Abstract
To investigate the association between the expression of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) and the clinicopathological features in lepidic and invasive components of adenocarcinoma of the lung, we performed immunostaining for type IV collagen, various MMPs, and TIMPs in 27 cases of invasive adenocarcinomas and 5 cases of atypical adenomatous hyperplasia of alveolar epithelial cells (AAH). Mean extent of lepidic growth was 61% and the survival was significantly better in cases with 50% or more lepidic component. The preservation of type IV collagen in lepidic areas correlated inversely with lymphatic or vascular invasion (P = 0.02 and 0.002, respectively). Five-year survival was reduced in cases showing destruction of type IV collagen (P = 0.004) or expression of MMP-2 (P = 0.008) in lepidic areas. MMP-2 co-localized with MT-1-MMP (its activating enzyme) and TIMP-2 in neoplastic cells. Reactivity for other MMPs and TIMPs did not correlate with destruction of type IV collagen or prognosis. Type IV collagen was preserved in all cases of AAH. MMP-2, but not MT-1-MMP, was expressed in two of the five cases of AAH. Immunostaining for type IV collagen MMP-2 is useful in evaluating the prognosis of the lung.
Criteria for the subclassification of adenocarcinoma of the lung have changed with the 1999 World Health Organization (WHO)/International Association for the Study of Lung Cancer (IASLC) proposal for histological typing of lung and pleural tumors. 1 In the 1981 WHO classification, lung adenocarcinomas were divided into the following four subtypes: acinar, papillary, bronchioloalveolar, and solid with mucin formation. 2 In this classification, 2 there was no specification about the extent of lepidic growth required for the diagnosis of bronchioloalveolar carcinoma (BAC). In the 1999 WHO/IASLC classification, it was recognized that the majority of pulmonary adenocarcinomas show a mixture of the four major subtypes mentioned above and it was proposed that such tumors be classified as adenocarcinoma, mixed subtype. 1 The criteria for BAC were restricted to tumors with a pure lepidic growth pattern without vascular, stromal, or pleural invasion. 1 The rationale for this change was based on a study by Noguchi and colleagues, 3 who found that BACs showing no invasive growth had a 100% 5-year survival, in contrast to 75% for adenocarcinomas showing both lepidic and invasive growth. The 1999 WHO/IASLC panel emphasized the need for detailed morphological studies of the invasiveness of pulmonary adenocarcinoma with careful clinical correlation. 1 The need for better understanding of the relationship between the lepidic and invasive components of pulmonary adenocarcinoma led us to perform an immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in a series of these lesions.
Recent studies of the activity of MMPs and TIMPs in invasive neoplasms have indicated that these enzymes play important roles in the degradation of connective tissue that is associated with the development of metastases. 4-8 In particular, MMP-2 and MMP-9 (gelatinase A and gelatinase B) are involved in the spread of carcinomas. These MMPs lyse partially degraded fibrillar collagens as well as elastin, but also have a high degree of affinity for type IV collagen, a major component of epithelial basement membranes, which are lysed during metastatic invasion. Galateau-Salle and colleagues 9 conducted immunohistochemical studies of the reactivity of bronchial squamous preneoplastic and neoplastic lesions from cigarette smokers, including basal cell hyperplasia, squamous metaplasia, dysplasia, carcinoma in situ, and invasive squamous cell carcinoma, for MMPs, TIMPs, and type IV collagen. They concluded that bronchial squamous preneoplastic lesions show increased reactivity for MMPs, in association with destructive changes in the epithelial basement membranes, before the development of actual neoplasia or invasion of the subjacent connective tissue.
Most of the MMPs are produced in the form of biologically inactive proenzymes, which need to be activated to become biologically functional. Several mechanisms can result in the activation of MMP-2. The most important of these involves the action of a membrane-type 1 matrix metalloproteinase (MT-1-MMP), which cleaves a portion of the carboxy terminus of pro-MMP-2. 10 The development of antibodies against MT-1-MMP has made it possible to evaluate the expression of both MMP-2 and its activating enzyme in the same tissue section, thus providing new information on the relationship between MMPs and the degradation of basement membranes.
Atypical adenomatous hyperplasia (AAH) was added to the group of preinvasive lesions in the 1999 WHO/IASLC classification, because it is thought to be a precursor of adenocarcinoma. 1 It is a millimeter-sized nodular lesion, in which the alveoli and respiratory bronchioles are lined by slightly atypical pneumocytes. 11,12 AAH is distinguished from nonmucinous BAC in that it has less cytological atypia, cellular crowding, and overlapping of nuclei, as well as less prominent nucleoli, and smaller cell size. 13
The significance of central scarring in adenocarcinomas has been debated for several decades. Initially, it was thought that adenocarcinomas arose in association with pre-existing scars, and the concept of scar carcinoma was advanced. 14,15 However, subsequent studies provided morphological, 16-18 clinical, and collagen analyses that favored the concept that the scar in most lung carcinomas was caused by tumor invasion. 19,20 The mechanisms proposed for this scarring include vascular 17 or airway 18 occlusion by tumor resulting in alveolar collapse and a dense fibrosing scar.
It has been reported that pulmonary adenocarcinomas with central scarring have a poorer prognosis than those without central scarring. 3 However, only a few studies have compared the results of staining for type IV collagen and MMPs in AAH and in BACs versus the invasive components of pulmonary adenocarcinomas. 7,8 In the present study, we have compared the preservation of the integrity of type IV collagen with the expression of MMPs and TIMPs, particularly of MMP-2 and MT-1-MMP, in both the lepidic and the invasive areas of pulmonary adenocarcinomas. We have also investigated the association between the expression of MMPs and the clinicopathological features and prognosis of these tumors.
Materials and Methods
Tissue Samples
The tissues studied consisted of 27 invasive adenocarcinomas with lepidic areas and 5 AAH lesions, obtained by lobectomy or more localized resection by thoracoscopy, without neoadjuvant chemotherapy or radiotherapy. These procedures were performed at the National Defense Medical College Hospital in Japan from 1989 to 1999. The study was approved by the Committee on Human Research of the National Defense Medical College. All lesions appeared to have originated from peripheral areas of the lungs, and were fully resectable. The patients were 10 men and 17 women and their mean age was 66 years (range, 44 to 81 years). The patients with AAH were three women and two men, with a mean age of 65 years (range, 52 to 74 years). Clinical and pathological data on these patients are summarized in Table 1 ▶ . The TNM classification was used for clinical staging. 21 The tumors were classified according to the 1999 WHO classification. 1
Table 1.
