Abstract
Infantile hemangiomas are endothelial tumors that grow rapidly in the first year of life and regress slowly during early childhood. Although hemangiomas are well-known vascular lesions, little is known about the mechanisms that cause the excessive endothelial cell proliferation in these most common tumors of infancy. To investigate the molecular basis of hemangioma, we isolated endothelial cells from several proliferative-phase lesions and showed that these cells are clonal and exhibit abnormal properties in vitro (E. Boye, Y. Yu, G. Paranya, J. B. Mulliken, B. R. Olsen, J. Bischoff: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 2001, 107:745–752). Here, we analyzed mRNA expression patterns of genes required for angiogenesis, including members of the vascular endothelial growth factor (VEGF)/VEGF receptor family and the angiopoietin/Tie family, in hemangioma-derived and normal endothelial cells. KDR, Flt-1, Tie1, Tie2, and angiopoietin-2 (Ang2) were strongly expressed in cultured hemangioma-derived endothelial cells and in hemangioma tissue. In contrast, there was little expression of angiopoietin-1 (Ang1) or VEGF. We found Tie2 mRNA and protein up-regulated with a concomitant increase in cellular responsiveness to Ang1 in most hemangioma-derived endothelial cells. Ang2 mRNA was down-regulated in response to serum in hemangioma-derived endothelial cells, but not in normal endothelial cells, suggesting altered regulation. These findings implicate Tie2 and its ligands Ang1 and Ang2 in the pathogenesis of hemangioma.
Infantile hemangiomas occur in 5 to 10% of Caucasian infants by 1 year of age. 1 Usually, these cutaneous endothelial tumors are small and pose no threat or complications. However, a subset grows dramatically so as to destroy tissue, impair function, or threaten life. Hemangioma typically appears soon after birth, grows rapidly during the first year of life (proliferating phase), slowly regresses from 1 to 7 years (involuting phase), and thereafter becomes a fibrous residuum (involuted phase). 2 In ∼20% of cases, hemangiomas are multiple. The relationship of these multifocal lesions to each other and how they might differ from the more common single lesion remains unknown. Children with endangering or life-threatening hemangiomas are treated with corticosteroid or interferon-α. 3,4 However, not all hemangiomas respond to these drugs and neurological toxicity has been associated with interferon-α administration. 5,6 The recent demonstration of ectopic expression of type 3 iodothyronine deiodinase in hemangioma, which in at least one child caused hypothyroidism, 7 underscores the need for novel therapy.
Histological studies of hemangiomas provide important insights into possible mechanisms for the aberrant localized angiogenesis. Analysis of proliferating, involuting, and involuted phase specimens reveals dramatic changes in cell and tissue morphology that occur throughout the natural life-span of hemangioma. Proliferating hemangiomas are composed of densely packed cells with little connective tissue and barely discernible vessel lumen. 8 Smoller and Apfelberg 9 suggested these regions contain a primitive cell type capable of giving rise to endothelial cells and pericytes. Transformation to more organized and mature vascular channels occurs during the involuting phase. The involuted phase is characterized by a few remaining thin-walled vessels, which resemble normal capillaries, surrounded by regions of fibrofatty tissue. 8 Clearly, understanding the cellular and biochemical mechanisms that control the growth and regression of blood vessels in hemangioma would provide critical insights into this particular tumor and angiogenesis in general.
Much has been learned by studying the expression of angiogenic regulators during the proliferating, involuting, and involuted phases of hemangioma. Two potent stimulators of angiogenesis, basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are up-regulated in proliferative phase hemangioma, 10,11 whereas tissue inhibitor of metalloproteinase-1, an angiogenesis inhibitor, is increased in involutive phase specimens. 10 Monocyte chemoattractant protein-1, a chemokine shown to stimulate angiogenesis, 12 is up-regulated in proliferating compared to involuting hemangiomas. 13 The cell adhesion molecules VE-cadherin, E-selectin, and ICAM-3 have also been shown to be expressed in hemangioma with the latter two, E-selectin and ICAM-3, increased in proliferating endothelial cells. 14-16 Extracellular matrix proteins implicated in blood vessel development, such as type IV collagen, laminin, and fibronectin are also present. 17,18 Overall, the consensus of these studies is that proteins that promote angiogenesis or reflect the angiogenic/proliferative phenotype are up-regulated in proliferating hemangioma.
Many theories have been proposed for the pathogenesis of hemangioma, including the idea that a defect in the local environment may drive endothelial cell proliferation. 19 Our recent study strongly indicates that hemangioma constitute clonal expansion of endothelial cells in which somatic mutations have occurred. 20 However, the cause(s) of involution is still a mystery; the endothelial defect seems to be overridden as the child grows, perhaps by angiogenic inhibitors produced locally or delivered systemically by the environment.
As a first step in unraveling molecular alterations in hemangioma, we examined mRNA expression patterns of the VEGF-receptors and the Tie receptors as well as the cognate ligands. These receptors were chosen because of the abundance of experimental data demonstrating their fundamental roles in physiological and pathological angiogenesis. 21 We found that, with the exception of Tie2, receptors were expressed at normal levels with no obvious changes in transcript size. Coincident with elevated Tie2, increased cellular responsiveness to angiopoietin-1 (Ang1) and altered regulation of angiopoietin-2 (Ang2) were evident in hemangioma-derived endothelial cells (HemECs) compared to normal human microvascular endothelial cells. These changes may contribute to the aberrant angiogenesis in hemangioma, and suggest a role for angiopoietin/Tie pathway in the pathogenesis of hemangioma.
Materials and Methods
Tissue Procurement
HemECs were isolated from ten surgically resected cutaneous proliferating hemangiomas obtained in accordance with a protocol approved by the Committee on Clinical Investigation, Children’s Hospital, Boston, MA. The patient’s ages at the time of resection, location of the lesion(s), and relevant clinical information has been previously described. 20 The numbers of HemECs reflect independent isolates. Normal human female skin endothelial cells (HFSECs) were acquired from age-matched infants under the same protocol. Human dermal microvascular endothelial cells (HDMECs) were isolated from discarded neonatal foreskins obtained in accordance with the Institutional Review Board at the Brigham and Women’s Hospital, Boston, MA.
