Abstract
The β-1,6-N-acetylglucosaminyltransferase (β1,6GnT) gene family encodes enzymes playing crucial roles in glycan synthesis. Important changes in β1,6GnT expression are observed during development, oncogenesis, and immunodeficiency. The most characterized β1,6GnTs in this gene family are the human (h) C2GnT-L and h-IGnT, which have core 2 [Galβ1→3(GlcNAcβ1→6)GalNAc] and I branching [GlcNAcβ1→3(GlcNAcβ1→6)Gal] activities, respectively. Recently, h-C2GnT-M was shown to be unique in forming core 2, core 4 [GlcNAcβ1→3(GlcNAcβ1→6)GalNAc], and I structures. To date, the β1,6GnT gene family has been characterized only in mammals. Here, we describe that bovine herpesvirus type 4 (BHV-4) encodes a β1,6GnT expressed during viral replication and exhibiting all of the core 2, core 4, and I branching activities. Sequencing of the BHV-4 genome revealed an ORF, hereafter called BORFF3–4, encoding a protein (pBORFF3–4) exhibiting 81.1%, 50.7%, and 36.6% amino acid identity with h-C2GnT-M, h-C2GnT-L, and h-IGnT, respectively. Reverse transcriptase-PCR analysis revealed that BORFF3–4 is expressed during BHV-4 replication. Expression of BORFF3–4 in Chinese hamster ovary cells directed the expression of core 2 branched oligosaccharides and I antigenic structures on the cell surface. Moreover, a soluble form of pBORFF3–4 had core 4 branching activity in addition to core 2 and I branching activities. Finally, infection of a C2GnT-negative cell line with BHV-4 induced expression of core 2 branched oligosaccharides. This study extends the β1,6GnT gene family to a viral gene and provides a model to study the biological functions of a β1,6GnT in the context of viral infection.
The β-1,6-N-acetylglucosaminyltransferase (β1,6GnT) gene family encodes enzymes playing crucial roles in glycan synthesis (1, 2). β1,6GnTs are involved in the synthesis of GlcNAc (β1→6)Gal(NAc) linkages, and their activities are distinguished by their acceptor substrate specificities. To date, three activities have been described for the β1,6GnT gene family: the synthesis of the O-glycan core 2 and core 4 structures, and the synthesis of the I structure (Table 1).
Table 1.
Structures and Ab reactivity mediated by β1,6GnTs
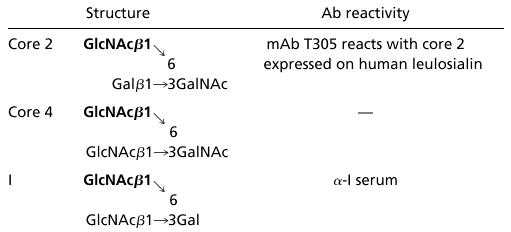 |
Core 2, core 4, and I structures are synthesized by addition of β1,6-linked GlcNAc (bold) by C2GnT, C4GnT, and IGnT, respectively. Further galactosylation yields oligosaccharides reactive with mAb T305 (for core 2 oligosaccharides) or with α-I serum (for I reactive oligosaccharides).
The core 2 structure is produced by core 2 β1,6GnT (C2GnT) activity by using core 1 (Galβ1→3GalNAc) as an acceptor substrate (3, 4). Importantly, sialyl Lex and sulfated sialyl Lex present in core 2 branched oligosaccharides have been shown to be preferential ligands for P- and L-selectin (5–9). The core 4 β1,6GnT (C4GnT) activity generates core 4 from core 3 (GlcNAcβ1→3GalNAc). Core 4 is mainly expressed in mucin-producing tissues (10).
In contrast to core 2 and core 4, which are O-glycan structures, the I structure is found in N-glycans, O-glycans, and glycolipids. I β1,6GnT (IGnT) activity converts linear poly-N-acetyllactosamine (Galβ1→4GlcNAcβ1→3)n to branched polyN-acetyllactosamine, Galβ1→4GlcNAcβ1→3(Galβ1→4GlcNAcβ1→6)Gal→R (11, 12). These linear and branched poly-N-acetyllactosamines represent, respectively, the i and I antigenic structures. The I structure is present on adult human erythrocytes and many mucins, whereas the i structure is expressed on fetal human erythrocytes (1, 13).
The most characterized β1,6GnTs encoded by the β1,6GnT gene family are the human (h) C2GnT-leukocyte-type (h-C2GnT-L) (14), h-IGnT (15), and the h-C2GnT-mucin-type (h-C2GnT-M) (16). h-C2GnT-L and h-IGnT have core 2 and I branching activities, respectively. Recently, h-C2GnT-M was shown to be an exception to the one-enzyme, one-linkage rule by forming core 2, core 4, and I structures.
