Abstract
Background
The purpose of this study was to investigate the anterior cingulate cortex (ACC) Glutamate/Glutamine (Glx) to creatine ratio (Glx/Cr) in two groups of children with bipolar disorder (BPD): those exhibiting manic symptoms requiring treatment and those being stably treated with the atypical antipsychotic risperidone. Atypical antipsychotics have been shown to increase serum glutamate levels and ACC Glx/Cr in subjects with schizophrenia. In this study, we hypothesized that the children with BPD in need of treatment would have lower Glx/Cr compared with the children with BPD being stably treated with risperidone.
Methods
Proton MR spectra were acquired, at 1.5 T, from the ACC of eighteen subjects with a DSM IV diagnosis of BPD: ten (11.10 ± 3.48 years; five female) were manic and not medicated with any antipsychotic and eight (10.88 ± 2.99 years; one female) were medicated with the atypical antipsychotic risperidone.
Results
Children with BPD exhibiting manic symptoms requiring treatment had lower Glx/Cr than children with BPD being stably treated with the atypical antipsychotic risperidone. The children treated with risperidone also had significantly lower YMRS and CGI-Mania scores than the children not treated with risperidone. Both YMRS and CGI-Mania scores correlated negatively with ACC Glx/Cr levels.
Limitations
The cross-sectional design, small sample size, the use of Glx rather than Glutamate or glutamine and the use of Cr ratios rather than absolute concentrations are limitations of this study.
Conclusions
Children with mania have lower Glx/Cr levels than children with BPD being stably treated with the atypical antipsychotic risperidone. Mania may be associated with reduced glutamate/glutamine levels in the ACC: other imaging studies have shown mania associated with hypometabolism in the ACC. These reductions in glutamate/glutamine may be increased following successful treatment with glutamatergic agents.
Background
The lifetime prevalence of bipolar disorder (BPD) is between 1 and 3 %; and BPD can first manifest itself in childhood and adolescence (Geller et al., 1994, Lewinsohn et al., 1995). Glial cell abnormalities have been indicated in the prefrontal cortex and anterior cingulate cortex (ACC) in affective illnesses including BPD and Major Depression (MDD) (Rajkowska et al., 2001, Ongur et al., 1998). Glia provide a pathway for neuronal glutamate synthesis (Cooper et al., 2003, Sanacora et al., 2003) and abnormalities in glial cell function may lead to reductions in glutamate and/or glutamine levels. Using proton Magnetic Resonance Spectroscopy (MRS), Rosenberg et al (Rosenberg et al., 2005) measured reduced glutamate levels in the ACC of adolescents diagnosed with MDD, relative to healthy comparison subjects. The Glx peak in proton MRS is composed of glutamate (Glu), glutamine (Gln) and γ-amino butyric acid (GABA). At field strengths less than 2.0 T it is difficult to resolve the resonances of Glu, Gln and GABA (Novotny et al., 2003) and the composite peak is usually referred to as Glx. Brain GABA concentrations are on the order of 1 μmole/g; Glu is of the order of 8–13 μmole/g (Cooper et al., 2003); and the Glu to Gln ratio in vivo had been shown to range from ~ 2.4 to 3.8 (Gruetter et al., 2003). Therefore, alterations in Glx, measured using proton MRS, are often attributed to alterations in Glu.
Atypical antipsychotics have been shown to be effective in the treatment of BPD in children and adolescents (Biederman et al., 2005, Delbello et al., 2002, Chang and Ketter, 2000). Studies have shown that atypical antipsychotics increase serum glutamate levels in subjects with schizophrenia (Goff et al., 2002, Evins et al., 1997). In addition to atypical antipsychotics increasing serum glutamate levels, Goff et al demonstrated that the atypical antipsychotic olanzapine increased the ACC Glx to creatine ratio (Glx/Cr) in subjects for whom olanzapine significantly improved negative symptoms (Goff et al., 2002).
The purpose of this study was to look at two groups of children and adolescents with BPD: (1) a group being assessed for treatment due to symptoms of mania (measured using the Young Mania Rating Scale (YMRS)) and (2) a group of children being successfully treated with the atypical antipsychotic risperidone. We hypothesized that the children with unstable illness would have lower Glx/Cr compared with the children being stably treated with the atypical antipsychotic risperidone.
