Abstract
Macrophages play a central role in the pathogenesis of peripheral neuropathy but the role of resident endoneurial macrophages is undefined because no discriminating markers exist to distinguish them from infiltrating hematogenous macrophages. We identified and characterized resident endoneurial macrophages during Wallerian degeneration in radiation bone marrow chimeric rats created by transplanting wild-type Lewis rat bone marrow into irradiated TK-tsa transgenic Lewis rats. In such animals, resident cells carry the transgene, whereas hematogenous cells do not. As early as 2 days after sciatic nerve crush and before the influx of hematogenous macrophages, resident transgene-positive endoneurial macrophages underwent morphological and immunophenotypic signs of activation. At the same time, resident macrophages phagocytosing myelin were found, and proliferation was detected by bromodeoxyuridine incorporation. Continuous bromodeoxyuridine feeding revealed that resident endoneurial macrophages sequentially retracted their processes, proliferated, and expressed the ED1 antigen, rendering them morphologically indistinguishable from hematogenous macrophages. Resident endoneurial macrophages thus play an early and active role in the cellular events after nerve lesion before hematogenous macrophages enter the nerve. They may thus be critically involved in the pathogenesis of peripheral neuropathy particularly at early stages of the disease and may act as sensors of pathology much like their central nervous system counterparts, the microglial cells.
The pathogenesis of peripheral neuropathy is intimately linked with endoneurial macrophage function. In autoimmune polyneuropathies including Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy, macrophage-mediated demyelination is considered the pathological hallmark of these diseases. 1 In addition, macrophages express major histocompatibility complex (MHC) antigens 2 and co-stimulatory B7 molecules 3 and may thus serve as local antigen-presenting cells in the peripheral nervous system. Other functions include the expression of regulatory proinflammatory cytokines and chemokines, the elaboration of cytotoxic substances, and a contribution to disease remission by producing anti-inflammatory mediators such as transforming growth factor-β1 and interleukin-10. 4 They are thus involved in many pathogenetic steps from the initiation of an autoimmune response to effector functions and disease remission. In Wallerian degeneration after peripheral nerve trauma and during primary or secondary neuropathic axonal damage, macrophages phagocytose and remove degenerating myelin in a complement-depending manner, paving the way for successful axonal regeneration. 5 As in inflammatory neuropathies, they secrete regulatory, trophic, and toxic molecules including cytokines and free radicals and are thus intimately involved in the evolution of the cellular response during axonal degeneration and regeneration.
Endoneurial macrophages are not a homogenous cell population. 6 In addition to hematogenous macrophages entering the nerve in large numbers during disease, a population of resident endoneurial macrophages exists in the normal peripheral nerve that accounts for up to 9% of the entire endoneurial cell population. 6-8 This endoneurial location makes them key candidates for an early response to disease similar to the enigmatic role of microglial cells of the central nervous system. 9 However, the biological role of resident endoneurial macrophages during disease in vivo is essentially unknown as there are no existing cellular markers that may discriminate them from infiltrating hematogenous macrophages. Early studies using nerve explants into the peritoneal cavity that were contained in Millipore chambers not allowing access of peritoneal macrophages, very little or no phagocytosis by resident endoneurial macrophages was found, and nonresident macrophages were required for myelin removal. 10,11 However, experiments in peripheral nerve organ cultures without added macrophages suggested that resident endoneurial macrophages may phagocytose myelin to a limited degree and increase in number. 12 Their possible counterparts in the central nervous system, the microglial cells, respond extremely rapidly to a wide variety of pathological stimuli and thus seem to be the primary local cells involved in immunosurveillance of the brain. 9 It may thus be hypothesized that resident endoneurial macrophages provide a functionally significant contribution to the macrophage response during peripheral nerve disorders, and the characterization and study of functional properties of resident endoneurial macrophages could provide important clues to our understanding of peripheral nerve disease.
One possibility to discriminate between hematogenous and resident macrophages in laboratory animals is the induction of bone marrow chimerism. Such chimeras are created by lethally irradiating recipient animals and transplanting donor bone marrow that carries a discriminating cellular marker allowing the distinction between local and bone marrow-derived cells. Radiation bone marrow chimeric rats based on different MHC haplotypes were successfully used to investigate the physiological turnover of resident endoneurial macrophages in the normal peripheral nerve. 13 However, the detection of the discriminating MHC haplotype required the stimulation of experimental animals with high-dose cytokines to up-regulate MHC molecules. TK-tsa transgenic Lewis rats carry 250 to 300 copies of a functionally silent DNA construct in all cell nuclei that may be constitutively detected by in situ hybridization. 14 In the present study we created radiation bone marrow chimeric rats by transplanting wild-type Lewis rat bone marrow into irradiated TK-tsa transgenic Lewis rats to identify resident endoneurial macrophages and investigate their role in a model of Wallerian degeneration. In such animals, all resident tissue cells carry the TK-tsa transgene whereas bone marrow-derived blood cells do not. Co-localization of the transgene with macrophage markers thus unequivocally identifies transgene-positive endoneurial macrophages as resident endoneurial macrophages.
Materials and Methods
Radiation Bone Marrow Chimeric Rats
TK-tsa transgenic Lewis rats 14 and wild-type Lewis rats were obtained from BRL, Füllinsdorf, Switzerland, and Charles River, Sulzfeld, Germany, respectively. Bone marrow chimeric rats were created as described previously. 15,16 Recipient TK-tsa transgenic Lewis rats were lethally irradiated with 1000 rads using a cobalt γ radiation source. Donor bone marrow cells were prepared by extruding bone marrow from the long bones of wild-type Lewis rats using a syringe. Bone marrow cells were then suspended in phosphate-buffered saline, filtered through a 70-μm nylon mesh, washed, and centrifuged at 1200 rpm twice for 10 minutes, resuspended in buffer, and counted. Subsequently, ∼10 7 donor bone-marrow cells in a volume of 400 μl were injected into the tail vein of recipient irradiated TK-tsa transgenic Lewis rats. Rats were allowed to recover for 3 months to establish mature chimerism before further experiments were performed. For control purposes, chimeras were created the opposite way, ie, TK-tsa transgenic bone marrow was transplanted into wild-type Lewis rats. Unless otherwise stated, the experiments presented in the present article were performed in chimeras where TK-tsa transgenic Lewis rats served as recipients and wild-type Lewis rats as donors.
