Abstract
Inflammatory bowel disease (IBD) consisting of ulcerative colitis (UC) and Crohn’s (CD) typically displays a waxing and waning course punctuated by disease flares that are characterized by transepithelial migration of neutrophils (PMN) and altered barrier function. Since epithelial barrier function is primarily regulated by the apical most intercellular junction referred to as the tight junction (TJ), our aim was to examine expression of TJ and adherens junction (AJ) proteins in relation to PMN infiltration in mucosal tissue samples from patients with active IBD. Expression of epithelial intercellular TJ proteins (occludin, ZO-1, claudin-1, and JAM) and subjacent AJ (β-catenin and E-cadherin) proteins were examined by immunoflourescence/confocal microscopy, immunohistochemistry, and Western blotting. Colonic mucosa from patients with UC revealed dramatic, global down-regulation of the key TJ transmembrane protein occludin in regions of actively transmigrating PMN and in quiescent areas in the biopsy samples. Significant decreases in occludin expression were observed at the protein and mRNA levels by Western and Northern blotting. In contrast, expression of other TJ and AJ proteins such as ZO-1, claudin-1, JAM, β-catenin, and E-cadherin were down-regulated only in epithelial cells immediately adjacent to transmigrating PMN. Analysis of inflamed mucosa from Crohn’s disease patients mirrored the results obtained with UC patients. No change in TJ and AJ protein expression was observed in colonic epithelium from patients with collagenous colitis or lymphocytic colitis that are respectively characterized by a thickened subepithelial collagen plate and increased intraepithelial lymphocytes. These results suggest that occludin expression is diminished in IBD by mechanisms distinct from those regulating expression of other intercellular junction proteins. We speculate that down-regulation of epithelial occludin may play a role in enhanced paracellular permeability and PMN transmigration that is observed in active inflammatory bowel disease.
The intestinal epithelium serves as a protective barrier separating luminal contents from the underlying tissue compartments. Epithelial barrier function is regulated in large part by the apical most intercellular junction, referred to as the tight junction (TJ). In turn, TJ integrity is intimately linked to a subjacent adherens junction (AJ). 1
Patients with inflammatory bowel disease (IBD) encompassing both ulcerative colitis (UC) and Crohn’s disease (CD) typically present with relapsing diarrhea. Diarrhea has been attributed to increased paracellular permeability in the epithelial lining from both the acutely inflamed and chronically damaged areas of the intestine in patients with IBD. 2,3 Although data pertaining to intestinal permeability changes are not completely consistent, the majority of studies suggest that most patients with CD as well as UC have a defect in epithelial barrier function. 4,5,6 Disease activity in IBD is linked to an influx of transepithelial migration of neutrophils (PMN) into the mucosal epithelium (cryptitis) and subsequently into the intestinal lumen resulting in the formation of so-called crypt abscesses. In vitro studies using human intestinal epithelial cell lines such as T84 have shown that PMN migration across epithelial monolayers occurs via a paracellular route. 7 Furthermore, it is apparent that migration of PMN across epithelial monolayers causes transient and rapidly reversible perturbations of TJs. 8,9
Paracellular permeability across epithelial cell monolayers is regulated primarily by TJs that encircle the apical poles of epithelial cells thereby establishing distinct microenvironments on both sides of the polarized epithelium. The TJ is comprised of a complex of proteins that are linked to the underlying actin cytoskeleton in the apical perijunctional F-actin ring. Cytoskeletal affiliations of the TJ are believed to play a very important role in regulating TJ function in diverse physiological and pathological states. 10 Transmembrane proteins of TJs include occludin, 11,12 junction adhesion molecule (JAM), 13 and members of the claudinfamily. 14 Juxtaposed to the TJ membrane is an electron dense area referred to as the terminal plaque that contains the scaffolding protein, ZO-1. ZO-1 is an important linker protein in TJs that affiliates with transmembrane protein, occludin, and other cytoplasmic proteins such as ZO-2, ZO-3, and actin. 15,16
The adherens junction (AJ) is immediately subjacent to the TJ and is important in maintaining integrity of other intercellular junctions, thereby playing an essential role in cell-cell recognition and cell sorting. 17 The AJ protein complex includes a single-spanning transmembrane glycoprotein, cadherin (E-cadherin in epithelial cells), cytoplasmic proteins, and catenins (α, β, γ) that associate with the underlying actin cytoskeleton. 18-20
Since enhanced paracellular permeability and PMN infiltration into the intestinal mucosa are a central feature of active inflammatory bowel disease, our objective was to determine the relationship between expression of key epithelial intercellular junction proteins and PMN infiltration in mucosal biopsies from patients with active IBD.
Materials and Methods
Study Population
Colorectal mucosal tissue samples from patients with chronic active colitis (n = 20) were obtained from the Emory Epithelial Pathobiology Research Group frozen tissue bank (ulcerative colitis, n = 11; Crohn’s disease, n = 9). In addition, normal control colorectal mucosa consisted of biopsies obtained at colonoscopy (n = 11) and resection specimens performed for failure of medical treatment or for colon cancer (n = 18). These samples were used for determining expression of epithelial intercellular junction proteins by immunofluorescence labeling/confocal microscopy, Western blotting, and Northern blotting.