Methods
Subjects
The Institutional Reviews Boards at McLean Hospital and Massachusetts General Hospital approved this study. All parents signed a written informed consent form and all children signed a written informed assent form. Subjects were recruited through the Pediatric Psychopharmacology Research Program at Massachusetts General Hospital.
To be included in the study subjects could be male or female aged between 6 and 17 years old. Each subject met criteria for DSM-IV TR bipolar I disorder, based on clinical assessment by board certified child and adolescent psychiatrist. None of the children studied had a learning disability. All of the subjects studied had co-morbid ADHD.
Fulfillment of the inclusion and exclusion criteria was obtained through a psychiatric evaluation and questionnaires, a detailed medical history, information on consumptive habits, a drug screen, laboratory analyses, and a description of presenting and preexisting conditions were conducted at the beginning of the study.
Diagnostic Assessment
A complete psychiatric history was obtained from all subjects using the Kiddie Schedule for Affective Disorders (K-SADS-E). Cognitive function (IQ, performance, memory, attention, and executive functions) was assessed using a brief neuropsychological assessment battery. Social functioning was assessed using the Social Adjustment Inventory for Children and Adolescents (SAICA) and the Global Assessment of Functioning (GAF), and family functioning was assessed with the Family Environment Scale (FES). Symptoms of mania were assessed with the Young Mania Rating Scale (YMRS) and symptoms of depression using the Children’s Depression Rating Scale (CDRS). The clinical global impressions (CGI) scale was also used as an assessment tool.
1H Magnetic Resonance Spectroscopy
Imaging and spectroscopy was performed using a 1.5 T General Electric Signa scanner (GEMS, Milwaukee, WI) and a quadrature proton head coil. A T2 weighted axial image set was acquired for spectral localization (Figure 1(a)) and a 3D coronal volume scan was acquired for image segmentation. Water suppressed localized spectra were acquired, from a region covering the ACC, using a 12 x 12 spectroscopic imaging (MRSI) grid (field of view: 24 cm x 24 cm; nominal voxel size: 2 cm x 2 cm x 1.2 cm; Inplane (axial) thickness: 12 cm) and the Probe-P pulse sequence (echo time = 30 ms; repetition time = 2s; 128 averages) (Figure 1(b)). Spectra were analyzed using SAGE_IDL (SAGE: Dev 2003.1 and IDL: Version 5.6) and LCModel (McLean et al., 2000, Provencher, 2001). The basis set used for this study included Alanine, Aspartate. Creatine, GABA, Guanidoacetate, Glucose, Glutamate, Glutamine, Glycerophosphocholine, Phosphocholine, Inositol, Lactate, N-acetyl Aspartate, N-acetyl aspartyl glutamate, scyllo-Inositol and Taurine.
Figure 1.
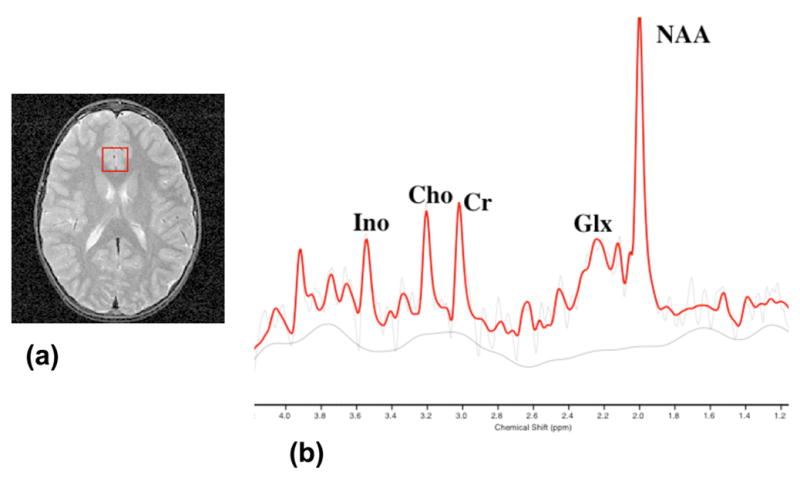
(a) A 2 cm x 2 cm x 1.2 cm (superior inferior) voxel in the anterior cingulate cortex of a child with BPD; and (b) a proton spectrum (TE=30 ms; TR = 2s) from that voxel with an LCModel fit.
