Abstract
Previous studies have provided strong evidence for a role for neutrophils in mediating pathology during reperfusion of ischemic tissues. CXC chemokines including interleukin-8, KC/Groα, and macrophage inflammatory protein (MIP)-2, direct neutrophils to tissue sites of inflammation. In the current study we tested the efficacy of antibodies to KC/Groα and MIP-2 in inhibiting neutrophil infiltration into kidneys during reperfusion after 1 hour of warm ischemia using a mouse model. KC mRNA and protein were produced within 3 hours after reperfusion of the ischemic kidneys. MIP-2 mRNA and protein were twofold to fourfold lower than KC and were at low levels until 9 hours after reperfusion. Only 60% of mice subjected to ischemia/reperfusion injury survived to day 3 after reperfusion. Treatment with rabbit neutralizing antibodies to both KC and MIP-2 inhibited neutrophil infiltration into ischemic kidneys during reperfusion, restored renal function as assessed by decreased serum creatinine and urea nitrogen levels to near normal levels, and resulted in complete survival of treated animals. Finally, treatment with both antibodies significantly reduced histologically graded pathology of kidneys subjected to ischemia/reperfusion injury. Collectively, the results indicate the efficacy of neutralizing the chemokines directing neutrophils into ischemic kidneys during reperfusion to inhibit this infiltration and attenuate the resulting pathology.
The induction and length of ischemia/reperfusion injury have a critical impact on the eventual outcome of solid organ allografts. 1 The decreased success of transplantation with increased ischemia/reperfusion to the graft as well as the greater success of living unrelated renal transplants when compared to those from mismatched cadaver transplants emphasize the importance of this tissue inflammation on organ function. 2 A key factor associated with ischemia/reperfusion injury of solid organs is neutrophil infiltration and activation in the ischemic tissue. 3-10 Studies in several animal models have shown beneficial effects by depleting neutrophils before reperfusion of ischemic organs or tissues. 5-12 These results predict that inhibition of neutrophil recruitment to ischemic tissues during reperfusion should also attenuate the tissue pathology.
Leukocyte trafficking into tissue sites of inflammation is primarily directed by chemokines, a superfamily of chemoattractant cytokines. 13,14 More than 50 chemokine proteins have been identified and are grouped into four families based on a cysteine motif in the amino terminal area of the protein. The CXC chemokines include several neutrophil chemoattractants such as interleukin-8, Groα, of which KC is the murine homologue, and macrophage inflammatory protein-2 (MIP-2). In addition, three interferon-γ-induced CXC chemokines including IP-10, Mig, and ITAC are potent chemoattractants for antigen-activated T cells. The CC chemokines are chemotactic for a variety of leukocytes including monocytes, macrophages, dendritic cells, and lymphocytes. Representative CC chemokines include monocyte chemotactic protein-1, MIP-1α, and MIP-1β. The presence of chemokine gene expression and/or protein during ischemia/reperfusion injury has been shown in several model systems. 5,15-24 However, the role of specific chemokines in mediating leukocyte infiltration into kidneys subjected to ischemia/reperfusion injury remains unclear. The ability to inhibit leukocyte recruitment and decrease inflammation through neutralization of specific chemokines is now being tested in many models of inflammation. 25-31 The important role of neutrophils in ischemia/reperfusion injury raises the possibility that tissue injury could be prevented by inhibiting neutrophil recruitment to the ischemic tissue through inhibition of chemokines that direct neutrophil infiltration into the tissue. The goal of the current study was to test if antibodies reactive to the neutrophil chemoattractants KC (Groα) and MIP-2 would inhibit neutrophil infiltration and tissue pathology during reperfusion of ischemic kidneys in a mouse model.
Materials and Methods
Experimental Animals
C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Adult males, 8 to 12 weeks old, were used throughout the study.
In Situ Ischemia Surgery
Mice were given 20 U heparin sodium intraperitoneally 30 minutes before surgery. Mice were anesthetized with phenobarbital and ether and kept warm under a 60-W light bulb until surgery. The abdominal cavity was opened with a midline incision and the unilateral or bilateral renal pedicle was tied with 6-0 silk suture nontraumatically. The abdominal cavity was flushed with warm (37°C) Ringer’s solution and the wound was temporarily closed with 4-0 silk suture. Except for ligation of renal pedicles, sham-operated mice were treated in an identical manner. Intraperitoneal temperature was maintained at 32°C. Kidneys were subjected to ischemia for 60 minutes with varying times of reperfusion. Just before reperfusion 500 μl of 6% hetastarch solution or 250 to 500 μl of mouse antiserum was given intravenously and the renal pedicles were untied and reperfused. Immediate and complete reperfusion was confirmed visually and the wound was closed. After recovery from anesthesia, mice were given access to food and water ad libitum.
