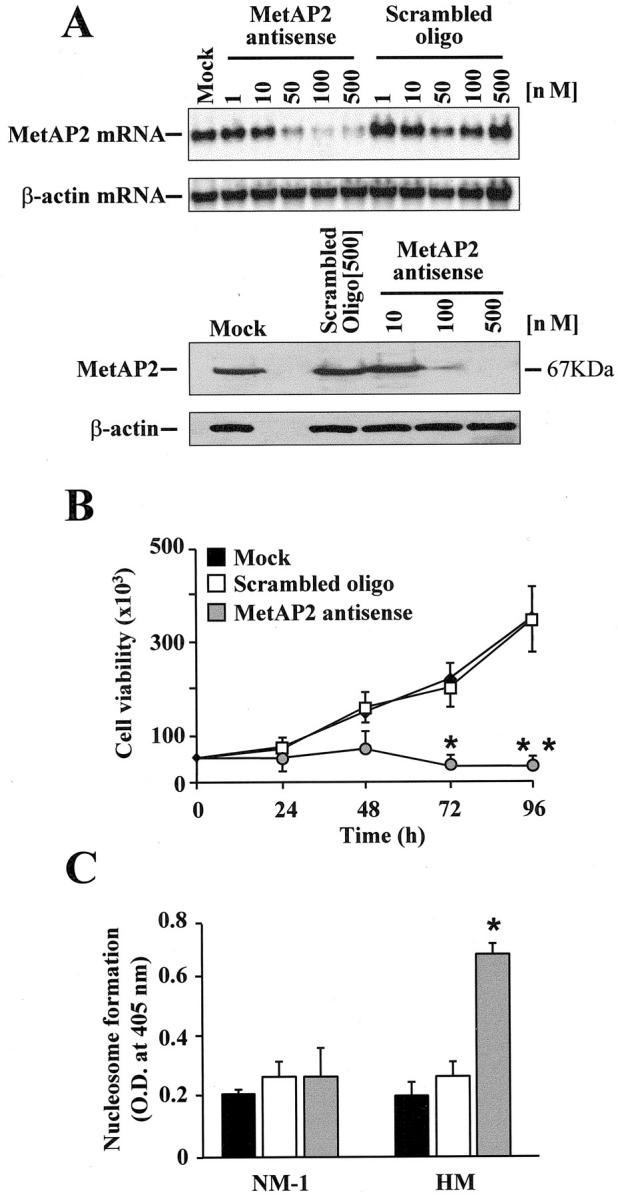
Impact of MetAP2 anti-sense oligonucleotide on mesothelioma cell viability and nucleosome formation. A: Mesothelioma cells (HM) were incubated with increasing concentrations of either a specific MetAP2 anti-sense or a scrambled oligonucleotide. After 24 hours total RNA was extracted and blotted with a specific probe for MetAP2. MetAP2 protein levels were determined by Western blotting after 48 hours exposure to the oligonucleotides. β-actin was used as an internal control. B: HM cells were treated for different periods of time with either lipofectin alone (mock) or with 100 nmol/L of the MetAP2 anti-sense or of a scrambled oligonucleotide. At the end of the incubation, cell viability was assessed by trypan blue exclusion. Data points depict mean values ± SD from two experiments with quadruplicate determinations (*, P = 0.02; **, P < 0.001). C: Normal mesothelial (NM-1) and HM cells were treated as indicated in the legend to B. Nucleosome formation in the presence of lipofectin alone, the MetAP2 anti-sense, or the scrambled oligonucleotide was determined after 96 hours using the cell death detection ELISAPLUS kit as indicated in the Materials and Methods section. Results are the average ± SD from three experiments with duplicate determinations. (*, P = 0.018).