NAA: N-acetyl aspartate.
Glx: Glutamate/Glutamine
Cho: Choline containing compounds.
Ino: Inositol containing compounds.
For this study we are unable to give absolute values because we have not measured T2 values in any of the subjects (in part, due to the time constraints of scanning children); or corrected for coil environment differences. Metabolite resonance areas were thus calculated as a ratio to the Cr resonance area and used for the statistical analysis. This is a convention, which corrects for partial volume effects and for imperfect excitation of the volume of interest. Metabolite ratios also help control for slight differences in scanner accuracy over time. The in vivo proton detectable Cr peak consists of creatine plus phosphocreatine, the sum of whose concentrations remains somewhat constant under different metabolic conditions (Huppi et al., 1995).
Image Segmentation
The SPGR coronal images were segmented into gray matter, white matter and CSF using a semi-automated software package, MRX (GE/BWH/MIT v3.0) (Kikinis et al., 1992). The segmented images were edited further by applying the voxel dimensions used for MRSI; and thus the gray matter, white matter and CSF content of each voxel was calculated (Renshaw et al., 1997)
Statistical Analysis
Statistical analysis was performed using SPSS 11.0 for Macintosh OSX. Linear regression, MANOVA and Pearson correlations were used. Results were considered significant for p < 05.
Results
Eighteen subjects with a DSM IV TR diagnosis of Bipolar 1 Disorder were recruited: ten (11.1 ± 1.0 years; 5 female) were not medicated with any antipsychotic and eight (10.9 ± 1.1 years; 1 female) were medicated with the atypical antipsychotic risperidone (see Table 1). The subjects with BPD, not treated with risperidone, were currently displaying manic or mixed symptoms (with or without psychotic features). All of these subjects had YMRS scores greater than 15. Of the BPD subjects treated with risperidone all had commenced risperidone treatment when presenting with manic or mixed symptoms (with or without psychotic features), and a YMRS score greater than 15. The mean dose of risperidone was 2.09 ± 1.25 mg/day and the mean duration of treatment was 101.14 ± 138.59 weeks (see Table 2). Three of the subjects treated with risperidone were responders and five were in remission.
Table 1.
Sex, age, CGI-Mania and YMRS for subjects treated without (No) and with (Yes) risperidone. Risperidone dose (mg/day), duration of risperidone treatment and risperidone response are included for the subjects treated with risperidone. Response: a 30 % decrease in YMRS. Remission: YMRS ≤ 10 and CGI: 1 or 2
| Subject | Risperidone | Sex | Age (Years) | CGI_Mania | YMRS | |||
|---|---|---|---|---|---|---|---|---|
| 1 | No | Male | 9 | 6 | 29 | |||
| 2 | No | Male | 12 | 5 | 25 | |||
| 3 | No | Male | 7 | 5 | 35 | |||
| 4 | No | Female | 12 | 5 | 39 | |||
| 5 | No | Male | 6 | 5 | 31 | |||
| 6 | No | Male | 9 | 5 | 31 | |||
| 7 | No | Female | 10 | na | 41 | |||
| 8 | No | Female | 15 | 5 | 36 | |||
| 9 | No | Female | 15 | na | 33 | |||
| 10 | No | Female | 16 | 3 | 23 | Dose (mg/day) | Duration (Weeks) | Response |
| 1 | Yes | Male | 9 | 2 | 14 | 1 | 8 | Responder |
| 5 | Yes | Male | 6 | 3 | 16 | 2 | 8 | Responder |
| 6 | Yes | Male | 9 | 3 | 11 | 1 | 8 | Responder |
| 11 | Yes | Female | 10 | 1 | 3 | 2 | 104 | Remitter |
| 12 | Yes | Male | 14 | 1 | 0 | 5 | 364 | Remitter |
| 13 | Yes | Male | 11 | 1 | 5 | 2 | 8 | Remitter |
| 14 | Yes | Male | 13 | 1 | 4 | 2 | 208 | Remitter |
| 15 | Yes | Male | 15 | 3 | 5 | 2 | 50 | Remitter |
Table 2(a).