Antibodies and Antisera
Rabbit antiserum to mouse KC and to mouse MIP-2 were generated as previously reported. 25,32,33 The specificity of the antisera was confirmed by immunoblotting to recombinant proteins. The neutralizing ability of these antisera has been shown both in vitro and in vivo. 25,32,33 For the chemokine neutralization studies, groups of mice received 250 μl of KC-antiserum (KCAS), 250 μl of MIP-2 antiserum (MIP2AS), or both KCAS and MIP2AS instead of hetastarch just before reperfusion. Control animals received 500 μl of normal rabbit serum (NRS). Anti-mouse Ly-6G monoclonal antibody (mAb) (RB6-8C5) was purified from culture supernatant using protein G-Sepharose. To deplete mice of neutrophils, animals received 150 μg of RB6-8C5 intraperitoneally on days −2 and −1 before the ischemia subjected on day 0. Previous studies have demonstrated that this treatment results in the absence of thioglycollate-mediated neutrophil infiltration into the peritoneal cavity. 25
Histology
For immunohistology, kidney halves were embedded in OCT compound (Sakura Finetek U.S.A., Torrence, CA) and immediately frozen in liquid nitrogen. Coronal sections were cut at 8-μm thickness and mounted onto slides. Slides were dried overnight and fixed in acetone for 10 minutes and air-dried. Slides were immersed in phosphate-buffered saline (PBS) for 10 minutes and in 0.03% H2O2 in PBS for 10 minutes at room temperature to eliminate endogenous peroxidase activity. Endogenous biotin activity was blocked with Biotin Blocking System (DAKO, Carpinteria, CA). Slides were stained for 1 hour at room temperature with RB6-8C5 diluted at 10 μg/ml in 0.05% Tris-HCl buffer with 1% bovine serum albumin. Control slides were incubated with rat IgG as the primary Ab. After three washes in PBS for 5 minutes each, slides were incubated for 20 minutes with biotinylated rabbit anti-rat IgG, diluted 1:300 in the same buffer. After three washes in PBS, slides were incubated with streptavidin-horseradish peroxidase (DAKO) for 20 minutes. The substrate-chromogen solution was prepared by dissolving a 3,3′-diaminobenzidine 10-mg tablet (Sigma Chemical Co., St. Louis, MO) in 15 ml of PBS and 12 μl of 30% H2O2 was added just before use. After three washes in PBS for 5 minutes each, the 3,3′-diaminobenzidine solution was applied to the slides and incubated for 2 to 3 minutes. After a wash in dH2O, slides were counterstained with hematoxylin, rinsed with dH2O, and immersed in 37 nmol/L NH4OH for 10 seconds. Finally, the slides were dehydrated, coverslipped, and viewed with a light microscope. Images were captured using Image Pro Plus (Media Cybernetics, Silver Spring, MD). The number of neutrophils was counted in 10 random fields/slide and five slides/kidney for four different kidneys at ×200 magnification. For morphology experiments, mice were systemically perfused with 10% formalin after 1× PBS through the left ventriculum on harvest. Then the kidneys were fixed with 10% buffered formalin. Paraffin-embedded sections were prepared and stained with hematoxylin and eosin (H&E) or periodic acid staining (PAS). Morphological changes as a result of ischemia/reperfusion injury were graded using the scoring system shown in Table 1 ▶ . Each tubulus was scored and average scores were calculated for each captured field.
Table 1.
Histological Grading of Renal Pathology after Ischemia/Reperfusion Injury
| Grade | Injury | Pathology description |
|---|---|---|
| 0 | None | Normal tubule |
| 1 | Slight | Mild blebbing, loss of brush |
| 2 | Mild | Intensive blebbing, mild vacuolization |
| 3 | Moderate | Shrunken nuclei, intensive vacuolization |
| 4 | Severe | Necrotic/apoptotic cells |
| Denudation/rupture of basement membrane | ||
| 5 | Necrosis | Total necrosis of the tubule |
Renal Function Measurement
Mice were anesthetized with ether and bled from the postorbital plexus using a heparin-coated microcapillary tube. The serum was stored at −70°C until measurement. Serum urea nitrogen and serum creatinine were measured using the Urea Nitrogen Kit (Sigma Chemical Co.) and the Creatinine Kit (Sigma Diagnostics, Inc., St. Louis, MO), respectively, according to the manufacturer’s protocol.
RNA Extraction
Kidneys were retrieved at various times after reperfusion and immediately frozen in liquid nitrogen. Grafts were pulverized in liquid nitrogen and homogenized in 1 ml of Trizol reagent (Life Technologies, Inc., Grand Island, NY). After phase separation and precipitation with isopropanol, the RNA was resuspended in diethylpyrocarbonate-treated H2O (DEPC-H2O) and the concentration determined by spectrophotometry.
In Vitro Transcription
The multiprobe templates set, mCK-5 includes templates for macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-2, IP-10, and monocyte chemoattractant protein (MCP)-1 (BD PharMingen, San Diego, CA). Template cDNAs for KC (120 to 459) were generated in this laboratory and linearized for in vitro transcription. Template cDNA for murine GAPDH was purchased from BD Pharmingen. 32P-UTP-radiolabeled antisense riboprobes for RNase protection assay (RPA) were synthesized and purified using the RiboQuant in vitro transcription kit (BD PharMingen) according to the manufacturer’s instructions.