Bcl-2 Expression Is Down-Regulated by MetAP2 Inhibition
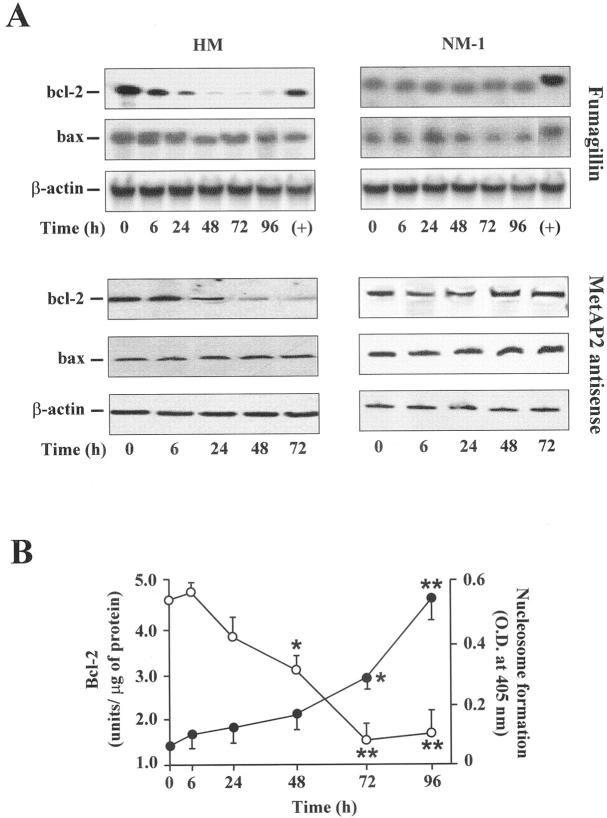
Products of genes belonging to the Lcl-2 family are main regulators of apoptosis. In particular, Bcl-2 prevents programmed cell death, whereas Bax can accelerate this process. 23 Therefore, we asked whether a dysregulation of these genes was involved in mesothelioma cell apoptosis after MetAP2 inhibition. To this end, bcl-2 and bax mRNA levels were determined by Northern blot in cells treated with either fumagillin or the MetAP2 anti-sense oligonucleotide. As displayed in Figure 6A ▶ , fumagillin, as well as the MetAP2 anti-sense, time-dependently reduced bcl-2 expression, whereas they did not modify bax mRNA levels in HM cells. The scrambled oligonucleotide was ineffective (results not shown). In contrast, MetAP2 inhibition did not determine significant changes in either bcl-2 or bax mRNA levels in NM-1 cells. A time-dependent inverse correlation between nucleosome formation and Bcl-2 protein expression was also denoted in HM cells (Figure 6B) ▶ .
Figure 6.
MetAP2 inhibition reduces Bcl-2 expression in mesothelioma cells. A: Total RNA was extracted from malignant (HM) and normal (NM-1) mesothelial cells treated with either 0.5 μg/ml of fumagillin or 100 nmol/L of MetAP2 anti-sense oligonucleotide for varying periods of time and hybridized with bcl-2-, bax-, and β-actin-specific probes. RNA from HeLa cells expressing bcl-2 and bax was used as a positive control (+). Results are from a representative experiment (n = 3). B: Bcl-2 protein levels (open circle) and nucleosome formation (filled circle) were determined in fumagillin-treated HM cells as described in Experimental Procedures. Data points represent the mean ± SD from three separate experiments with duplicate determinations. (*, P < 0.05; **, P < 0.001).
MetAP2 Regulates Telomerase Activity in Mesothelioma Cells
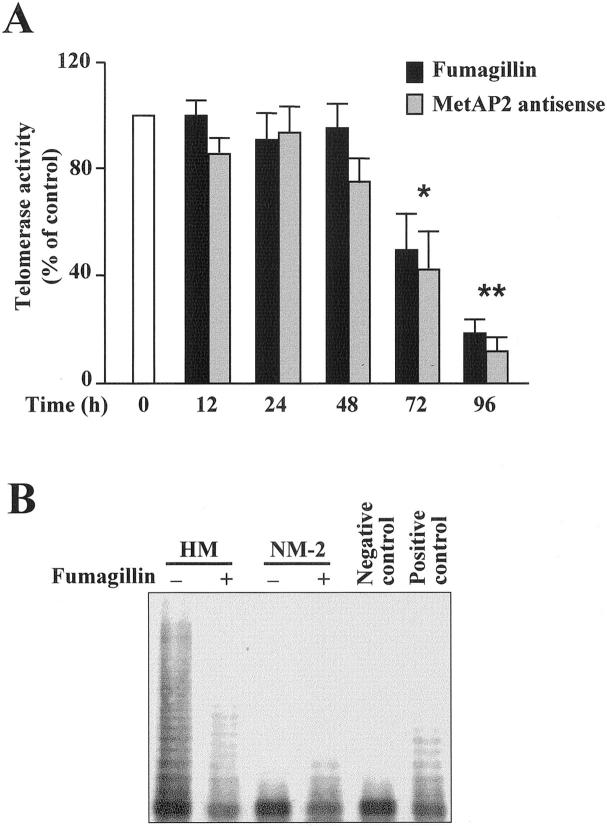
Recently, a close relationship between bcl-2 expression and telomerase activity in cancer cells has been denoted. 24 Therefore, we hypothesized that MetAP2 inhibition might have an impact also on telomerase activity. Consistent with previous results, 25 mesothelioma cells displayed a strong telomerase activity, whereas normal mesothelial cells did not. When mesothelioma cells were exposed to fumagillin or the MetAP2 anti-sense a significant time-dependent reduction in telomerase activity was observed (Figure 7A) ▶ . A quantitative polymerase chain reaction-ELISA-TRAP assay showed that telomerase activity was reduced by ∼55% after 72 hours and by ∼75% after 96 hours of exposure to fumagillin (P = 0.025 and P < 0.001, respectively). Consistently, fumagillin-treated HM cells also exhibited a lower number of DNA fragments, typical of telomerase activity, compared to untreated cells (Figure 7B) ▶ . However, telomerase mRNA levels were not modified after exposure of HM cells to fumagillin (results not shown).