Mean ± standard deviation for the metabolite ratios, YMRS, and CGI-Mania in a group of subjects treated, without (no) and with (yes) risperidone. Also included is the mean ± standard deviation dose and duration of risperidone treatment, for those treated with risperidone.
| Risperidone | NAA/Cr | Cho/Cr | Ins/Cr | Glx/Cr* | YMRS** | CGI-Mania*** | Dose (mg/day) | Duration (Weeks) |
|---|---|---|---|---|---|---|---|---|
| No (10) | 0.98 ± 0.09 | 0.24 ± 0.03 | 0.62 ± 0.10 | 1.37 ± 0.30 | 32.3 ± 5.7 | 4.88 ± 0.84 | na | na |
| Yes (8) | 1.05 ± 0.14 | 0.25 ± 0.05 | 0.64 ± 0.11 | 1.75 ± 0.27 | 7.3 ± 5.7 | 1.88 ± 0.99 | 2.09 ± 1.25 | 101.14 ± 138.59 |
p < 0.05;
p < 0.000;
p < 0.000.
Three of the subjects were scanned before and 8 weeks after treatment with risperidone. All of the subjects had a co-morbid diagnosis of ADHD. Additional co-morbid diagnoses include (not treated with risperidone, treated with risperidone): Alcohol Abuse (n = 2, n = 0), Substance Abuse (n = 3, n = 0), OCD (n = 2, n = 0), generalized anxiety disorder (n = 3, n = 3), post traumatic stress disorder (n = 1, n = 2), Oppositional Defiant Disorder (n = 9. n = 5), Conduct Disorder (n = 5, n = 2), Autism (n = 0, n = 1) and Pervasive Developmental Disorder (n = 1, n = 0).
Eight youths were medicated at the time of the study with medications other than risperidone. Five subjects who were not receiving risperidone treatment were medicated with: clonidine (n = 2), a selective serotonin reuptake inhibitor (n = 1), atomoxetine (n = 1) and a stimulant (n = 1). Three subjects who were receiving medication with risperidone were additionally treated with a selective serotonin reuptake inhibitor (n = 1), bupropion (n=1), atomoxetine (n = 1) and a stimulant (n = 1).
All the spectra in this study were fit using LCModel. LCModel gives a time domain fit and a standard deviation (SD%) based on the Cramer-Rao lower bound. The SD% is a measure of the reliability of the fit. A standard deviation of 100 % means the metabolite would need to double in order for a change to be seen. All included spectra in this study had SD % less than 12 % for NAA, Cr, Cho and a SD % less than 16 % for Ins and Glx.
Table 2 shows the means and standard deviations for all of the measured metabolite ratios (NAA/Cr, Cho/Cr, Ins/Cr and Glx/Cr) and the YMRS and CGI-Mania scales for those subjects treated without and with risperidone.
Multivariate linear regression analysis with Glx/Cr as the dependant variable and age, sex and risperidone status as independent variables showed that risperidone had a significant positive effect on Glx/Cr (df = (3, 17), B = 0.33, t = 2.19, p < 0.05). Significant results were not seen for any of the other metabolite ratios.
Both the YMRS and CGI-Mania scores were significantly different (MANOVA) between the children not receiving risperidone treatment and those receiving risperidone treatment: df = (1, 17), F = 85.22, p < 0.000 and df= (1, 15), F = 42.89, p < 0.000, respectively. The YMRS score and CGI-Mania scores correlated negatively with Glx/Cr (r = −0.59. p < 0.01, and r = −0.55, p < 0.03; respectively).
Tissue segmentation data was acquired from 14 subjects (9 not treated with risperidone, and 5 treated with risperidone). One of the subjects not treated with risperidone and three of the subjects treated with risperidone did not have the SPGR volume scan data necessary to segment the data. There were no significant differences between grey matter, white matter and CSF contributions to the spectroscopic voxels for the two groups (Table 1).