Additional archived intestinal mucosal tissue from Emory surgical pathology files was analyzed for TJ and AJ protein expression by immunohistochemistry. Formalin-fixed, paraffin-embedded samples of colorectal mucosa from patients with chronic active colitis (n = 10) and chronic inactive colitis (n = 2) (ulcerative colitis, n = 10; Crohn’s disease, n = 2) were analyzed. Controls included intestinal tissue from normal mucosa (n = 9) and mucosa showing features of collagenous colitis (n = 3) or lymphocytic colitis (n = 2).
Pathological Analysis
Hematoxylin and eosin stained sections of both frozen and permanent samples were reviewed by two gastrointestinal pathologists (A.N., S.V.W.) for the presence of chronic colitis and degree of disease activity. Histological grading of activity was based on a modified protocol by Truelove and Richards. 21 Disease activity was defined as identification of PMN within colonic or ileal epithelium and was graded as mild, moderate, or severe depending on increasing numbers of PMN within the epithelium (crypt and surface epithelial cells). According to this classification, we identified 19 patients with chronic active ulcerative colitis (mild activity, n = 7; moderate activity, n = 4; severe activity, n = 8) and 11 patients with chronic active Crohn’s disease (mild activity, n = 3; moderate activity, n = 3; severe activity, n = 5).
Immunohistochemistry
Immunofluorescence Labeling of Frozen Tissue Sections
Five-μm frozen sections of colonic mucosa cut with a cryostat, were mounted on ethanol-sterilized glass coverslips, air dried, and stored at −80°C. Tissue sections were fixed in either ethanol (20 minutes at −20°C), methanol (20 minutes at −20°C), 3% paraformaldehyde (30 minutes at room temperature), or 3% buffered formalin (5 minutes at room temperature) followed by incubation with sodium borohydride to quench autofluorescence (1 hour at 4°C). Paraformaldehyde and formalin fixed tissue were permeabilized with 1% Triton X-100 for 10 minutes at room temperature. Tissue sections were incubated with respective primary antibodies to TJ and AJ proteins for 60 minutes in a humidity chamber, washed in Hanks’ balanced salt solution (HBSS), incubated with fluoresceinated secondary antibodies (Jackson Labs, PA), mounted in p-phenylenediamine glycerol (1:1) and analyzed by confocal microscopy (Zeiss laser confocal microscope, Emory University, Atlanta, GA). The following antibodies were used at concentrations of 1 to 5 μg/ml: ZO-1, β-catenin mAb (Transduction Labs, KY), occludin and claudin-1 polyclonal antibodies (Zymed, CA). JAM mAb (clone J10.4) were generated in our laboratory. 13 E-cadherin hybridoma supernatant ((clone HECD.1 (1:1600)) was used for these studies.
Immunohistochemistry of Paraffin-Embedded Tissue Samples
Formalin-fixed, paraffin-embedded tissue sections (5 μm) on glass slides were deparaffinized in xylene and rehydrated through graded alcohol solutions. Sections were incubated with 3% H2O2 for 20 minutes, and immunohistochemistry was performed on an automated instrument (Ventana ES, Ventana Medical Systems, Tucson, AZ) as previously described. 22 Thus, sections were incubated with antibodies to the respective TJ and AJ proteins (see above) followed by peroxidase conjugated secondary antibodies and the bound complexes were visualized by using 3,3-diaminobenzidine as a substrate. Identical reaction times permitted accurate comparison of all samples. The slides were then counter-stained with Mayer’s hematoxylin. A negative control in which the primary antibody was omitted was included in each test run. Staining intensity was expressed as no change compared to healthy control, <50% reduction (diminished expression) and >50% reduction (strongly diminished expression).
Immunoblotting
Intestinal mucosa was stripped from the underlying submucosal tissue, harvested in HBSS+ containing 1% Triton X-100 and protease inhibitors (leupeptin, chymostatin, aprotinin; 10 μg/ml, phenylmethylsulfonyl fluoride; 1.25 mmol/L). Protein concentration in tissue lysates was determined by using the Pierce BCA protein assay (Pierce, Rockford, IL). Samples were mixed with an equal volume of sodium dodecyl sulfate (SDS) sample buffer and analyzed for TJ/AJ protein expression by SDS-polyacrylamide gel electrophoreseis and immunoblots using standard protocol. 23 10 μg of total protein was loaded in each lane of the SDS-polyacrylamide gel. Western blots with a pan-cytokeratin mAb were also done so as to normalize epithelial protein loading between samples.
