Abstract
CD2AP, an adapter protein containing multiple SH3 domains, plays a critical role in kidney function. Mice lacking CD2AP die soon after birth because of kidney failure. In the kidney, CD2AP is expressed in glomerular podocytes, which suggests that it may play a role in a specialized adhesion complex known as the slit diaphragm. One of the major components of the slit diaphragm is nephrin, a podocyte-specific protein. Here we demonstrate that CD2AP localizes to the slit diaphragm in podocytes using immunoelectron microscopy and that nephrin and CD2AP co-immunoprecipitate from a podocyte cell line. The specificity of this interaction was verified by mapping studies, which demonstrated that a novel domain at the C terminus of CD2AP interacts with the C-terminal portion of the nephrin cytoplasmic domain. These studies lend further support to the idea that CD2AP plays a role in the structural integrity of the slit diaphragm.
The CD2-associated protein (CD2AP) is an 80-kd protein that was originally cloned as a protein involved in T cell activation. 1 It contains three consecutive SH3 domains at the N terminus followed by a proline-rich domain. Near the C terminus is a coiled-coiled domain and a potential monomeric actin binding domain. CD2AP is found in all tissues, but is expressed primarily in epithelial cells. 2
CD2AP knockout mice were generated and found to die of kidney failure. 3 Soon after birth, CD2AP KO mice become proteinuric. Three to four weeks after birth, the mice develop nephrotic syndrome. All of the mice die by six to eight weeks of age. Examination of kidneys from knockout animals demonstrated that the primary lesion involves the glomerular epithelial cell, or podocyte. This is consistent with the fact that CD2AP is expressed predominantly in podocytes of the kidney. 2
The expression of CD2AP in podocytes suggested that CD2AP might be involved in a specialized structure in the podocyte known as the slit diaphragm. The slit diaphragm spans the spaces between individual podocyte foot process and maintains a distance of approximately 40 nm between each of the foot processes at the opening of the urinary space. 4 Based on the localization of ZO-1 in the slit diaphragm, it has been proposed that the slit diaphragm functions as a specialized tight junction. 5 Electron microscopic studies suggest that the slit diaphragm forms a grid or net-like structure, which could play a role in blood filtration. 6 Recently, a novel podocyte protein, nephrin, was identified as one of the major components of the slit diaphragm. 7-9 Nephrin, a transmembrane protein of approximately 180 kd, is thought to interact with itself and possibly other proteins, to form the slit-diaphragm. Mutations in the nephrin gene are the major cause of congenital nephrotic syndrome in humans. 10
As CD2AP is expressed in podocytes and as CD2AP knockout mice exhibit congenital nephrotic syndrome, CD2AP may be linked in function to nephrin. Consistent with this hypothesis, we showed previously that nephrin and CD2AP have very similar patterns of expression in the podocyte. 2 In addition, we showed that CD2AP/nephrin complexes can be detected when both proteins are over-expressed in Hela cells or in yeast. 3
Here we present further evidence supporting the idea that CD2AP interacts with nephrin. First, we show that CD2AP localizes to the slit diaphragm in mouse kidney. Second, we show that endogenous CD2AP/nephrin complexes can be detected in a podocyte cell line. Lastly, we demonstrate the specificity of this interaction by mapping the domains required for nephrin and CD2AP association both in vivo and in vitro.