Discussion
Children with BPD exhibiting manic symptoms requiring treatment had lower Glx/Cr than children with BPD being stably treated with the atypical antipsychotic risperidone. The children treated with risperidone also had significantly lower YMRS and CGI-Mania Severity scores than the children not treated with risperidone.
The Glx peak in proton MRS is composed primarily of glutamate (Glu) and glutamine (Gln): Glu is of the order of 8–13 μmole/g (Cooper et al., 2003); and the Glu to Gln ratio in vivo had been shown to range from ~ 2.4 to 3.8 (Gruetter et al., 2003). Glial cell abnormalities have been indicated in the frontal cortex in major psychiatric illnesses including BPD and MDD (Rajkowska et al., 2001). Glia provide a pathway for neuronal glutamate synthesis (Cooper et al., 2003, Sanacora et al., 2003, Lebon et al., 2002). Reduced glial function could account for reduced glutamate or glutamine synthesis. Alterations in N-methyl-D-aspartate (NMDA) receptor glutamatergic inputs have been demonstrated in the ACC in both schizophrenia and bipolar disorder (Woo et al., 2004). A number of studies suggest that glutamatergic mechanisms may be involved in the efficacy of atypical antipsychotics (Goff and Coyle, 2001, Heresco-Levy, 2003); in particular through the action of atypical antipsychotics on the NMDA glutamate receptors. Thus the increased Glx/Cr measured in this study, in subjects treated with risperidone, is perhaps due to increased glutamate synthesis secondary to risperidone treatment.
The children treated with risperidone, who had greater Glx/Cr levels, had significantly lower scores on the YMRS and CGI-Mania scales, that is, they were less manic. In deed, both the YMRS and CGI-Mania scales correlated negatively with Glx/Cr. Functional Magnetic Resonance Imaging (fMRI) studies have demonstrated abnormalities in corticolimbic regions in BPD (Blumberg et al., 2003, Chang et al., 2004, Gruber et al., 2004, Altshuler et al., 2005, Blumberg et al., 2005, Strakowski et al., 2005); in particular decreased activation, compared with healthy comparison subjects, on tasks involving the ACC (Gruber et al., 2004, Blumberg et al., 2005). The manic state has been shown to be associated with decreased ACC (Altshuler et al., 2005) (and prefrontal cortex (Blumberg et al., 2003)) activation. Blumberg et al also observed decreased activation in the ACC of unmedicated subjects with BPD compared with medicated subjects with BPD; the medicated subjects having an increased activation similar to healthy comparison subjects (Blumberg et al., 2005). In this current study those subjects who were more manic (those not treated with risperidone) had reduced ACC Glx/Cr levels than the less manic subjects (those treated with risperidone). Hypoglutamagergic ACC metabolism may be associated with manic symptoms, which are alleviated by risperidone treatment.
Seven youths in this study were medicated with medications other than risperidone; including two subjects treated with atomoxetine and two subjects treated with a stimulant. Atomoxetine and stimulants may work by altering glutamatergic function (Carrey et al., 2002). Also, abnormal glutamate function in terminal areas of dopaminergic neurons have been hypothesized to contribute to the development of ADHD (Russell, 2003); and altered Glx and Glx/Cr has been measure in subjects with ADHD (Courvoisie et al., 2004, MacMaster et al., 2003). However, in this study an equal number of subjects treated with and without risperidone were treated with atomoxetine/and or a stimulant; and all of the subjects with BPD had co-morbid ADHD.
A limitation of this study is that only three of the subjects studied had measurements before and after risperidone treatment. However, of those three subjects two exhibited increased Glx/Cr (33 % and 36 %) and one showed only a small decrease (2.5 %) in Glx/Cr following risperidone treatment. All three subjects showed clinical improvement (YMRS decreases of 45 %, 64 % and 51% respectively). This study also had a number of other limitations, in particular, a small sample size, different gender composition of the two groups, the use of Glx rather than Glu, the use of Cr ratios rather than absolute concentrations, the presence of concurrent medications, and the absence of a healthy control comparison group.
In conclusion, children with mania have lower Glx/Cr levels than children with BPD being stably treated with the atypical antipsychotic risperidone. Mania may be associated with reduced glutamatergic function in the ACC; and those reductions in glutamate may be increased following successful treatment with glutamatergic agents.