Sciatic Nerve Injury
The right sciatic nerve was exposed under deep ether anesthesia and crushed just distal to the sciatic notch in a standardized way for 10 seconds with a fine forceps. TK-tsa transgenic rats carrying Lewis rat bone marrow were sacrificed 1, 2, 3, 4, 7, 14, and 28 days after crush injury. There were 21 rats in the experimental group, three animals for each time point examined. A total of 40 control rats comprising Lewis rat chimeras carrying TK-tsa bone marrow (8 rats), nonchimeric TK-tsa transgenic rats (8 rats), and nonchimeric wild-type Lewis rats (24 rats) were examined without crush injury and 1, 4, 7, and 14 days after crush injury, with additional time points for wild-type Lewis rats. The control experiments were performed to exclude any influence of chimera production, the direction of chimerism and the presence of the transgene on Wallerian degeneration, and endoneurial macrophage numbers and their response to peripheral nerve injury. All animal experiments were approved by the veterinary office at the Bezirksregierung Münster, Germany.
Assessment of Cell Proliferation
Rats received 75 mg/kg of bromodeoxyuridine (BrdU; Sigma, Deisenhofen, Germany) intraperitoneally 2 hours before sacrifice to detect proliferating cells. An additional group of chimeric animals, killed 7 days after crush injury, received drinking water containing 1 mg/ml BrdU for the entire period between crush injury and sacrifice to mark all cells that had undergone proliferation during that time.
Fixation and Tissue Preparation
Rats were perfused through the left ventricle under deep ether anesthesia for 1 minute with a 6% hydroxyethyl-starch solution (HAES sterile; Fresenius, Bad Homburg, Germany) followed by 4% phosphate-buffered paraformaldehyde at pH 7.4 for 10 minutes. Relevant tissues were postfixed in the same fixative for a further 3 hours.
Methyl Methacrylate Embedding
Sciatic nerve tissue 7-mm distal from the crush site and from the contralateral control side as well as splenic tissue was embedded in methyl methacrylate as described previously. 16 Polymerization was allowed for 48 hours at −20°C under vacuum. Series of transverse adjacent 0.5-μm semithin sections were cut on a Reichert-Jung ultracut ultramicrotome (Leica, Wetzlar, Germany), transferred onto coated glass slides, and dried at 35°C for 2 hours.
TK-tsa Probe for in Situ Hybridization
A plasmid containing the sequence of the TK-tsa transgene 14 was used as a template to produce digoxigenin-labeled DNA probes by polymerase chain reaction according to standard protocols. Four regions of the transgene were amplified by different sets of primer pairs (Table 1) ▶ , yielding a probe cocktail with four labeled probes of ∼200 bp each. Labeling efficiency was tested by dot-blot analysis of labeled polymerase chain reaction products in comparison with serial dilutions of a labeled standard sequence. All reagents used for the digoxigenin labeling and dot-blot procedures were purchased from Roche (Mannheim, Germany).
Table 1.
Nucleotide Sequences of Forward and Reverse Polymerase Chain Reaction Primers and Their Positions 14 as Used for the Synthesis of a Probe Cocktail for TK-tsa in Situ Hybridization
| Probe no. | Length | Nucleotide position | Forward primer | Reverse primer |
|---|---|---|---|---|
| 1 | 213 bp | 365–577 | 5′-CCTGGCTGTCTTCATCATCA-3′ | 5′-AAAGTGGTATTGCTTTGCTT-3′ |
| 2 | 216 bp | 671–886 | 5′-CCTGCAGTGTTTTAGGCACA-3′ | 5′-AGGATGTAAAGGGCACTGGA-3′ |
| 3 | 203 bp | 905–1107 | 5′-GCTCAAAGTTCAGCCCC-3′ | 5′-GCTACACTGTTTGTTGCCCA-3′ |
| 4 | 219 bp | 2005–2223 | 5′-CACTGCTCCCATTCATCAGTT-3′ | 5′-GATTGCTACTGCTTCGATTGC-3′ |
In Situ Hybridization
In situ hybridization was performed as previously described. 16 In brief, sections were cleared from methylmethacrylate by incubation in acetone and pretreated by microwave irradiation. Hybridization was performed in a humid chamber at 37°C for 14 hours in a mixture containing the labeled denatured DNA probe at 5 ng/μl, 5 mg/ml denatured salmon sperm DNA, 50% formamide, 10% dextran sulfate, and 0.02% sodium dodecyl sulfate (all from Sigma) in 2× standard saline citrate (SSC) (1× SSC = 0.15 mol/L sodium chloride, 30 mmol/L trisodium citrate, pH 7). Before hybridization, sections and probe were denatured again at 95°C on a hot plate for 5 minutes. Washes were in 2× SSC for 3 × 20 minutes and 50% formamide/1× SSC at 50°C for 15 minutes. Hybridized probe was detected by a horseradish peroxidase-conjugated anti-digoxigenin antibody (DAKO, Hamburg, Germany), tyramide amplification, and fluorescent Cy3-streptavidin (Amersham, Braunschweig, Germany) or A594-streptavidin (Molecular Probes, Leiden, The Netherlands). Sections were counterstained with 4,6-diamidino-2-phenylindol (DAPI; Vector Laboratories, Burlingame, CA). To estimate the sensitivity and specificity of in situ hybridization for each experiment, in situ hybridization on serial sections of splenic tissue from TK-tsa transgenic and wild-type Lewis rats was always included as a control.
Immunohistochemistry and Double-Labeling Immunohistochemistry
Deplasticized tissue sections were pretreated and incubated with primary monoclonal or polyclonal antibodies as summarized in Table 2 ▶ . Secondary anti-mouse or anti-rabbit horseradish peroxidase-conjugated antibodies (DAKO) were used, followed by tyramide amplification and fluorescent signal detection with A488-streptavidin or A594-streptavidin (Molecular Probes) as described previously. 16 Negative controls were performed without primary antibody or with an irrelevant primary antibody at comparable concentrations. Double-labeling immunohistochemistry on single sections using one polyclonal and one monoclonal antibody was achieved by sequentially blocking excess biotin sites with avidin and excess avidin sites with biotin (DAKO) as well as residual horseradish peroxidase activity with H2O2 for 10 minutes each before starting the detection procedure for the second primary antibody. Negative controls again included sections where one or both primary antibodies were omitted or replaced by irrelevant antibodies.