Materials and Methods
Podoycte Cell Culture and Co-Immunoprecipitation
The immortalized wild-type podocyte cell line 11 (MCP-5) was grown on collagen-A coated plates with RPMI 1640 (Gibco) containing 10 units/ml of mouse interferon-γ (a gift of Dr. R. Schreiber, Washington University) and 10% fetal calf serum (FCS, Hyclone) at 33°C until 80% confluency. Cells were used at early passage numbers as nephrin expression was lost at later passages. To induce their differentiation, IFN-g was removed and the temperature shifted to 37°C. Cells were incubated for at least 14 to 20 days before cell lysates were prepared. For co-immunoprecipitation experiments, 1 × 10 7 cells were first washed with phosphate buffered saline. Cell lysates were then prepared and immunoprecipitates analyzed as described previously. 3
Generation of CD2AP and Nephrin Constructs
The VSV G/nephrin mutants, CT1, CT2, CT3, CT4, and CT5 were generated by inverse polymerase chain reaction (PCR) using the G/nephrin plasmid as template. 3 The CD2AP mutants, ΔSH3, ΔPro, Δ428–661, Δ428–600, and Δ601–661 were generated by inverse PCR using BS-myc-CD2AP 1 as template. The sequence of all constructs was confirmed by automated DNA sequencing (ABI Prism).
Protein Expression and Co-Immunoprecipitation Assays
Expression and co-immunoprecipitation assays were performed as described previously. 3 Briefly, HeLa cells were seeded in six-well plates and incubated for 4 to 6 hours at 37°C before infection with T7 vaccinia virus. 12 Infected cells were transfected with the indicated DNAs and harvested after overnight incubation. Cell lysates were prepared and immunoprecipitates analyzed as described previously. 3
In Vitro Nephrin/CD2AP Binding Assay
Glutathione S-transferase-nephrin (GST-nephrin) was generated by cloning the cytoplasmic domain of nephrin into the BamHI site of pGEX-KT. 13 The sequence was verified using automated sequencing and the expressed protein was verified by immunoblotting analysis. The myc-tagged CD2AP constructs were overexpressed in HeLa cells using the T7 vaccinia virus expression system 12 and cell lysates prepared using digitonin lysis buffer. 3 20 μg of GST-CT-Nep fusion protein was added to clarified lysates and incubated at 4°C overnight. GST-nephrin was precipitated with glutathione beads and washed three times. Bound proteins were analyzed by SDS-PAGE and immunoblotting.
Immunoelectron Microscopic Studies
Small pieces of wild-type and CD2AP knockout (KO) kidney cortices were fixed in 2% paraformaldehyde/0.2% glutaraldehyde in phosphate-buffered saline (PBS) at room temperature for 3 hours. Tissues were rinsed in PBS, equilibrated in 2 mol/L sucrose-polyvinylpyrrolidone buffer at 4°C for 48 hours, frozen and then stored in liquid nitrogen. 100 nm sections were cut on a cryo-ultramicrotome and blocked with 10% goat serum. Sections were incubated with either rabbit anti-CD2AP or rabbit anti-nephrin for 2 hours, rinsed, and then incubated with 12 nm gold particle-labeled goat anti-rabbit secondary antibody. After rinsing, sections were stained with uranyl acetate, embedded in methyl cellulose, and viewed on a Zeiss 902 electron microscope.
Results
Localization of CD2AP in the Foot Processes of Podocytes
Our previous immunofluorescence studies of CD2AP demonstrate that it is expressed in the podocyte foot processes in a pattern similar to the expression of nephrin. 2 If CD2AP and nephrin associate in vivo, we expect that CD2AP should, like nephrin, be localized to the slit diaphragm. Cryo-immunoelectron microscopy was performed to determine the localization of CD2AP in the podocyte. To control for non-specific staining of the CD2AP antibody, kidneys from the CD2AP KO mouse were also used.
Immunogold localization demonstrated abundant staining of CD2AP antibodies in wild-type kidney and little to no reactivity detected in CD2AP KO kidney (Figure 1) ▶ . The majority of CD2AP staining was on the lateral membranes of the podocyte foot processes, with some gold particles clearly labeling the vicinity of slit diaphragm (Figure 1, B–D) ▶ . Only occasional gold particles were detected in the cell body. We suspect that the absence of gold particles in the cell body and the abundance of gold particles near the slit diaphragm reflects the increased concentration of CD2AP in this location. The pattern of staining was specific as no staining was detected on the basal podocyte membrane (adjacent to the basement membrane) nor was any staining detected in CD2AP KO kidney (Figure 1, A) ▶ . Immuno-electron-microscopic studies using two different antibodies to nephrin showed a similar pattern of expression (data not shown). Thus, CD2AP appears to localize in podocytes to a location consistent with the slit diaphragm.