Clinical Features of 27 Patients with Pulmonary Adenocarcinoma with Bronchioalveolar Component and 5 Patients with Atypical Adenomatous Hyperplasia
| Case | Age (years)/sex | Location | Size (cm) | Stage (TNM) | Subtype | Growth pattern | Extent of lepidic growth (%) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma | ||||||||
| 1 | 77F | RLL | 4.0 | 3a (220) | NM | BAC, A, P | 20 | 10 (Dead) |
| 2 | 69F | LUL | 2.0 | 1a (100) | NM | BAC, A | 95 | 114 (Alive) |
| 3 | 59F | RUL | 3.3 | 1b (200) | NM | BAC, A | 90 | 110 (Alive) |
| 4 | 60M | RUL | 3.8 | 1b (200) | NM | BAC, A | 90 | 54 (Alive) |
| 5 | 44F | RUL | 2.9 | 3b (420) | NM | BAC, A, P | 25 | 38 (Dead) |
| 6 | 59F | RUL | 1.9 | 2a (110) | NM | BAC, A, P | 50 | 31 (Alive) |
| 7 | 70M | RLL | 5.0 | 1b (200) | M | BAC, A | 20 | 62 (Alive) |
| 8 | 78F | LLL | 6.5 | 1b (200) | NM | BAC, A, P | 10 | 12 (Dead) |
| 9 | 75M | RUL | 3.4 | 3a (310) | M | BAC, A, P, S | 25 | 25 (Dead) |
| 10 | 68M | RLL | 2.2 | 1a (100) | NM | BAC, A | 90 | 37 (Alive) |
| 11 | 60F | RUL | 2.0 | 1a (100) | M | BAC, A, P, S | 20 | 36 (Alive) |
| 12 | 65M | RUL | 2.9 | 3a (120) | NM | BAC, A, S | 30 | 16 (Dead) |
| 13 | 81M | LUL | 5.0 | 1b (200) | M | BAC, A | 90 | 22 (Alive) |
| 14 | 50F | RUL | 1.9 | 1a (100) | NM | BAC, A | 95 | 19 (Alive) |
| 15 | 49F | RLL | 1.9 | 1a (100) | M | BAC, A | 90 | 19 (Alive) |
| 16 | 59F | RUL | 2.7 | 1a (100) | NM | BAC, A | 30 | 19 (Alive) |
| 17 | 79M | RUL | 2.6 | 1a (100) | NM | BAC, A | 95 | 18 (Alive) |
| 18 | 58M | LLL | 4.0 | 2b (210) | NM | BAC, A, P, S | 75 | 17 (Alive) |
| 19 | 78F | RUL | 0.7 | 1a (100) | NM | BAC, A | 95 | 17 (Alive) |
| 20 | 76F | LLL | 1.6 | 1a (100) | M | BAC, A | 80 | 12 (Alive) |
| 21 | 73M | RUL | 0.9 | 1a (100) | NM | BAC, A | 75 | 12 (Alive) |
| 22 | 62F | LLL | 4.5 | 1b (200) | M | BAC, A | 90 | 12 (Alive) |
| 23 | 72F | LUL | 3.5 | 3a (220) | NM | BAC, A | 25 | 11 (Dead) |
| 24 | 62F | LLL | 2.2 | 1a (100) | NM | BAC, A | 50 | 10 (Alive) |
| 25 | 72M | RML | 2.0 | 1a (100) | NM | BAC, A | 90 | 9 (Alive) |
| 26 | 67F | RML | 1.8 | 1a (100) | NM | BAC, A, P | 60 | 8 (Alive) |
| 27 | 64F | RUL | 3.3 | 1b (200) | NM | BAC, A, P | 60 | 6 (Alive) |
| Atypical adenomatous hyperplasia | ||||||||
| 1 | 74F | RML | 0.8 | |||||
| 2 | 68M | RLL | 0.2 | |||||
| 3 | 52F | LUL | 0.2 | |||||
| 4 | 65M | RUL | 0.6 | |||||
| 5 | 64F | RUL | 0.3 |
Abbreviations: RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; NM, nonmucinous type; M, mucinous type; BAC, bronchiloalveolar component; A, acinar pattern; A, P, acinar and papillary patterns; A, S, acinar and solid pattern; A, P, S, acinar, papillary and solid with mucin formation. All categories were classified according to the criteria of the World Health Organization.1 TNM, T, tumor depth; N, lymph node metastasis; M, distant metastasis.
Preparation of Tissues
For histological study, tissues obtained from the largest cut surface of each tumor were fixed with buffered 10% formalin and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin and eosin (H&E). Each tumor was classified according to subtype (nonmucinous, mucinous, and mixed), growth pattern, and extent of lepidic growth. The extent of lepidic growth was estimated as a percentage of the overall tumor. The periodic acid-methenamine silver (PAM)-staining method was used to evaluate the basement membranes of the alveolar walls. 22
Immunohistochemical Staining
Sections of paraffin-embedded tissues were used for the immunohistochemical staining procedures described below. The primary antibodies used in the study consisted of mouse monoclonal antibodies against the following components: MMP-1 (dilution, 1:500), MMP-3 (1:200), MMP-7 (1:100), MMP-9 (1:200), MT-1-MMP (1:100), MT-2-MMP (1:100), MT-3-MMP (1:100) (Chemicon International Inc., Temecula, CA), and type IV collagen (1:100) (DAKO, Carpinteria, CA) and rabbit polyclonal antibodies against MMP-2 (1:500), TIMP-1 (1:500) and TIMP-2 (1:100) prepared in the laboratory of Dr. W. Stetler-Stevenson (National Cancer Institute, National Institutes of Health, Bethesda, MD).
Sections were deparaffinized, rehydrated, treated with 0.4% pepsin (Sigma, St. Louis, MO) in 0.01 N HCl at 37°C (15 minutes for MMP-1, MMP-2, MMP-7, MT-1-MMP, and MT-2-MMP, and 30 minutes for MMP-9, TIMP-1, and TIMP-2). Pretreatment with 0.04% protease (P-5380, Sigma) was used for the demonstration of type IV collagen. No pretreatment was used to stain for MMP-3 and MT-3-MMP. The sections were treated with 0.3% hydrogen peroxide in methanol for 30 minutes at room temperature to block endogenous peroxidase activity. Then they were washed with phosphate-buffered saline (PBS) (0.01 mol/L, pH 7.2). The tissues to be reacted with monoclonal or polyclonal antibodies were incubated with 10% normal horse or goat serum, respectively, for 30 minutes to block nonspecific immunoglobulin binding. The sections were subsequently incubated for 2 hours at room temperature with the primary antibodies. After four washes with PBS (15 minutes each), the color was developed using the EnVision System (DAKO) and Vectastain kit (Vector Laboratories, Burlingame, CA) according to the manufacturers’ instructions. Then they were counterstained with hematoxylin and mounted. Negative immunohistochemical control procedures included: 1) omission of the primary antibody and 2) replacement of the primary antibody by normal mouse or rabbit IgG in appropriate concentrations. These control procedures gave negative results.
We observed both peripheral lepidic and central collapsed areas in the tumors examined. In both types of areas, the distribution of reactive tumor cells was graded as: 0, none; 1, 1 to 10%; 2, 10 to 50%; and 3, >50%. The intensity of the staining was graded as: 0, negative; 1, mild; 2, moderate; and 4, strong. Furthermore, we calculated the sum of these two parameters to evaluate the overall expression of MMPs and TIMPs, and created an immunohistochemical score based on this sum: 0, negative; 1 to 2, low; 3 to 4, moderate; and 5 to 6, high. Type IV collagen was rated as: + (normal) and − either partially (mainly in areas of lepidic growth) or completely (mainly in areas of central scars) destroyed.