Cell Culture
HemECs, HFSECs, and HDMECs were isolated as described, 20,22 and grown on 1% gelatin-coated dishes in endothelial cell basal medium (Clonetics, San Diego, CA), 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), 1×glutamine-penicillin-streptomycin (GIBCO-BRL, Rockville, MD), and 2 ng/ml bFGF (Scios Nova Inc., Mountain View, CA) in 5% CO2 at 37°C. Cells were passaged 1:3 every 4 to 6 days and used between passage 5 to 10.
cDNA Probe Synthesis
Ang2 probe was generated by digestion of Ang2 cDNA construct pKS+/hTL2 23 with BlpI and HindIII, or by reverse transcriptase-polymerase chain reaction (RT-PCR) using 5′-AGCTG TGATCTTGTCTTGGC-3′ (forward primer) and 5′-GTTCAAGTCTCGTGGTCTGA-3′ (reverse primer) 24 from RNA of HDMECs. Ang1 probe was cut from cDNA construct pKS/hTL1 23 with NotI and EcoRI. Flt1 and KDR probes were inserts of human cDNA (GenBank accession nos. AF063657 and AF063658) corresponding to a BamHI-BglII fragment of 1.36 kb, and a EcoRI-BsmI fragment of 1 kb, respectively. XhoI-XbaI, EcoRI-HindIII, and KpnI-SacI cDNA fragments were used to probe Tie2, VEGF, and VE-cadherin transcripts, respectively. Tie1 probe was amplified by RT-PCR using 5′-CTTCCAGACAAGGTCACACACAC-3′ (forward primer), and 5′-GTCACAA GTGCCACCATTCTGAC-3′ (reverse primer) from HDMECs RNA. bFGF probe was PCR amplified using primers 5′-TCTAGGTAAGCTTCACTGG-3′ and 5′-AGTGTGTG CTAACCGTTAC-3′ from bFGF cDNA.
Northern Blot Analysis
Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA), and Northern blot analyses were performed as described. 25 Briefly, total RNA (10 μg) was electrophoresed through 1.2% formaldehyde-agarose gel and then transferred to nylon membrane (Midwest Scientific, St. Louis, MO). Radioactive cDNA probes were generated by using RediPrime DNA labeling kit (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) and [α-32P] dCTP (Dupont-NEN, Boston, MA), and then purified with Quick spin columns (Boehringer Mannheim, Indianapolis, IN). After UV cross-linking, membrane was prehybridized, hybridized at 65°C with indicated cDNA probes in hybridization solution containing 0.5 mol/L Na2HPO4/.7 H2O, pH 7.2, 7% sodium dodecyl sulfate, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 8.0, and 1% bovine serum albumin (BSA), and washed at 65°C in buffer containing 40 mmol/L Na2HPO4/.7 H2O, pH 7.2, 1 mmol/L EDTA, and 1% sodium dodecyl sulfate. Membrane was autoradiographed using Hyperfilm MP (Amersham Pharmacia Biotech). Signals were quantified using Scion Image Software (www.scioncorp.com).
Western Blot Analysis
Cells were lysed in buffer containing 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 2 mmol/L EDTA, 2 mmol/L EGTA, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin, and 1 mmol/L phenylmethyl sulfonyl fluoride. Proteins were separated on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA), and analyzed by immunoblotting with anti-Tie-2 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1 μg/ml or anti-actin polyclonal antibodies (Sigma, St. Louis, MO) at 1:100 dilution. Antigen-antibody complexes were visualized by using enhanced chemiluminescence Western-blotting detection reagent (Amersham Pharmacia Biotech).
Migration Assay
Cell migration assays were performed using a modified Boyden chamber (Neuroprobe, Gaithersburg, MD). Briefly, polycarbonate polyvinylpyrrolidone-free membranes with a pore size of 8 μm (Neuroprobe) were coated overnight with 100 μg/ml of collagen type I (Collaborative Biomedical Products, Bedford, MA) in 0.2 N acetic acid and air-dried. Test substances diluted in EBM and 0.1% BSA were placed in the lower chamber. Cells collected from confluent cultures were resuspended in EBM and 0.1% BSA, and seeded in the upper chamber at 10,000 cells/well. The apparatus was incubated for 4 hours at 37°C with 5% CO2 to allow cells to migrate. After incubation, the membranes were fixed in formalin (Fisher, Pittsburgh, PA) and stained with Hematoxylin Gill No.3 (Polysciences, Inc., Warrington, PA). Nonmigrated cells were scraped off. Each condition was performed in quadruplicate. Migration was quantified by counting cells in five random high-power fields, each of which corresponded to 1.25 mm2. Therefore, the migration data represents the mean ± SD of 20 high-power fields from four different wells.
Endothelial Cell Survival Assay
The assay was performed as previously described. 26 Briefly, HemECs or HDMECs were plated at 1.2 × 10 5 cells/well in 24-well plates in EBM containing 10% FBS. After 24 hours, cells were washed in phosphate-buffered saline (PBS) and incubated in EBM containing 10% FBS without or with 2 ng/ml bFGF, 10 ng/ml VEGF165 (R&D Systems, Indianapolis, IN), 100 ng/ml Ang1* or Ang2 (Regeneron Pharmaceuticals, Tarrytown, NY) at the indicated concentrations. After 5 days, viable cells were stained with Trypan Blue (Life Technologies, Inc., Grand Island, NY) and counted using a hemocytometer.
Regulation of Ang2 mRNA
Cells at ∼80% confluency were serum-starved for 24 hours in EBM containing 0.1% BSA, then treated for 8 hours with 5% FBS, 10 ng/ml of VEGF, 10 ng/ml of bFGF, 20 ng/ml of epidermal growth factor (R&D systems), 100 ng/ml of platelet-derived growth factor (PDGF) (R&D systems), 10 ng/ml of transforming growth factor-β1 (R&D systems), 50 ng/ml of tumor necrosis factor-α (Sigma), 100 nmol/L of phorbol 12-myristate 13-acetate (PMA) (Sigma), 1 μg/ml of endostatin (EntreMed, Rockville, MD), and 100 ng/ml of TNP-470 (TAP Holdings Inc., Deerfield, IL). For dose-response experiments, serum-starved cells were treated with 0%, 2%, 5%, or 10% FBS for 8 hours. For time course experiment, serum-starved cells were incubated with 5% FBS for 0, 2, 8, or 24 hours. At the end of incubation, RNA was isolated from the treated cells and analyzed by Northern blotting.