RNA Isolation and Northern Blotting
RNA was isolated from stripped intestinal mucosa (as above) using the Trizol-method (Life Technologies, Gaithersburg, MD) according to the manufacturer’s specifications. 20 μg of total RNA was separated in a 1% denaturing formaldehyde agarose gel and transferred to a nylon membrane. [32P]dCTP (Amersham)-labeled DNA probes were generated from the entire occludin coding region by random oligonucleotide-priming using the NEBlot-Kit (NEB). Unincorporated nucleotides were removed using a G-50 spin column (Amersham-Pharmacia, Piscataway, NJ), and the probe was hybridized for one hour to the membrane-immobilized RNA at 68°C using ExpressHyb buffer (Clontech, Palo Alto, CA) according to the manufacturer’s recommendation. After washing at 50°C the membrane was developed using a PhosphoImager. To normalize for the amount of RNA, a 32P-labeled probe directed against glyceraldehyde-3-phosphate dehydrogenase was included.
Statistical Analysis
For statistical analysis Student’s unpaired t-test was used. Statistical significance was considered if p < 0.05.
Results
Global Down-Regulation of Occludin in Colonic Epithelial Cells of Patients with Chronic Active Inflammatory Bowel Disease
Idiopathic inflammatory bowel disease is associated with intestinal epithelial damage, crypt architectural irregularity, and relapsing acute inflammation characterized by transepithelial migration of PMN. Histology of colonic mucosa from control and IBD patients was characterized by hematoxylin and eosin staining of mucosal tissue sections (Figure 1a ▶ -d). Since increased paracellular permeability has been reported in intestinal epithelium from patients with IBD, and TJs are a primary determinant of epithelial paracellular permeability, we initially analyzed the expression of TJ transmembrane protein, occludin and its cytoplasmic plaque protein, ZO-1 in colonic mucosal sections from control and UC patients by immunofluorescence labeling and confocal microscopy. To further characterize expression of these proteins relative to active inflammation, tissue sections were double-labeled for occludin/ZO-1 versus the β2- integrin CD11b/CD18 as a leukocyte specific marker strongly expressed on PMN. In control biopsies, occludin and ZO-1 were localized to the apical region of the lateral plasma membrane representing the region of TJs in surface and crypt epithelial cells (Figure 1, e, f, i, j) ▶ . In mucosal tissue from patients with chronic active ulcerative colitis, occludin-staining was globally down-regulated, both in epithelium with active inflammation (Figure 1h) ▶ and in crypts where transmigrating PMN were not identified (Figure 1g) ▶ . Thus, even in regions with only mild active inflammation, occludin staining was markedly diminished (Figure 1g) ▶ and was almost absent in areas with increased PMN transmigration and crypt abscess formation (Figure 1h) ▶ . Neither in controls nor in the UC population was there a difference in occludin expression in surface versus crypt epithelial cells. Occludin expression in colonic tissue samples from patients with Crohn’s disease paralleled that of UC patients. However, as determined by immunhistochemical analysis (Table 1 ▶ ) inactive IBD as well as in other intestinal disorders such as lymphocytic colitis and collagenous colitis, no change in occludin expression relative to control healthy tissue was observed.
Figure 1.
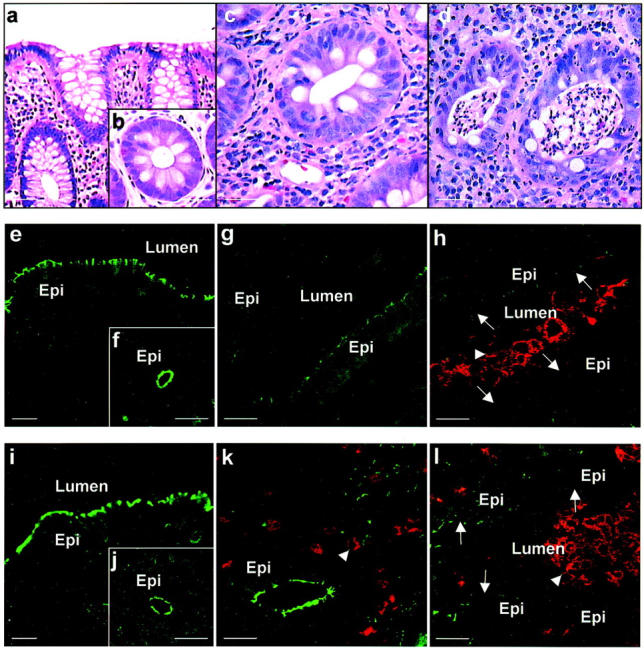
Differential down-regulation of TJ proteins occludin and ZO-1 in chronic active IBD. Hematoxylin and eosin staining of colonic mucosa from control non-IBD (a, b) and UC (c, d) patients was used to evaluate the histology of tissue sections. Classic features of crypt architectural distortion during mild inflammation (c) and PMN-transmigration with crypt abscesses during active inflammation (d) consistent with ulcerative colitis were noted. To analyze expression of TJ proteins in epithelial cells relative to active inflammation, mucosal tissue sections were double labeled for occludin or ZO-1 (green) and CD11b/CD18 as a marker for PMN (red). Labeled sections were analyzed by confocal microscopy. Occludin was identified in TJs of normal control surface (e) and crypt (f) epithelial cells and was markedly down-regulated to absent in tissue sections from UC patients. Decreased intensity of occludin staining in epithelial cells was observed in regions of active inflammation (h) and in areas not exposed to transmigrating PMN (g). In contrast to occludin, ZO-1 expression in colonic tissues from normal (i, j) and IBD (k, l) patients is down-regulated only in epithelial cells exposed to transmigrating PMN (l). Arrows: tight junctions; arrowhead: PMN; Lumen, lumen; Epi, epithelial cell. Scale bar, 15 μm.