Dual Labeling for Confocal Microscopy
For immunofluorescent staining, sections were deparaffinized, treated with pepsin or protease as described above, washed, and incubated with the mixture of 10% normal horse serum and 10% normal goat serum for 30 minutes at room temperature. They were then incubated overnight at 4°C with the mixture of a mouse monoclonal antibody (type IV collagen, MT-1-MMP, or TIMP-2) and a rabbit polyclonal antibody (MMP-2). In the mixture, the antibodies were diluted 1:20 for type IV collagen, 1:20 for MT-1-MMP, 1:20 for TIMP-2, and 1:100 for MMP-2. After washing with PBS, the sections were incubated with a mixture of the two secondary antibodies [fluorescein isothiocyanate-conjugated horse anti-mouse IgG, diluted 1:100 (FI-2000; Vector), and Texas red-conjugated goat anti-rabbit IgG, diluted 1:100 (TI-1000; Vector)] for 1 hour at room temperature. After washing, the nuclei were counterstained with 4′6-diamidino-2-phenylindole (DAPI-containing mounting medium H-1200; Vector), mounted, and examined with a confocal microscope (model TCS-4D/DMIRBE; Leica, Heidelberg, Germany) equipped with argon and argon-krypton lasers. In the preparations stained as described above, a green fluorescence indicated either type IV collagen, MT-1-MMP, or TIMP-2; a red fluorescence, MMP-2; a yellow fluorescence, co-localization of the red and green signals; and a blue fluorescence, nuclear DNA. A yellow autofluorescence, not indicative of co-localization, was observed in elastic fibers and in some macrophages and red blood cells. This autofluorescence was recognized by its presence in unstained sections and in immunohistochemical-negative control preparations.
Statistical Analysis
We compared the extent of lepidic growth and the preservation of type IV collagen with the expression of MMPs in the samples of adenocarcinoma with BAC component and AAH. The chi-square test was performed to determine the significance of the relationships between the expression of MMPs and the clinicopathological features, ie, tumor size, lymph node metastasis, lymphatic invasion, and vascular invasion. Survival was compared between patients whose tumors showed greater or equal to versus less than 50% lepidic areas. Survival rates were estimated by the method of Kaplan and Meier, and comparisons of these rates were made with the log-rank test. A P value of <0.05 was considered to be statistically significant.
Results
Clinical Features
The TNM and staging information on the 27 patients in the study is summarized in Table 1 ▶ . Thirteen tumors were stage 1a, 7 were stage 1b, 4 were stage 3a, and 1 each were stage 2a, 2b, and 3b. The total follow-up time for all patients was 70.5 years with a mean for individual patients of 2.3 years (range, 0.48 to 9.34 years). The 78-year-old female patient with a stage 1 tumor who died, developed tumor recurrence in multiple bones and she died from tumor 12 months after surgery.
Pathological Findings
The mean tumor size was 2.9 cm (range, 0.7 to 6.5 cm). Data on the location of the tumors are summarized in Table 1 ▶ . The 27 adenocarcinomas were classified as the mixed subtype because they showed mixtures of lepidic components and other subtypes of invasive lesions. The lepidic component consisted of atypical pneumocytes that proliferated along slightly thickened alveolar walls in a lepidic manner. The mean extent of lepidic growth was 61% (range, 10 to 95%). The invasive components consisted of varying combinations of the acinar, papillary, and solid with mucin formation subtypes of adenocarcinoma. In the invasive areas the epithelial proliferation was associated with destruction of alveolar basement membranes and proliferation of stromal cells (Figure 1, A and B) ▶ . Inflammatory cells adjacent to the tumor cells and areas of invasion of pulmonary lymphatics or blood vessels were recognized in 13 cases.
Figure 1.
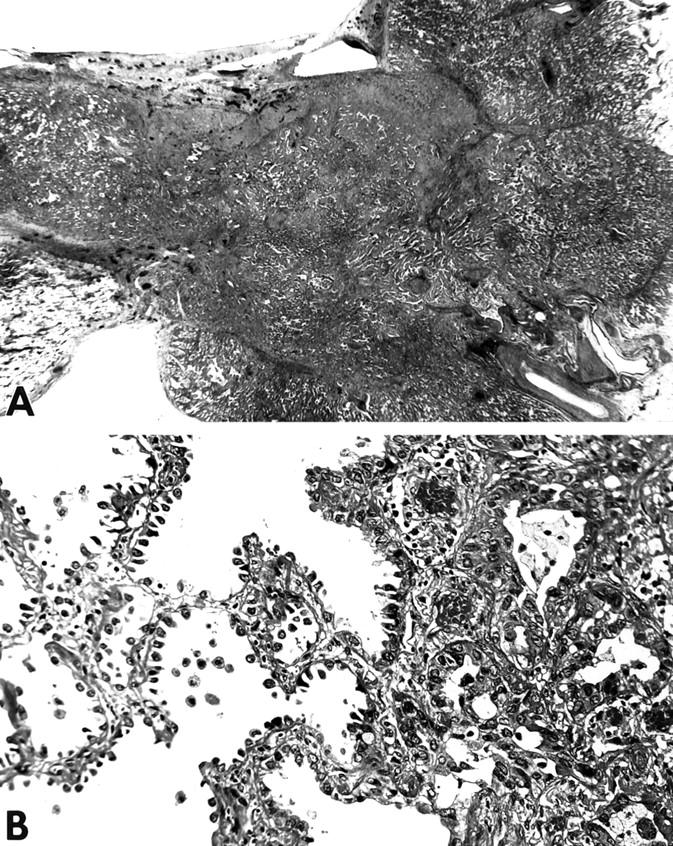
Histology of pulmonary adenocarcinoma showing lepidic growth. A: Low-magnification photomicrograph showing typical pulmonary adenocarcinoma. Lepidic growth is recognized at the periphery (top left) of the tumor and acinar growth and scarring are present in the central area. H&E stain; original magnification, ×6. B: There is prominent proliferation of pneumocytes along the slightly thickened alveolar walls in the area of lepidic growth (left). In an invasive area (right), the tumor shows an acinar pattern and invades the stroma. H&E stain; original magnification, ×80.
In all AAH cases, we observed pneumocytes proliferating in a lepidic manner along slightly thickened alveolar walls, without associated inflammation or scarring (Figure 2, A and B) ▶ . Two distinct types of basement membranes (epithelial and endothelial) were clearly recognized in lepidic areas of alveolar walls in sections stained by the PAM method (Figure 3, A and B) ▶ . In invasive areas, these basement membranes were abnormally shaped or absent.
Figure 2.