Table 2.
List of Primary Antibodies, Their Sources, Specificities, Dilutions, and Pretreatment Conditions on Methyl Methacrylate-Embedded Semithin Sections as Used in the Present Study
| Antibody (source) | Specificity | Dilution | Incubation, minutes | Pretreatment |
|---|---|---|---|---|
| ED1 (Serotec), monoclonal | Activated macrophages | 1 :100 | 90 | None |
| Iba1, polyclonal 17 | All macrophages | 1 :100 | 180 | None |
| NR4 (Dako), monoclonal | 68-kd neurofilament | 1 :20 | 90 | Microwave 20 minutes, citrate buffer |
| MBP (Serotec), polyclonal | Myelin basic protein | 1 :25 | 90 | Microwave 20 minutes, citrate buffer |
| BrdU (Dako), monoclonal | Proliferating cells | 1 :50 | 120 | Microwave 30 minutes, citrate buffer |
All incubations were at room temperature. Iba1, ionized calcium-binding adaptor molecule 1; MBP, myelin basic protein; BrdU, bromodeoxyuridine.
Double-Labeling in Situ Hybridization and Immunohistochemistry on Single Sections
Combined in situ hybridization and immunohistochemistry on single sections was done as described previously. 16 In brief, sections were first hybridized as described above. The primary antibody for the immunohistochemistry procedure was co-incubated with the horseradish peroxidase-conjugated anti-DIG antibody needed to detect the hybridized in situ hybridization probe. After incubation with fluorescent streptavidin to detect the in situ hybridization signal, excessive biotin, avidin, and horseradish peroxidase was blocked as described above, followed by the immunohistochemistry protocol as described with a different fluorescent marker. Negative controls included sections where the primary antibody or the specific DNA probes were omitted.
Co-Localization of DNA and Multiple Antigens on Adjacent Sections
For this purpose, series of adjacent 0.5-μm semithin sections were prepared and processed for in situ hybridization and immunohistochemistry as needed. Most series consisted of up to eight adjacent sections of which up to four were used for in situ hybridization to increase the sensitivity of detecting transgene-positive resident cells. Individual cells were identified on each section using landmark histological features as a reference.
Image Acquisition and Processing
Sections were examined under a Leica DM fluorescence microscope and images were digitized and transferred to a PC using a Diagnostic Instruments SPOT II camera system (Visitron, München, Germany). Merging of fluorescent signals was done by Adobe Photo Shop 4.0 or the SPOT II software.
Quantitative Studies
To compare the various control groups and the experimental group of TK-tsa transgenic Lewis rats transplanted with wild-type Lewis rat bone marrow, the number of proliferating cells, the total number of endoneurial cell nuclei, and the total number of endoneurial macrophages were quantified on transverse sections of uninjured and injured sciatic nerve at selected time points. Two randomly selected fields covering approximately two thirds of the nerve area were analyzed in three different animals per group. Only Iba1-positive macrophages clearly associated with a nucleus were counted.
The degree of the physiological turnover of resident endoneurial macrophages was assessed in uninjured sciatic nerves at the mid-thigh level from chimeric TK-tsa transgenic Lewis rats transplanted with wild-type Lewis rat bone marrow. Transverse sections double-stained for Iba1 (ionized calcium-binding adaptor molecule 1) 17 and the TK-tsa transgene were analyzed. Iba1-positive endoneurial macrophages in clear association with a nucleus were counted on randomly selected fields, and the percentage of cells with and without hybridization for the TK-tsa transgene was determined. Control sections from TK-tsa transgenic, nonchimeric rats were included with each in situ hybridization experiment to determine the sensitivity of in situ hybridization on single sections.
Results
Sensitivity of TK-tsa in Situ Hybridization and Characterization of Chimerism
The sensitivity of TK-tsa in situ hybridization was studied on single sections as well as serial tissue sections from TK-tsa transgenic rats. In brain, 44% of the cell nuclei hybridized with the TK-tsa probe when one single section was analyzed. When analyzing four adjacent serial sections, 90% of cell nuclei were TK-tsa-positive on at least one of the four sections. In peripheral nerve, 31% of nuclei hybridized on single sections and 77% positive nuclei were found when four serial sections were analyzed. In wild-type rats, no specific in situ hybridization signal for the TK-tsa transgene was found.
The effectiveness of chimerism induced by bone marrow transplantation into irradiated rats was studied by in situ hybridization for the TK-tsa transgene, with results similar to those described in a previous study from our laboratory. 16 In chimeras where TK-tsa transgenic rats served as recipients and wild-type rats as donors as used in all following experiments, the majority of parenchymal brain and peripheral nerve cell nuclei hybridized with the TK-tsa transgene on at least one section when four serial sections were investigated. These results were similar to those in nonchimeric TK-tsa transgenic rats used as controls. In spleen, only a few cells, mainly endothelial cells and putative stromal cells in the red pulp, were TK-tsa-positive whereas most cells within the follicles did not hybridize, indicating their derivation from bone marrow cells. In control chimeras where wild-type rats served as recipients and TK-tsa transgenic rats as bone marrow donors, opposite results were found in spleen, whereas most brain and sciatic nerve cell nuclei did not contain the transgene. There was no evidence of inflammatory autoimmune disease in any of the chimeric animals.
Wallerian Degeneration in Radiation Bone Marrow Chimeric Rats and Controls
To control for possible differences in the cellular response to peripheral nerve injury in chimeric TK-tsa transgenic and wild-type Lewis rats, the kinetics of axonal and myelin breakdown and local cellular reactions were compared in normal and injured nerves. Quantitative studies of endoneurial cell nuclei, proliferating endoneurial cell nuclei, and endoneurial macrophages revealed no significant difference between the four groups of animals (Tables 3 and 4) ▶ ▶ . Qualitative assessments of the density and breakdown of NF4-positive axonal profiles, axonal regeneration as revealed by the occurrence of small, thinly myelinated axonal profiles distal to the lesion, and myelin breakdown as detected by immunohistochemistry for myelin basic protein gave similar results in all four groups. There was also no difference in endoneurial macrophage morphology and the frequency of macrophages containing myelin basic protein indicating myelin phagocytosis (data not shown). In summary, no significant difference of selected features of Wallerian degeneration could be found between the two types of bone marrow chimeras, TK-tsa transgenic Lewis rats and normal wild-type Lewis rats.