Table 1.
Changes in TJ and AJ Protein Expression During Chronic Active IBD
| Protein | Inactive IBD | Active IBD | |
|---|---|---|---|
| Epithelium away from PMN | Epithelium adjacent to PMN | ||
| Occludin | → | ↓ | ↓↓ |
| Claudin-1 | → | → | ↓↓ |
| ZO-1 | → | → | ↓↓ |
| JAM | → | → | ↓↓ |
| β-Catenin | → | → | ↓↓ |
| E-cadherin | → | → | ↓↓ |
→, no changes compared to healthy control; ↓, diminished expression; ↓↓, strongly diminished expression.
Since we observed down-regulation of occludin in inflammed mucosa from IBD patients, we extended the analysis to other TJ and AJ proteins. The TJ cytoplasmic plaque protein, ZO-1 was localized by immunofluorescence labeling and confocal microscopy. ZO-1 was also distributed in the apical region of the lateral membrane representing TJs of surface and crypt epithelial cells in control samples (Figure 1, i and j) ▶ . In contrast to the global decrease observed for occludin, ZO-1 staining was diminished to absent only in areas adjacent to intraepithelial PMN (Figure 1l) ▶ . In particular, no change in ZO-1 staining was observed in areas of epithelial cells lacking intraepithelial PMN (Figure 1k ▶ and Table 1 ▶ ). The pattern of ZO-1 expression in tissue sections of patients with CD was identical to that observed in the UC samples (data not shown). Figure 1k ▶ highlights distinct distribution of ZO-1 in TJs of epithelial cells that is comparable to control mucosal samples (Figure 1, i and j) ▶ even though PMN are seen in the lamina propria but are not transmigrating across the epithelium. We also examined the pattern of ZO-1 staining in endothelial cells and observed no difference in IBD versus control tissues even in areas of active inflammation. Occludin and ZO-1 staining in frozen sections from IBD and control tissues was also identical to that observed in archived paraffin embedded tissue sections stained by immunoperoxidase labeling (not shown).
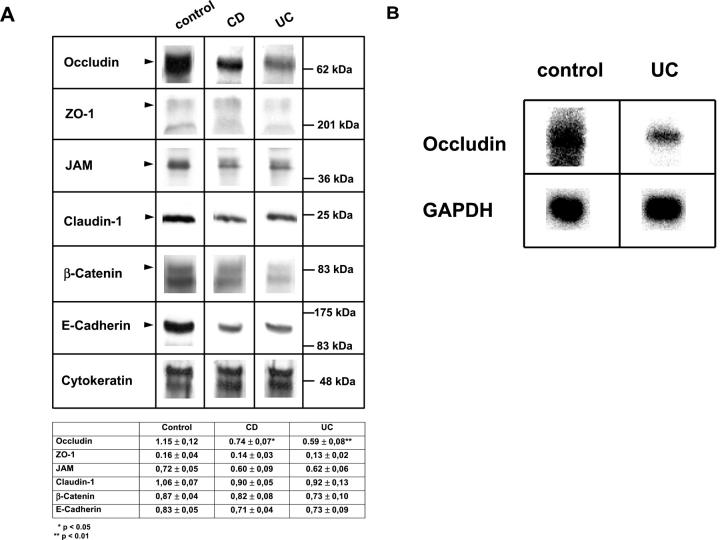
To further analyze occludin and ZO-1 protein expression in colonic epithelium from control and IBD patients we performed Western blots from mucosal tissue lysates of patients undergoing colonic resection. Thus, colonic mucosa from individuals with chronic active colitis and normal colonic mucosa (five patients each) was used for Western blots. The mucosal tissue was mechanically separated from the underlying submucosa. To normalize epithelial protein loading between samples, Western blots were probed with a pan-cytokeratin mAb. Results from these experiments demonstrated consistent and significant reduction of occludin expression in patients with UC as well as with CD (Figure 2A) ▶ . These findings are consistent with the immunofluorescence results showing diffuse down-regulation of occludin in the intestinal mucosa of IBD patients. Levels for ZO-1, JAM, claudin-I, β-catenin, and E-cadherin all showed a tendency of diminished protein expression. However, these levels did not reach significance in either CD or UC (Figure 2A) ▶ . Since we observed a global down-regulation of occludin in intestinal epithelial cells of UC patients we evaluated occludin mRNA expression in these mucosal tissue samples by performing a Northern blot analysis. As shown in Figure 2B ▶ , we observed marked down-regulation of occludin in chronic active UC samples compared to controls.
Figure 2.