In Situ Hybridization
Details of in situ hybridization have been published previously. 27 Briefly, slides were passed through xylene, graded alcohols, 0.2 mol/L HCl, Tris/EDTA with 3 μg/ml proteinase K, 0.2% glycine, 4% paraformaldehyde in phosphate-buffered saline, pH 7.4, 0.1 mol/L triethanolamine containing 1/200 (v/v) acetic anhydride, and 2× standard saline citrate (SSC). Slides were hybridized overnight at 50°C with 35S-labeled anti-sense riboprobes in the following mixture: 0.3 mol/L NaCl, 0.01 mol/L Tris, pH 7.6, 5 mmol/L EDTA, 50% formamide, 10% dextran sulfate, 0.1 mg/ml yeast tRNA, and 10 mmol/L dithiothreitol. VEGF sense probe was used as a negative control for hybridization. Posthybridization washes included 2×SSC/50% formamide/10 mmol/L dithiothreitol at 50°C; 4×SSC/10 mmol/L Tris/1 mmol/L EDTA with 20 μg/ml ribonuclease A at 37°C; and 2×SSC/50% formamide/10 mmol/L dithiothreitol at 65°C and 2×SSC. Slides were then dehydrated through graded alcohols containing 0.3 mol/L of ammonium acetate, dried, coated with Kodak NTB 2 emulsion (Eastman-Kodak, Rochester, NY) and stored in the dark at 4°C for 2 weeks. The emulsion was developed with Kodak D19 developer and the slides were counterstained with hematoxylin. Probes for VEGF, Flt1, KDR, Ang1, Ang2, Tie1, and Tie2 have been described previously. 28-30
Immunohistochemistry
OCT-embedded tissues were sectioned at 5 to 6 μm thickness on Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA). After being fixed in cold acetone for 10 minutes, sections were washed in PBS and treated with 1% H2O2 for 10 minutes to block endogenous peroxidases. Immunochemical staining was performed using R. T. U. Vectastain kit (Vector Laboratories, Burlingame, CA), according to the manufacturer’s instructions, and AEC chromagen (DAKO, Carpinteria, CA). Anti-Tie2 monoclonal antibody HTEK (Regeneron Pharmaceuticals, Inc.) was used at 1:600. Mouse IgG control at the same concentration was used as a negative control.
Results
Increased Expression of Tie2 in HemECs
Because infantile hemangiomas are composed of tortuous and disorganized microvessels, 10 we first examined mRNA expression levels of a key regulator of vessel growth and remodeling, Tie2, in five HemEC cultures isolated from different proliferative phase hemangioma lesions. The ages of the children at time of resection ranged from 3.5 to 24 months. 20 Levels were compared to normal human microvascular endothelial cells isolated from infant skin (HFSECs and HDMECs) and human skin fibroblasts as a negative control. Tie-2 mRNA was detected in all endothelial cells but was present at higher levels in four of five HemECs (Figure 1A ▶ , top). HemEC-12, isolated from a lesion from a 4-month-old child, expressed Tie2 at levels similar to those detected in normal human microvascular endothelial cells. Consistently, Tie2 protein levels were up-regulated in the same HemECs (Figure 1A ▶ , bottom). Similar results were obtained when more than three different preparations of HFSECs and HDMECs were compared in separate experiments (data not shown).
Figure 1.
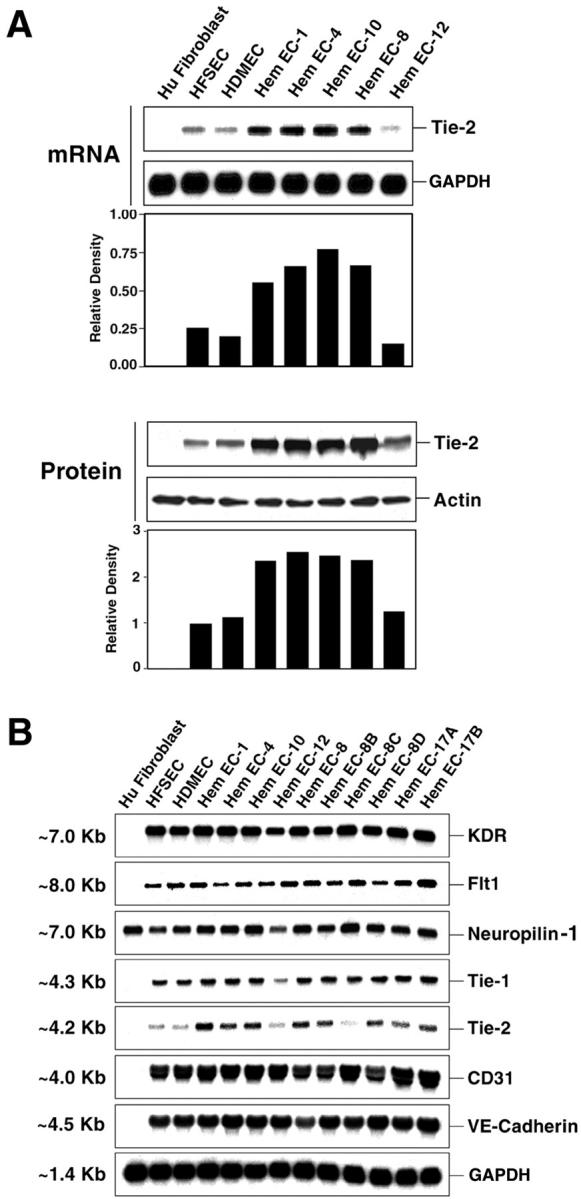
Tie-2 mRNA and protein are increased in HemECs. A: Total RNA and total cellular protein lysates were prepared from subconfluent human fibroblasts, normal human ECs (HFSECs and HDMECs), and HemECs cultured under the same conditions. Numbers of HemECs reflect independent primary cultures isolated from individual patient specimens. RNA samples were analyzed by Northern blotting with 32P-labeled cDNA probes for human Tie-2 and GAPDH as RNA-loading controls. Protein lysates were analyzed by Western blotting with polyclonal anti-human Tie-2 antibodies. Equivalent protein loading was demonstrated by probing the same blot with polyclonal anti-actin antibodies. Signals were quantified using Scion Image software program. Relative density, which was normalized to GAPDH for RNA and actin for protein, is shown. B: Total RNA from cultured HFSECs, HDMECs, or HemECs was analyzed by Northern blotting with 32P-labeled cDNA probes for human KDR, Flt-1, neuropilin-1, Tie-1, Tie-2, CD31/PECAM-1, VE-cadherin, and GAPDH. Size of transcript is presented in kb.
The RNA analysis was expanded to include five more HemECs and to examine other angiogenic factors including Tie1, Tie2, and VEGF-receptor family members KDR, Flt-1, and neuropilin-1. In addition, levels of VE-cadherin and CD31/PECAM-1, two endothelial adhesion molecules, were examined. In contrast to increased Tie2 mRNA in 8 of 10 HemECs, levels of Tie1, KDR, Flt-1, neuropilin-1, VE-cadherin, and CD31/PECAM-1 were expressed at remarkably consistent levels in all HemECs and normal human endothelial cells (ECs) (Figure 1B) ▶ . As expected, only neuropilin-1 was detected in human skin fibroblasts. HemEC-8, -8B, -8C, and -8D were isolated from distinct lesions from a single patient. Similarly, HemEC17A and HemEC17B represent cells isolated from distinct lesions from a single patient. Two lesions, HemEC-12 and HemEC-8C, expressed normal levels of Tie-2 (Figure 1, A and B) ▶ . Importantly, endothelial cells from hemangioma tissue and from normal human skin were isolated and cultured using identical techniques and conditions. 20,22 Thus, variations in Tie2 mRNA and protein levels cannot be attributed to differences in culture conditions. This is supported by the remarkably consistent levels of Tie1, KDR, Flt-1, neuropilin-1, VE-cadherin, and CD31 (Figure 1B) ▶ . The specific increase in Tie2 in HemECs over several other angiogenic molecules suggests that regulation of Tie2 expression is altered and its increase may contribute to the abnormal angiogenesis that underlies hemangioma.