Figure 7.
MetAP2 inhibition decreases telomerase activity in mesothelioma cells. A: Mesothelioma (HM) cells were exposed to either 0.5 μg/ml of fumagillin or 100 nmol/L of MetAP2 anti-sense for the indicated periods of time. Telomerase activity in cell extracts (0.1 μg) was determined as described in Experimental Procedures. Values are expressed as percentage of the telomerase activity present in untreated or scrambled oligo-treated HM cells (white bar) and represent the mean ± SD from three experiments with duplicate determinations (*, P = 0.015; **, P < 0.001). B: TRAP assay of mesothelioma (HM) and normal mesothelial (NM-2) cell extracts from cultures treated for 96 hours with either vehicle or 0.5 μg/ml of fumagillin. Negative control refers to HM cell extracts that were incubated with 200 μg/ml of RNase before the TRAP assay. Positive control was 0.02 μg of HeLa cell extract. Results are from one experiment representative of three.
Bcl-2 Overexpression Antagonizes the Effect of MetAP2 Inhibition on Telomerase Activity and Nucleosome Formation
To further analyze the relationship between bcl-2 deregulation and reduced telomerase activity after MetAP2 inhibition, we generated HM stable transfectants overexpressing Bcl-2. Figure 8A ▶ shows Bcl-2 protein levels in two of four of these clones. Clones 1 and 2 expressed threefold to fivefold higher Bcl-2 protein levels compared to either parental HM cells or a clone expressing an unrelated PKR/neo gene (HM-PKneo cells). Bcl-2 overexpression did not appreciably change basal telomerase activity. However, exposure of HM-bcl-2.2 to fumagillin did not give the inhibitory effect on telomerase activity seen with either HM or HM-PKneo cells (Figure 8B) ▶ . Moreover, fumagillin did not induce apoptosis in the Bcl-2-overexpressing HM cells (Figure 8C) ▶ .
Figure 8.
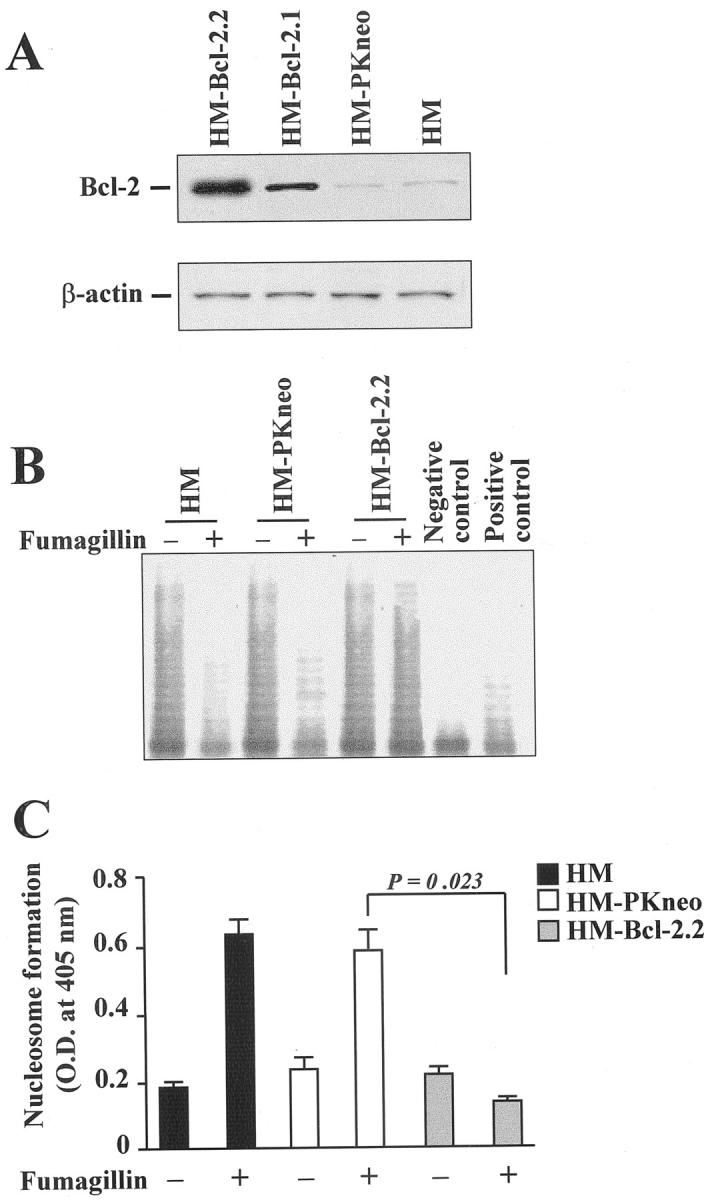