Expression of β1,6GnT products changes during development, immunodeficiency, and oncogenesis. During T cell development, the synthesis of the core 2 branch is highly regulated. For example, quiescent T cells in the peripheral blood express simpler O-glycans, whereas core 2 branched O-glycans appear when T cells are activated by mitogens (4). Immunodefiencies, such as AIDS (17) and Wiskott–Aldrich syndrome (15, 18), are associated with aberrant expression of core 2 O-glycans on T cells. Changes in C2GnT expression have also been observed during oncogenesis. An increase in expression of core 2 branched oligosaccharides by cancer cells has been reported in leukemia, colonic carcinoma, and cells transfected with T24Hras (17, 19–22).
To date, the β1,6GnT gene family has been characterized only in mammals (1, 2). In this report, we present data demonstrating that bovine herpesvirus type 4 (BHV-4) encodes a functional β1,6GnT. Bovine herpesvirus type 4 belongs to the Gammaherpesvirinae subfamily (23). Gammaherpesviruses are lymphotropic viruses represented by Epstein–Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (HHV-8). The genome of BHV-4 is a double-stranded 145-kb DNA molecule consisting of a 110-kb central unique coding sequence, called L-DNA, flanked by two stretches of tandem repeats (24, 25). These stretches are designated polyrepetitive DNA (prDNA). After primary infection of cattle, BHV-4 replicates in mucosal cells and then spreads to mononuclear blood cells, which support both replication and latent infection (26). BHV-4 is the etiological agent of certain diseases of the respiratory and genital tracts of cattle, and has been isolated from a variety of lesions, including neoplasms, as well as from healthy tissues (reviewed by ref. 27).
Here, we show that BHV-4 encodes a multipotential β1,6GnT highly homologous to h-C2GnT-M that is expressed during viral replication. This study shows that the β1,6GnT gene family includes a viral gene and provides a model to study the biological functions of a β1,6GnT in the context of viral infection.
Materials and Methods
Cells and Virus Strain.
The Chinese hamster ovary (CHO)-Py⋅leu (CHO-Py⋅leu) cell line stably expressing human leukosialin (CD43) was grown in Ham's F12 medium (GIBCO/BRL) supplemented with 10% FCS (GIBCO/BRL) as described previously (14). Madin Darby bovine kidney (MDBK, ATCC CCL-22), embryonic bovine trachea (EBTr, ATCC CCL-44), embryonic bovine lung (EBL, German Collection of Microorganisms and Cell Cultures DSMZ ACC192), and bovine turbinate (BT, ATCC CRL-1390) cell lines were cultured in minimum essential medium (GIBCO/BRL) containing 10% FCS. The V. test strain of BHV-4, which was isolated from a case of orchitis (28), was used throughout this study.
Antibodies.
The mouse mAb 1G10 (PharMingen) reacts with human leukosialin independently of the expression of core 2 oligosaccharides on the target protein. In contrast, mAb T305 reacts only with human leukosialin-expressing core 2 oligosaccharides (15) (Table 1). The mAb 35 is raised against the BHV-4 glycoprotein complex gp6/gp10/gp17 (29). A human serum (Ma) reacting with I antigen [anti (α)-I serum] (30) was also used in this study (Table 1).
Construction of Vectors Encoding Bovine ORF F3–4 (BORFF3–4).
A cDNA fragment encoding BORFF3–4 of the BHV-4 V. test strain was prepared by PCR using viral genomic DNA as a template. The 5′ primer 5′-CCGGATCCATGAAGATGGCTGGGTGGAAG-3′ for PCR contained a 5′ BamHI site and nucleotides 1–21 (nucleotides 1–3 encode the initiation methionine, see Fig. 1), whereas the reverse primer 5′-CCATGCATTCAAAGTTCAGTCCCATAGATGG-3′ contained the 3′ end of the coding sequence (nucleotides 1301–1320), the stop codon, and an NsiI site. The PCR product was then subcloned into pGEM-T (Promega), resulting in pGEM-T-BORFF3–4. A fragment encoding BORFF3–4 was excised from pGEM-T-BORFF3–4 by BamHI/SpeI digestion and ligated into BamHI and XbaI sites of pcDNA3.1/Hygro(+) (Invitrogen), resulting in pcDNA3.1-BORFF3–4.
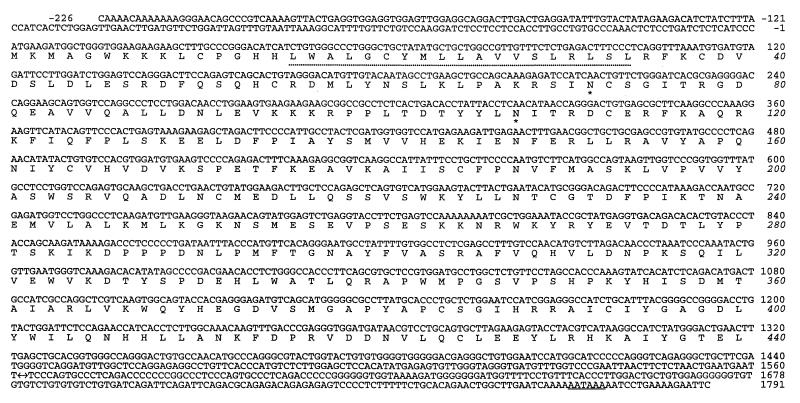
Figure 1.