Table 3.
Number of Endoneurial Cellular Nuclei and Endoneurial Macrophages in Wild-Type Lewis Rats (LW), Chimeric TK-tsa Transgenic Lewis Rats Transplanted with Wild-Type Bone Marrow (LW/TK), Chimeric Wild Type Lewis Rats Transplanted with TK-tsa Transgenic Bone Marrow (TK/LW), and Nonchimeric TK-tsa Transgenic Rats (TK)
| LW | LW/TK | TK/LW | TK | |
|---|---|---|---|---|
| Cell nuclei | ||||
| Normal | 17.28 | 15.78 | 14.50 | 16.64 |
| 7 days after lesion | 24.21 | 25.14 | 27.28 | 24.35 |
| Macrophages | ||||
| Normal | 0.93 | 0.79 | 0.71 | 0.79 |
| 7 days after lesion | 10.28 | 10.85 | 11.28 | 10.21 |
Transverse sections from normal and injured sciatic nerves were examined. Mean values from three animals per group, area size 100 μm2.
Table 4.
Frequency of Proliferating Cells in Transverse Sections from Normal and Injured Sciatic Nerve from Wild Type Lewis Rats (LW) and Chimeric TK-tsa Transgenic Lewis Rats Transplanted with Wild Type Bone Marrow (LW/TK)
| Control | Day | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | 14 | 28 | ||
| LW | 0.00 | 2.33 | 8.67 | 20.33 | 4.67 | 2.67 | 2.33 | 0.67 |
| LW/TK | 0.00 | 1.67 | 7.00 | 18.00 | 6.00 | 3.67 | 1.67 | 0.67 |
Mean values from three animals per group in percent.
Resident Endoneurial Macrophages in the Normal Sciatic Nerve
Macrophages detected by Iba1 antibody 17 were distributed throughout the endoneurium in the normal sciatic nerve (Figure 1 ▶ ; A, C, and D; Figure 4A ▶ ). In cross-sections they appeared triangular, sometimes with tiny, slim processes, and frequently co-localized with the TK-tsa transgene (Figure 1, D and E) ▶ . Some endoneurial macrophages filled the gap between myelinated axons and seemed to attach to the myelin sheaths. Others were located close to endothelial cells of endoneurial capillaries. Quantitative studies revealed that 5% of the total endoneurial cell population expressed the Iba1 macrophage antigen. The physiological turnover of resident endoneurial macrophages with bone marrow-derived cells in the sciatic nerve was determined in uninjured nerves from radiation bone marrow chimeric rats matured for 3 months. Co-localization of the Iba1 antigen with the TK-tsa transgene on multiple adjacent sections revealed that 41% of all Iba1-positive endoneurial macrophages were TK-tsa-positive on at least one of four sections, whereas the others were not. Considering the sensitivity of the in situ hybridization procedure of 77% on four adjacent sections as shown above, a calculated number of 53% of endoneurial macrophages were TK-tsa transgenic and thus resident endoneurial macrophages without turnover from the blood within the 3 months since bone marrow transplantation. We did not observe any morphological differences between TK-tsa-positive and TK-tsa-negative resident endoneurial macrophages. In control animals without peripheral nerve injury, these Iba1-positive macrophages did not express the ED1 antigen present on activated macrophages using MMA-embedded tissue.
Figure 1.
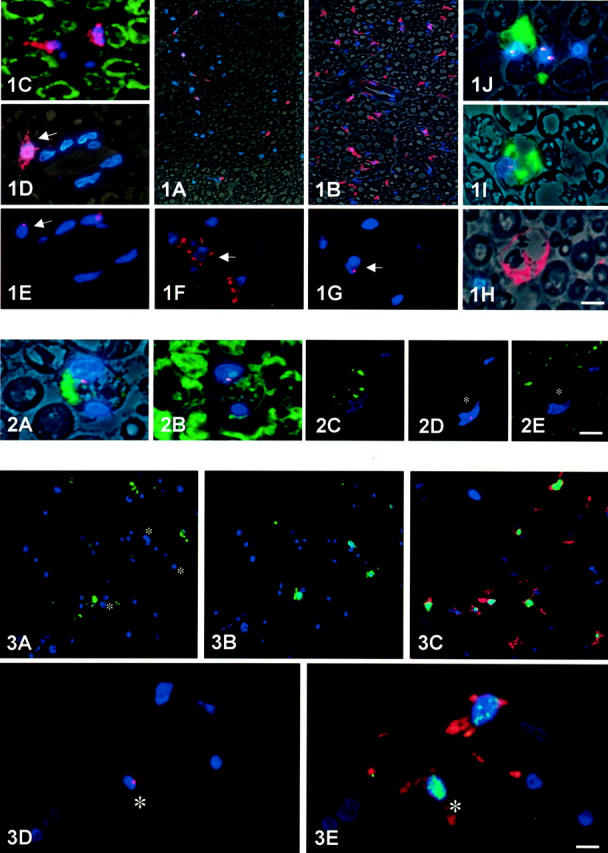
Resident endoneurial macrophages in normal nerves and early changes during Wallerian degeneration. In normal sciatic nerve (A,C, D and E), immunocytochemistry using the polyclonal macrophage antibody against Iba1 (A, C, and D; red color) reveals low numbers of resident endoneurial macrophages. They frequently are small, have a triangular shape, and carry tiny slim processes. They are positioned around blood vessels or within the endoneurium between myelin sheaths as revealed by phase contrast microscopy (A and D) or double-labeling immunocytochemistry for myelin basic protein (C, green color). D and E: Co-localization with the TK-tsa transgene on serial semithin sections revealed that some of them carry the transgene (red spot in E), whereas others had undergone physiological turnover from the blood and are TK-tsa-negative. Three days after sciatic nerve crush, there is also a marked increase in number of endoneurial macrophages (B). As early as 2 days after sciatic nerve crush (F–J) and more pronounced after 3 days (B), Iba1-positive endoneurial macrophages begin retracting their processes, become slightly thicker (F), and sometimes already take up a rounded appearance (H). Co-localization of Iba1 (F) with the TK-tsa transgene (red spot in G) on adjacent serial sections revealed that many of these activated macrophages are resident. I and J: Also as early as 2 days after lesion, resident endoneurial macrophages, identified by in situ hybridization for the TK-tsa transgene (red spots), were found that newly express the ED1 antigen (green color) present on activated macrophages. These cells exhibit an activated phenotype and are large and round. Cross-sections from sciatic nerve 7-mm distal from a crush lesion. Blue nuclear counterstain with DAPI. Scale bars: 30 μm (A and B); 10 μm (C–J).