A: Immunoblot analysis of TJ and AJ protein expression in tissues from patients with chronic active IBD. Mucosal colonic tissue from control and IBD patients was analyzed for TJ and AJ protein expression by performing Western blots. Equal protein was loaded in each lane as determined by protein analysis and Western blots for cytokeratin. Data are representative of results obtained from five control patients and five patients with active IBD. Densitometry results from these patients were presented as mean ± SEM. B: Transcriptional down-regulation of occludin in chronic active UC. RNA was isolated from tissue of normal controls and patients with chronic active UC and transferred to a nitrocellulose membrane. Hybridization with a radioactive labeled probe for full length occludin reveals a signal that is markedly diminished in patients with active UC. Data are representative for results obtained from three control patients and two patients with chronic active UC.
Differential Down-Regulation of ZO-1, Claudin-1, and JAM Exclusively in Epithelial Cells Adjoining Transmigrating PMN in Active IBD
To confirm our Western blot results suggesting slight reduction in expression of most TJ/AJ proteins with the exception of occludin, we performed a morphological analysis of their expression patterns in tissue samples of control and IBD patients. Archived intestinal tissue embedded in paraffin blocks was analyzed by immunohistochemistry. We first analyzed distribution of TJ proteins, JAM, and claudin-1 in control and IBD tissue sections. Focal high concentrations of JAM were seen in the apical region of the lateral membrane representing TJs of control intestinal epithelial cells (Figure 3a) ▶ . A smaller pool of JAM was distributed in the lateral membrane below TJs. Analysis of JAM expression in intestinal epithelium from patients with chronic active UC revealed a pattern that was identical to that of ZO-1. Thus we observed decreased intensity of JAM expression exclusively in epithelial cells adjoining transmigrating PMN (Figure 3c) ▶ . Non-inflamed epithelium without transmigrating PMN even in areas adjoining crypt abscesses showed normal JAM expression (Figure 3b) ▶ . There was no difference in JAM distribution between surface and crypt epithelium (data not shown). The staining pattern of JAM again contrasts with that of occludin which was observed to be diffusely down-regulated in both the acutely inflamed and non-inflamed regions of the mucosa. JAM expression in colonic tissue from patients with lymphocytic and collagenous colitis was comparable to control normal tissue (Table 1 ▶ ). In addition, JAM expression in intercellular junctions of endothelial cells was indistinguishable in control versus IBD intestinal mucosal tissue sections. Given the differential expression patterns of occludin versus ZO-1 and JAM in IBD tissue sections, we analyzed distribution of another TJ transmembrane protein, claudin-1, by immunoperoxidase labeling. Interestingly, claudin-1 expression paralleled that of JAM in intestinal tissue sections from control and IBD patients. Thus, claudin-1 was concentrated in TJs and a small pool was additionally distributed in the subjacent lateral membranes of surface and crypt epithelial cells (Figure 3d) ▶ . Comparable to JAM, claudin-1 expression in chronic active UC was decreased only in areas with PMN transmigration both in the crypt epithelium (cryptitis, crypt abscess) (Figure 3f) ▶ and surface epithelium (data not shown). In areas away from active inflammation (PMN transmigration), claudin-1 staining was indistinguishable from that in control tissue sections (Figure 3e) ▶ . Patients with chronic inactive UC as well as disease controls such as collagenous colitis and lymphocytic colitis revealed the same distribution of claudin-1 as normal controls. However, claudin-1, JAM, and ZO-1 expression patterns in tissue sections from CD patients was analogous to that observed for UC.
Figure 3.
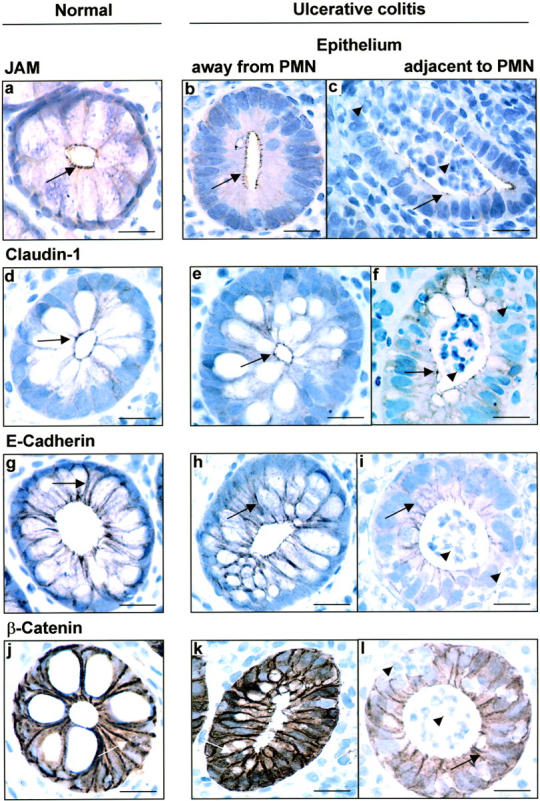
Decreased expression of TJ and AJ proteins in IBD colonic mucosa is restricted to epithelium with actively transmigrating PMN. Immunohistochemistry was used to map the distribution of TJ and AJ proteins, JAM (a, b, c), claudin-1 (d, e, f), E-cadherin (g, h, i), and β-catenin (j, k, l) in normal and actively inflammed IBD colonic mucosa. JAM and claudin-1 in normal mucosa are expressed in focal concentrations at the apical region of the lateral membranes of epithelial cells consistent with its localization in TJs. Additional pool of these proteins are identified in the lateral membrane subjacent to the TJ in the AJ. The AJ proteins, β-catenin and E-cadherin, are abundantly expressed in lateral membranes of normal epithelial cells. In active UC, expression of all four proteins was diminished exclusively in epithelial cells adjacent to actively transmigrating PMN (arrowheads). However, no change in expression or distribution of these TJ and AJ proteins was observed in epithelial cells not exposed to transmigrating PMN. Arrows, TJ, AJ; arrowhead, PMN. Scale bar, 15 μm