Nucleotide and deduced amino acid sequences of BORFF3–4. The nucleotide sequence represents the right end (1,787 bp) of the L-DNA and the first 230 bp of the prDNA of BHV-4 V. test strain genome. The junction between the L-DNA and the prDNA is marked with a bidirectional arrow at nucleotides 1561–1562. The sequences are numbered relative to the translation initiation site. A consensus sequence for polyadenylation signal is underlined. The signal/membrane-spanning domain is denoted by a dotted line, and two potential N-glycosylation sites are marked with asterisks.
Construction of Vector Encoding a Soluble Chimeric Form of BORFF3–4 Translation Product.
A cDNA fragment encoding the putative catalytic domain plus the stem region of BORFF3–4 translation product (pBORFF3–4) was generated by PCR using pcDNA3.1-BORFF3–4 as a template. The sequence of the forward primer was 5′-CCGGATCCCCTCAGGTTTAAATGTGATGTAG-3′, corresponding to the BamHI site and nucleotides 99–121 of BORFF3–4 (Fig. 1). The 3′ primer was 5′-CCATGCATTCAAAGTTCAGTCCCATAGATGG-3′. The cDNA fragment encoding amino acids 34–440 of pBORFF3–4 was subcloned into pGEM-T, resulting in pGEM-T-TrBORFF3–4. A fragment encoding TrBORFF3–4 was then excised by BamHI/SpeI digestion and ligated in frame into BamHI and XbaI sites of pcDNA3-A, resulting in pcDNA3-A⋅TrBORFF3–4. pcDNA3-A is a pcDNA3.1-derived vector harboring a sequence encoding a signal peptide and the IgG binding domain of protein A (16).
Vectors.
The pcDNA3.1-C2GnT-M and pcDNA3-A⋅C2GnT-M vectors encode h-C2GnT-M and a chimeric h-C2GnT-M, respectively (16). The pcDSRα-leukosialin vector encodes human leukosialin (16).
Indirect Immunofluorescent Staining.
Cells were fixed in PBS containing 4% paraformaldehyde (wt/vol) for 10 min on ice and then 20 min at 20°C. Staining (incubation and washes) of fixed samples was performed in PBS containing 10% FCS (vol/vol) (PBSF). When permeabilization was required after fixation, the staining was performed in PBSF containing 0.2% saponin (wt/vol) (Sigma). The mAbs T305 (1/100), 1G10 (1/100), and 35 (1/1000) and the α-I serum (1/200) were used as primary Abs at the indicated dilutions. The mAbs and the α-I serum were detected with Alexa Fluor 488 goat α-mouse IgG (H + L) (Alexa-GAM, 1/200, Molecular Probes) and R-phycoerythrin goat α-human IgM (μ-chain-specific) Abs (PE-GAH, 1/64, Sigma), respectively.
Confocal Microscopy Analysis.
Confocal microscopy analyses were performed with a TCS SP confocal microscope (Leica) as described previously (31).
Enzyme Assays of Chimeric pBORFF3–4.
These assays were carried out exactly as described previously (16). Briefly, pcDNA3-A⋅TrBORFF3–4, pcDNA3-A⋅C2GnT-M, or pcDNA3-A was transiently transfected into CHO cells. The chimeric enzymes were then adsorbed to IgG-Sepharose from the concentrated cell culture supernatant and used as the enzyme source for the assays. As acceptors, Galβ1→3GalNAcα→p-nitrophenol and GlcNAcβ1→3GalNAcα→p-nitrophenol (Toronto Research Chemicals, Downsview, ON, Canada) were used for assaying C2GnT and C4GnT activities, respectively. Galβ1→4GlcNAcβ1→3Galβ1→4GlcNAcβ1→6Manα1→6Manβ→octyl and GlcNAcβ1→3Galβ1→4GlcNAcβ1→6Manα1→Manβ1→octyl were used for assaying IGnT activity. Because addition of N-acetylglucosamine occurs at the underlined galactose residues, the two acceptors serve for centrally acting IGnT (cIGnT) and predistally acting IGnT (dIGnT), respectively (32). CHO cells transfected with pcDNA3-A vector were used as a negative control. The radioactivity obtained with the negative control was subtracted from the values obtained in experiments with the enzyme. The former was less than 0.1% of the latter when considering reactions with the highest incorporation.
Reverse Transcriptase (RT)-PCR.
Cytoplasmic RNA was isolated from mock-infected and BHV-4-infected MDBK cells 24 h after infection (multiplicity of infection) of 10 plaque-forming units per cell by using RNeasy Mini Kit (Qiagen, Chatsworth, CA), and then further purified by using High Pure RNA Isolation Kit (Roche Molecular Biochemicals). RT reactions were performed on 150 ng of RNA by using First-Strand cDNA Synthesis Kit (Amersham Pharmacia). The 5′-NotI-d(T)18-3′ primer provided in the kit was used for these reactions. Finally, PCRs were performed on the first strand with the forward primer 5′-CCGGATCCATGAAGATGGCTGGGTGGAAG-3′, corresponding to nucleotides 1–21 of BORFF3–4 (Fig. 1), and the reverse primer 5′-NotI-d(T)18-3′.