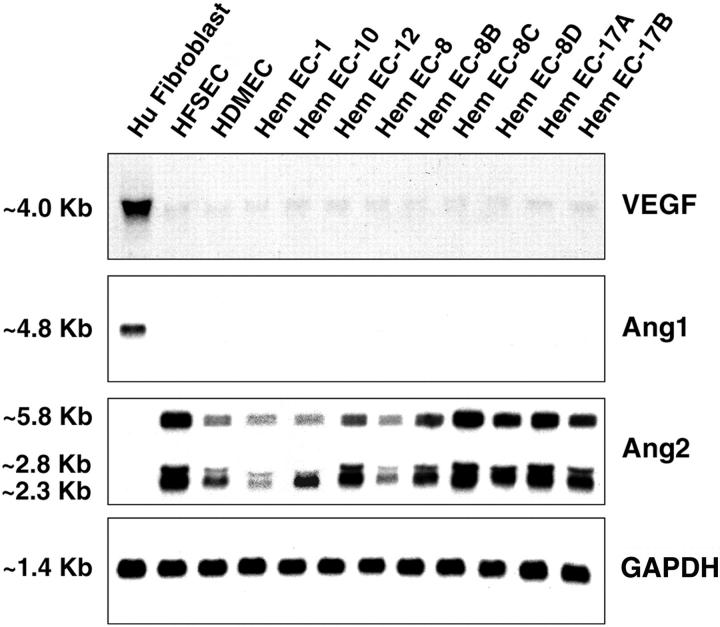
Next, we examined whether mRNA expression of VEGF, Ang1, and Ang2 was dysregulated in HemECs. Ectopic expression of these factors by the endothelial cells that comprise hemangioma might contribute to the pathological angiogenesis of this tumor. VEGF transcripts were detected at very low levels in normal human ECs and HemECs, but at high levels in human fibroblasts (Figure 2) ▶ . Similarly, Ang1 mRNA was not detected in normal ECs or HemECs, but was abundant in human fibroblasts. Ang2 transcripts of 2.3, 2.8, and 5.8 kb 31 were detected in all endothelial cultures but not in human fibroblasts. These patterns are entirely consistent with current knowledge about expression of these ligands by cultured endothelial cells. Taken together, Tie2 is the only factor among all examined that is differentially expressed between HemECs and normal endothelial cells under basal conditions. To gain further insight, we studied the biological role and regulation of the angiopoietin/Tie2 system in HemECs in more detail below.
Figure 2.
Analysis of soluble angiogenic factors VEGF, Ang1, and Ang2 in HemECs. Total RNA was isolated from cultured human fibroblasts, normal human ECs (HFSECs and HDMECs), and HemECs. Northern blot analysis was performed with indicated 32P-labeled cDNA probes. GAPDH level showed uniformity of RNA loading. Size of transcripts is presented in kb.
Functional Consequence of Increased Tie2
Binding of Ang1 to its receptor Tie2 is known to stimulate endothelial cell migration, endothelial sprouting, and endothelial cell survival. 32 We examined whether elevated Tie2 expression affected the response of HemEC to Ang1. Cellular migration in response to 0, 10, and 100 ng/ml of Ang1* was measured using the modified Boyden chamber assay (Figure 3A) ▶ . Compared to HDMECs, HemEC-1 showed increased migration in response to 10 and 100 ng/ml of Ang1*. HemEC-1 also exhibited increased survival in response to Ang1* compared to HDMECs (Figure 3B) ▶ . Other survival factors, bFGF, and VEGF promoted an approximately twofold increase in survival of both HemECs and HDMECs, but did not show any preferential effect. Similar results in both migration and cell survival assays were observed in two other independent HemECs (data not shown). Increased cellular migration and survival in response to Ang1 is consistent with increased signaling through elevated Tie-2 levels.
Figure 3.
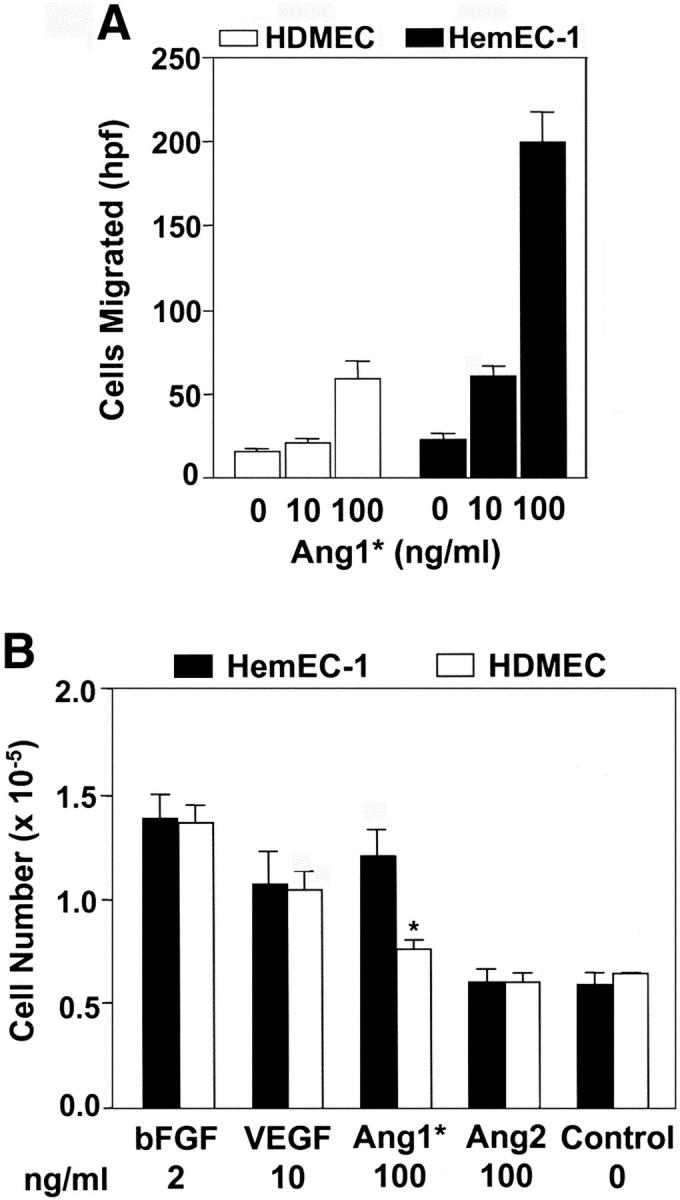
Enhanced migration and survival of HemECs in response to Ang1. A: Migration of HemEC-1 (filled bars) and HDMECs (open bars) in response to 0, 10, and 100 ng/ml Ang1*. All experiments were in quadruplicate. Cells migrated/high-power field were counted in five random fields, and values are presented as means ± SD. B: HemEC-1 (filled bars) and HDMECs (open bars) were plated at 1.2 × 105/well and incubated in EBM containing 10% FBS without or with bFGF, VEGF, Ang1*, or Ang2 for 5 days. Each condition was performed in triplicate. Cell numbers were determined by hemocytometer and presented as means ± SD. The differences between HemEC-1 and HDMECs cultured in presence of Ang1* were statistically significant (P < 0.05).