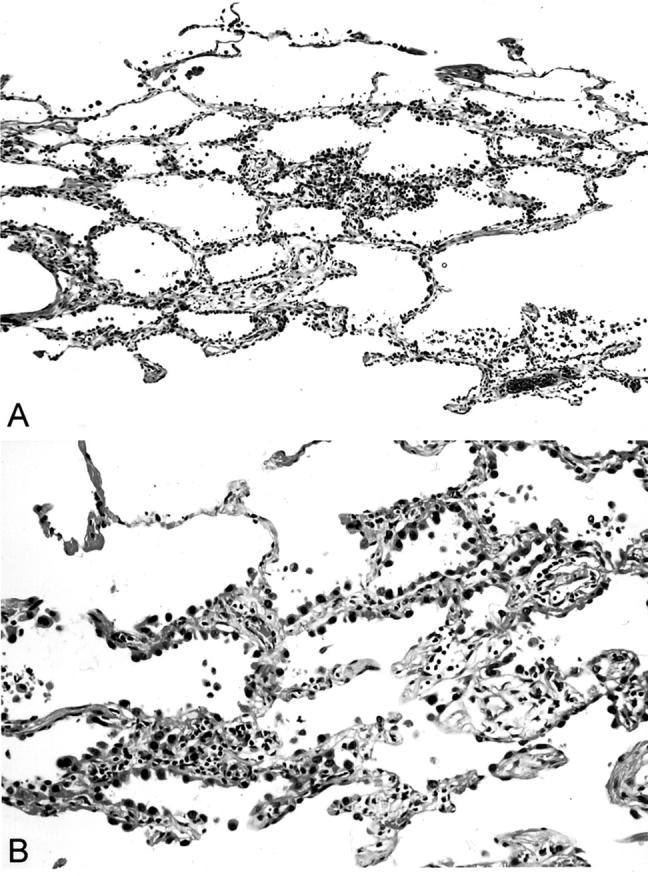
Histology of atypical AAH. A: The lesion consists of bronchioloalveolar proliferation of atypical pneumocytes. There is mild interstitial thickening and little inflammation. H&E stain; original magnification, ×48. B: There is less cytological atypia and cell crowding in AAH than in bronchioloalveolar carcinoma. H&E stain; original magnification, ×250.
Figure 3.
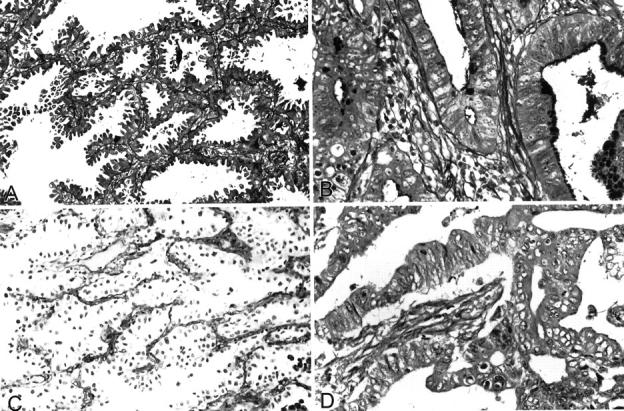
Alterations of the epithelial basement membranes in pulmonary adenocarcinoma showing lepidic growth. A: The PAM stain shows intact basement membrane in this lepidic area. B: The basement membrane is markedly destroyed in this area of invasive adenocarcinoma. PAM stain. C and D: Immunostaining for type IV collagen. The basement membrane is intact in lepidic area (C) and markedly destroyed basement membrane in area of invasive adenocarcinoma (D). Peroxidase method and hematoxylin counterstain; original magnifications, ×250.
Immunohistochemical Staining
Type IV Collagen
In all tissue samples, the immunoreactivity for type IV collagen was localized in the basement membranes of alveolar epithelial cells, endothelial cells, and smooth muscle cells. There was a close correspondence between the results obtained using the PAM stain and the immunoperoxidase or immunofluorescence methods for type IV collagen. Immunostaining for type IV collagen showed that the epithelial basement membranes in areas of lepidic growth were not damaged in 16 cases (Figure 3C) ▶ but were partially destroyed in the other 11. There was a negative correlation between the preservation of type IV collagen in basement membranes and the occurrence of lymph node metastasis (P = 0.03), as well as lymphatic (P = 0.02) and vascular invasion (P = 0.002). Furthermore, the 5-year survival was lower in cases showing destruction of type IV collagen than in those in which this component was well preserved in lepidic areas (20% versus 100%, P = 0.004). The basement membranes were either fragmented or completely destroyed in most invasive areas (Figure 3D) ▶ . Preservation of type IV collagen was observed in all areas of AAH.
MMP-2
The reactivity for MMP-2 in the cytoplasm of the tumor cells varied from negative to strong. This reactivity was mild to strong in 26 of the 27 cases of lepidic areas and mild to strong in all cases having invasive areas (Figure 4, A and B) ▶ . The staining for MMP-2 was significantly stronger in invasive areas than in lepidic areas (P < 0.001). The MMP-2 score correlated with the occurrence of lymph node metastasis (P = 0.01) and vascular invasion (P = 0.003). In contrast, there was a negative correlation between the expression of MMP-2 and the preservation of type IV collagen (P = 0.02). Two of the five cases of AAH showed high expression of MMP-2. The other three cases were unreactive for this enzyme.
Figure 4.
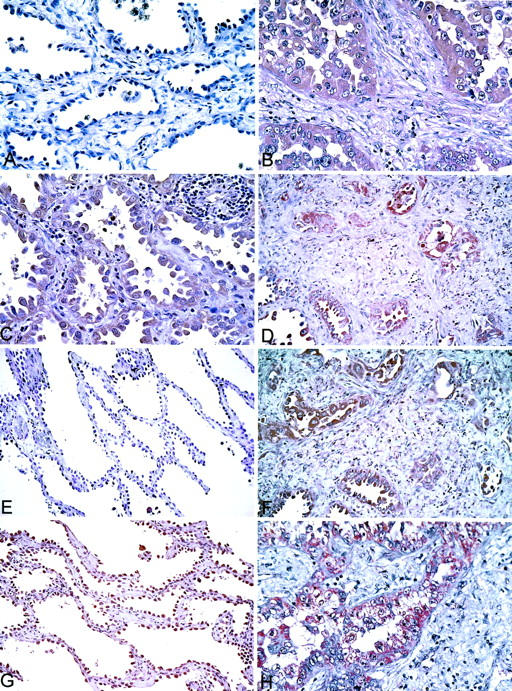
Immunostaining of lepidic and invasive areas for MMPs and TIMPs. Peroxidase method and hematoxylin counterstain. A and B: MMP-2. The tumor cells in a lepidic area are negative (A), but are positive in area of invasive adenocarcinoma (B). C and D: MT-1-MMP. A positive reaction is evident in the tumor cells in a BAC component (C) and in an area of invasive adenocarcinoma (D). E and F: TIMP-1. Area of BAC (E) is negative, whereas area of invasive adenocarcinoma (F) is positive. G and H: TIMP-2. Area of BAC (G) is weakly positive. The reaction is much stronger in area of invasive adenocarcinoma (H). Original magnifications: ×160 (A–D, F, and H); ×80 (E and G).