Results
The Genome of BHV-4 Contains an ORF Encoding a Protein Highly Homologous to h-β1,6GnTs.
Recently, the overall genomic organization of the BHV-4 V. test strain was determined by partial sequencing (33). This study identified four ORFs in the F region located at the right end of the L-DNA. These ORFs were designated BORFF1 to -4 (from left to right) for bovine ORF of the F region. Database searches performed at the time of this study did not reveal any amino acid sequence homology between BORFF3 or BORFF4 and any known protein (33).
In the present study, we have carefully sequenced a region of the BHV-4 V. test strain genome corresponding to the right end of the L-DNA, the junction between the L-DNA and the prDNA, and the beginning of the prDNA (Fig. 1). Our sequencing revealed a potentially important discrepancy with the previously reported sequence (33). Indeed, based on the sequence presented in Fig. 1, BORFF3 and BORFF4 are replaced by a single ORF because of a frame shift. This longer ORF of 1,320 bp, which we designate BORFF3–4, encodes a protein of 440 amino acid residues (with a predicted molecular mass of 50,701 Da) that we term pBORFF3–4. A hydropathy plot predicts that pBORFF3–4 has type II membrane topology.
Interestingly, database searches revealed that pBORFF3–4 is highly homologous to human β1,6GnTs (Fig. 2). The amino acid sequence of pBORFF3–4 has 81.1%, 50.7%, and 36.6% identity with the sequences of h-C2GnT-M, h-C2GnT-L, and h-IGnT, respectively. The percent identity (81%) observed between pBORFF3–4 and h-C2GnT-M is much higher than the highest percent identity observed among the three human β1,6GnTs (49.8% observed between h-C2GnT-M and h-C2GnT-L). These results suggest that pBORFF3–4 is a viral homologue of h-C2GnT-M.
Figure 2.
Homology among pBORFF3–4, h-C2GnT-M, h-C2GnT-L, and h-IGnT. The four proteins were compared by using a clustal alignment program. The amino acid residues are numbered with respect to the translation initiation methionine. Identical residues are indicated by boxes. Dashes represent gaps in the sequence.
BORFF3–4 Is Expressed During BHV-4 Replication Cycle.
The structural homology of pBORFF3–4 and h-C2GnT-M suggests that these proteins could also be functional homologues. Before analyzing the enzymatic activity of pBORFF3–4, we determined whether BORFF3–4 is transcribed during BHV-4 infection, thereby meeting the criteria for being a gene. To do so, we used RT-PCR as described in Materials and Methods. PCR was performed on first-strand cDNA made from infected and mock-infected MDBK cells. In contrast to mock-infected cells, infected cells gave rise to a 1.8-kb PCR product. When RT was omitted from the reactions, the product seen in infected cells was not detected, indicating that the latter did not result from amplification of contaminant viral DNA (data not shown). To verify that the RT-PCR 1.8-kb product was related to BORFF3–4, it was cloned and partially sequenced (approximately 0.5 kb of each extremity). Sequencing confirmed that the 1.8-kb product was related to BORFF3–4 and enabled us to characterize the 3′ end of BORFF3–4 transcripts. The latter consists of genomic sequence up to base 1788 (Fig. 1), followed by a poly(A) tail. A consensus sequence for polyadenylation is present at bases 1769–1774. Taken together, these results demonstrate that BORFF3–4 is expressed during BHV-4 replication cycle.
BORFF3–4 Encodes a Functional β1,6GnT Forming Core 2 and I Structures.
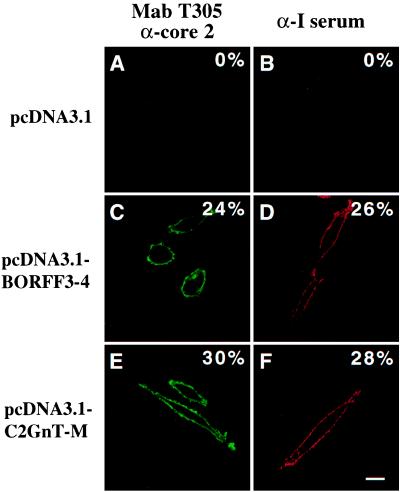
Because pBORFF3–4 is highly homologous to h-C2GnT-M, we analyzed whether it could direct the expression of core 2 branched oligosaccharides and I antigen on the cell surface. After transfection with pcDNA3.1-BORFF3–4 or pcDNA3.1-C2GnT-M, CHO-Py⋅leu cells showed strong staining both with mAb T305, which reacts with leukosialin-expressing core 2 oligosaccharides (Fig. 3 C and E), and with α-I serum (Fig. 3 D and F). In contrast, no staining was observed when CHO-Py⋅leu cells were transfected with pcDNA3.1 under the same conditions (Fig. 3 A and B). These results indicate that, like h-C2GnT-M, pBORFF3–4 has both core 2 and I branching activities.