RNase Protection Assay
Renal expression of the chemokine genes and chemokine receptor genes was quantified by RPA using RiboQuant RPA Kit (BD PharMingen) according to the manufacturer’s protocol. In brief, 10 μg of sample RNA was hybridized overnight at 56°C with 32P-labeled riboprobes. Then the samples were treated with RNase A/T1 cocktail and with proteinase K. After extraction and precipitation, the samples were run on a denaturing 5% polyacrylamide gel. The gel was transferred to filter paper, dried, and then exposed to an X-ray film with a Storage Phosphor Screen (Molecular Dynamics, Sunnyview, CA). The intensity of each signal was measured using ImageQuant (Molecular Dynamics, Sunnyview, CA) and standardized to the GAPDH signal for each sample. The data are expressed as the mean signal ratio ± SD for each group.
Protein Preparation and Enzyme-Linked Immunosorbent Assay (ELISA)
Kidneys were harvested at several time points, cut into halves, and frozen in liquid nitrogen. The kidneys were pulverized in liquid nitrogen and dissolved in 500 μl of PBS with 0.01 mol/L ethylenediaminetetraacetic acid and a proteinase inhibitor cocktail (10 μg/ml phenylmethyl sulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 100 μg/ml Pefabloc SC, and 100 μg/ml chymostatin) and then 1 ml of 1.5% Triton X-100 in PBS was added. After incubation with agitation for 30 minutes at 4°C, samples were centrifuged at 12,000 × g for 10 minutes. The supernatant was collected and stored at −20°C until use. Total protein concentration was determined using the DC Protein Kit (BioRad, Richmond, CA). KC and MIP-2 were quantitated by sandwich ELISA using Quantikine M Kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical Analysis
Differences in cellular infiltration and renal function between treatment groups were analyzed by one-way factorial analysis of variance using StatView (Abacus Concepts, Inc., Berkeley, CA) and a P < 0.05 was considered a significant difference. Differences in viability between treatment groups of animals subjected to renal ischemia/reperfusion injury were analyzed by log-rank test and a P < 0.01 was considered a significant difference between treatment groups.
Results
Expression of Chemokines in Kidneys at Early Times after Ischemia/Reperfusion Injury
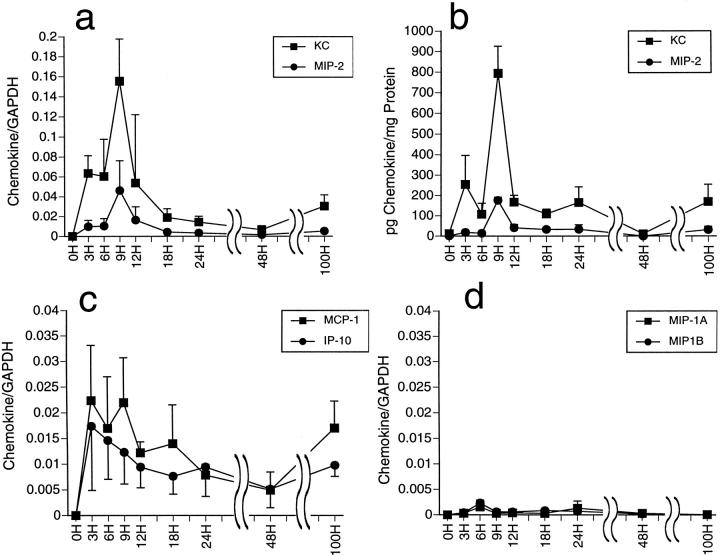
To begin to examine the roles of neutrophil chemoattractants after renal ischemia/reperfusion injury, we first tested the temporal expression of mRNA encoding neutrophil and macrophage chemoattractants in kidneys subjected to ischemic insult. After 60 minutes of ischemia, mice were treated with 500 μl of 6% hetastarch and the kidneys were harvested serially up to 100 hours after reperfusion and tissue RNA was isolated. After reperfusion, some mice died with high levels of serum creatinine (>5 mg/dL) indicating renal failure as the cause of death. These mice were excluded from this experiment. Expression of chemokine genes was tested by RNase protection assay. Expression of the neutrophil chemoattractants KC and MIP-2 were evident at low levels within 3 hours of reperfusion and reached peak levels at 9 hours after reperfusion before declining to baseline levels by 48 hours (Figure 1a) ▶ . Within the first 18 hours after reperfusion, levels of KC mRNA expression were twofold to fourfold higher than expression of MIP-2. Protein levels of KC and MIP-2 in the kidneys were quantitated by ELISA and closely followed the patterns of mRNA expression with greater production of KC than MIP-2 (Figure 1b) ▶ . The macrophage chemoattractant MCP-1 and the T-cell chemoattractant IP-10 were expressed at lower levels than KC and MIP-2 reaching peak levels at 3 to 9 hours after reperfusion and declining to low levels by 48 hours (Figure 1c) ▶ . Expression of MIP-1α and MIP-1β was undetectable except for low levels at a single time point, 6 hours after reperfusion (Figure 1d) ▶ .
Figure 1.