MT-1-MMP
Reactivity for MT-1-MMP in areas of lepidic growth was observed in 23 of the 27 cases of adenocarcinoma (Figure 4C) ▶ , but in 0 of the 5 cases of AAH. In invasive areas, this reactivity for MT-1-MMP was found in 25 of the 27 patients (Figure 4D) ▶ . The score of the reactivity for MT-1-MMP was greater in invasive areas than in lepidic areas.
Other MMPs
The reactivity for other MMPs varied considerably in the cytoplasm of the tumor cells in both lepidic and invasive areas, ranging from negative to strong for MMP-3, MMP-9, MT-2-MMP, and MT-3-MMP, and from mild to strong for MMP-1 and MMP-7. The reaction for other MMPs in areas of AAH ranged from mild to moderate for MMP-3, MMP-7, MT-2-MMP, and MT-3-MMP, moderate to strong for MMP-1, and from negative to mild for MMP-9. No significant correlation was found between the expression of any of these MMPs and the clinicopathological features of the tumors.
TIMP-1 and TIMP-2
The staining for TIMP-1 (Figure 4, E and F) ▶ and TIMP-2 (Figure 4, G and H) ▶ ranged from negative to strong in lepidic and invasive areas. The reaction of AAH cells was mild for TIMP-1 and ranged from negative to mild for TIMP-2. The staining for TIMP-2 was significantly stronger in invasive areas than in lepidic areas (P < 0.001). Furthermore, the expression of TIMP-2 correlated significantly with that of MMP-2 in invasive areas (P = 0.046). No other correlations were found between these reactivities and the clinicopathological features of the tumors. Data on the expression of MMP-2, MT-1-MMP, TIMP-1, TIMP-2, and type IV collagen are summarized in Table 2 ▶ .
Table 2.
Expression of MMP-2, MT-1-MMP, TIMP-1, TIMP-2, and Type IV Collagen in Lepidic and Invasive Areas of Pulmonary Adenocarcinomas with Bronchioloalveolar Component and Atypical Adenomatous Hyperplasia
| Case | Type IV collagen | MMP-2 | MT-1-MMP | TIMP-1 | TIMP-2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LEP | INV | LEP | INV | LEP | INV | LEP | INV | LEP | INV | |
| Adenocarcinoma | ||||||||||
| 1 | − | − | 2 | 3 | 1 | 1 | 1 | 3 | 2 | 3 |
| 2 | + | − | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 |
| 3 | + | − | 1 | 2 | 0 | 1 | 0 | 2 | 1 | 2 |
| 4 | + | − | 1 | 2 | 1 | 1 | 2 | 3 | 1 | 2 |
| 5 | − | − | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 |
| 6 | + | − | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 1 |
| 7 | + | − | 1 | 1 | 0 | 0 | 1 | 3 | 1 | 2 |
| 8 | − | − | 2 | 3 | 1 | 3 | 0 | 2 | 1 | 1 |
| 9 | − | − | 2 | 3 | 1 | 2 | 0 | 2 | 0 | 3 |
| 10 | + | − | 1 | 3 | 2 | 2 | 0 | 0 | 0 | 1 |
| 11 | + | − | 2 | 3 | 1 | 1 | 1 | 1 | 0 | 0 |
| 12 | − | − | 2 | 3 | 1 | 2 | 1 | 2 | 0 | 2 |
| 13 | + | − | 1 | 3 | 2 | 2 | 1 | 2 | 1 | 3 |
| 14 | − | − | 2 | 2 | 1 | 3 | 1 | 3 | 0 | 2 |
| 15 | + | + | 0 | 1 | 2 | 2 | 2 | 3 | 2 | 3 |
| 16 | − | − | 2 | 3 | 2 | 2 | 3 | 3 | 2 | 3 |
| 17 | + | + | 1 | 3 | 1 | 2 | 3 | 3 | 0 | 2 |
| 18 | − | − | 3 | 3 | 2 | 3 | 1 | 3 | 1 | 3 |
| 19 | + | − | 1 | 2 | 3 | 3 | 1 | 3 | 1 | 2 |
| 20 | + | − | 1 | 2 | 2 | 2 | 0 | 1 | 0 | 1 |
| 21 | − | − | 2 | 3 | 2 | 1 | 3 | 3 | 2 | 3 |
| 22 | + | + | 1 | 3 | 1 | 3 | 2 | 3 | 1 | 3 |
| 23 | − | − | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 3 |
| 24 | + | − | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 2 |
| 25 | + | − | 2 | 3 | 3 | 3 | 1 | 3 | 2 | 3 |
| 26 | − | − | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| 27 | + | − | 1 | 3 | 0 | 1 | 1 | 3 | 3 | 3 |
| Atypical adenomatous hyperplasia | ||||||||||
| 1 | + | 2 | 0 | 1 | 1 | |||||
| 2 | + | 0 | 0 | 1 | 0 | |||||
| 3 | + | 2 | 0 | 1 | 0 | |||||
| 4 | + | 1 | 0 | 1 | 0 | |||||
| 5 | + | 0 | 0 | 1 | 1 | |||||
Note: Staining was graded as 0, absent; 1, mild; 2, moderate; 3, strong: −, type IV collagen was destroyed; +, remained.
Abbreviations: LEP, lepidic area; INV, invasive area.
Immunofluorescence Staining
The destruction of type IV collagen in lepidic and invasive areas was readily recognized in sections subjected to dual staining for type IV collagen and MMP-2 (Figure 5, A and B) ▶ . In agreement with the results of the immunoperoxidase method, the tumor cells in both lepidic and invasive areas were reactive for MMP-2. Type IV collagen was preserved in lepidic areas of some cases in which MMP-2 was strongly expressed. MMP-2 and MT-1-MMP were co-localized in the cytoplasm of the tumor cells (Figure 5, C and D) ▶ . Both MMP-2 and TIMP-2 were strongly expressed in the cytoplasm of carcinoma cells in invasive areas (Figure 5E) ▶ . The expression of MMP-2, MT-1-MMP, and TIMP-2 showed accentuation in the basal aspect of the carcinoma cells.
Figure 5.