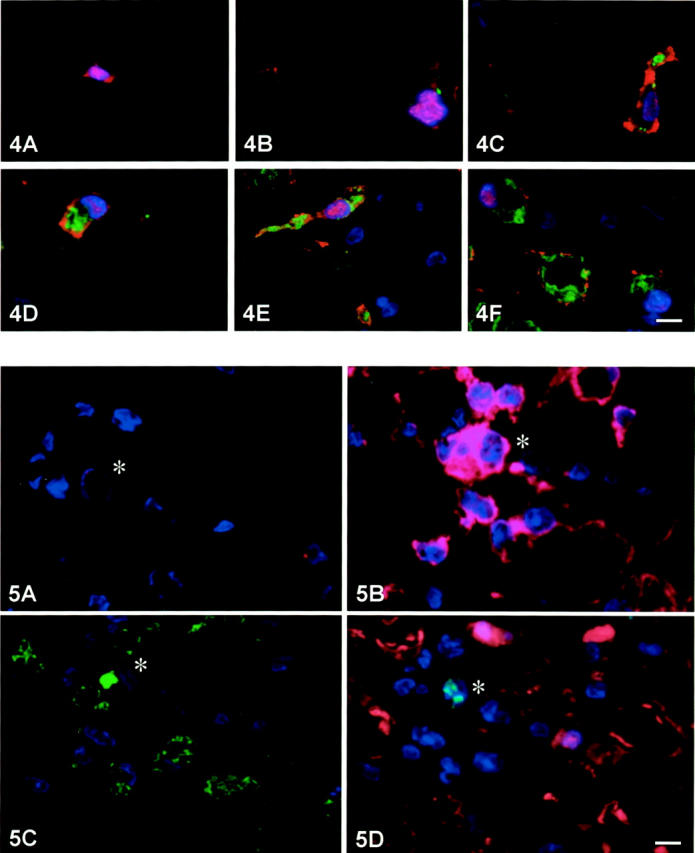
Figure 4.

Evolution of cellular changes in macrophages during Wallerian degeneration. Cross-sections through sciatic nerve 7-mm distal to a crush lesion. A–F: Co-localization of ED1 (green) and Iba1 (red) in a normal nerve (A) and 2 (B), 3 (C), 4 (D), 7 (E), and 28 days (F) after crush lesion in representative macrophages. There is a progressive expression of the ED1 antigen concomitant with a change of morphology toward large phagocytic macrophages. Blue nuclear counterstain with DAPI. Scale bar, 5 μm.
Early Morphological Alterations and de Novo Expression of ED1 Antigen in Identified Resident Endoneurial Macrophages during Wallerian Degeneration
One day after crush injury, the first time point examined in this study, no alterations of endoneurial macrophages in the distal nerve stump were observed. Two days after crush injury, the cell bodies of most Iba1-positive endoneurial macrophages began to enlarge while their cell processes appeared retracted and thicker (Figure 1, F and H) ▶ . These changes were more pronounced at days 3 and 4, and their number increased (Figure 1B) ▶ . Co-localization with the TK-tsa transgene on serial sections revealed that many of these activated endoneurial macrophages carried the TK-tsa transgene and thus were resident endoneurial cells (Figure 1, F and G) ▶ . At later time points, identification of such cells became increasingly difficult as macrophage numbers rapidly increased because of influx of macrophages from the blood.
As early as 2 days after nerve injury, occasional macrophages were found that were highly immunoreactive for the lysosomal macrophage antigen ED1 (Figure 1, I and J) ▶ , a feature not found in methyl methacrylate-embedded normal nerves. These cells always had large, rounded cell bodies with retracted processes in contrast to the slim appearance of quiescent endoneurial macrophages. Co-localization with the TK-tsa transgene on the same or adjacent sections again revealed that many of those cells contained the transgene in their genome and thus were resident endoneurial macrophages (Figure 1, I and J) ▶ . At 3 and 4 days, their numbers slightly increased. At all later time points, occasional cells of this type were always found but, much like Iba1-positive resident macrophages, they were increasingly difficult to detect among the many hematogenous macrophages infiltrating the nerve. However, ED1-positive resident macrophages carrying the TK-tsa transgene were still identified at 28 days after nerve crush, the latest time point examined.
Myelin Phagocytosis by Identified Resident Endoneurial Macrophages during Wallerian Degeneration
To further investigate features of macrophage activation in identified resident endoneurial macrophages, we studied myelin phagocytosis by co-localizing myelin basic protein as a marker for myelin-containing debris with the macrophage-specific antigens Iba1 and ED1 and the TK-tsa transgene. Two days after crush injury, ED1-positive, TK-tsa-positive resident endoneurial macrophages were found that contained myelin debris (Figure 2) ▶ , indicating early myelin phagoytosis by identified resident endoneurial macrophages. Their numbers increased at days 3 and 4. At days 7 and 14, a large number of ED-1-positive macrophages containing myelin debris were found, but the majority of those macrophages were now TK-tsa-negative and thus of hematogenous origin. At day 28, there were still many postphagocytotic, mostly TK-tsa-negative macrophages but immunoreactivity for myelin basic protein was lost in the debris. Phagocytosis was never found in Iba1-positive, ED1-negative macrophages.
Figure 2.