Dysregulated Expression of Ang2 in HemECs
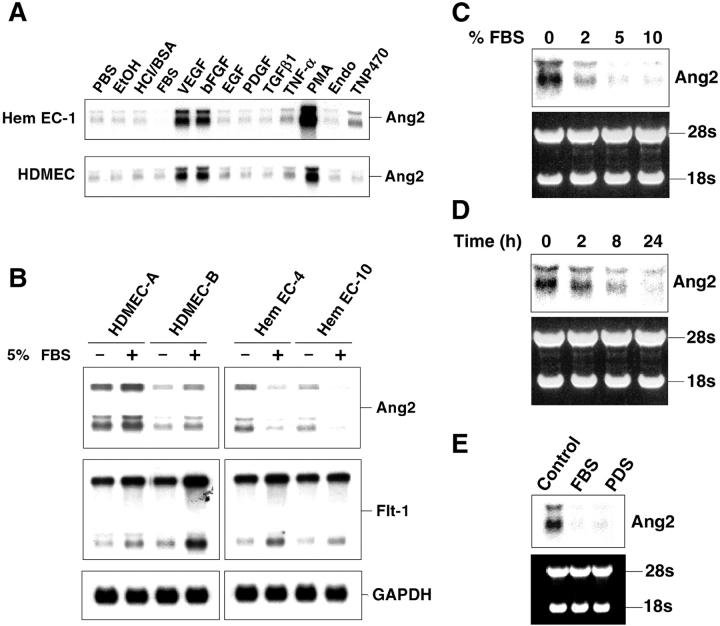
Regulation of Tie2 and Ang2 expression in cultured endothelial cells has been studied previously in an effort to understand the mechanism by which Ang2 regulates angiogenesis through Tie2. For example, Tie2 gene expression is stimulated by hypoxia and proinflammatory cytokines, tumor necrosis factor-α, and interleukin-1β. 33 Ang2 mRNA is known to be up-regulated by VEGF, bFGF, hypoxia, and PMA. 24,31,34 We compared expression of Tie2 and Ang2 mRNA in response to various growth factors, serum, cytokines, and angiogenic inhibitors in normal HDMECs and HemECs (Figure 4A) ▶ . As expected, Tie2 mRNA was slightly enhanced by incubation for 8 hours in serum-free medium containing tumor necrosis factor-α, but no difference in expression was observed between HemECs and HDMECs (data not shown). VEGF, bFGF, or PMA up-regulated Ang2 mRNA (Figure 4A) ▶ in both HemECs and HDMECs. All three Ang2 transcripts were modulated in a similar manner by these factors. However, incubation in 5% FBS caused a down-regulation of Ang2 in HemEC-1, but not in HDMECs (Figure 4A ▶ , arrow). This apparent dysregulated Ang2 expression in response to serum was further characterized by examining two different HDMEC preparations and two additional HemECs. As shown in Figure 4B ▶ , the down-regulation in response to FBS was also observed in HemEC-4 and HemEC-10, but not in two different isolations of HDMECs. Flt-1 was slightly increased and the control transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was unchanged by 5% FBS, indicating the effect on Ang2 was specific. Down-regulation of Ang2 in HemECs in response to serum was both concentration- and time-dependent, and occurred in response to plasma-derived serum (Figure 4 ▶ ; C, D, and E). This finding indicates that the down-regulation occurs in serum devoid of PDGF, which is consistent with the fact that purified PDGF did not affect Ang2 gene expression (Figure 4A) ▶ . These results suggest that regulation of Ang2 gene expression is altered in HemECs, and underscore that an autonomous endothelial cell defect occurs in hemangioma and supports our previous studies showing altered HemEC behavior in vitro. 20
Figure 4.
Regulation of Ang2 mRNA in response to exogenous angiogenic factors. A: Serum-starved HemEC-1 and HDMECs were incubated for 8 hours in medium with 5% FBS, 10 ng/ml VEGF, 10 ng/ml bFGF, 20 ng/ml epidermal growth factor, 100 ng/ml PDGF, 10 ng/ml transforming growth factor-β1, 50 ng/ml tumor necrosis factor-α, 100 nmol/L PMA, 1 μg/ml endostatin, or 100 ng/ml TNP-470. Northern blot analysis was performed with a 32P-labeled cDNA probe for human Ang2. PBS, ethanol (EtOH), and 4 mmol/L of HCl/0.01% BSA were used as carrier controls at same dilution as corresponding factors. B: Effect of 5% FBS on Ang2 expression was compared in two preparations of HDMECs, HemEC-4, and HemEC-10 after 8 hours of incubation. Northern blots were probed with 32P-labeled cDNA probes for Ang-2, Flt-1, and GAPDH. C: Dose-response of Ang2 expression to FBS in HemECs. Serum-starved HemEC-1 were incubated with medium containing 0, 2, 5, or 10% FBS for 8 hours. D: Time course of 5% FBS-induced down-regulation of Ang2 RNA at 0, 2, 8, and 24 hours. E: Ang2 expression in HemECs in response to plasma-derived serum. HemEC-1 was incubated with medium containing no serum, 5% FBS, or 5% plasma-derived serum for 8 hours. Uniformity of loading and RNA integrity was indicated by ethidium bromide-stained 28S and 18S rRNAs for C, D, and E.
High-Level Expression of Tie2 in Hemangioma Tissue
To determine whether mRNA expression patterns observed in cultured HemECs could be confirmed in hemangioma tissue, we performed in situ hybridization on histological tissue specimens from a set of four proliferating phase specimens using probes directed against human Tie2, Tie1, Ang1, and Ang2, as well as VEGF, Flt-1, KDR, (Figure 5) ▶ . A bright-field from a representative proliferative phase lesion is shown in Figure 5A ▶ , with corresponding dark-fields in Figure 5, B to H ▶ . In contrast to Ang1 (Figure 5E) ▶ , there were strong expression of Ang2 (Figure 5F) ▶ , Tie1 (Figure 5G) ▶ , and Tie2 (Figure 5H) ▶ , which was consistent with expression patterns observed in cultured HemECs. Also, similar to what we found in cultured HemECs, levels of VEGF transcripts were very low in hemangioma tissue (Figure 5B) ▶ , consistent with low levels detected by quantitative TaqMan RT-PCR (n = 5, unpublished data), whereas VEGF receptors, Flt-1 (Figure 5C) ▶ and KDR (Figure 5D) ▶ were abundantly expressed. These results confirm our in vitro studies on HemECs and demonstrate that Tie2 is highly expressed in proliferating hemangioma. In addition to Tie2 mRNA, Tie2 antigen was readily detected in proliferating hemangioma, with abundant protein on vascular channels as well as in some interstitial cells (Figure 6A) ▶ . Sections stained with control mouse IgG were negative (data not shown). Expression in normal skin, shown for comparison, was confined to the lining of blood vessels (Figure 6B) ▶ .