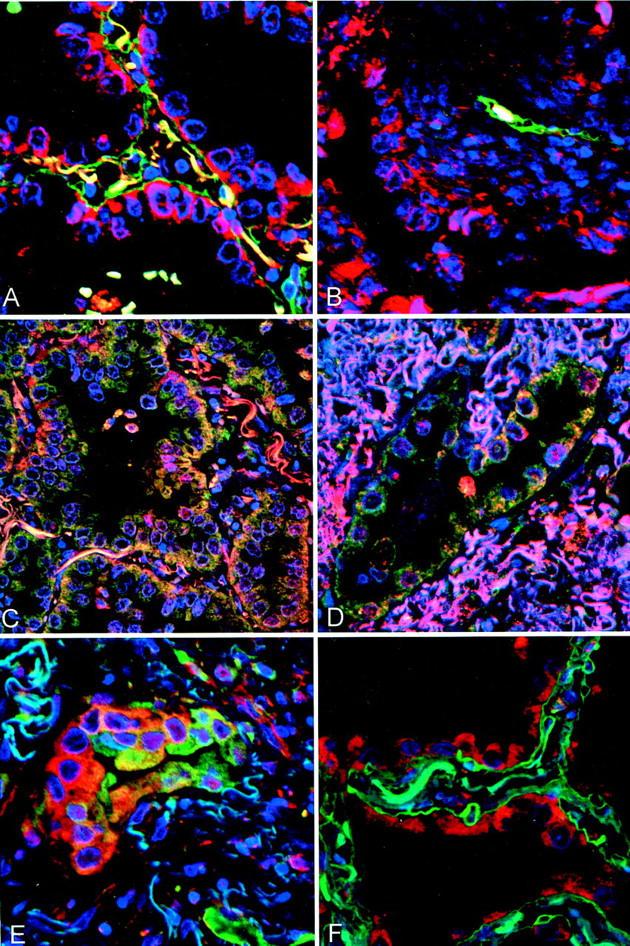
Confocal microscopic images of dual immunofluorescent labeling (red and green) and nuclear counterstaining with 4′6-diamidino-2-phenylindole (blue). A and B: Type IV collagen (green) and MMP-2 (red) in adenocarcinoma. Lepidic area (A) shows intact basement membrane, highlighted by type IV collagen, and expression of MMP-2 in BAC tumor cells, with accentuation in the basal aspect of the cytoplasm along the basement membrane. Invasive adenocarcinoma (B) shows expression of MMP-2 by tumor cells and marked loss of type IV collagen in destroyed basement membrane. C and D: MMP-2 (red) and MT-1-MMP (green) in adenocarcinoma. Areas of lepidic growth (C) and invasive adenocarcinoma (D) show yellow fluorescence, indicative of co-localization of the two reactions, in the cytoplasm of the tumor cells. This reactivity is accentuated in the basal aspect of the cytoplasm along the basement membrane. E: TIMP-2 (green) and MMP-2 (red) in adenocarcinoma. There is considerable variation in the expression of these two components. Some tumor cells (left) appear more red and others (right) show more green. Some cells show yellow staining indicative of co-localization of the two reactions. F: Type IV collagen (green) and MMP-2 (red) in AAH. Type IV collagen in the basement membrane is intact. The pneumocytes express MMP-2, without accentuation in the basal aspect along the basement membrane (such as that seen in lepidic areas of adenocarcinomas). Original magnifications, ×400.
In all AAH cases, type IV collagen was preserved, regardless of the expression of MMP-2 (Figure 5F) ▶ . No accentuation of the latter along the basement membrane was observed.
Correlation with Prognosis
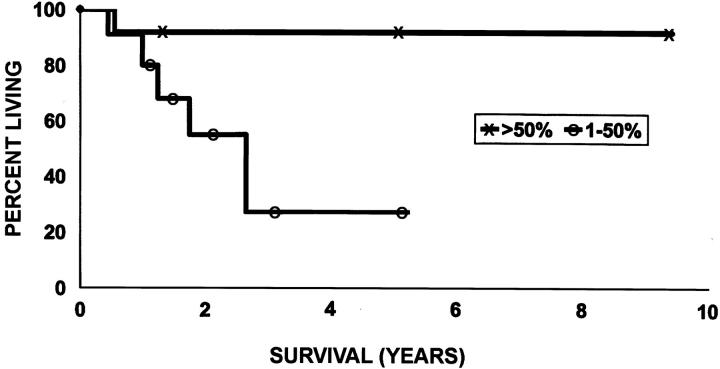
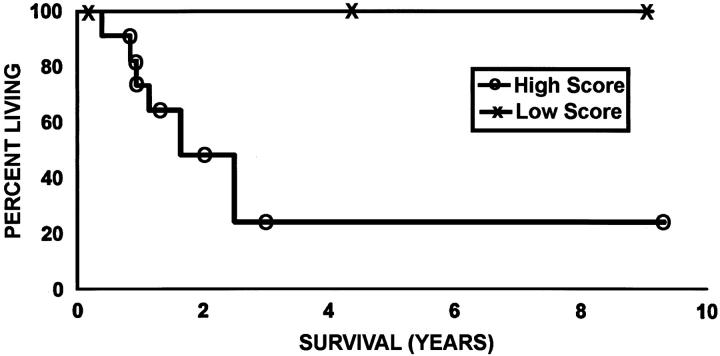
The prognosis was significantly better in cases showing >50% lepidic growth than in those showing <50% (P = 0.05) (Figure 6) ▶ . The 5-year survival was reduced in cases showing a moderate or high expression of MMP-2 compared with those showing negative or low expression of this enzyme in lepidic areas (24% versus 100%, P = 0.008) (Figure 7) ▶ .
Figure 6.
Kaplan-Meier curve showing survival according to the extent of lepidic growth. The survival of patients with tumor showing >50% lepidic growth is significantly better than that of patients with 1 to 50% (P = 0.05).
Figure 7.
Kaplan-Meier curve showing survival according to MMP-2 score in lepidic areas. Survival is better in patients with low MMP-2 score than in those with a high score (P = 0.008).
Discussion
The present study shows that the expression of MMP-2 and MT-1-MMP in invasive pulmonary adenocarcinomas with a BAC component correlates with the destruction of type IV collagen in the epithelial basement membranes and with the clinicopathological features of the tumors. Furthermore, the expression of MMP-2 and MT-1-MMP was accentuated in the basal aspect of the cytoplasm of carcinoma cells along the basement membrane, indicating an attempt to lyse this membrane. This feature was not seen in AAH. In addition, accumulation of MMP-2 was observed at the edges of nests of invasive tumor cell, suggesting a leading edge of invasion. Other areas of the same nests of tumor cells, showed increased expression of TIMP-2. This change would seem to represent a trailing edge and would favor the accumulation of extracellular matrix. Our results further demonstrate that the cases showing >50% lepidic growth in histological sections had a better prognosis than those in which the extent of this alteration did not exceed 50%. These data support the concept that stromal involvement and metastases of pulmonary adenocarcinomas with a BAC component do not occur without invasive growth that is associated with extensive destruction of type IV collagen. This is in accord with the concept that the epithelial basement membrane plays the role of a protective barrier that prevents invasion of stroma and lymphatic or blood vessels by the tumor. These findings are also consistent with those of the study of Noguchi and colleagues, 3 who found a reduced survival in patients with pulmonary adenocarcinomas with lepidic growth and invasion compared to those with purely lepidic growth.
Expression of MMP-2 has been observed in a variety of human tumors, and the importance of this enzyme in tumor cell invasion and metastasis has been widely recognized. Using a substrate capture enzyme-linked immunosorbent assay, Garbisa and colleagues 6 found a good correlation of serum levels of MMP-2 with lung cancer metastasis and response to therapy. Kawano and colleagues 7 reported that high levels of MMP-2 were expressed in most types of lung tumors. Kitamura and colleagues 8 studied expression of MMP-2 in 48 peripherally located adenocarcinomas of the lung and 33 cases of AAH. They observed a positive reaction for MMP-2 in areas of lepidic growth in 17% of the cases and in invasive areas in 38%; the reaction was negative in AAH cases. In our study, some degree of reactivity for MMP-2 was observed in areas of lepidic growth in 26 cases (96.3%), in invasive areas of all 27 cases (100%) as well as in 2 of 5 cases of AAH. These results are comparable to those obtained by Galateau-Salle and colleagues, 9 who found that MMP-2 is expressed in preneoplastic as well as in neoplastic bronchial squamous lesions.