Expression Pattern of Adherens Junction Proteins in Active IBD
Since the adherens junction (AJ) is immediately subjacent to TJs of epithelial cells, and its function is intimately linked to that of the TJ, we determined expression of AJ proteins in tissue sections from IBD and control patients. Paraffin-embedded mucosal samples were stained by immunoperoxidase labeling with antibodies to the AJ transmembrane protein, E-cadherin, and its linker protein, β-catenin. E-cadherin was predominantly distributed in the lateral membrane of epithelial cells (Figure 3g) ▶ . In mucosal tissue samples of UC patients with active PMN transepithelial migration, E-cadherin expression was decreased only in the lateral membranes of epithelial cells exposed to transmigrating PMN (Figure 3i) ▶ . Epithelia distant to transmigrating PMN showed a normal E-cadherin staining pattern in the lateral membranes (Figure 3h) ▶ . No significant differences between crypt and surface epithelium were observed in the UC samples. Analogous to E-cadherin, β-catenin was also distributed in the lateral membranes of intestinal epithelial cells of control mucosal biopsies (Figure 3j) ▶ . In chronic active UC, we observed markedly diminished expression for β-catenin that was restricted to sites of PMN transepithelial migration (Figure 3l) ▶ . The surface epithelium and crypts lacking transmigrating PMN in UC samples showed a staining pattern that did not differ from that of normal controls (Figure 3k) ▶ . Mucosal tissue sections from patients with chronic active CD revealed an identical staining pattern to that observed in UC (Table 1) ▶ . In addition, expression pattern of β-catenin and E-cadherin in non-IBD patients such as lymphocytic and collagenous colitis did not differ from that of normal controls.
Further analysis of E-cadherin and β-catenin expression in UC mucosal tissue samples by Western blot revealed a slight decrease in expression of these proteins compared to control non-IBD tissue (Figure 2A) ▶ . However, densitometric analyses did not reveal any statistically significant differences.
Discussion
Idiopathic inflammatory bowel disease (IBD) is associated with an underlying change in epithelial barrier function in a subgroup of patients. Disease activity in IBD is characterized by migration of PMN across epithelial cells. Although transepithelial migration of PMN has been extensively reported in both IBD and non-IBD inflammatory disorders of the intestine and altered TJ structure in active UC has been described, 6 the relationship of migrating PMN to epithelial intercellular junctions is less well characterized. Our studies have focused on analysis of TJ and AJ protein expression in intestinal mucosal tissue obtained from patients with IBD. Immunofluorescence labeling revealed a global down-regulation of occludin in IBD colonic epithelium encompassing both areas with active inflammation and regions distant from intraepithelial PMN. This pattern of occludin loss was specific to IBD and was not observed in other intestinal disorders such as lymphocytic and collagenous colitis. In contrast to occludin, reduced staining intensity for all other TJ and AJ proteins was observed exclusively in epithelial cells adjoining transmigrating PMN. This lack of global down-regulation of claudin-1, JAM, ZO-1, E-cadherin, and β-catenin would suggest that loss of these intercellular junction proteins is a transient process that is rapidly reversible after PMN transmigration. This is consistent with the observation that mucosal wounds rapidly reseal after PMN transmigration. 9 Analysis of TJ/AJ protein expression by Western blots of whole mucosal lysates revealed significant down-regulation of the TJ transmembrane protein occludin in CD as well as in UC. In contrast, densitometric analysis of Western blots for all other junctional proteins examined revealed only slightly diminished protein levels that were not statistically different from controls. The discrepancy is likely related to the ability of immunofluorescence labeling to more accurately discriminate between focal regions of TJ/AJ protein down-regulation in epithelial cells directly exposed to transmigrating PMN and the transient nature of such events. Microscopic analysis clearly demonstrated the lack of global down-regulation of ZO-1, claudin-1, JAM, β-catenin, and E-cadherin in chronic active UC that also held true for chronic active CD. Thus, this method of analysis appears to be more informative for mapping TJ/AJ protein expression compared to analyses of mucosal tissue lysates by Western blot.