Figure 1.
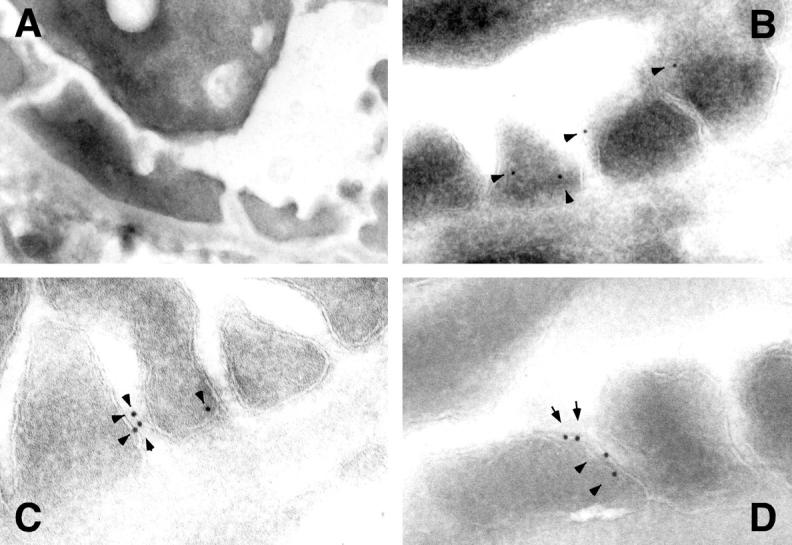
Immunoelectron microscopic localization of CD2AP in mouse podocytes. Immunoelectron microsopic localization of CD2AP in a 6-week-old normal mouse was performed using affinity purified CD2AP and secondary antibody conjugated with 10-nm gold particles. The immunogold particles (arrowheads) were found predominantly along the lateral borders of the podocyte foot processes (B–D). Many of the particles localize in a region near or at the slit diaphragm. Original magnification, ×20,000. No gold particles were seen in CD2AP KO kidney (A).
Co-Precipitation of CD2AP with Nephrin in a Podocyte Cell Line
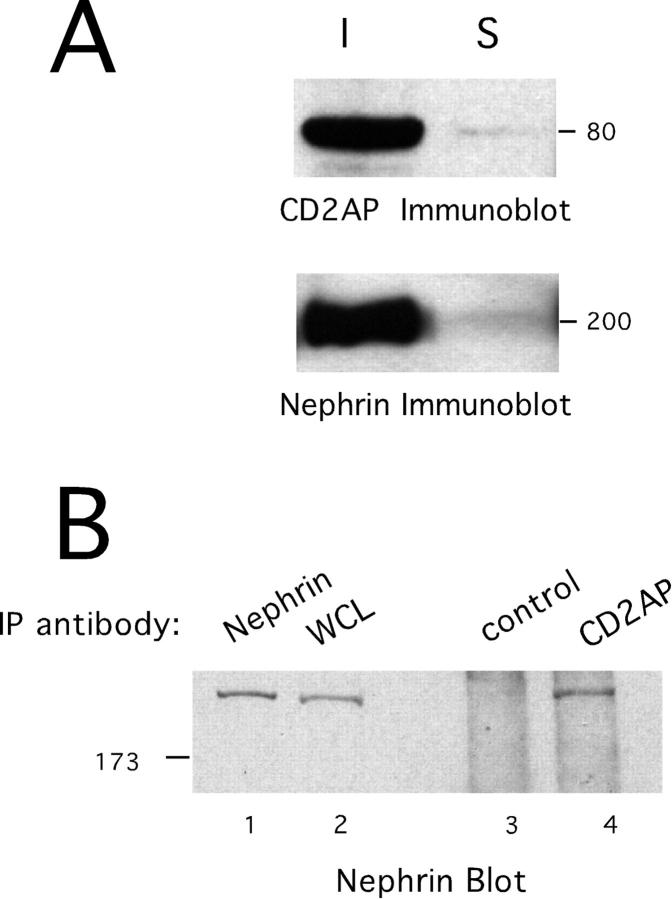
In our previous work, we were unable to test whether nephrin and CD2AP associate in the kidney because neither protein was soluble in our lysis solutions using weak detergents like digitonin or CHAPS (Figure 2A) ▶ . We therefore tested whether CD2AP and nephrin could be detected in a podocyte cell line. These cells were derived from a transgenic mouse that expresses a temperature-sensitive form of SV40 large T antigen under the control of an interferon inducible promoter. 11 At the permissive temperature, in the presence of γ-interferon, cells grow continuously in culture. At the non-permissive temperature and after interferon removal, cells stop growing, differentiate, and acquire a podocyte-like morphology.