Temporal expression of chemokine genes and proteins in renal ischemia/reperfusion injury. Kidneys were subjected to 1 hour of warm ischemia. The mice were injected with 6% hetastarch and the kidneys were harvested serially until 100 hours after reperfusion. (a, c, and d) Total RNA was extracted and tested for chemokine mRNA levels by ribonuclease protection assay. The intensity of chemokine mRNA signals is plotted as a ratio to the GAPDH signal and the mean ratio of four kidneys in each group is shown ± SD. b: Tissue protein was also extracted for quantitation of KC and MIP-2 protein levels by sandwich ELISA.
Neutrophil Infiltration after Renal Ischemia/Reperfusion Is Inhibited by Neutralization of KC or MIP-2
Neutrophil infiltration into kidneys after ischemia/reperfusion injury and the role of the neutrophil chemoattractants KC and MIP-2 in this infiltration were directly tested. Kidneys were subjected to ischemia for 60 minutes and in place of hetastarch groups of mice were treated with KCAS, MIP2AS, or both KCAS and MIP2AS before reperfusion. Control mice were given NRS before reperfusion. Kidneys were harvested at 12 hours and 24 hours after reperfusion and frozen sections were prepared and stained with antibodies to detect tissue infiltration by neutrophils. At 12 hours after reperfusion, neutrophil infiltration into kidneys from NRS-treated animals was clearly evident, primarily within the corticomedullary junction (Figure 2, a and c) ▶ . In contrast, treatment with both KCAS plus MIP2AS resulted in a striking decrease in neutrophil infiltration into this area of the ischemic kidney tissue after reperfusion (Figure 2, b and d) ▶ . Similar results were observed in both groups at 24 hours after reperfusion. Random microscopic fields of sections from all groups were examined and the numbers of neutrophils per field were counted. When compared to kidneys from NRS-treated control mice, treatment with either KCAS or MIP2AS significantly inhibited neutrophil infiltration into ischemic kidneys at both 12 hours and 24 hours after reperfusion (Figure 3) ▶ . Treatment with KCAS was slightly, although not significantly, more effective than MIP2AS in inhibiting neutrophil infiltration into the ischemic kidneys. However, treatment with both antisera was more effective than treatment with either antiserum alone.
Figure 2.

Inhibition of neutrophil infiltration into kidneys after ischemia/reperfusion by neutralization of KC and MIP-2. After 1 hour of warm ischemia, mice were treated with normal rabbit serum (a and c) or with antisera to KC and MIP-2 (b and d) and kidneys were harvested at 12 hours after reperfusion. Frozen sections were prepared and stained with anti-Ly-6G mAb, RB6.8C5, for immunohistochemistry to detect neutrophils (brown staining). Original magnifications: ×50 (a and b); ×200 (c and d).
Figure 3.
Inhibition of neutrophil infiltration into kidneys after ischemia/reperfusion by neutralization of KC and/or MIP-2. After 1 hour of warm ischemia, mice were treated with normal rabbit serum (NRS) or with antisera to KC, to MIP-2, or to both KC and MIP-2 just before reperfusion. Kidneys were harvested at 12 hours (black bars) and 24 hours (white bars) after reperfusion. Frozen sections were prepared and stained with anti-Ly-6G mAb, RB6.8C5, for immunohistochemistry to detect neutrophils as in Figure 2 ▶ . The numbers of neutrophils were counted for 10 fields/slide and five slides/kidney for four kidneys at ×200 magnification. Mean number of neutrophils per field ± SD is shown for each group. *, P < 0.001 when compared to NRS group.
The survival of mice subjected to bilateral ischemia/reperfusion was compared between groups of animals treated with NRS or KCAS plus MIP2AS just before reperfusion. A third group was treated with anti-Ly6G mAb RB6.8C5 to deplete neutrophils before the induction of bilateral renal ischemia. In animals treated with NRS, 41.2% of the animals died within 3 days after bilateral ischemia and reperfusion (Figure 4) ▶ . In contrast, all mice either treated with KCAS plus MIP2AS or depleted of neutrophils survived after the injury.
Figure 4.
Treatment with antisera to both KC and MIP-2 or depletion of neutrophils inhibits mortality induced by ischemia/reperfusion injury. After 1 hour of warm ischemia, mice were treated with normal rabbit serum (filled triangle) or with antisera to KC and MIP-2 (filled square) just before reperfusion. In addition a group of animals was depleted of neutrophils by treatment with 100 μg of anti-Ly-6G mAb, RB6.8C5, on 3 consecutive days before renal ischemia and received hetastarch just before reperfusion (filled diamond). Viability of the animals in each group was monitored. Treatment with antisera to KC and MIP-2 or neutrophil depletion resulted in a significant difference in survival when compared to control, NRS-treated animals. P < 0.001 by log-rank analysis.