Phagocytosis of myelin by resident endoneurial macrophages. A and B: Adjacent semithin serial sections double-stained for the TK-tsa transgene (red spots) and either ED1 (A) or myelin basic protein (B). A representative ED1-positive resident macrophage is shown that has phagocytosed myelin debris. The specificity of the stain is documented by semithin serial sections through another resident macrophage (C–E, asterisk) stained for ED1 (C), the TK-tsa transgene (D, red spot), and myelin basic protein (E). This resident macrophage does not contain myelin debris. Two (A and B) and 3 (C–E) days after crush lesion. A and B show additional phase contrast to delineate the morphology. Blue nuclear counterstain with DAPI. Scale bars, 10 μm (A and B); 15 μm (C).
Proliferation of Identified Resident Endoneurial Macrophages during Wallerian Degeneration
To investigate whether resident endoneurial macrophages contribute to the increase in macrophage numbers after crush injury, cell proliferation was assessed by nuclear BrdU incorporation. Proliferating cell profiles were found from day 2 onwards with maximum mitotic activity on day 3. Co-localization of nuclear BrdU incorporation with the macrophage antigen ED1 revealed many ED1-negative proliferating cells, whereas ED1-positive macrophages were never found to proliferate (Figure 3, A and B) ▶ . However, when the macrophage antigen Iba1 was co-localized with BrdU, many Iba1-positive macrophages were found to incorporate BrdU between days 2 and 7, with maximum mitotic activity at day 3 (Figure 3C) ▶ . Co-localization of Iba1-positive proliferating cells with the Schwann cell marker S-100 revealed that these cells were always S-100-negative and displayed morphology different from Schwann cells. Co-localization with the TK-tsa transgene on multiple serial sections revealed that many proliferating Iba1-positive, S-100-negative macrophages carried the transgene thus indicating that they were resident endoneurial macrophages (Figure 3, D and E) ▶ .
Figure 3.

Proliferation of resident endoneurial macrophages. A and B: Adjacent serial semithin sections stained with ED1 (A) and BrdU to mark proliferating cells (B) reveal no proliferation of ED1-positive macrophages 3 days after crush injury. Note that proliferating cells (green color in B) are always ED1-negative (nuclei marked with asterisks in A). C: Co-localization of Iba1 (red) and BrdU (green) reveals many proliferating macrophages. D and E: Co-localization of Iba1 (red) and BrdU (green) on one semithin section (D) and the TK-tsa transgene on another adjacent semithin section (E, red spot) reveals that identified resident endoneurial macrophages proliferate 3 days after crush injury. Blue nuclear counterstain with DAPI. Scale bars, 30 μm (A and B); 20 μm (C); 10 μm (D and E).
Evolution of Cellular Changes in Proliferating Macrophages after Crush Injury
To clarify the apparent discrepancy between our above finding of abundant proliferation in Iba1 but not ED1-positive resident macrophages, we first co-localized the two antigens at various time points. Although occasional Iba1-positive, rounded and enlarged macrophages expressed ED1 at a high level as early as 2 days after injury, the majority of Iba1-positive macrophages revealed a much slower increase in ED1 immunoreactivity. At day 2, usually only a few small ED1-immunoreactive granules were found that then gradually increased in size until, at day 28, nearly the entire cell appeared to be filled with ED1-immunoreactive material. At the same time, the macrophages retracted their processes as described above and became large and rounded with much thicker processes or no processes at all (Figure 4) ▶ . We then fed additional chimeric rats with BrdU continuously for 7 days after crush injury to detect proliferating cells within that time period. In such animals we found ED1-positive, Iba1-positive macrophages that had incorporated BrdU into their nuclei. Additional co-localization with the TK-tsa transgene revealed that many of these cells were TK-tsa transgenic, resident macrophages (Figure 5) ▶ . These cells typically were very large rounded macrophages containing postphagocytotic vacuoles. There were still occasional Iba1-positive macrophages that had undergone proliferation as indicated by BrdU incorporation but did not express the ED1 antigen and appeared in a more ramified morphology.
Figure 5.

Evolution of cellular changes in macrophages during Wallerian degeneration. A–D: Co-localization of the TK-tsa transgene (A, red spot), Iba1 (B), ED1 (C), BrdU (D, green), and S-100 (D, red) on adjacent serial sections from an animal fed BrdU continuously for 7 days after crush lesion. The cell marked with an asterisk is positive for the TK-tsa transgene, Iba1, ED1, and BrdU, but negative for S-100, indicating its identity as a resident endoneurial macrophage that has proliferated within the 7 days between lesioning and sacrifice. As ED1-positive macrophages do not proliferate, the cell must first have proliferated and subsequently expressed the ED1 antigen. Blue nuclear counterstain with DAPI. Scale bar, 5 μm.
Discussion
The existence of a population of resident macrophages in the peripheral nerve has been recognized for more than 20 years 7,8 but their physiological and pathological functions remained uncertain to the present day. The use of a bone marrow chimera experimental model now enabled us for the first time to identify resident endoneurial macrophages in the lesioned peripheral nerve and to characterize their response to injury. We found that resident endoneurial macrophages distal to a sciatic nerve crush lesion rapidly change their shape, newly express the lysosomal macrophage activation antigen ED1, proliferate, and phagocytose myelin. We further found that this response occurred extremely early after injury and before the influx of hematogenous macrophages into the lesioned nerve.
Our findings became possible through the availability of TK-tsa transgenic Lewis rats as partners for the creation of bone marrow chimerism. The functionally silent TK-tsa transgene is constitutively present in all cells of the transgenic animals and can readily be detected by in situ hybridization. 14 The ease of handling, however, does depend on the use of methyl methacrylate as embedding medium. 16 This resin proved to be very useful for our work, allowing highly sensitive histochemical stains with superior morphological resolution and the co-localization of DNA and multiple antigens on adjacent serial sections. One drawback is that not all transgenic cells hybridize on one single semithin section. Therefore, absence of the TK-tsa in situ hybridization signal on one section does not necessarily identify this cell as hematogenous as the transgene may be located elsewhere in the nucleus and picked up only on an adjacent serial section. The technology used in our experiments is thus an excellent tool for the study of individual cells, but quantitative studies are extremely difficult to perform and require complex corrective calculations. Studies aiming at establishing a different chimera system allowing easier quantification are underway. Another point of consideration is that resident endoneurial macrophages undergo physiological turnover from the blood that was reported to amount to 60% of all endoneurial macrophages after 3 months, 13 correlating well with the calculated figure of 53% TK-tsa transgenic resident macrophages without turnover as found in our present study. Therefore, only those resident macrophages can be identified as resident in a lesion model that did not undergo turnover from the blood since bone marrow transplantation. The frequency of resident endoneurial macrophages after a lesion is thus underestimated by the rate of preceding physiological turnover.