It is known that PMN can rapidly migrate across epithelia, induce transient opening of intercellular junctions and not cause appreciable morphological discontinuities. 9 It has been postulated that down-regulation of TJ proteins during PMN transepithelial migration might be induced by the migrating leukocytes themselves either by stimulating epithelial cells to reorganize their TJ and AJ components or simply by mechanical forces. 24 Physical migration of PMN from the basolateral to the apical surfaces of epithelial cells is preceeded by a fall in the transepithelial resistance to passive ion flow suggesting signaling events between PMN and the basolateral aspects of epithelial cells. 25
Since PMN have been documented to release proteases that induce artifactual degradation of intercellular junction proteins in vascular endothelium, we examined the effect of different tissue fixatives on the staining pattern of these intercellular junction proteins. However, IBD and non-IBD tissue samples treated with four different fixatives (ethanol, methanol, paraformaldehyde, and formalin + sodium borohydride) revealed an identical pattern of TJ/AJ protein expression. Moreover, no significant differences in staining intensity of TJ/AJ proteins was observed in unfixed frozen tissue sections in which suspensions of PMN had been allowed to adhere to in high density before processing. It therefore appears that pre- and post-fixative protein degradation of TJ/AJ proteins by PMN-released proteases in tissue sections cannot account for the diminished TJ/AJ protein expression we observed in our studies.
Given that occludin was the only TJ/AJ protein that was diffusely down-regulated in areas with and without PMN epithelial infiltration, we determined its expression at the transcriptional level. Indeed, by Northern blot analysis, markedly diminished RNA levels of occludin were observed in intestinal tissue from patients with chronic active UC. Global decreases in occludin expression in the colonic mucosa of IBD patients would suggest that there are PMN-independent mechanisms accounting for occludin down-regulation. A possible mechanism accounting for altered intercellular junction protein expression in IBD involves the influence of cytokines on epithelial cells. Indeed, it has been shown that proinflammatory cytokines may alter barrier function during intestinal inflammation. 26 interferon-γ (IFN-γ) as well as interleukin-4 (IL-4) are cytokines that have previously been shown to influence epithelial barrier function and may be likely candidates that may modulate transepithelial migration of PMN. 27,28 It has been reported that IFN-γ decreases barrier function in T84 cells by diminishing ZO-1 expression levels. 29 In addition, tumor necrosis factor-α (TNF-α) that is known to play a role in the pathology of active IBD decreases epithelial barrier function and tight junction complexity of a human intestinal epithelial cell line as analyzed by freeze-fracture electron microscopy. 30 Recently, it has been demonstrated that expression of the human occludin promoter is down-regulated by TNF-α as well as IFN-γ. 31 Given that both TNF-α and IFN-γ play an important role in the pathophysiology of intestinal inflammation in IBD 32,33 , and their influence on the occludin promoter activity, it is reasonable to suspect that these or other cytokines are likely to account for our observations showing global down-regulation of occludin at the protein and transcription level. It is conceivable that some of the cytokines that may play a role in barrier function during inflammation are secreted by PMNs thereby regulating junction proteins. It is well known that PMNs secrete a variety of inflammatory cytokines after activation. 34 Further studies are therefore necessary to clarify whether cytokines play a predominant role in diminished barrier function and decreased tight junction expression during chronic active IBD. In general, it is conceivable that a combination of these factors, local effects induced by neutrophils as well as cytokine mediated mechanisms, is responsible for diminished expression of junction proteins in active IBD. This would be consistent with our observation that changes in expression of different junction proteins in active IBD are not uniform. Whether the observed changes in occludin expression may contribute to increased paracellular permeability that has been described in a subgroup of patients with CD and UC remains to be determined. Impedance analysis of intestinal mucosal tissue from mice lacking occludin expression failed to reveal differences when compared to normal mice. 35 However, these studies lacked precise determination of paracellular permeability as defined by flux of paracellular solutes. In addition, susceptibility of knock-out versus wild type mice to colitis was not examined. Thus, the precise role of occludin in regulation of paracellular permeability remains to be determined.
In conclusion, our study provides evidence that PMN transepithelial migration during chronic active UC is associated with a diffuse global down-regulation of occludin and a reduction of other TJ and AJ proteins specifically at sites of active inflammation. Global down-regulation of occludin in IBD-patients may account for increased paracellular permeability that is observed in a subgroup of IBD patients. Further studies are necessary to elucidate factors that are responsible for differential down-regulation of occludin and other TJ and AJ proteins in IBD.
Footnotes
Address reprint requests to Asma Nusrat, M.D., Department of Pathology, Emory University, 1639 Pierce Drive, Woodruff Memorial Research Building, Atlanta, GA 30322. E-mail: anusrat@emory.edu
Supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft) (Ku 1328/1–1; T.K.), the American Digestive Health Foundation (S.V.W.), the National Institutes of Health (HL54229 and HL60540 to C.P.; DK53202 to A.N.), the Crohn’s & Colitis Foundation of America (A.N.), and the Arthritis Foundation (A.N.).