Table 2(b).
Mean ± standard deviation for mean voxel tissue in a group of subjects treated without (no) and with (yes) risperidone.
| Grey % | White % | CSF % | ||
|---|---|---|---|---|
| Risperidone | No (9) | 74.31 ± 6.23 | 14.58 ± 5.37 | 7.71 ± 3.86 |
| Yes (5) | 78.16 ± 8.36 | 14.38 ± 7.33 | 6.44 ± 3.10 |
Acknowledgments
The authors would like to acknowledge the following for support: National Institute of Mental Health (MH01798) and Johnson and Johnson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALTSHULER L, BOOKHEIMER S, PROENZA MA, TOWNSEND J, SABB F, FIRESTINE A, BARTZOKIS G, MINTZ J, MAZZIOTTA J, COHEN MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. American Journal of Psychiatry. 2005;162:1211–3. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- BIEDERMAN J, MICK E, WOZNIAK J, ALEARDI M, SPENCER T, FARAONE SV. An open-label trial of risperidone in children and adolescents with bipolar disorder. Journal of Child & Adolescent Psychopharmacology. 2005;15:311–7. doi: 10.1089/cap.2005.15.311. [DOI] [PubMed] [Google Scholar]
- BLUMBERG HP, DONEGAN NH, SANISLOW CA, COLLINS S, LACADIE C, SKUDLARSKI P, GUEORGUIEVA R, FULBRIGHT RK, MCGLASHAN TH, GORE JC, KRYSTAL JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–13. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- BLUMBERG HP, LEUNG HC, SKUDLARSKI P, LACADIE CM, FREDERICKS CA, HARRIS BC, CHARNEY DS, GORE JC, KRYSTAL JH, PETERSON BS. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Archives of General Psychiatry. 2003;60:601–9. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- CARREY N, MACMASTER FP, SPARKES SJ, KHAN SC, KUSUMAKAR V. Glutamatergic changes with treatment in attention deficit hyperactivity disorder: a preliminary case series. J Child Adolesc Psychopharmacol. 2002;12:331–6. doi: 10.1089/104454602762599871. [DOI] [PubMed] [Google Scholar]
- CHANG K, ADLEMAN NE, DIENES K, SIMEONOVA DI, MENON V, REISS A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–92. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- CHANG KD, KETTER TA. Mood stabilizer augmentation with olanzapine in acutely manic children. Journal of Child & Adolescent Psychopharmacology. 2000;10:45–9. doi: 10.1089/cap.2000.10.45. [DOI] [PubMed] [Google Scholar]
- COOPER J, BLOOM F, ROTH R. The Biochemocal Basis of Neuropharmacology. 8. New York: Oxford University Press; 2003. Amino Acid Transmitters. [Google Scholar]
- COURVOISIE H, HOOPER SR, FINE C, KWOCK L, CASTILLO M. Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: preliminary findings. J Neuropsychiatry Clin Neurosci. 2004;16:63–9. doi: 10.1176/jnp.16.1.63. [DOI] [PubMed] [Google Scholar]
- DELBELLO MP, SCHWIERS ML, ROSENBERG HL, STRAKOWSKI SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41:1216–23. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- EVINS AE, AMICO ET, SHIH V, GOFF DC. Clozapine treatment increases serum glutamate and aspartate compared to conventional neuroleptics. Journal of Neural Transmission. 1997;104:761–6. doi: 10.1007/BF01291892. [DOI] [PubMed] [Google Scholar]
- GELLER B, FOX LW, CLARK KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry. 1994;33:461–8. doi: 10.1097/00004583-199405000-00003. [DOI] [PubMed] [Google Scholar]
- GOFF DC, COYLE JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. American Journal of Psychiatry. 2001;158:1367–77. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- GOFF DC, HENNEN J, LYOO IK, TSAI G, WALD LL, EVINS AE, YURGELUN-TODD DA, RENSHAW PF. Modulation of brain and serum glutamatergic concentrations following a switch from conventional neuroleptics to olanzapine. Biological Psychiatry. 2002;51:493–7. doi: 10.1016/s0006-3223(01)01321-x. [DOI] [PubMed] [Google Scholar]