Figure 5.
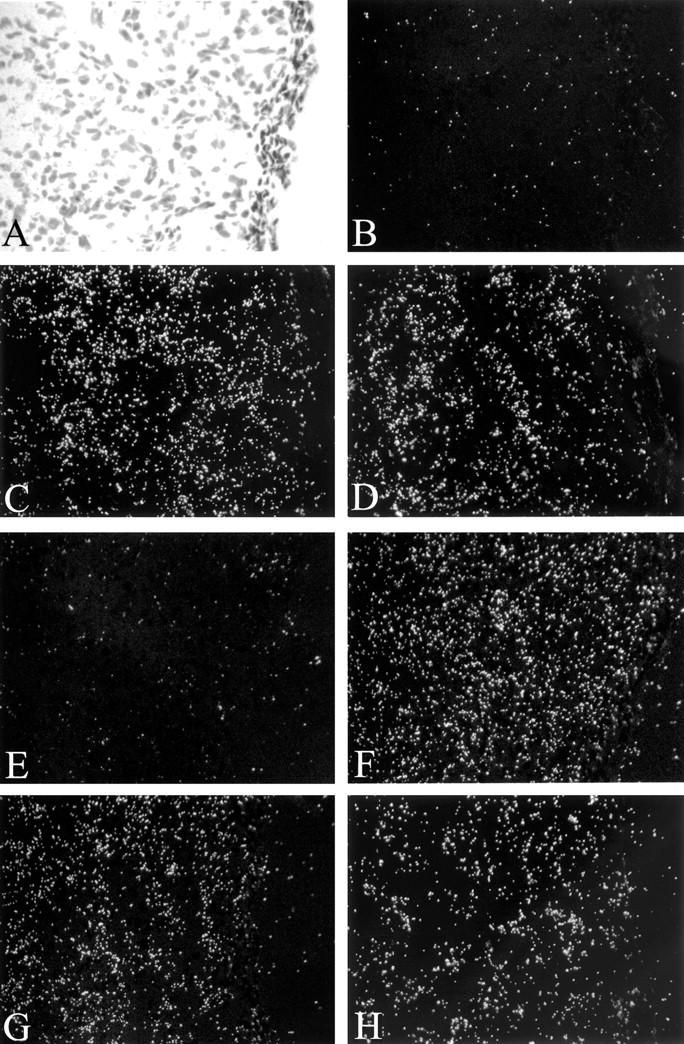
In situ hybridization of proliferative phase hemangioma. Bright-field photomicrograph (A) and dark-field photomicrographs of corresponding areas labeled by in situ hybridization for VEGF (B), Flt-1 (C), KDR (D), Ang1 (E), Ang2 (F), Tie1 (G), and Tie2 (H) mRNAs. Note strong expression of Flt-1, KDR, Ang2, Tie1, and Tie2 mRNAs, and in contrast weak expression of VEGF or Ang1. Original magnification, ×200.
Figure 6.
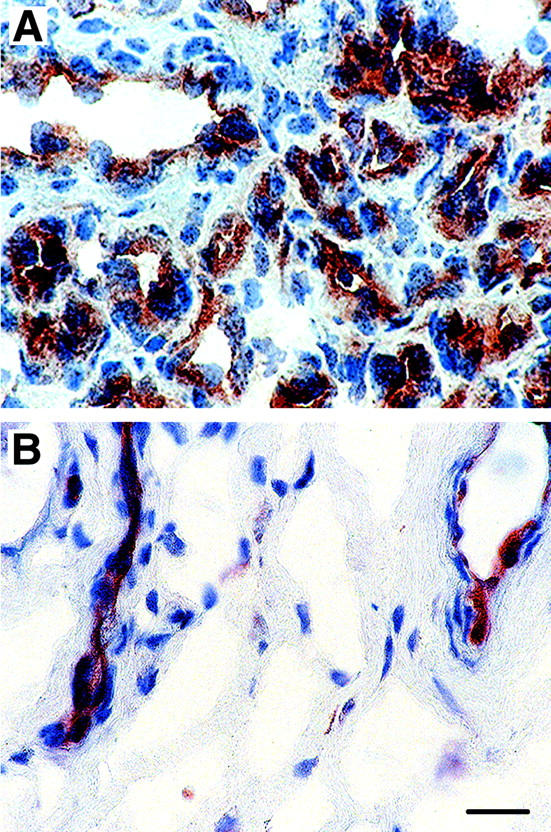
Immunohistochemical detection of Tie2. Frozen sections of proliferative phase hemangioma (A) and normal human skin (B) were immunostained with a mouse anti-human Tie2 monoclonal antibody. Original magnification, ×630. Scale bar, 16 μm.
Discussion
We demonstrate here that Tie2 is specifically increased in HemECs (Figure 1) ▶ , and that this increase corresponds to enhanced cellular responses to the Tie2 agonist Ang1 (Figure 3) ▶ . In contrast, levels of other tyrosine kinase receptors known to be critical for angiogenesis, such as KDR, Flt-1, and Tie1, are expressed at very similar levels to those detected in normal human microvascular endothelial cells, cultured under the same conditions (Figure 1B) ▶ . No evidence of strong VEGF or Ang1 expression was found in HemECs (Figure 2) ▶ . Regulation of Ang2 expression seems to be altered in HemECs, as shown by the down-regulation of Ang2 mRNA in response to serum (Figure 4) ▶ . Our results strongly suggest a role for the angiopoietin/Tie2 signaling pathways in the pathogenesis of hemangioma.
The angiopoietin/Tie system plays critical roles in embryonic vascular development and maintenance of normal adult vasculature. 35-37 Overexpression of Ang1 in mice can increase vascularization in the skin, 38 whereas delivery of soluble Tie2 can inhibit tumor angiogenesis. 39 Both findings demonstrate that activation of angiopoietin/Tie2 signaling pathway can lead to excessive angiogenesis. Defects in Tie2 are known to affect vascular development in humans. An activating mutation in the intracellular kinase domain of human Tie2 gene has been implicated in one form of inherited cutaneo-mucosal venous malformations, demonstrating the critical function of Tie2 in proper vascular development. 40 We are currently sequencing the Tie2 gene from hemangioma to determine whether hemangioma is associated with any Tie2 mutations. Further studies are needed to fully understand how the elevated Tie2 seen in HemECs contributes to pathogenesis of infantile hemangioma in vivo. We speculate that increased activity in the angiopoietin/Tie2 pathway, as a consequence of somatic mutation(s) in a gene that regulates this pathway in endothelial cells, contributes to the formation of excessive and disorganized vasculature in infantile hemangioma. It is worth noting that Tie2 levels in 2 of 10 HemECs were similar to that in normal controls (Figure 1) ▶ . This suggests that other factors independent of the angiopoietin/Tie system can contribute to pathogenesis of hemangioma.