Tissue Injury after Renal Ischemia/Reperfusion Is Inhibited by Neutralization of KC or MIP-2
Kidneys were subjected to bilateral ischemia for 1 hour, were treated with 6% hetastarch just before reperfusion, and at several time points after reperfusion peripheral blood was drawn to assay levels of serum creatinine and urea nitrogen. Mice that died from renal failure earlier than the designated time point were not used in this experiment. After bilateral renal ischemia, serum creatinine and urea nitrogen reached peak levels 24 to 48 hours after reperfusion (Figure 5, a and b) ▶ . To test the effects of neutrophil infiltration on renal function, in place of hetastarch groups of mice were treated with either NRS or KCAS plus MIP2AS just before reperfusion and levels of serum creatinine and urea nitrogen were compared at several time points after reperfusion. NRS-treated mice that died were excluded from this experiment. In addition, a group of mice treated with RB6.8C5 to deplete neutrophils before bilateral renal ischemia was treated with hetastarch just before reperfusion and the serum levels of creatinine and urea nitrogen were compared with the other two groups. As shown in Figure 5, c and d ▶ , renal function in mice treated with NRS deteriorated rapidly with high levels of serum urea nitrogen and creatinine observed at 48 hours after reperfusion. These levels did not approach the levels observed in sham-operated control mice (data not shown) until day 30 after ischemia/reperfusion. In contrast, renal function was preserved by treatment with either KCAS plus MIP2AS or by neutrophil depletion when tested as early as 48 hours after reperfusion and this function was maintained through day 30.
Figure 5.
Treatment with antisera to both KC and MIP-2 or depletion of neutrophils inhibits deterioration of renal function after ischemia/reperfusion injury. a and b: After 1 hour warm renal ischemia, mice received 500 μl of hetastarch just before reperfusion. Peripheral blood was drawn from groups of four mice at the indicated time points after reperfusion and serum creatinine (a) and urea nitrogen (b) concentrations were determined. c and d: Groups of four mice were treated with normal rabbit serum (NRS) or with antisera to KC and MIP-2 just before reperfusion. In addition a group of animals was depleted of neutrophils by treatment with 100 μg of anti-Ly-6G mAb, RB6.8C5, on 3 consecutive days before renal ischemia. These mice received 6% hetastarch just before reperfusion. Peripheral blood was drawn from all mice and levels of serum urea nitrogen (c) or creatinine (d) at 48 hours, day 15, and day 30 after reperfusion were quantitated. The data represent the mean level ± SD for each group. At 48 hours after reperfusion levels of serum creatinine and urea nitrogen were significantly higher in NRS-treated animals than in those treated with KCAS plus MIPAS or depleted of neutrophils before reperfusion. *, P < 0.01.
To directly assess tissue damage in ischemic kidneys in this model, animals were subjected to unilateral renal ischemia for 60 minutes and were treated with NRS or KCAS plus MIP2AS just before reperfusion. After 48 hours of reperfusion the ischemic and nonischemic, contralateral kidneys were harvested. Frozen sections were prepared and stained with H&E and PAS. Tubular necrosis was clearly evident in ischemic kidneys from NRS-treated animals when compared to the nonischemic, contralateral kidney (Figure 6, a ▶ versus c). Intense vacuolization in proximal tubules as well as cast formation was also present in ischemic renal tissue from the NRS-treated animals. In ischemic kidneys from KCAS plus MIP2AS-treated animals, cast formation was evident although there was little necrosis and vacuolization (Figure 6b) ▶ . Using the criteria detailed in the Materials and Methods, the average injury score for ischemic kidneys from the NRS-treated group was 2.69 ± 0.630 (Figure 7) ▶ . Treatment with KCAS and MIP2AS significantly inhibited renal tissue injury with a score of 1.06 ± 0.35. However, the score of KCAS and MIP2AS treatment group remained significantly higher than the average score for the nontreated, contralateral kidneys (0.06 ± 0.06).
Figure 6.
Renal morphology in ischemic kidneys from mice treated with neutralizing KC and MIP-2 antisera after renal ischemia. For each test animal, one kidney was subjected to 60 minutes of ischemia. Groups of mice were treated either with NRS (a) or with both anti-MIP-2 and KC antisera (b) at reperfusion. Kidneys subjected to ischemia (a and b) and control contralateral kidneys (c) were harvested at 48 hours after reperfusion. Formalin-fixed, paraffin-embedded sections were prepared and stained with H&E and PAS. Original magnification, ×200.
Figure 7.
Preserved renal morphology by neutralizing KC and MIP-2 after renal ischemia. For each test animal, one kidney was subjected to 60 minutes of ischemia. Groups of four mice were treated either with NRS or with both anti-MIP-2 and KC antisera at reperfusion. Kidneys subjected to ischemia and control contralateral kidneys were harvested at 48 hours after reperfusion. H&E- and PAS-stained sections were graded for the degree of injury with the score shown in Table 1 ▶ . *, P < 0.0001; **, P < 0.005.
Discussion
Neutrophils quickly infiltrate ischemic tissues after reperfusion. 3,4 Tissue damage is mediated through neutrophil–mediated oxidant stress and the release of granules containing proteolytic enzymes. 34-37 Studies using liver, hindlimb, intestinal, and myocardial models of ischemia have directly tested the role of neutrophils in tissue injury during reperfusion. 5-10 Antibody-mediated depletion of neutrophils before reperfusion of ischemic tissues in these models attenuates tissue damage and preserves at least partial organ function. Similarly, depletion of neutrophils in rat models of warm renal ischemia/reperfusion attenuated tissue pathology and resulted in protection of renal function. 11,12 The results of the current report demonstrate the infiltration of neutrophils into kidneys after 60 minutes of warm ischemia. Reperfusion of the ischemic kidneys is followed by tissue destruction and in many animals mortality ensues. Depletion of neutrophils before the induction of renal ischemia prevents tissue injury and the death of the animals. Furthermore, depletion of neutrophils protected renal function as assessed by serum creatinine and urea nitrogen levels that were similar to sham-operated animals.