Bcl-2 overexpression protects mesothelioma cells from telomerase reduction and apoptosis induced by MetAP2 inhibition. A: Western blot relative to Bcl-2 protein expression in two clones of HM cells overexpressing Bcl-2 (HM-Bcl-2.1 and HM-Bcl-2.2) and vector-transfected HM cells (HM-PKneo). Bcl-2 band migrates at ∼30 kd. Forty μg of protein was loaded in each lane. β-actin was used as internal control. B: TRAP assay of HM, HM-PKneo, and HM-bcl-2.2 cell extracts from cultures treated for 96 hours with either vehicle or 0.5 μg/ml of fumagillin. Positive and negative controls were obtained as described in the legend to Figure 7 ▶ . C: HM cells, HM-PKneo, or HM-Bcl-2.2 clones were exposed to either vehicle or 0.5 μg/ml of fumagillin for 96 hours and nucleosome formation was assessed. Results are the mean ± SD from three separate experiments.
Caspase Activity Is Involved in MetAP2-Dependent Apoptosis
The cysteine proteases, caspases, are the best known effectors of programmed cell death in most, if not all, mammalian cell types. 26 To determine whether caspases were involved in the apoptotic pathways triggered by MetAP2 inhibition, HM cells were exposed to the broad-spectrum irreversible caspase inhibitor, zVAD-fmk. 26 As shown in Figure 9A ▶ , zVAD-fmk abrogated the fumagillin-induced nucleosome formation, although it did not prevent telomerase inhibition (results not shown). Consistently, the MetAP2 anti-sense oligonucleotide significantly enhanced caspase activity in HM cells, but not in the HM-bcl-2.2 clone and this effect was blunted by zVAD-fmk (Figure 9B) ▶ .
Figure 9.
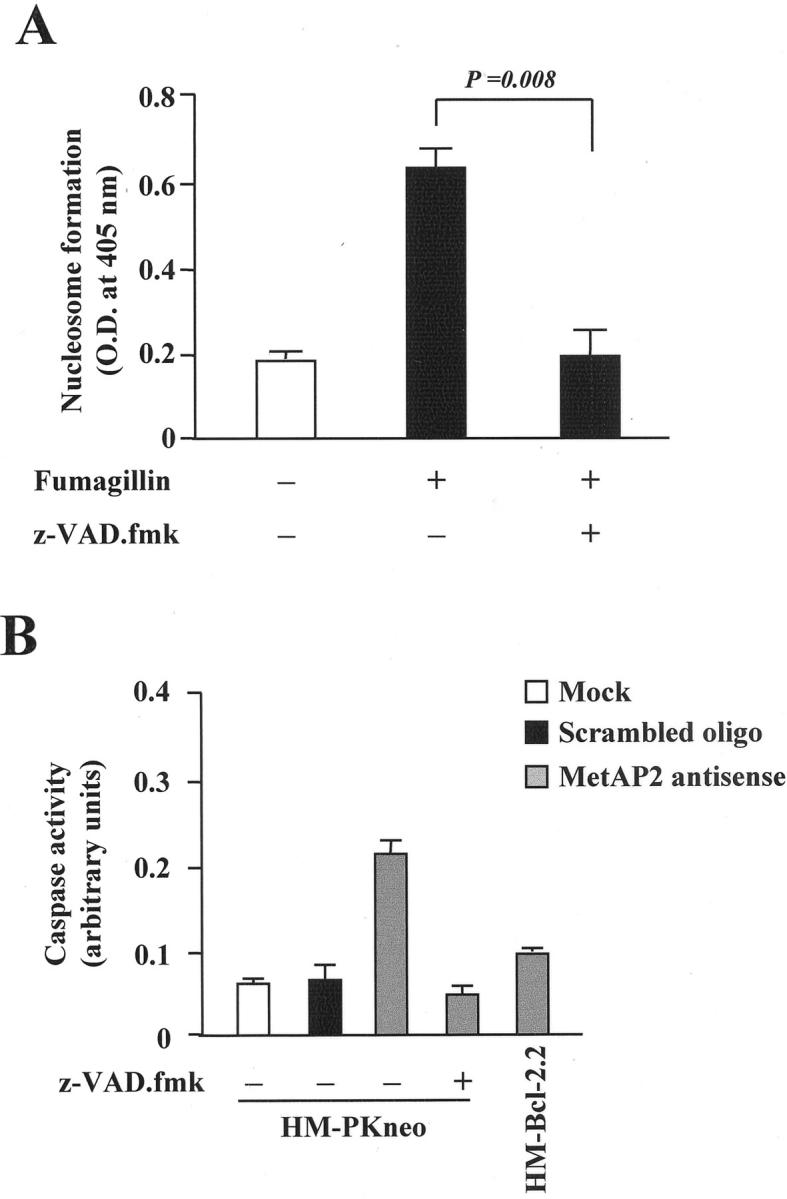
Bcl-2 overexpression and zVAD-fmk protect mesothelioma cells from caspase 3 activation and apoptosis induced by MetAP2 inhibition. A: HM cells were exposed to either vehicle or 0.5 μg/ml of fumagillin for 96 hours either in the presence or in the absence of 100 nmol/L of zVAD-fmk. Nucleosome formation was determined as indicated in Materials and Methods. Results are the mean ± SD from four separate experiments. B: HM cells, HM-PKneo, or HM-Bcl-2.2 clones were incubated with 100 nmol/L of MetAP2 anti-sense oligonucleotide either in the presence or the absence of 100 nmol/L of zVAD-fmk. Caspase 3 activity was determined as indicated in Materials and Methods.