Figure 3.
BORFF3–4 encodes a functional β1,6GnT forming core 2 and I structures. CHO-Py⋅leu cells were transfected with pcDNA3.1 (A and B), pcDNA3.1-BORFF3–4 (C and D), or pcDNA3.1-C2GnT-M (E and F) by using FuGENE 6 transfection reagent (Roche). Twenty-four hours after transfection, the cells were treated for indirect immunofluorescent staining of fixed samples. mAb T305 reacting with core 2 oligosaccharides in leukosialin (A, C, and E) and α-I serum (B, D, and F) were used as primary Abs and were revealed by Alexa-GAM and PE-GAH secondary Abs, respectively. The percentage of positive cells estimated by examination of 600 randomly selected cells is presented in each panel. (Bar, 10 μm.)
A Soluble Chimeric Form of pBORFF3–4 Has Core 2, Core 4, and I Branching Activities.
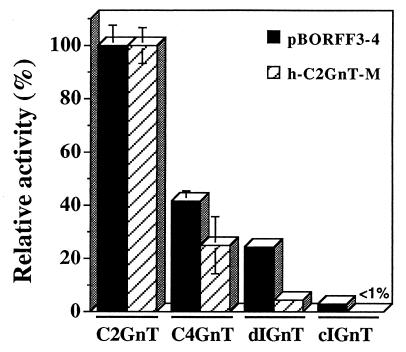
By using an in vitro assay, h-C2GnT-M was recently shown to have core 4 branching activity in addition to core 2 and I branching activities (16). Here, we used an identical approach to test whether pBORFF3–4 also has core 4 activity. To this end, the pcDNA3-A⋅TrBORFF3–4 vector expressing a soluble chimeric form of pBORFF3–4 was constructed as described in Materials and Methods. Transfection of this vector into CHO-Py⋅leu cells and immunofluorescent staining with mAb T305 and α-I serum (Table 1) revealed that the chimeric form retains core 2 and I branching activities exhibited by the full-length protein (data not shown). In vitro enzyme assays showed that chimeric pBORFF3–4 exhibited strong activity toward GlcNAcβ1→3GalNAcα→p-nitrophenol, indicating that pBORFF3–4 has C4GnT activity as well (Fig. 4). The use of acceptors specific for dIGnT and cIGnT activities revealed that BORFF3–4 has both activities and that the former is predominant (Fig. 4). These results indicate that pBORFF3–4 has core 2, core 4, and I branching activities as h-C2GnT-M.
Figure 4.
A soluble chimeric form of pBORFF3–4 has core 2, core 4, and I branching activities. Soluble chimeric forms of pBORFF3–4 (filled bars) and h-C2GnT-M (hatched bars) were assayed for C2GnT, C4GnT, dIGnT, and cIGnT activities as described in Materials and Methods. The results are expressed relative to C2GnT activity, which is defined as 100%. The C2GnT activity was 45.3 pmol/h per ml and 84.0 pmol/h per ml for chimeric pBORFF3–4 and chimeric h-C2GnT-M, respectively. Bars represent standard errors for duplicate measures.
Replication of BHV-4 Directs the Expression of Core 2 Branched Oligosaccharides.
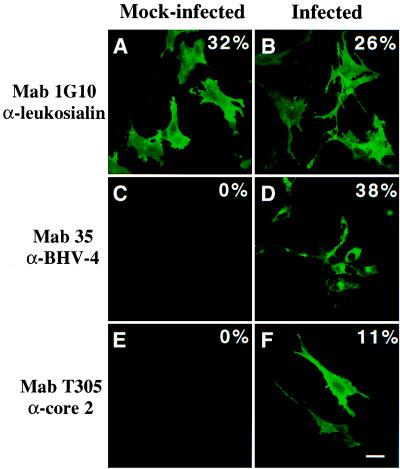
The results of the RT-PCR analysis presented above indicate that BORFF3–4 is expressed during BHV-4 replication, suggesting that pBORFF3–4 enzymatic activities could be detected in BHV-4-infected cells. To test such a hypothesis, we required a cell line that does not express C2GnT and/or IGnT activities and that can support BHV-4 replication. The MDBK, EBTr, EBL, and BT cell lines, all known to support BHV-4 replication, were tested for expression of C2GnT and IGnT activities by immunofluorescent assays. Surprisingly, all uninfected cell lines expressed IGnT activity, and only the EBTr cell line was negative for C2GnT activity (data not shown). The EBTr cell line was then used to determine whether core 2 branched oligosaccharides are expressed during BHV-4 replication cycle (Fig. 5). Staining with mAb 1G10, which reacts with human leukosialin independently of the expression of core 2 branched oligosaccharides on the target protein, revealed that both mock-infected and infected EBTr cells expressed human leukosialin as the result of the transfection with pcDSRα-leukosialin (Fig. 5 A and B). Staining with mAb T305, which reacts only with human leukosialin expressing core 2 branched oligosaccharides (Table 1), revealed positive cells in BHV-4-infected cells but not in mock-infected cells (Fig. 5, compare E and F). Infection of the cells was controlled by staining with mAb 35, which reacts with the BHV-4 glycoprotein complex gp6/gp10/gp17 (Fig. 5D). Together, these results indicate that production of core 2 branched oligosaccharides occurred during BHV-4 replication cycle.