A great deal of interest has centered on strategies to inhibit neutrophil trafficking into ischemic tissue after reperfusion. Many studies have tested the effect of using antagonists to neutrophil cell surface molecules that mediate arrest in the vascular endothelium, a requirement for infiltration of the tissue. Use of antibodies to the adhesion molecule, ICAM-1 during reperfusion of ischemic tissues has shown considerable success in preventing tissue injury. 37,38 Treatment with soluble P-selectin ligand also inhibited tissue injury after renal ischemia/reperfusion in a rat model. 4,39 These results are supported by studies comparing renal ischemia/reperfusion injury in wild-type versus ICAM-1−/− mice where there was decreased neutrophil infiltration and tissue injury in ischemic kidneys from the ICAM-1-deficient animals. 40
Another key factor directing the recruitment of leukocytes to inflammatory tissue sites are chemokines. Studies by many laboratories have demonstrated the ability to inhibit leukocyte tissue infiltration through the use of anti-chemokine antibodies in animal models of tissue pathology. 25-31 The results of the current report demonstrate a direct role for two neutrophil chemoattractants in the tissue injury observed after renal ischemia/reperfusion injury of mice. Both Groα and MIP-2 mRNA and protein appeared shortly after reperfusion of the ischemic kidneys. Although the two chemokines appeared and declined with identical temporal patterns, the levels of Groα mRNA and protein were threefold to fourfold higher than MIP-2 at the peak time point of production, 9 hours after reperfusion. It is possible that different renal constituent cells produce KC and MIP-2 after reperfusion of ischemic kidneys and account for the difference in KC versus MIP-2 production. For example, two different interferon-γ-induced chemokines, IP-10 and Mig, are produced in entirely separate tissue locations of allogeneic skin grafts during acute rejection. 41 In contrast to the temporal appearance of KC and MIP-2 observed in ischemic kidneys after reperfusion, induction of MIP-2 was observed earlier than Groα during reperfusion of ischemic liver in a mouse model. 22 The mechanisms accounting for these differences in neutrophil chemoattractant production in the renal versus liver models are unclear at this time.
Inhibition of neutrophil infiltration into ischemic kidneys and decreased tissue pathology were observed using antibodies to KC and MIP-2. The antibodies to MIP-2 were not quite as effective as the antibodies to KC in inhibiting this infiltration, which is consistent with the lower levels of MIP-2 observed in the ischemic renal tissue shortly after reperfusion. However, the ability of the anti-MIP-2 antibodies alone to significantly inhibit neutrophil infiltration indicates functions for both Groα and MIP-2 in optimal recruitment of neutrophils into the kidney and raises the possibility that these functions are distinct for each chemokine. In support of this, administration of both antisera before reperfusion of ischemic kidneys yielded more effective inhibition of the pathology than either antiserum alone. Although not previously tested in renal models, neutralization of neutrophil chemoattractants has been shown to attenuate tissue injury in models of lung, hindlimb, and myocardial ischemia/reperfusion. 5,16,17,22
Although the tissue pathology is strikingly decreased, there remains considerable tissue injury in ischemic kidneys from animals treated with antibodies to both Groα and MIP-2 when viewed histologically including cast formation within the epithelium. Treatment with both antibodies resulted in few detectable neutrophils in the ischemic renal tissue. In addition to neutrophils, however, other mechanisms may mediate tissue pathology during reperfusion of ischemic tissue. 42-44 Several studies have implicated a role for complement in tissue damage after ischemia/reperfusion that is independent of neutrophil infiltration. 16,18,20,45 Macrophages have been implicated as mediators of tissue injury in ischemic organs after reperfusion. 15,19 However, we observed very low levels of MCP-1 after reperfusion of ischemic kidneys although macrophage infiltration was not examined during the course of the current studies. Similarly, we observed low and sustained levels of the T-cell chemoattractant IP-10 beginning at shortly after reperfusion of the ischemic kidneys. Several reports have indicated a role for T cells in mediating tissue damage after renal ischemia/reperfusion injury. 46-48
In summary, the current studies have shown that the neutrophil chemoattractants Groα and MIP-2 are produced shortly after reperfusion of kidneys subjected to 1 hour of warm ischemia. Associated with this chemokine production is neutrophil infiltration into the ischemic kidneys. This infiltration is mediated by these two chemokines as administration of antibodies to both chemokines just before reperfusion almost completely inhibits the neutrophil infiltration and maintains renal function. These results support the use of strategies directed at blocking neutrophil chemokine receptors to attenuate tissue damage during ischemia and solid organ transplantation.
Footnotes
Address reprint requests to Robert L. Fairchild, Ph.D., NB3-79, Lerner Research Inst., Cleveland Clinic Fdn., 9500 Euclid Ave., Cleveland, OH 44195-0001. E-mail: fairchr@ccf.org.