Discussion
In the present report we show for the first time that the MetAP2 gene is highly expressed in human mesothelioma cells and that its inhibition results in a proliferative block and cell death by apoptosis (Figures 1 to 3) ▶ ▶ ▶ . Recently, MetAP2 has been identified as the molecular target of the angiostatic agents fumagillin and ovalicin 9,10 and it has been documented that its inhibition by these drugs determines endothelial cell-cycle arrest in the late G1 phase and blockage of angiogenesis. 27 For this reason, considering the relevance of neovascularization in cancer growth and invasiveness, the fumagillin analog, TNP-470, is being used for treatment of a variety of neoplasms. 3,4 Consistent with previous findings, 28 our present results indicate that MetAP2 may regulate tumor growth also by a direct effect on cancer cell survival. However, the observation that normal mesothelial cells showed very low MetAP2 mRNA and protein levels (Figure 4) ▶ raises the question whether the MetAP2 gene is up-regulated as a part of the molecular events that take place during malignant transformation of mesothelial cells. If this is the case, MetAP2 could be proposed as a marker of mesothelial cell malignancy and a selective pharmacological target. In this respect, it is noteworthy that all of the mesothelioma cell lines tested displayed very high MetAP2 mRNA levels regardless of the histotype (Figure 3) ▶ . However, additional studies are needed to establish the clinical significance of MetAP2 expression in mesothelioma. On the other hand, fumagillin inhibited DNA synthesis in both HUVECs and mesothelioma cells (Figure 1) ▶ , but it induced apoptosis only in mesothelioma cells (Figure 2) ▶ . These results suggest that the MetAP2 signaling may involve different pathways in normal versus malignant cells.