Figure 5.
Replication of BHV-4 directs the expression of core 2 branched oligosaccharides in infected cells. Subconfluent monolayers of EBTr cells were transfected with pcDSRα-leukosialin by using FuGENE 6 transfection reagent. Twenty-four hours after transfection, the cells were mock infected (A, C, and E) or infected (B, D, and F) with BHV-4 at a multiplicity of infection of 10. Twenty-four hours after infection, the cells were treated for indirect immunofluorescent staining of fixed and permeabilized samples. mAb 1G10 reacting with leukosialin (A and B), mAb 35 reacting with BHV-4 glycoprotein complex gp6/gp10/gp17 (C and D), and mAb T305 raised against leukosialin expressing core 2 oligosaccharides (E and F) were used as primary Abs and were revealed by Alexa-GAM Abs. The percentage of positive cells estimated by examination of 600 randomly selected cells is presented in each panel. (Bar, 10 μm.)
Discussion
In the present study, we show that BHV-4 encodes a viral homologue of h-C2GnT-M. The viral enzyme has all of the core 2, core 4, and I branching activities reported for h-C2GnT-M (16) and is expressed during viral replication. Thus, we have shown that β1,6GnT can be encoded by a virus.
The β1,6GnT encoded by BHV-4 is a structural and functional homologue of h-C2GnT-M. A structural comparison of pBORFF3–4 and h-C2GnT-M reveals that the amino acid sequence of the two proteins is 81.1% identical, whereas the highest percent identity observed among human β1,6GnTs is only 49.8% (calculation from Fig. 2). Because BORFF3–4 is probably derived from a bovine gene, it will be interesting to determine the homology between pBORFF3–4 and bovine C2GnT-M when the latter is identified. During the last decade, the number of reported viral genes homologous to cellular host genes increased drastically, leading to the concept that acquisition of host cellular genes by some viruses conferred selective advantages during evolution. Surprisingly, there are very few examples of glycosyltransferases encoded by viruses. However, it is most likely that the growing list of available complete nucleotide sequences of large DNA viruses will lead to the characterization of new viral glycosyltransferases. Recent sequencing data of poxviruses support this idea (34, 35).
BORFF3–4 is expressed during the BHV-4 replication cycle. This conclusion is supported by identification of BORFF3–4 transcripts and detection of C2GnT activity (Fig. 5) in BHV-4-infected cells. Characterization of the 3′-end of BORFF3–4 transcripts revealed that the polyadenylation signal is located in the prDNA of the viral genome (Fig. 1). This observation indicates that the prDNA, in addition to its roles in cleavage and encapsidation of replicated concatemeric viral DNA (36), contributes to expression of an ORF encoded by the L-DNA.
Viruses infecting bacteria and insects encode glycosyltransferases. Some bacteriophages encode glucosyltransferases catalyzing the addition of glucose to hydroxymethyl groups of modified cytosines in their DNA genome (37). This DNA glucosylation protects the phage genome against the host restriction endonuclease system. Other bacteriophages that require O-antigenic polysaccharide chains as receptors for adsorption and infection of the host bacterium encode O-acetyltransferase, which causes specific O-antigenic conversions of their receptor (38). These conversions could prevent the binding of phages to already infected bacteria. Baculovirus Autographa californica nuclear polyhedrosis virus encodes an ecdysteroid glucosyltransferase catalyzing the transfer of glucose from UDP-glucose to ecdysteroid insect molting hormones. Expression of this enzyme allows the virus to block molting and pupation of infected insect larvae (39). It is noteworthy that these viral glycosyltransferases are involved in interactions of the virus with its host cell or its host organism.
BORFF3–4 is highly conserved among BHV-4 strains. In this report, we present the BORFF3–4 sequence of the BHV-4 V. test strain. However, we and others (M. Goltz, W. Zimmermann, and H.-J. Buhk, personal communication) have sequenced this gene from other strains (data not shown), demonstrating that pBORFF3–4 is highly conserved among different strains, suggesting that this protein is important in the biology of BHV-4 infection.
Several hypotheses that are not mutually exclusive could be made concerning the role of pBORFF3–4 in the biology of BHV-4 infection. The first is that this enzyme is required for posttranslational modification of a structural viral protein. Gammaherpesviruses replicate in different cell types because of their complex biology. In the case of BHV-4, after initial replication in mucosal cells, the virus spreads to mononuclear blood cells, where it can establish latency, and eventually replicates after reactivation (26). Interestingly, mucosal cells but not mononuclear blood cells express C2GnT-M (16). BHV-4 replication in mononuclear blood cells may require pBORFF3–4 activities.