Supported by National Institutes of Health grants AI40459 (to R. L. F.) and GM 50401 (to D. G. R.).
References
- 1.Tilney NL, Guttmann RD: Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation 1997, 64:945-947 [DOI] [PubMed] [Google Scholar]
- 2.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S: High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 1995, 333:333-336 [DOI] [PubMed] [Google Scholar]
- 3.Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR: Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int 1997, 51:1463-1468 [DOI] [PubMed] [Google Scholar]
- 4.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL: The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest 1997, 99:2682-2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel G, Wilke CA, Strieter RM: Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat: the role of epithelial neutrophil activating protein. J Clin Invest 1995, 95:134-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN: Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol 1987, 253:H699-H703 [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H, Farhood A, Smith CW: Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J 1990, 4:3355-3359 [PubMed] [Google Scholar]
- 8.Punch J, Rees R, Cashmer B, Oldham K, Wilkins E, Smith DJ: Acute lung injury following reperfusion after ischemia in the hindlimbs of rats. J Trauma 1991, 31:760-767 [DOI] [PubMed] [Google Scholar]
- 9.Romsom JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR: Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 1983, 67:1016-1023 [DOI] [PubMed] [Google Scholar]
- 10.Seekamp A, Mulligan MS, Till GO, Ward PA: Requirements for neutrophil products and L-arginine following ischemia-reperfusion of rat hind limbs. Am J Pathol 1993, 142:1217-1226 [PMC free article] [PubMed] [Google Scholar]
- 11.Hellberg PO, Kallskog TO: Neutrophil-mediated post-ischemic tubular leakage in the rat kidney. Kidney Int 1989, 36:555-561 [DOI] [PubMed] [Google Scholar]
- 12.Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, Valeri CR, Shepro D, Hechtman HB: Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol 1989, 256:F794-F802 [DOI] [PubMed] [Google Scholar]
- 13.Lukacs NW, Hogaboam C, Campbell E, Kunkel SL: Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol 1999, 72:102-120 [DOI] [PubMed] [Google Scholar]
- 14.Rollins BJ: Chemokines. Blood 1997, 90:909-928 [PubMed] [Google Scholar]
- 15.Azuma M, Nadeau KC, Takada M, Mackenzie HS, Tilney NL: Cellular an molecular predictors of chronic renal dysfunction after initial ischemia/reperfusion injury of a single kidney. Transplantation 1997, 64:190-197 [DOI] [PubMed] [Google Scholar]
- 16.Bless NM, Warner RL, Padgaonkar VA, Lentsch AB, Czermak BJ, Schmal H, Friedl HP, Ward PA: Roles for C-X-C chemokines and C5a in lung injury after hindlimb ischemia-reperfusion. Am J Physiol 1999, 276:L57-L63 [DOI] [PubMed] [Google Scholar]
- 17.Boyle EM, Kovacich JC, Hebert CA, Canty TG, Chi E, Morgan EN, Pohlman TH, Verrier ED: Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. J Thorac Cardiovasc Surg 1998, 116:114-121 [DOI] [PubMed] [Google Scholar]
- 18.Ivey CL, Williams FM, Collins PD, Jose PJ, Williams TJ: Neutrophil chemoattractants generated in two phases during reperfusion of ischemic myocardium in the rabbit. Evidence for a role of C5a and interleukin-8. J Clin Invest 1995, 95:2720-2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S: Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest 2000, 80:1127-1136 [DOI] [PubMed] [Google Scholar]
- 20.Kilgore KS, Park JL, Tanhehco EJ, Booth EA, Marks RM, Lucchesi BR: Attenuation of interleukin-8 expression in C6-deficient rabbits after myocardial ischemia/reperfusion. J Mol Cell Cardiol 1998, 30:75-85 [DOI] [PubMed] [Google Scholar]
- 21.Lemay S, Rabb H, Postler G, Singh AK: Prominent and sustained up-regulation of GP130-signaling cytokines and of the chemokine MIP-2 in murine renal ischemia-reperfusion injury. Transplantation 2000, 69:959-963 [DOI] [PubMed] [Google Scholar]
- 22.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ: Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 1998, 27:507-512 [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Mier G, Toledo-Pereya LH, McDuffie JE, Warner RL, Ward PA: P-selectin and chemokine response after liver ischemia and reperfusion. J Am Coll Surg 2000, 191:395-402 [DOI] [PubMed] [Google Scholar]
- 24.Yoshidome H, Lentsch AB, Cheadle WG, Miller FN, Edwards MJ: Enhanced pulmonary expression of CXC chemokines during hepatic ischemia/reperfusion-induced lung injury in mice. J Surg Res 1999, 81:33-37 [DOI] [PubMed] [Google Scholar]
- 25.DiIulio NA, Engeman TM, Armstrong D, Tannenbaum C, Hamilton TA, Fairchild RL: Groα-mediated recruitment of neutrophils is required for elicitation of contact hypersensitivity. Eur J Immunol 1999, 29:3485-3495 [DOI] [PubMed] [Google Scholar]