Figure 2.
Association of CD2AP and nephrin in podocyte cell line. A: Insolubility of nephrin and CD2AP from purified glomeruli. Glomeruli were purified from mouse kidneys by sieving. Lysates were prepared with digitonin based lysis buffer. A portion of the soluble material (S) and a portion of the insoluble material (I) was analyzed by SDS-PAGE and immunoblotting with antibodies to CD2AP and nephrin. B: Lysate were prepared from a podocyte cell line. Equal amounts of the clarified lysate were incubated with either pre-immune rabbit serum (C) or affinity purified CD2AP antibody (CD2AP). Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with anti-nephrin. Either whole cell lysate (-) or anti-nephrin immunoprecipitate (Nep) were loaded as positive controls.
Lysates were prepared from early passage podocyte cells. Immunoblotting of cell lysates demonstrated that both nephrin and CD2AP were expressed in these cells (Figure 2 ▶ , and data not shown). It should be noted that the expression level of nephrin was not high and immunofluorescence staining showed that the protein is localized mainly at intracellular membranes. However, three different nephrin antibodies immunoblotted a band of between 180–190 kd in size and this band was similar in molecular mobility to nephrin immunoblotted from kidney. Importantly, both proteins were solubilized in a lysis buffer containing 1% digitonin.
Immunoblotting of CD2AP immunoprecipitates with an anti-nephrin antibody showed association of CD2AP with nephrin (Figure 2B ▶ , lane 4). This was specific to CD2AP, as immunoprecipitates prepared with a control antibody showed no nephrin reactivity (Figure 2B ▶ , lane 3). Thus, endogenous CD2AP/nephrin complexes are present in a cultured podocyte cell line.
CD2AP Interacts with the C Terminus of the Nephrin Cytoplasmic Domain
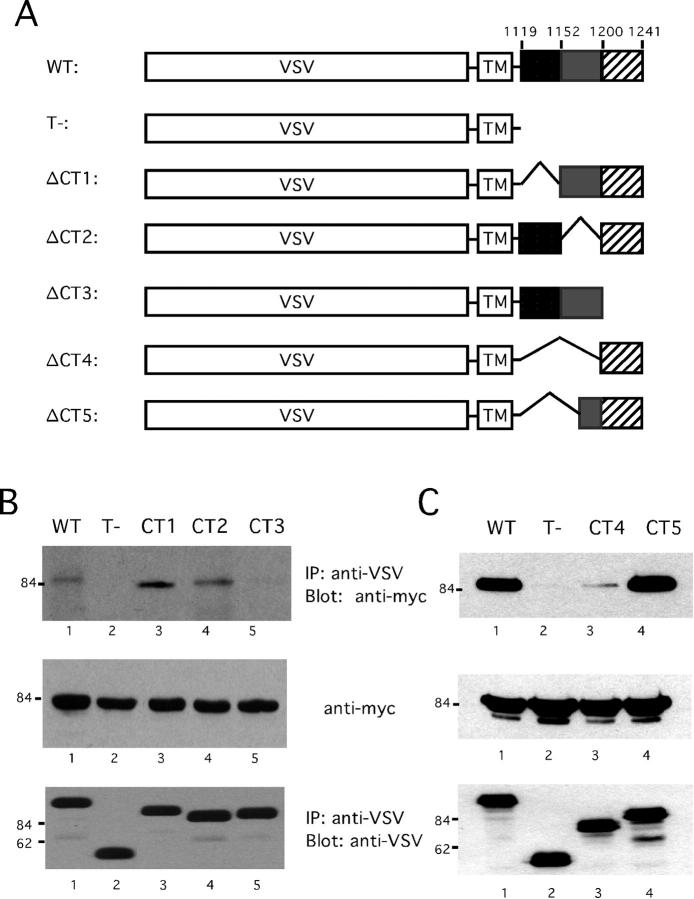
To confirm the specificity of the interaction between nephrin and CD2AP, we next focused on identifying the domains of nephrin and CD2AP critical for their interaction. Previously we showed that co-expression of CD2AP with a chimeric protein containing the cytoplasmic domain of nephrin fused to the extracellular and transmembrane domains of the VSV G protein in HeLa cells resulted in the formation of nephrin/CD2AP complexes. 3 We used this system to identify the portion of the nephrin cytoplasmic domain that interacts with CD2AP. We initially generated three mutated nephrin constructs. CT1 lacks the first third, CT2 lacks the second third, and CT3 lacks the last third of the nephrin cytoplasmic domain (Figure 3A) ▶ .