Although Ang2 is known as a naturally occurring antagonist of Ang1, the physiological roles of Ang2 are complex and depend on local conditions, such as presence of other growth factors. In vivo, Ang2 promotes tumor angiogenesis in the presence of VEGF, but facilitates tumor vessel regression in absence of VEGF. 41 Like Ang1, high doses of Ang2 induce Tie2 autophosphorylation in vitro, and act as an apoptosis survival factor for endothelial cells through PI3-kinase/Akt pathway. 42 Here, we showed that Ang2 was abundantly expressed in proliferating infantile hemangiomas, suggesting a role for Ang2 in neovascularization of hemangioma through an autocrine loop. Previously, high levels of Ang2 mRNA were also observed in highly vascularized glioblastoma and hepatocellular carcinoma. 43,44 Further study of the mechanism or consequences of the down-regulation of Ang2 by serum in HemECs, and how Ang2 functions in the presence of low expression of Ang1 and VEGF, will provide insight into the role of Ang2 in the pathological angiogenesis of hemangioma.
There are conflicting reports on expression of VEGF in infantile hemangioma. Takahashi and colleagues 10 detected VEGF protein by immunohistochemical staining with a rabbit polyclonal anti-VEGF. They reported significant staining for VEGF in the cytoplasm of endothelial cells and pericytes in proliferating hemangioma but no immunoreactive product in several involuting or involuted specimens. Chang and colleagues 11 also examined VEGF mRNA expression by in situ hybridization. They reported 1660 ± 371 positive cells/mm 2 in proliferative phase hemangioma (n = 10) and greatly reduced expression in involutive phase specimens (n = 3). In this study, we found very weak VEGF mRNA expression by three different methods: Northern blot of cultured HemECs isolated from proliferating hemangiomas (n = 9), in situ hybridization of proliferating phase hemangiomas (n = 4), and TaqMan RT-PCR of proliferating hemangiomas (n = 5) (data not shown). These apparent discrepancies may be attributable to age difference and heterogeneity in hemangioma specimens, which may result in variations in expression levels of growth factors such as VEGF. Even low levels of VEGF, produced locally or deposited in hemangioma from exogenous cells, are likely to contribute to growth of hemangioma, given its well-known angiogenic effect.
To our knowledge, this is the first study on expression of these tyrosine kinase receptors in infantile hemangiomas. The abundant expression of several key regulators of angiogenesis, KDR, Flt-1, neuropilin-1, Tie2, Tie1, and Ang2 in hemangioma tissue (Figure 5) ▶ most likely reflects a proliferative endothelial phenotype. The fact that, with the exception of Tie2, the expression patterns in normal human ECs and HemECs are similar reflects the fact that in vitro culture conditions induce a proliferative phenotype. However, cultured HemECs retain properties in vitro that seem related to the development of hemangioma, as demonstrated by increased responses to Ang1 and dysregulated expression of Ang2. In addition, we showed previously that HemECs are clonal, exhibit increased proliferation and migration, and are stimulated by the angiogenesis inhibitor endostatin. 20 These unique properties of cultured HemECs will be critical in elucidating the molecular defect(s) that cause infantile hemangioma.
In summary, this study has identified the angiopoietin/Tie2 system as molecular regulators associated with the pathogenesis of infantile hemangiomas. The increased Tie2 levels, enhanced response to Ang1, and dysregulated Ang2 gene expression in HemECs may contribute to the abnormal growth and remodeling of vasculatures in hemangiomas. Targeting angiopoietin/Tie pathway may prove to be effective anti-angiogenic therapy for preventing hemangioma progression.
Acknowledgments
We thank Dr. George Yancopoulos (Regeneron Pharmaceuticals, Inc. Tarrytown, NY) for providing recombinant Ang1* and Ang2 as well as cDNA constructs of the two factors; 23 Dr. Shay Soker (Children’s Hospital, Boston) for partial cDNA constructs of human VEGF and neuropilin-1; Dr. Björn Olsen (Harvard Medical School, Boston) for human Tie2 cDNA construct; Dr. Elisabetta Dejana (Milan, Italy) for human VE-cadherin cDNA construct; Dr. Peter Newman (the Blood Center of Southeastern Wisconsin, Wisconsin, MI) for human CD31/PECAM-1 cDNA construct; Dr. Ellis Neufeld (Children’s Hospital, Boston) for human skin fibroblasts. We thank Drs. Fong-Ying Tsai and Michael Donovan (Millennium Pharmaceutical Inc., Cambridge, MA) for VEGF TaqMan analysis of hemangioma specimens; Gretchen Paranya for superb technical assistance with human endothelial cells; and Kristin Gullage for preparation of the figures.
Footnotes
Address reprint requests to Joyce Bischoff, Ph.D., Surgical Research Laboratory, Children’s Hospital, 300 Longwood Ave., Boston, MA 02115. E-mail: joyce.bischoff@tch.harvard.edu.
Supported by grants from the Gackstatter Foundation and the John Butler Mulliken Foundation.