Expression of MT-1-MMP, the cell-surface activator of pro-MMP-2, has been found in tumor cells in a variety of neoplasms, such as primary carcinomas of the stomach, 23 pancreas, 24 breast, 25 thyroid, 26 adrenal cortex, 27 liver, 28 and lung 29 in association with activation of MMP-2. Tokuraku and colleagues 30 reported that expression of MT-1-MMP correlated with activation of MMP-2 and with lymph node metastasis in carcinoma of the lung. However, we are not aware of studies of the expression of MT-1-MMP in other preneoplastic lesions.
We found no correlation between the expression of other MMPs (MMP-1, MMP-3, MMP-7, and MMP-9) and the clinicopathological features of the adenocarcinomas. Shima and colleagues 31 reported that the tissue levels of MMP-3 correlated with vascular invasion and distant metastases in squamous cell carcinoma of the esophagus. Kossakowska and colleagues 32 found that the levels of MMP-9 correlated with the histological grades of human malignant lymphomas. Using quantitative zymography, Davies and colleagues 5 observed that levels of MMP-9 and activated MMP-2 correlated closely with tumor grade and invasiveness in transitional cell carcinoma of the bladder. These differences suggest that the expression of these markers may be influenced by the localization and histological type of tumor, the condition of adjacent normal tissues, and other factors.
TIMP-1 and TIMP-2 have been identified as protective factors for type IV collagen and fibrillar collagens. Kinoshita and colleagues 33 reported that the addition of TIMP-2 inhibited the processing of pro-MMP-2 in a dose-dependent manner, and that TIMP-1 had only 10% or less of the inhibitory effect of TIMP-2. In our study, the expression of TIMP-1 was not found to correlate with the preservation of type IV collagen. The finding of increased TIMP2 expression in the invasive components of adenocarcinomas, compared to those in lepidic areas, provides evidence that the invading tumor cells promote the accumulation of extracellular matrix. This is consistent with the concept that the accumulation of extracellular matrix that results in the scar in pulmonary adenocarcinomas is promoted by TIMP-2 expression by the invasive tumor cells. It is possible that protective factors other than TIMP-2 also were associated with type IV collagen in such cases. Suzuki and colleagues 34 and Yokose and colleagues 35 recently highlighted the clinical importance of fibrous scars in pulmonary adenocarcinomas, in which they found a strong prognostic correlation with tumor scar size. They found a 100% 5-year survival in patients whose tumors had a scar size less than 5 mm whereas survival was significantly reduced in patients with larger scars. Thus, TIMP-2 expression by tumor cells and the development of pulmonary scars may relate to prognosis in pulmonary adenocarcinoma, even though in these sets of data we did not demonstrate a correlation between survival and the reactivity for TIMP-2.
Strong expression of MMP-2 was recognized in two of our five cases of AAH, but MT-1-MMP was not expressed in areas of AAH in any of these cases. The antibody that we used for the immunolocalization of MMP-2 does not distinguish between the proenzyme and the activated form of MMP-2. However, two observations suggest that the MMP-2 detected in our two cases of AAH was inactive: 1) the finding of an intact epithelial basement membrane in the areas of reactivity for MMP-2, and 2) the absence of MT-1-MMP from these areas. These findings are in contrast with those in invasive adenocarcinomas, in which we observed co-expression of MMP-2 and MT-1-MMP, frequently in association with damage to the type IV collagen in the basement membranes. Therefore, these observations suggest that the expression of MT-1-MMP is associated with progression of the preneoplastic lesion of AAH to adenocarcinoma. This progression would provide a potential mechanism for eventual invasion of the stroma by the neoplastic cells.
In conclusion, evaluation of the extent of the lepidic and invasive components of pulmonary adenocarcinoma, as demonstrated in histological sections by the PAM stain and in immunohistochemical preparations stained for type IV collagen and MMP-2, correlates with the prognosis in pulmonary adenocarcinomas. The development of scars in pulmonary adenocarcinoma is promoted by increased expression of TIMP-2 by the tumor cells as they grow invasively.
Footnotes
Address reprint requests to William D. Travis, M.D., Chairman of the Department of Pulmonary and Mediastinal Pathology, Armed Forces Institute of Pathology, 6825 16th St., NW, Washington, DC 20306. E-mail: travis@afip.osd.mil.
Victor J. Ferrans is deceased.
References
- 1.Travis WD, Colby TV, Corrin B, Shimosato Y, Branhamera (Eds): Histologic typing of lung and pleural tumors. World Health Organization International Histological Classification of Tumours. Berlin, Springer, 1999
- 2.Sobin LH, Yesner R (Eds): Histological typing of lung tumors. International Histological Classification of Tumors, vol 1, ed 2. Geneva, World Health Organization, 1981
- 3.Noguchi M, Morikawa A, Kawasaki M, Matsuno Y, Yamada T, Hirohashi S, Kondo H, Shimosato Y: Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995, 75:2844-2852 [DOI] [PubMed] [Google Scholar]
- 4.Kleiner DE, Stetler-Stevenson WG: Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 1999, 43(Suppl):S42-S51 [DOI] [PubMed] [Google Scholar]
- 5.Davies B, Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR: Activity of type IV collagenases in benign and malignant breast disease. Br J Cancer 1993, 67:1126-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbisa S, Scagliotti G, Masiero L, Francesco C, Caenazzo C, Onisto M, Micela M, Stetler-Stevenson WG, Liotta LA: Correlation of serum metalloproteinase levels with lung cancer metastasis and response to therapy. Cancer Res 1992, 52:4548-4549 [PubMed] [Google Scholar]
- 7.Kawano N, Osawa H, Ito T, Nagashima Y, Hirahara F, Inayama Y, Nakatani Y, Kimura S, Kitajima H, Koshikawa N, Miyazaki K, Kitamura H: Expression of gelatinase A, tissue inhibitor of metalloproteinases-2, matrilysin, and trypsin (ogen) in lung neoplasms: an immunohistochemical study. Hum Pathol 1997, 28:613-622 [DOI] [PubMed] [Google Scholar]