The capacity of resident endoneurial macrophages to phagocytose myelin was previously demonstrated in organotypic sciatic nerve cultures, allowing the study of Wallerian degeneration in the absence of hematogenous macrophages. 12 In this paradigm, marked phagocytic activity was demonstrated by resident macrophages, but removal of myelin was more effectively performed by peritoneal macrophages added to the cultures, as in another model of nerve explants. 18 Similar results were obtained in vivo in animals depleted from peripheral macrophages by irradiation or toxic silica. 18,19 It was thus concluded that the contribution of resident endoneurial macrophages to myelin phagocytosis may be minor.
Our own in vivo results now indicate that not so much the quantity but rather the time course of myelin phagocytosis may highlight the functional relevance of phagocytosis by resident endoneurial macrophages. Myelin phagocytosis was demonstrated as early as 2 days after injury. It was found to be one of the first features of macrophage activation observed and occurred several days before the influx of hematogenous macrophages that begins around day 4, consistent with previous data. 20,21 Although hematogenous macrophages may be more effective in removing myelin, resident macrophages do it earlier. Phagocytosis and processing of antigen is a prerequisite for antigen presentation to T cells in inflammatory conditions. Endoneurial macrophages constitutively express MHC II molecules, 22 and strong up-regulation occurs after lesions and during inflammatory neuropathy. 20,22,23 Co-stimulatory B7-1 molecules are present on endoneurial macrophages during inflammatory neuropathies. 3 Endoneurial macrophages are thus equipped to at least potentially present antigen, and an early phagocytotic capacity as demonstrated in our work may be a prerequisite for antigen processing. However, antigen presentation is a feature of autoimmune neuropathies and not required for the cellular cascade during Wallerian degeneration as studied in the present work. It is conceivable that early phagocytosis may be a general and nonspecific feature of resident endoneurial macrophage activation independent of the cause of nerve damage. From our present knowledge it is unlikely that early phagocytosis is needed to remove myelin because this is much better done by hematogenous macrophages. 5 Rather, it may represent the alertness of resident endoneurial macrophages to pathology and the potential to present antigen.
Another feature of early activation of resident endoneurial macrophages is proliferation. This was an unexpected finding as Schwann cells are considered to be the main proliferating cell type after nerve injury. Iba1-positive proliferating cells did not co-localize with the Schwann cell marker S-100, thus excluding cross-reactivity of Iba1 with Schwann cells and proving their identity as proliferating macrophages. An increase in endoneurial macrophage numbers was also found in earlier studies using explant cultures but proliferation was not formally shown. 12 However, microglial cells of the central nervous system are known to proliferate rapidly in response to injury. 9,24 Using long-term BrdU supplementation for 7 days we could now identify full-blown activated ED1-positive macrophages that had incorporated BrdU and carried the TK-tsa transgene. These macrophages are phenotypically indistinguishable from hematogenous macrophages infiltrating the nerve in large numbers at this time point. However, the presence of the TK-tsa transgene unequivocally identifies them as resident macrophages, and BrdU incorporation documents preceding proliferation. It thus seems that the high numbers of macrophages in the degenerating peripheral nerve found at later stages during Wallerian degeneration are not exclusively derived from a hematogenous source. Rather, resident macrophages may provide a considerable and as yet unrecognized contribution, and both resident and hematogenous macrophages may feed the total endoneurial macrophage pool during Wallerian degeneration.
One of the most striking features is the extremely rapid response of resident endoneurial macrophages that involves changes in morphology and immunophenotype, proliferation, and phagocytosis. This pattern has remarkable similarities to the response of resident microglial cells, the local macrophage system of the brain. Microglia respond to a wide variety of stimuli with a graded response that ranges from a slight up-regulation of complement receptors to the differentiation into full-blown macrophages. In particular, microglial cells proliferate peaking around 2 and 3 days after a lesion, 25 just as resident endoneurial macrophages do in our system. Early activation and proliferation of a pool of resident macrophages/microglia may thus be a general feature both in the central and peripheral nervous system. However, there are also marked differences between resident endoneurial macrophages and microglia. Resident endoneurial macrophages undergo turnover from the blood 13 whereas microglial cells do not. 26 Furthermore, they constitutively express the ED2 antigen 22 whereas microglia do not. They thus more closely resemble the perivascular macrophages of the brain 15,27 that undergo an exchange from the blood 26 and also express the ED2 antigen. 28 The role of perivascular macrophages of the brain during disease is primarily undetermined but antigen presentation is one of their important capabilities. 15 Resident macrophages of the peripheral nervous system thus seem to represent a local macrophage population that carries features of both microglial cells and perivascular cells of the brain. However, it is presently undetermined whether the entire population of resident macrophages undergoes turnover from the blood or whether a truly local population persists. We also observed occasional cells that express the ED1 antigen very early and phagocytose, whereas others become activated more slowly, proliferate, and later begin to express the ED1 antigen. It is thus conceivable that resident endoneurial macrophages are not a homogenous cell population but rather comprise different subsets with different turnover characteristics and activation patterns.
In summary, our results document an extremely rapid response of resident endoneurial macrophages to injury that precedes the influx of blood-derived macrophages and resembles the microglial response of the brain to injury. Much like microglial cells of the brain, resident endoneurial macrophages may be primarily involved in surveillance of the peripheral nervous system and as sensors of pathological events. 24 By virtue of their location and rapid response, they may act primarily as regulating cells, whereas later arriving hematogenous macrophages may take the role of effector cells of tissue destruction and repair.