In mesothelioma cells MeAP2 seems to control two key regulators of proliferation and programmed cell death, namely Bcl-2 and telomerases. In fact, MetAP2 inhibition caused a time-dependent down-regulation of the anti-apoptotic gene bcl-2, whereas it did not alter the expression of the pro-apoptotic gene, bax (Figure 6) ▶ . These results represent the first observation of bcl-2 regulation by MetAP2 and are consistent with the findings of membrane blebbing, cytoplasm vacuolization, and mitochondrial body formation in mesothelioma cells exposed to fumagillin (Figure 3) ▶ . It is in fact known that Bcl-2 maintains mitochondrial integrity by regulating the opening of the transition pore, this preventing the release into the cytosol of the caspase activators, AIF and cytochrome c. 29 However, how MetAP2 regulates bcl-2 expression remains unknown. MetAP2 is not a transcription factor, therefore, it is unlikely that it may have a direct impact on bcl-2 gene expression. Instead, MetAP2 controls the posttranslational processing required for protein myristoylation. 7,8 Several proteins including members of the src tyrosine kinase family, cyclic AMP-dependent kinase, the protein phosphatase calcineurin, and the α subunits of numerous GTP-binding proteins are modified by the covalent attachment of myristic acid to a glycine residue, which is exposed after removal by MetAPs of the initial amino-terminal methionine. 8 Myristoylation regulates the localization, stability, and degradation rate of these proteins. Thus, it could be hypothesized that in mesothelioma cells MetAP2 inhibition may alter the function of proteins involved in the control of bcl-2 expression. Another possibility is that, because the MetAP2 inhibitor TNP-470 induces p53 and p21 expression 30 and, at least in selected systems, p53 down-regulates bcl-2 expression, 31,32 the impact of MetAP2 inhibition on bcl-2 mRNA may be in relation with the regulation of p53. In this regard, interactions between the p53 and Lcl-2 pathways are extremely interesting in the mesothelioma model because it has been shown that SV40 large T-antigen, which is likely to be involved in the pathogenesis of human mesothelioma, binds and inactivates p53. 33 Thus, in mesothelioma cells MetAP2 inhibition could restore p53 function and this may have a downstream impact on bcl-2 expression. This hypothesis also awaits experimental documentation.
In addition to bcl-2, MetAP2 inhibition regulated telomerase activity in mesothelioma cells (Figure 7) ▶ . Telomeres are structures containing (TTAGGG)n repeats localized at the ends of chromosomes. Their main function is to protect chromosomes from rearrangements, degradation, and fusions. In normal somatic cells, telomere length decreases with aging. In contrast, tumor cells express high levels of telomerase, a ribonucleic acid-protein complex that prevents telomere loss. Moreover, telomerase inhibition may alter cancer cell growth. 24 Recently, telomerase activity was detected in >90% of mesothelioma, but not in normal mesothelial cells, indicating that telomerase re-activation may occur during the development of this tumor. 25 This observation, together with our present results showing that mesothelioma cells express high MetAP2 mRNA and protein levels (Figure 4) ▶ and that in these cells MetAP2 inhibition significantly reduced telomerase activity (Figure 7) ▶ , seems to indicate that a close relationship may exist between MetAP2 expression and telomerase re-activation in human mesothelioma. Whether or not this is a general phenomenon during carcinogenesis remains to be determined. However, this previously unappreciated MetAP2 effect on telomerase activity may be relevant to selectively direct the pharmacological effects of MetAP2 inhibitors toward actively replicating cells, such as cancer cells, that express high telomerase activity.
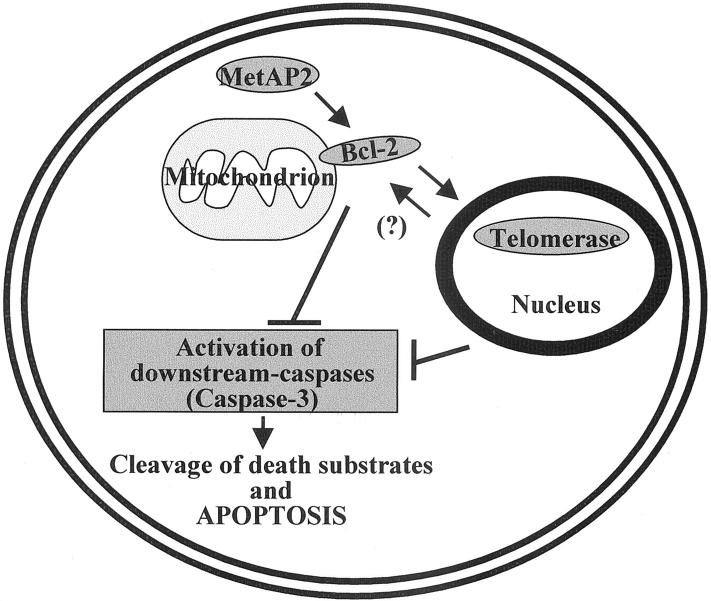
It has been reported that telomerase activity is increased by bcl-2 overexpression in human cancer cells with low expression of this gene. 24 Moreover, bcl-2 overexpression in pheochromocytoma cells protects them from the apoptosis induced by telomerase inhibitors. 34 We observed that bcl-2 overexpression in mesothelioma cells did not change their already quite high basal telomerase activity, however, it did counteract the effects of MetAP2 inhibition on telomerase activity and apoptosis (Figure 8) ▶ . Taken together, these results indicate that in mesothelioma cells Lcl-2 may function as a downstream effector of MetAP2 actions on apoptosis and telomerase activity. On the other hand, members of the caspase family are in many circumstances ultimately responsible for DNA fragmentation and apoptosis. 31,32 Because the caspase inhibitor zVAD-fmk abrogated mesothelioma cell apoptosis induced by MetAP2 inhibition and a MetAP2 anti-sense oligonucleotide increased caspase activity (Figure 9) ▶ , it may be concluded that caspases are involved in MetAP2 regulation of mesothelioma cell survival. However, the MetAP2 anti-sense did not modify caspase activity in Bcl-2 overexpressing tumor clones (Figure 9) ▶ and zVAD-fmk did not reverse the fumagillin-dependent inhibition of telomerase activity (results not shown). Taken together these findings indicate that MetAP2 acts upstream of Bcl-2, whereas Bcl-2 site of action is likely to be upstream of that of telomerases and caspases. On the other hand, telomerases seem to act upstream of caspases. A schematic representation of this pathway is shown in Figure 10 ▶ .