References
- 1.Holmdahl K: Cutaneous hemangiomas in premature and mature infants. Acta Paediatr 1955, 44:370-379 [DOI] [PubMed] [Google Scholar]
- 2.Mulliken JB: Cutaneous vascular anomalies. Semin Vasc Surg 1993, 6:204-218 [PubMed] [Google Scholar]
- 3.Ezekowitz RAB, Mulliken JB, Folkman J: Interferon-alpha-2a therapy for life-threatening hemangioma of infancy. N Engl J Med 1992, 326:1456-1463 [DOI] [PubMed] [Google Scholar]
- 4.Chang E, Boyd A, Nelson CC, Crowley D, Law T, Keough KM, Folkman J, Ezekowitz AB, Castle VP: Successful treatment of infantile hemangioma with interferon-alpha-2b. J Pediatr Hematol Oncol 1997, 19:237-244 [DOI] [PubMed] [Google Scholar]
- 5.Barlow CF, Priebe CJ, Mulliken JB, Barnes PD, Macdonald D, Folkmam J, Ezekowitz RA: Spastic diplegia is a complication of interferon-alpha 2a treatment of hemangiomas of infancy. J Pediatr 1998, 132:527-530 [DOI] [PubMed] [Google Scholar]
- 6.Greinwald JH, Burke DK, Bonthius DJ, Bauman NM, Smith RJH: An update on the treatment of hemangiomas in children with interferon alfa-2a. Arch Otolaryngol Head Neck Surg 1999, 125:21-27 [DOI] [PubMed] [Google Scholar]
- 7.Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HPW, Fishman SJ, Larsen PR: Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med 2000, 343:185-189 [DOI] [PubMed] [Google Scholar]
- 8.Mulliken JB, Young AE: Vascular Birthmarks: Hemangiomas and Malformations. 1988:pp 64-72 W.B. Saunders Co., Philadelphia
- 9.Smoller BR, Apfelberg DB: Infantile (juvenile) capillary hemangioma: a tumor of heterogenous cellular elements. J Cutan Pathol 1993, 20:330-336 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Mulliken JB, Kozakewich HPW, Rogers RA, Folkman J, Ezekowitz RAB: Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest 1994, 93:2357-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang J, Most D, Bresnick S, Mehrara B, Steinbach DS, Reinisch J, Longaker MT, Turk AE: Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plast Reconstr Surg 1999, 103:1-10 [DOI] [PubMed] [Google Scholar]
- 12.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ: Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000, 96:34-40 [PubMed] [Google Scholar]
- 13.Isik FF, Rand RP, Gruss JS, Benjamin D, Alpers CE: Monocyte chemoattractant protein-1 RNA expression in hemangiomas and vascular malformations. J Surg Res 1996, 61:71-76 [DOI] [PubMed] [Google Scholar]
- 14.Martin-Padura I, Castellarnau CD, Uccini S, Pilozzi E, Natali PG, Nicotra MR, Ughi F, Azzolini C, Dejana E, Ruco L: Expression of VE (vascular endothelial)-cadherin and other endothelial-specific markers in hemangiomas. J Pathol 1995, 175:51-57 [DOI] [PubMed] [Google Scholar]
- 15.Kräling BM, Razon MJ, Boon LM, Zurakowski D, Seachord C, Darveau RP, Mulliken JB, Corless CL, Bischoff J: E-selectin is present in proliferating endothelial cells in human hemangiomas. Am J Pathol 1996, 148:1181-1191 [PMC free article] [PubMed] [Google Scholar]
- 16.Verkarre V, Patey-Mariaud de Serre N, Vazeux R, Teillac-Hamel D, Chretien-Marquet B, Le Bihan C, Leborgne M, Fraitag S, Brousse N: ICAM-3 and E-selectin endothelial expression differentiate two phases of angiogenesis in infantile hemangioma. J Cutan Pathol 1999, 26:17-24 [DOI] [PubMed] [Google Scholar]
- 17.Jang YC, Arumugam S, Ferguson M, Gibran NS, Isik FF: Changes in matrix composition during the growth and regression of human hemangiomas. J Surg Res 1998, 80:9-15 [DOI] [PubMed] [Google Scholar]
- 18.Tan ST, Velickovic M, Ruger BM, Davis PF: Cellular and extracellular markers of hemangioma. Plast Reconstr Surg 2000, 106:529-538 [DOI] [PubMed] [Google Scholar]
- 19.Bielenberg DR, Bucana CD, Sanchez R, Mulliken JB, Folkman J, Fidler IJ: Progressive growth of infantile cutaneous hemangiomas is directly correlated with hyperplasia and angiogenesis of adjacent epidermis and inversely correlated with expression of endogenous angiogenesis inhibitor, IFN-beta. Int J Oncol 1999, 14:401-408 [DOI] [PubMed] [Google Scholar]
- 20.Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 2001, 107:745-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale NW, Yancopoulos GD: Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 1999, 13:1055-1068 [DOI] [PubMed] [Google Scholar]
- 22.Kräling BM, Bischoff J: A simplified method for growth of human microvascular endothelial cells results in decreased senescence and continued responsiveness to cytokines and growth factors. In Vitro Cell Dev Biol 1998, 33:308-315 [DOI] [PubMed] [Google Scholar]
- 23.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM: Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 1998, 273:18514-18521 [DOI] [PubMed] [Google Scholar]
- 24.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y: Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 1999, 274:15732-15739 [DOI] [PubMed] [Google Scholar]
- 25.Church GM, Gilbert W: Genomic sequencing. Proc Natl Acad Sci USA 1984, 81:1991-1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ: Identification of Tek/Tie2 binding partners. J Biol Chem 1999, 274:30896-30905 [DOI] [PubMed] [Google Scholar]
- 27.French-Constant C, Van de Water L, Dvorak HF, Hynes RO: Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 1989, 109:903-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown LF, Berse B, Tognazzi K, Manseau EJ, Van de Water L, Senger DR, Dvorak HF, Rosen S: Vascular permeability factor RNA and protein expression in human kidney. Kidney Int 1992, 42:1457-1461 [DOI] [PubMed] [Google Scholar]
- 29.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF: Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res 1993, 53:4727-4735 [PubMed] [Google Scholar]
- 30.Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD: Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol 2000, 156:2179-2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandriota SJ, Pepper MS: Regulation of angiopoietin-2 RNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 1998, 83:852-859 [DOI] [PubMed] [Google Scholar]
- 32.Jones N, Dumont DJ: Tek/Tie2 signaling: new and old partners. Cancer Metastasis Rev 2000, 19:13-17 [DOI] [PubMed] [Google Scholar]
- 33.Willam C, Koehne P, Jurgensen JS, Grafe M, Wagner KD, Bachmann S, Frei U, Eckardt KU: Tie2 receptor expression is stimulated by hypoxia and pro-inflammatory cytokines in human endothelial cells. Circ Res 2000, 87:370-377 [DOI] [PubMed] [Google Scholar]
- 34.Krikun G, Schatz F, Finlay T, Kadner S, Mesia A, Gerrets R, Lockwood CJ: Expression of angiopoietin-2 by human endometrial endothelial cells: regulation by hypoxia and inflammation. Biochem Biophys Res Commun 2000, 275:159-163 [DOI] [PubMed] [Google Scholar]
- 35.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y: Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376:70-74 [DOI] [PubMed] [Google Scholar]
- 36.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD: Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87:1171-1180 [DOI] [PubMed] [Google Scholar]
- 37.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD: Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277:55-60 [DOI] [PubMed] [Google Scholar]
- 38.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD: Increased vascularization in overexpressing angiopoietin-1. Science 1998, 282:468-471 [DOI] [PubMed] [Google Scholar]
- 39.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peter KG: Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest 1997, 100:2072-2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vikkula M, Boon LM, Carraway KL, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR: Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 1996, 87:1181-1190 [DOI] [PubMed] [Google Scholar]
- 41.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ: Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284:1994-1998 [DOI] [PubMed] [Google Scholar]
- 42.Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY: Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Oncogene 2000, 19:4549-4552 [DOI] [PubMed] [Google Scholar]
- 43.Stratmann A, Risau W, Plate KH: Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol 1998, 153:1459-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka S, Mori M, Sakamoto Y, Makuuchi M, Sugimachi K, Wands JR: Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest 1999, 103:341-345 [DOI] [PMC free article] [PubMed] [Google Scholar]