Acknowledgments
We thank Dr. Monika Bradl for a plasmid containing the TK-tsa sequence and Antje Stöber and Margret Lindermann for excellent technical assistance.
Footnotes
Address reprint requests to Dr. Reinhard Kiefer, Dept. of Neurology, Universitätsklinikum Münster, Albert-Schweitzer-Str. 33, D-48129 Münster, Germany. E-mail: kieferr@uni-muenster.de.
Supported by the Deutsche Forschungsgemeinschaft (Ki 532/3-1 and -2) and the Innovative Medizinische Forschung Program, Medical Faculty, Westfälische Wilhelms-Universität Münster.
References
- 1.Prineas JW: Acute idiopathic polyneuritis. Lab Invest 1972, 26:133-147 [PubMed] [Google Scholar]
- 2.Pollard JD, Baverstock J, McLeod JG: Class II antigen expression and inflammatory cells in the Guillain-Barré syndrome. Ann Neurol 1987, 21:337-341 [DOI] [PubMed] [Google Scholar]
- 3.Kiefer R, Dangond F, Mueller M, Toyka KV, Hafler DA, Hartung HP: Enhanced B7 costimulatory molecule expression in inflammatory human sural nerve biopsies. J Neurol Neurosurg Psychiatry 2000, 69:362-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiefer R, Kieseier BC, Stoll G, Hartung H-P: The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol 2001, 64:109-127 [DOI] [PubMed] [Google Scholar]
- 5.Brück W: The role of macrophages in Wallerian degeneration. Brain Pathol 1997, 7:741-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin JW, George R, Ho T: Macrophage systems in peripheral nerves. A review. J Neuropathol Exp Neurol 1993, 52:553-560 [DOI] [PubMed] [Google Scholar]
- 7.Arvidson B: Cellular uptake of exogenous horseradish peroxidase in mouse peripheral nerve. Acta Neuropathol (Berlin) 1977, 37:35-41 [DOI] [PubMed] [Google Scholar]
- 8.Oldfors A: Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol (Berlin) 1980, 49:43-49 [DOI] [PubMed] [Google Scholar]
- 9.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW: Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 2000, 30:77-105 [DOI] [PubMed] [Google Scholar]
- 10.Beuche W, Friede RL: The role of non-resident cells in Wallerian degeneration. J Neurocytol 1984, 13:767-796 [DOI] [PubMed] [Google Scholar]
- 11.Scheidt P, Friede RL: Myelin phagocytosis in Wallerian degeneration. Properties of Millipore diffusion chambers and immunohistochemical identification of cell populations. Acta Neuropathol (Berlin) 1987, 75:77-84 [DOI] [PubMed] [Google Scholar]
- 12.Hann Bonnekoh PG, Scheidt P, Friede RL: Myelin phagocytosis by peritoneal macrophages in organ cultures of mouse peripheral nerve. A new model for studying myelin phagocytosis in vitro. J Neuropath Exp Neurol 1989, 48:140-153 [DOI] [PubMed] [Google Scholar]
- 13.Vass K, Hickey WF, Schmidt RE, Lassmann H: Bone marrow-derived elements in the peripheral nervous system. Lab Invest 1993, 69:275-282 [PubMed] [Google Scholar]
- 14.Kääb G, Brandl G, Marx A, Wekerle H, Bradl M: The myelin basic protein-specific T cell repertoire in (transgenic) Lewis rat/SCID mouse chimeras: preferential V beta 8.2 T cell receptor usage depends on an intact Lewis thymic microenvironment. Eur J Immunol 1996, 26:981-988 [DOI] [PubMed] [Google Scholar]
- 15.Hickey WF, Kimura H: Perivascular microglia are bone marrow derived and present antigen in vivo. Science 1988, 239:290-292 [DOI] [PubMed] [Google Scholar]
- 16.Mueller M, Wacker K, Hickey WF, Ringelstein EB, Kiefer R: Colocalization of multiple antigens and specific DNA: a novel method using methyl methacrylate embedded semithin serial sections and catalyzed reporter deposition. Am J Pathol 2000, 157:1829-1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito D, Imai Y, Ohsawe K, Nakajima K, Fukuuchi Y, Kohsaka S: Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res 1998, 57:1-9 [DOI] [PubMed] [Google Scholar]
- 18.Beuche W, Friede RL: Myelin phagocytosis in Wallerian degeneration of peripheral nerves depends on silica-sensitive Bg/Bg-negative and Fc-positive monocytes. Brain Res 1986, 378:97-106 [DOI] [PubMed] [Google Scholar]
- 19.Perry VH, Tsao JW, Feam S, Brown MC: Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur J Neurosci 1995, 7:271-280 [DOI] [PubMed] [Google Scholar]
- 20.Stoll G, Griffin JW, Li CY, Trapp BD: Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol 1989, 18:671-683 [DOI] [PubMed] [Google Scholar]
- 21.Taskinen HS, Roytta M: The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol (Berlin) 1997, 93:252-259 [DOI] [PubMed] [Google Scholar]
- 22.Monaco S, Gehrmann J, Raivich G, Kreutzberg GW: MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J Neurocytol 1992, 21:623-634 [DOI] [PubMed] [Google Scholar]
- 23.Schmidt B, Stoll G, Hartung H-P, Heininger K, Schäfer B, Toyka KV: Macrophages but not Schwann cells express Ia antigen in experimental autoimmune neuritis. Ann Neurol 1990, 28:70-77 [DOI] [PubMed] [Google Scholar]
- 24.Kreutzberg GW: Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996, 19:312-318 [DOI] [PubMed] [Google Scholar]
- 25.Graeber MB, Tetzlaff W, Streit WJ, Kreutzberg GW: Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett 1988, 85:317-321 [DOI] [PubMed] [Google Scholar]
- 26.Hickey WF, Vass K, Lassmann H: Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropath Exp Neurol 1992, 51:246-256 [DOI] [PubMed] [Google Scholar]
- 27.Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, Watanabe E, Luoma J, Yla Herttuala S, Fraser I, Gordon S, Kodama T: Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci USA 1996, 93:3269-3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graeber MB, Streit WJ, Kreutzberg GW: Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res 1989, 22:103-106 [DOI] [PubMed] [Google Scholar]