Figure 10.
Schematic representation of intracellular pathways controlled by MetAP2. MetAP2 positively regulates Bcl-2 expression. This, on one side contributes to the up-regulation of telomerase activity in the nucleus, and on the other inhibits caspase activation. On the other hand, telomerase activity down-regulates caspase activity. Whether telomerase activity may have also an impact on Bcl-2 expression in this model remains to be established.
In conclusion, the present results emphasizing the role played by MetAP2 in mesothelioma cell growth and apoptosis and characterizing some novel MetAP2-related intracellular pathways may contribute to expanding our current knowledge on the involvement of MetAP2 in the mechanisms of cancerogenesis. These findings may also provide the rationale for alternative therapeutic strategies in the treatment of human mesothelioma.
Footnotes
Address reprint requests to Mario Romano, M.D., Università “G. D’Annunzio”, Dipartimento di Medicina e Scienze dell’Invecchiamento, Sezione di Ematologia, Via dei Vestini, 31 66013 Chieti Italy. E-mail: mromano@unich.it.
Supported by grants from Associazione Italiana Ricerca contro il Cancro and Ministero dell’Universtà e Ricerca Scientifica, ex 60%, ex 40% (to A. P.).
References
- 1.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J: Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86:353-364 [DOI] [PubMed] [Google Scholar]
- 3.Dezube BJ, Von Roenn JH, Holden-Wiltse J, Cheung TW, Remick SC, Cooley TP: Fumagillin analog in the treatment of Kaposi’s sarcoma: a phase I AIDS Clinical. J Clin Oncol 1998, 16:1444-1449 [DOI] [PubMed] [Google Scholar]
- 4.Gleich LL, Zimmerman N, Wang YO, Gluckman JL: Angiogenic inhibition for the treatment of head and neck cancer. Anticancer Res 1998, 18:2607-2609 [PubMed] [Google Scholar]
- 5.Arfin SM, Kendall RL, Hall L, Weaver LH, Stewart AE, Matthews BW: Eukaryotic methionine aminopeptidase: two classes of cobalt-dependent enzymes. Proc Natl Acad Sci USA 1995, 92:7714-7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker KW, Bradshaw RA: Yeast methionine aminopeptidase I. J Biol Chem 1999, 274:13403-13409 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Chang YH: Evidence that the human homologue of a rat initiation factor-2 associated protein (p67) is a methionine aminopeptidase. Biochem Biophys Res Commun 1996, 227:152-159 [DOI] [PubMed] [Google Scholar]
- 8.Ray MK, Datta B, Chakraborty A, Chattopadhyay A, Meza-Keuthen S, Gupta NK: The eukaryotic initiation factor 2-associated 67-kDa polypeptide (p67) plays a critical role in regulation of protein synthesis initiation in animal cells. Proc Natl Acad Sci USA 1992, 89:539-543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sin N, Meng L, Wang MQW, Wen JJ, Bornmann WG, Crews CM: The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA 1997, 94:6099-6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith EC, Su Z, Turk BE, Chen S, Chang YW, Wu Z: Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol 1997, 4:461-471 [DOI] [PubMed] [Google Scholar]
- 11.Carbone M, Rizzo P, Procopio A, Giuliano M, Gephard M, Hansen M: Incidence of SV40-like sequences in human tumors. Oncogene 1996, 13:527-535 [PubMed] [Google Scholar]
- 12.Kumar-Singh S, Weyler J, Martin MJ, Vermeulen PB, Van Marck E: Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and -2, and TGF beta expression. J Pathol 1999, 189:72-78 [DOI] [PubMed] [Google Scholar]
- 13.Harvey P, Warn A, Dobbin S, Arakaki N, Daikuhara Y, Jaurand MC: Expression of HGF/SF in mesothelioma cell lines and its effects on cell motility, proliferation and morphology. Br J Cancer 1998, 77:1052-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta Y, Shridhar V, Bright RK, Kalemkerian GP, Du W, Carbone M: VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer 1999, 81:54-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JBJ: Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin Cancer Res 1999, 5:3364-3368 [PubMed] [Google Scholar]