References
- 1.Madara JL: Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 1998, 60:143-159 [DOI] [PubMed] [Google Scholar]
- 2.Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI: Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology 1989, 97:927-931 [DOI] [PubMed] [Google Scholar]
- 3.Sandle GI, Higgs N, Crowe P, Marsh MN, Venkatesan S, Peters TJ: Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology 1990, 99:97-105 [DOI] [PubMed] [Google Scholar]
- 4.Hollander D: The intestinal permeability barrier: a hypothesis to its regulation and involvement in Crohn’s disease. Scand J Gastroenterol 1992, 27:721-726 [DOI] [PubMed] [Google Scholar]
- 5.Söderholm JD, Peterson KH, Olaison G, Franzen LE, Weström B, Magnusson K-E, Sjödahl R: Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology 1999, 117:65-72 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD: Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116:301-309 [DOI] [PubMed] [Google Scholar]
- 7.Nash S, Stafford J, Madara JL: Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest 1987, 80:1104-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash S, Stafford J, Madara JL: The selective and superoxide-independent disruption of intestinal epithelial tight junctions during leukocyte transmigration. Lab Invest 1988, 59:531-537 [PubMed] [Google Scholar]
- 9.Nusrat A, Parkos CA, Liang TW, Carnes DK, Madara JL: Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology 1997, 113:1489-1500 [DOI] [PubMed] [Google Scholar]
- 10.Edens HA, Parkos CA: Modulation of epithelial and endothelial paracellular permeability by leucocytes. Adv Drug Del Rev 2000, 41:315-328 [DOI] [PubMed] [Google Scholar]
- 11.Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita S: Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol 1997, 73:222-231 [PubMed] [Google Scholar]
- 12.Wong V, Gumbiner BM: A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 1997, 136:399-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA: Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci 2000, 113:2363-2374 [DOI] [PubMed] [Google Scholar]
- 14.Morita K, Furuse M, Fujimoto K, Tsukita S: Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999, 96:511-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S: Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 1994, 127:1617-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM: The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 1998, 273:29745-29753 [DOI] [PubMed] [Google Scholar]
- 17.Gumbiner B: Generation and maintenance of epithelial cell polarity. Curr Opin Cell Biol 1990, 2:881-887 [DOI] [PubMed] [Google Scholar]
- 18.Gumbiner BM: Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996, 84:345-357 [DOI] [PubMed] [Google Scholar]
- 19.Telo P, Breviario F, Huber P, Panzeri C, Dejana E: Identification of a novel cadherin (vascular endothelial cadherin-2) located at intercellular junctions in endothelial cells. J Biol Chem 1998, 273:17565-17572 [DOI] [PubMed] [Google Scholar]
- 20.Dejana E, Del Maschio A: Molecular organization and functional regulation of cell to cell junctions in the endothelium. Thromb Haemost 1995, 74:309-312 [PubMed] [Google Scholar]
- 21.Truelove SC, Richards WCD: Biopsy studies in ulcerative colitis. Br Med J 1956, 3:1315-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh S, Murphy M, Silverman M, Odze R, Antonioli D, Goldman H, Loda M: p27 expression in inflammatory bowel disease-associated neoplasia: further evidence of a unique molecular pathogenesis. Am J Pathol 1999, 155:1511-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusrat A, Parkos CA, Bacarra AE, Godowski PJ, Delp-Archer C, Rosen EM, Madara JL: Hepatocyte growth factor/scatter factor effects on epithelia: regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest 1994, 93:2056-2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nusrat A, Turner JR, Madara JL: IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol 2000, 279:G851-G857 [DOI] [PubMed] [Google Scholar]
- 25.Parkos CA, Delp C, Arnaout MA, Madara JL: Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest 1991, 88:1605-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaoutzani P, Colgan SP, Cepek KL, Burkard PG, Carlson S, Delp-Archer C, Brenner MB, Madara JL: Reconstitution of cultured intestinal epithelial monolayers with a mucosal-derived T lymphocyte cell line: modulation of epithelial phenotype dependent on lymphocyte-basolateral membrane apposition. J Clin Invest 1994, 94:788-796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colgan SP, Parkos CA, Delp C, Arnaout MA, Madara JL: Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-γ in a highly polarized fashion. J Cell Biol 1993, 120:785-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colgan SP, Resnick MB, Parkos CA, Delp-Archer C, McGuirk D, Bacarra AE, Weller PF, Madara JL: IL-4 directly modulates function of a model human intestinal epithelium. J Immunol 1994, 153:2122-2129 [PubMed] [Google Scholar]
- 29.Youakim A, Ahdieh M: Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999, 276:G1279-G1288 [DOI] [PubMed] [Google Scholar]
- 30.Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD: Tumor necrosis factor-α (TNF-α) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci 1999, 112:137-146 [DOI] [PubMed] [Google Scholar]
- 31.Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD: Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci 2000, 113:2085-2090 [DOI] [PubMed] [Google Scholar]
- 32.Papadakis KA, Targan SR: Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology 2000, 119:1148-1157 [DOI] [PubMed] [Google Scholar]
- 33.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M: Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today 1997, 18:61-64 [DOI] [PubMed] [Google Scholar]
- 34.Cassatella MA: The production of cytokines by polymorphonuclear neutrophils. Immunol Today 1995, 16:21-26 [DOI] [PubMed] [Google Scholar]
- 35.Saitou M, Furuse M, Sasaki H, Schulzke J-D, Fromm M, Takano H, Noda T, Tsukita S: Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000, 11:4131-4142 [DOI] [PMC free article] [PubMed] [Google Scholar]