A second hypothesis is that pBORFF3–4 is involved in modification of interactions occurring between infected mononuclear blood cells and cells of the immune system, thus protecting the infected cells from the cellular immune response. As mentioned previously, the level of C2GnT activity and resulting core 2 branched oligosaccharides significantly increase in leukocytes of patients with immunodeficiencies, including Wiskott–Aldrich syndrome (15, 18) and AIDS (17). By using a transgenic model, it was also demonstrated that overexpression of C2GnT in T lymphocytes resulted in decreased T cell interactions with B cells and Ag-presenting cells, leading to impaired immune responses (40, 41). These results raise a possibility that BHV-4 evades the cellular immune response raised against infected mononuclear blood cells by expressing core 2 branched oligosaccharides on the cell surface.
In conclusion, this study extends the β1,6GnT gene family to a viral gene and provides a model to study the biological functions of a β1,6GnT in the context of viral infection. Future studies of this model will provide a synergy between the fields of virology and glycobiology. These studies should reveal the evolutionary advantage conferred to a virus expressing β1,6GnT activities and should facilitate the understanding of the biological functions of cellular β1,6GnTs in physiological and pathological conditions.
Acknowledgments
N.M.-G. and F.B. are Research Fellows of the Fonds National Belge de la Recherche Scientifique (FNRS). L.W. is a Research Director of the FNRS. A.V. is a Research Associate of the FNRS and the laureate of a Union International Contre le Cancer International Cancer Technology Transfer Fellowship (ICRETT). This work was supported by a Concerted Action awards program of the French Community of Belgium (ARC 98/03-220) and by U.S. National Cancer Institute Grant R37 CA33000. Purchase of the confocal microscope was supported by the following grants: FRFC 2.4532.98 from the FNRS, FNRS LOTTO 9.4592.97 from the Belgian National lottery, and ARC 98/03-220 from the French Community of Belgium.
Abbreviations
- β1,6GnT
β-1,6-N-acetylglucosaminyltransferase
- BHV-4
bovine herpesvirus type 4
- BORFF3–4
bovine ORF F3–4
- pBORFF3–4
protein encoded by BORFF3–4
- C2GnT
C4GnT, and IGnT, core 2, core 4, and I branching β1,6GnT, respectively
- cIGnT
centrally acting IGnT
- dIGnT
predistally acting IGnT
- h-C2GnT-L and -M
human core 2 leukocyte-type β1,6GnT and mucin-type, respectively
- prDNA
polyrepetitive DNA
- CHO
Chinese hamster ovary
- EBTr
embryonic bovine trachea
- α-
anti-
- RT
reverse transcriptase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF231105).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100058897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100058897
References
- 1.Fukuda M. In: Molecular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford: IRL; 1994. pp. 1–52. [Google Scholar]
- 2.Schachter H. In: Molecular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford: IRL; 1994. pp. 88–162. [Google Scholar]
- 3.Fukuda M, Carlsson S R, Klock J C, Dell A. J Biol Chem. 1986;261:12796–12806. [PubMed] [Google Scholar]
- 4.Piller F, Piller V, Fox R I, Fukuda M. J Biol Chem. 1988;263:15146–15150. [PubMed] [Google Scholar]
- 5.Hemmerich S, Leffler H, Rosen S D. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins P P, McEver R P, Cummings R D. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 7.Lowe B J. In: Molecular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford: IRL; 1994. pp. 163–205. [Google Scholar]
- 8.Bistrup A, Bhakta S, Lee J K, Belov Y Y, Gunn M D, Zuo F R, Huang C C, Kannagi R, Rosen S D, Hemmerich S. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraoka N, Petryniak B, Nakayama J, Tsuboi S, Susuki M, Yeh J-C, Izama D, Tanaka T, Miyasaka M, Lowe J B, Fukuda M. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- 10.Schachter H, Brockhausen I. In: Glycoconjugates: Composition, Structure, and Function. Allen H J, Kisailus E C, editors. New York: Dekker; 1992. pp. 263–332. [Google Scholar]
- 11.Fukuda M, Fukuda M N, Hakomori S. J Biol Chem. 1979;254:3700–3703. [PubMed] [Google Scholar]