- GRUBER SA, ROGOWSKA J, YURGELUN-TODD DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. Journal of Affective Disorders. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- GRUETTER R, ADRIANY G, CHOI IY, HENRY PG, LEI H, OZ G. Localized in vivo 13C NMR spectroscopy of the brain. NMR in Biomedicine. 2003;16:313–38. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERESCO-LEVY U. Glutamatergic neurotransmission modulation and the mechanisms of antipsychotic atypicality. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1113–23. doi: 10.1016/j.pnpbp.2003.09.007. [DOI] [PubMed] [Google Scholar]
- HUPPI PS, FUSCH C, BOESCH C, BURRI R, BOSSI E, AMATO M, HERSCHKOWITZ N. Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatric Research. 1995;37:145–50. doi: 10.1203/00006450-199502000-00003. [DOI] [PubMed] [Google Scholar]
- KIKINIS R, SHENTON ME, GERIG G, MARTIN J, ANDERSON M, METCALF D, GUTTMANN CR, MCCARLEY RW, LORENSEN W, CLINE H, et al. Routine quantitative analysis of brain and cerebrospinal fluid spaces with MR imaging. Journal of Magnetic Resonance Imaging. 1992;2:619–29. doi: 10.1002/jmri.1880020603. [DOI] [PubMed] [Google Scholar]
- LEBON V, PETERSEN KF, CLINE GW, SHEN J, MASON GF, DUFOUR S, BEHAR KL, SHULMAN GI, ROTHMAN DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–31. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWINSOHN PM, KLEIN DN, SEELEY JR. Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–63. [PubMed] [Google Scholar]
- MACMASTER FP, CARREY N, SPARKES S, KUSUMAKAR V. Proton spectroscopy in medication-free pediatric attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;53:184–7. doi: 10.1016/s0006-3223(02)01401-4. [DOI] [PubMed] [Google Scholar]
- MCLEAN MA, WOERMANN FG, BARKER GJ, DUNCAN JS. Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magnetic Resonance in Medicine. 2000;44:401–11. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- NOVOTNY EJ, JR, FULBRIGHT RK, PEARL PL, GIBSON KM, ROTHMAN DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54(Suppl 6):S25–31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- ONGUR D, DREVETS WC, PRICE JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROVENCHER SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- RAJKOWSKA G, HALARIS A, SELEMON LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder.[see comment] Biological Psychiatry. 2001;49:741–52. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- RENSHAW PF, LAFER B, BABB SM, FAVA M, STOLL AL, CHRISTENSEN JD, MOORE CM, YURGELUN-TODD DA, BONELLO CM, PILLAY SS, ROTHSCHILD AJ, NIERENBERG AA, ROSENBAUM JF, COHEN BM. Basal ganglia choline levels in depression and response to fluoxetine treatment: an in vivo proton magnetic resonance spectroscopy study. Biological Psychiatry. 1997;41:837–43. doi: 10.1016/S0006-3223(96)00256-9. [DOI] [PubMed] [Google Scholar]
- ROSENBERG DR, MACMASTER FP, MIRZA Y, SMITH JM, EASTER PC, BANERJEE SP, BHANDARI R, BOYD C, LYNCH M, ROSE M, IV, EY J, VILLAFUERTE RA, MOORE GJ, RENSHAW P. Reduced anterior cingulate glutamate in pediatric major depression: a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–4. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- RUSSELL VA. Dopamine hypofunction possibly results from a defect in glutamate-stimulated release of dopamine in the nucleus accumbens shell of a rat model for attention deficit hyperactivity disorder--the spontaneously hypertensive rat. Neuroscience & Biobehavioral Reviews. 2003;27:671–82. doi: 10.1016/j.neubiorev.2003.08.010. [DOI] [PubMed] [Google Scholar]
- SANACORA G, ROTHMAN DL, MASON G, KRYSTAL JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Annals of the New York Academy of Sciences. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- STRAKOWSKI SM, ADLER CM, HOLLAND SK, MILLS NP, DELBELLO MP, ELIASSEN JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. American Journal of Psychiatry. 2005;162:1697–705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- WOO TU, WALSH JP, BENES FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]