- 8.Kitamura H, Oosawa Y, Kawano N, Kameda Y, Hayashi H, Nakatani Y, Udaka N, Ito T, Miyazaki K: Basement membrane patterns, gelatinase A and tissue inhibitor of metalloproteinase-2 expressions, and stromal fibrosis during the development of peripheral lung adenocarcinoma. Hum Pathol 1999, 30:331-338 [DOI] [PubMed] [Google Scholar]
- 9.Galateau-Salle FB, Luna RE, Horiba K, Sheppard MN, Hayashi T, Fleming MV, Colby TV, Bennett W, Harris CC, Stetler-Stevenson WG, Liotta LA, Ferrans VJ, Travis WD: Matrix metalloproteinases and tissue inhibitors of metalloproteinases in bronchial squamous preinvasive lesions. Hum Pathol 2000, 31:296-305 [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370:61-65 [DOI] [PubMed] [Google Scholar]
- 11.Kodama T, Biyajima S, Watanabe S, Shimosato Y: Morphometric study of adenocarcinomas and hyperplastic epithelial lesions in the peripheral lung. Am J Clin Pathol 1986, 85:146-151 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Nagai K, Yoshida J, Yokose T, Kodama T, Takahashi K, Nishimura M, Kawasaki H, Yokozaki M, Nishiwaki Y: The prognosis of resected lung carcinoma associated with atypical adenomatous hyperplasia: a comparison of the prognosis of well-differentiated adenocarcinoma associated with atypical adenomatous hyperplasia and intrapulmonary metastasis. Cancer 1997, 79:1521-1526 [DOI] [PubMed] [Google Scholar]
- 13.Travis WD: Lung. Pathology of Incipient Neoplasia. 2001, :pp 295-318 J Albores-Saavedra. New York, Oxford University Press, Edited by DE Henson DE [Google Scholar]
- 14.Auerbach O, Garfinkel L, Parks VR: Scar cancer of the lung: increase over a 21 year period. Cancer 1979, 43:636-642 [DOI] [PubMed] [Google Scholar]
- 15.Bakris GL, Mulopulos GP, Korchik R, Ezdinli EZ, Ro J, Yoon BH: Pulmonary scar carcinoma. A clinicopathologic analysis. Cancer 1983, 52:493-497 [DOI] [PubMed] [Google Scholar]
- 16.Shimosato Y, Suzuki A, Hashimoto T, Nishiwaki Y, Kodama T, Yoneyama T, Kameya T: Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol 1980, 4:365-373 [DOI] [PubMed] [Google Scholar]
- 17.Kolin, Koutoulakis T: Role of arterial occlusion in pulmonary scar cancers. Hum Pathol 1988, 19:1161–1167 [DOI] [PubMed]
- 18.Kung IT, Lui IO, Loke SL, Khin MA, Mok CK, Lam WK, So SY: Pulmonary scar cancer. A pathologic reappraisal. Am J Surg Pathol 1985, 9:391-400 [DOI] [PubMed] [Google Scholar]
- 19.Madri JA, Carter D: Scar cancers of the lung: origin and significance. Hum Pathol 1984, 15:625-631 [DOI] [PubMed] [Google Scholar]
- 20.el-Torky M, Giltman LI, Dabbous M: Collagens in scar carcinoma of the lung. Am J Pathol 1985, 121:322-326 [PMC free article] [PubMed] [Google Scholar]
- 21.Sobin LH, Wittekind CH (Eds): UICC, TNM Classification of Malignant Tumors, ed 5. New York, John Wiley & Sons, 1997, pp 91–100
- 22.Yajima G, Aihara K: Periodic acid methenamine silver staining. Igaku no Ayumi 1971, 76:328-338 [Google Scholar]
- 23.Bando E, Yonemura Y, Endou Y, Sasaki T, Taniguchi K, Fujita H, Fushida S, Fujimura T, Nishimura G, Miwa K, Seiki M: Immunohistochemical study of MT-MMP tissue status in gastric carcinoma and correlation with survival analyzed by univariate and multivariate analysis. Oncol Rep 1998, 5:1483-1488 [DOI] [PubMed] [Google Scholar]
- 24.Iwamura T, Ohshio G, Mise M, Harada T, Suwa H, Okada N, Wang Z, Yoshitomi S, Tanaka T, Sato H, Arii S, Seiki M, Imamura M: Expression of membrane-type matrix metalloproteinase-1 in human pancreatic adenocarcinomas. J Cancer Res Clin Oncol 1998, 124:65-72 [DOI] [PubMed] [Google Scholar]
- 25.Ishigaki S, Toi M, Ueno T, Matsumoto H, Muta M, Koike M, Seiki M: Significance of membrane type 1 matrix metalloproteinase expression in breast cancer. Jpn J Cancer Res 1999, 90:516-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura H, Ueno H, Yamashita K, Shimada T, Yamamoto E, Noguchi N, Fujimoto N, Sato H, Seiki M, Okada Y: Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human thyroid carcinomas. Cancer Res 1999, 59:467-473 [PubMed] [Google Scholar]
- 27.Kjellman M, Enberg U, Höög A, Larsson C, Holst M, Farnebo L, Sato H, Bäckdahl M: Gelatinase A and membrane-type 1 matrix metalloproteinase mRNA: expressed in adrenocortical cancers but not in adenomas. World J Surg 1999, 23:237-242 [DOI] [PubMed] [Google Scholar]
- 28.Ogata R, Torimura, Kin M, Ueno T, Tateishi Y, Kuromatsu R, Shimauchi Y, Sakamoto M, Tamaki S, Sata M, Tanikawa K: Increased expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 with tumor dedifferentiation in hepatocellular carcinomas. Hum Pathol 1999, 30:443–450 [DOI] [PubMed]
- 29.Tsunezuka Y, Kinoh H, Takino T, Watanabe Y, Okada Y, Shinagawa A, Sato H, Seiki M: Expression of membrane-type matrix metalloproteinase I (MT1-MMP) in tumor cells enhances pulmonary metastasis in an experimental metastasis assay. Cancer Res 1996, 56:5678-5683 [PubMed] [Google Scholar]
- 30.Tokuraku M, Sato H, Murakami S, Okada Y, Watanabe Y, Seiki M: Activation of the precursor of gelatinase A/72 kDa type IV collagenase/MMP-2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int J Cancer 1995, 64:355-359 [DOI] [PubMed] [Google Scholar]
- 31.Shima I, Sasaguri Y, Kusukawa J, Yamana H, Fujita H, Kakegawa T, Morimatsu M: Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer 1992, 70:2747-2753 [DOI] [PubMed] [Google Scholar]
- 32.Kossakowska AE, Huchcroft SA, Urbanski SJ, Edwards DR: Comparative analysis of the expression patterns of metalloproteinases and their inhibitors in breast neoplasia, sporadic colorectal neoplasia, pulmonary carcinomas and malignant non-Hodgkin’s lymphomas in humans. Br J Cancer 1996, 73:1401-1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinoshita T, Sato H, Takino T, Itoh M, Akizawa T, Seiki M: Processing of a precursor of 72-kilodalton type IV collagenase/gelatinase A by a recombinant membrane-type 1 matrix metalloproteinase. Cancer Res 1996, 56:2535-2538 [PubMed] [Google Scholar]
- 34.Suzuki K, Yokose T, Yoshida J, Nishimura M, Takahashi K, Nagai K, Nishiwaki Y: Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg 2000, 69:893-897 [DOI] [PubMed] [Google Scholar]
- 35.Yokose T, Suzuki K, Nagai K, Nishiwaki Y, Sasaki S, Ochiai A: Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer 2000, 29:179-188 [DOI] [PubMed] [Google Scholar]