- 12.Piller F, Cartron J P, Maranduba A, Veyrieres A, Leroy Y, Fournet B. J Biol Chem. 1984;259:13385–13390. [PubMed] [Google Scholar]
- 13.Feizi T. Nature (London) 1985;314:53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 14.Bierhuizen M F, Fukuda M. Proc Natl Acad Sci USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piller F, Le Deist F, Weinberg K, Parkman R, Fukuda M. J Exp Med. 1991;173:1501–1510. doi: 10.1084/jem.173.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh J-C, Ong E, Fukuda M. J Biol Chem. 1999;274:3215–3221. doi: 10.1074/jbc.274.5.3215. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh O, Piller F, Fox R I, Fukuda M. Blood. 1991;77:1491–1499. [PubMed] [Google Scholar]
- 18.Higgins E A, Siminovitch K A, Zhuang D L, Brockhausen I, Dennis J W. J Biol Chem. 1991;266:6280–6290. [PubMed] [Google Scholar]
- 19.Brockhausen I, Kuhns W, Schachter H, Matta K L, Sutherland D R, Baker M A. Cancer Res. 1991;51:1257–1263. [PubMed] [Google Scholar]
- 20.Yang J M, Byrd J C, Siddiki B B, Chung Y S, Okuno M, Sowa M, Kim Y S, Matta K L, Brockhausen I. Glycobiology. 1994;4:873–884. doi: 10.1093/glycob/4.6.873. [DOI] [PubMed] [Google Scholar]
- 21.Vavasseur F, Yang J M, Dole K, Paulsen H, Brockhausen I. Glycobiology. 1995;5:351–357. doi: 10.1093/glycob/5.3.351. [DOI] [PubMed] [Google Scholar]
- 22.Yousefi S, Higgins E, Daoling Z, Pollex-Krüger A, Hindsgaul O, Dennis J W. J Biol Chem. 1991;266:1772–1782. [PubMed] [Google Scholar]
- 23.Bublot M, Lomonte P, Lequarre A-S, Albrecht J-C, Nicholas J, Fleckenstein B, Pastoret P-P, Thiry E. Virology. 1992;190:654–665. doi: 10.1016/0042-6822(92)90903-3. [DOI] [PubMed] [Google Scholar]
- 24.Ehlers B, Buhk H-J, Ludwig H. J Gen Virol. 1985;66:55–68. doi: 10.1099/0022-1317-66-1-55. [DOI] [PubMed] [Google Scholar]
- 25.Bublot M, Van Bressem M-F, Thiry E, Dubuisson J, Pastoret P-P. J Gen Virol. 1990;71:133–142. doi: 10.1099/0022-1317-71-1-133. [DOI] [PubMed] [Google Scholar]
- 26.Osorio F A, Reed D E. Am J Vet Res. 1983;44:975–980. [PubMed] [Google Scholar]
- 27.Thiry E, Lomonte P, Vanderplasschen A, Pastoret P-P. Lymphotropic Herpesviruses: Epstein–Barr Virus and Human Herpesvirus 8. Vol. 70. International Agency for Research on Cancer, Geneva: World Health Organization; 1997. pp. 439–445. [Google Scholar]
- 28.Thiry E, Pastoret P-P, Dessy-Doizé C, Hanzen C, Calberg-Bacq C M, Dagenaix L, Vindevogel H, Ectors F. Ann Méd Vét. 1981;125:207–214. [Google Scholar]
- 29.Vanderplasschen A, Goltz M, Lyaku J, Benarafa C, Buhk H-J, Thiry E, Pastoret P-P. Virology. 1995;213:328–340. doi: 10.1006/viro.1995.0006. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe K, Hakomori S, Childs R A, Feizi T. J Biol Chem. 1979;254:3221–3228. [PubMed] [Google Scholar]
- 31.Vanderplasschen A, Smith G L. J Virol. 1997;71:4032–4041. doi: 10.1128/jvi.71.5.4032-4041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu J, Nishikawa A, Fujii S, Gasa S, Taniguchi N. J Biol Chem. 1992;267:2994–2999. [PubMed] [Google Scholar]
- 33.Lomonte P, Bublot M, van Santen V, Keil G M, Pastoret P-P, Thiry E. J Gen Virol. 1995;76:1835–1841. doi: 10.1099/0022-1317-76-7-1835. [DOI] [PubMed] [Google Scholar]
- 34.Afonso C L, Tulman E R, Lu Z, Oma E, Kutish G F, Rock D L. J Virol. 1999;73:533–552. doi: 10.1128/jvi.73.1.533-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson R J, Hall D F, Kerr P J. J Virol. 1999;73:2376–2384. doi: 10.1128/jvi.73.3.2376-2384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broll H, Buhk H-J, Zimmermann W, Goltz M. J Gen Virol. 1999;80:979–986. doi: 10.1099/0022-1317-80-4-979. [DOI] [PubMed] [Google Scholar]
- 37.Vrielink A, Rüger W, Driessen H P C, Freemont P S. EMBO J. 1994;13:3413–3422. doi: 10.1002/j.1460-2075.1994.tb06646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma N K, Brandt J M, Verma D J, Lindberg A A. Mol Microbiol. 1991;5:71–75. doi: 10.1111/j.1365-2958.1991.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 39.O'Reilly D R, Miller L K. Science. 1989;245:1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- 40.Tsuboi S, Fukuda M. EMBO J. 1997;16:6364–6373. doi: 10.1093/emboj/16.21.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuboi S, Fukuda M. J Biol Chem. 1998;273:30680–30687. doi: 10.1074/jbc.273.46.30680. [DOI] [PubMed] [Google Scholar]