- 26.Gong J-H, Ratkay LG, Waterfield JD, Clark-Lewis I: An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med 1997, 186:131-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huffnagle GB, Strieter RM, Standiford TJ, McDonald RA, Burdick MD, Kunkel SL, Toews GB: The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J Immunol 1995, 155:4790-4797 [PubMed] [Google Scholar]
- 28.Koga S, Auerbach MD, Engeman TM, Novick AC, Toma H, Fairchild RL: T cell infiltration into class II MHC-disparate allografts and acute rejection is dependent on the IFN-γ-induced chemokine Mig. J Immunol 1999, 163:4878-4885 [PubMed] [Google Scholar]
- 29.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TNC, Salant DJ, Gutierrez-Ramos J-C: RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med 1997, 185:1371-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA: Role of macrophage inflammatory protein-1α (MIP-1α) in acute lung injury in rats. J Immunol 1995, 154:4793-4802 [PubMed] [Google Scholar]
- 31.Smith RE, Strieter RM, Phan SH, Lukacs NW, Huffnagle GB, Wilke CA, Burdick MD, Lincoln P, Evanhoff H, Kunkel SL: Production and function of murine macrophage inflammatory protein-1α in bleomycin-induced lung injury. J Immunol 1994, 153:4704-4712 [PubMed] [Google Scholar]
- 32.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG: Ratio of local to systemic chemokine concentration regulates neutrophil recruitment. Am J Pathol 2001, 158:715-721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Wollenberg GK, Remick DG: Differential local and systemic regulation of the murine chemokines KC and MIP-2. Shock 2001, 4:278-284 [DOI] [PubMed] [Google Scholar]
- 34.Formigli C, Lombardo LD, Adembri C, Brunelleschi S, Ferrari E, Novelli GP: Neutrophils as mediators of human skeletal muscle ischemia-reperfusion syndrome. Hum Pathol 1992, 23:627-634 [DOI] [PubMed] [Google Scholar]
- 35.Ganey PE, Bailie MB, Vancise S, Coligan ME, Madhukar BV, Robinson JP, Roth RA: Activated neutrophils from rat injured isolated hepatocytes. Lab Invest 1994, 70:53-60 [PubMed] [Google Scholar]
- 36.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW: Functional inactivation of neutrophils with a Mac- (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology 1993, 17:915-923 [PubMed] [Google Scholar]
- 37.Linas SL, Whittenburg D, Parsons PE, Repine JE: Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM-1. Kidney Int 1995, 48:1584-1591 [DOI] [PubMed] [Google Scholar]
- 38.Raab H, Mendiola CC, Saba SR, Dietz J, Smith CW, Bonventre JV, Ramirez G: Antibodies to ICAM-1 protect kidneys in severe ischemic reperfusion injury. Biochem Biophys Res Com 1995, 211:67-73 [DOI] [PubMed] [Google Scholar]
- 39.Takada M, Nadeau KC, Shaw GD, Tilney NL: Prevention of late renal changes after initial ischemia/reperfusion injury by blocking early selectin binding. Transplantation 1997, 64:1520-1525 [DOI] [PubMed] [Google Scholar]
- 40.Kelly KJ, Williams WW, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV: Intercellular adhesion molecule-1 (ICAM-1) knockout mice are protected against renal ischemia. J Clin Invest 1996, 97:1056-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watarai Y, Koga S, Paolone DR, Engeman RM, Tannenbaum C, Hamilton TA, Fairchild RL: Intra-allograft chemokine RNA and protein during rejection of MHC-matched/multiple minor histocompatibility-disparate skin grafts. J Immunol 2000, 164:6027-6033 [DOI] [PubMed] [Google Scholar]
- 42.Simpson R, Alon R, Kobzik L, Valeri CR, Shepro D, Hechtman HB: Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg 1993, 218:444-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steimle CN, Guynn TP, Morganroth ML, Bolling SF, Carr K, Deeb GM: Neutrophils are not necessary for ischemia-reperfusion lung injury. Ann Thorac Surg 1992, 53:64-73 [DOI] [PubMed] [Google Scholar]
- 44.Tullius SG, Tilney NL: Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation 1995, 59:513-518 [PubMed] [Google Scholar]
- 45.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH: Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 2000, 105:1363-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandraker A, Takada M, Nadeau KC, Peach R, Tilney NL, Sayegh MH: CD28–B7 blockade in organ dysfunction secondary to cold ischemia/reperfusion injury. Kidney Int 1997, 52:1678-1684 [DOI] [PubMed] [Google Scholar]
- 47.Goes N, Urmson J, Ramassar V, Halloran PF: Ischemic tubular necrosis induces an extensive local cytokines response. Evidence for induction of interferon-gamma, transforming growth factor-beta, granulocyte-macrophage colony-stimulating factor, interleukin-2 and interleukin-10. Transplantation 1995, 59:565-572 [PubMed] [Google Scholar]
- 48.Takada M, Chandraker A, Nadeau KC, Sayegh MH, Tilney NL: The role of B7 costimulatory pathway in experimental cold ischemia/reperfusion injury. J Clin Invest 1997, 100:1199-1203 [DOI] [PMC free article] [PubMed] [Google Scholar]