- 16.Strizzi L, Catalano A, Vianale G, Orecchia S, Casalini A, Tassi G, Procopio A: VEGF is an autocrine growth factor in human malignant pleural mesothelioma. J Pathol 2001, 193:468-475 [DOI] [PubMed] [Google Scholar]
- 17.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR: Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci USA 2000, 97:10214-10219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orengo AM, Spoletini L, Procopio A, Favoni RE, De Cupis A, Ardizzoni A, Castagneto B, Ribotta M, Betta PG, Ferrini S, Mutti L: Establishment of four new mesothelioma cell lines: characterization by ultrastructural and immunophenotypic analysis. Eur Respir J 1999, 13:527-534 [DOI] [PubMed] [Google Scholar]
- 19.Bennett CF, Chiang MY, Chan H, Shoemaker JE, Mirabelli CK: Cationic lipids enhance cellular uptake and activity of phosphorothioate antisense oligonucleotides. Mol Pharmacol 1992, 41:1023-1033 [PubMed] [Google Scholar]
- 20.Engelhardt M, Kumar R, Albanell J, Pettengell R, Han W, Moore MA: Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood 1997, 90:182-193 [PubMed] [Google Scholar]
- 21.Mandal M, Kumar R: Bcl-2 modulates telomerase activity. J Biol Chem 1997, 272:14183-14187 [DOI] [PubMed] [Google Scholar]
- 22.Di Pietro R, Robuffo I, Pucci AM, Bosco D, Santavenere E: Effects of TNF-α/colchicine combined treatment on Burkitt lymphoma cells: molecular and ultrastructural changes. Cytokine 1999, 2:144-150 [DOI] [PubMed] [Google Scholar]
- 23.Adams JM, Cory S: The Bcl-2 protein family: arbiters of cell survival. Science 1998, 281:1322-1326 [DOI] [PubMed] [Google Scholar]
- 24.Liu JP: Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J 1999, 13:2091-2104 [DOI] [PubMed] [Google Scholar]
- 25.Dhaene K, Wauters J, Weyn B, Timmermans JP, van Marck E: Expression profile of telomerase subunits in human pleural mesothelioma. J Pathol 2000, 190:80-85 [DOI] [PubMed] [Google Scholar]
- 26.Kidd VJ: Proteolytic activities that mediate apoptosis. Annu Rev Physiol 1998, 60:533-573 [DOI] [PubMed] [Google Scholar]
- 27.Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H: Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348:555-557 [DOI] [PubMed] [Google Scholar]
- 28.Yanase T, Tamura M, Fujita K, Kodama S, Tanaka K: Inhibitory effect of angiogenesis inhibitor TNP-470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res 1993, 53:2566-2567 [PubMed] [Google Scholar]
- 29.Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G: Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene 1998, 16:2265-2282 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Griffith EC, Sage J, Jacks T, Liu JO: Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21/WAF1/CIP1. Proc Natl Acad Sci USA 2000, 97:6427-6431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi J, Ookawa K, Kohno T, Tomizawa Y, Tsuchida S, Yokota J: Phenotypic alterations of small cell lung carcinoma induced by different levels of wild-type p53 expression. Cell Death Differ 1998, 5:148-155 [DOI] [PubMed] [Google Scholar]
- 32.Ju JF, Banerjee D, Lenz HJ, Danenberg KD, Schmittgen TC, Spears CP: Restoration of wild-type p53 activity in p53-null HL-60 cells confers multidrug sensitivity. Clin Cancer Res 1998, 4:1315-1322 [PubMed] [Google Scholar]
- 33.Carbone M, Rizzo P, Grimley PM, Procopio A, Mew DJ, Shridhar V: Simian virus-40 large-T antigen binds p53 in human mesotheliomas. Nat Med 1997, 3:908-912 [DOI] [PubMed] [Google Scholar]
- 34.Fu W, Begley JG, Killen MW, Mattson MP: Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem 1999, 274:7264-7271 [DOI] [PubMed] [Google Scholar]