Figure 3.
Mapping the binding site for CD2AP in nephrin. A: Three cytoplasmic truncation mutants of nephrin, CT1 (lacking residues 1119–1151), CT2 (lacking residues 1152–1200), and CT3 (lacking residues 1201–1241), fused with VSV-G were generated to define the CD2AP binding site. B: Full-length myc-CD2AP and nephrin wild-type (WT), tail-truncated (T-), or cytoplasm-truncated mutants, were cotransfected as indicated in HeLa cells. VSV-G fusion proteins were immunoprecipitated with anti-VSV monoclonal antibody and immunoprecipitates were subjected to immunoblotting with anti-Myc antibody to detect CD2AP (top). The expression levels of VSV G fusion proteins were determined by re-probing the same blot using anti-VSV polyclonal sera. The expression levels of myc-CD2AP were determined by immunoblotting a sample of the cell lysate with anti-myc.
Each of the mutated VSV G/nephrin constructs was co-expressed with myc-tagged CD2AP in HeLa cells. After lysis, immunoprecipitates prepared with antibodies to VSV G were immunoblotted with antibodies to myc (Figure 3B) ▶ . While CT1 and CT2 were both able to bind to CD2AP (Figure 3B ▶ , lanes 3 and 4), there was no CD2AP detected in CT3 immunoprecipitates (Figure 3B ▶ , lane 5). The last 41 residues of the nephrin cytoplasmic domain are therefore required for CD2AP association.
To test whether the last 41 residues were sufficient by themselves to bind to CD2AP, a VSV G/nephrin construct lacking all but the last 41 residues of the nephrin cytoplasmic domain was generated (CT4, Figure 3A ▶ ). CT4 bound poorly to CD2AP suggesting that the last 41 residues, while required, are not sufficient to fully reconstitute binding (Figure 3C ▶ , lane 3). We therefore generated another construct to test whether a larger fragment (the last 65 residues of nephrin) was sufficient to bind to CD2AP (CT5, Figure 3A ▶ ). CT5 was easily co-precipitated with CD2AP from cells overexpressing both proteins (Figure 3C ▶ , lane 4). Thus, while the last 41 residues of nephrin are required for CD2AP binding, a larger fragment of nephrin is required to fully reconstitute nephrin/CD2AP complex formation.
Nephrin Binds to the C-Terminal Domain of CD2AP
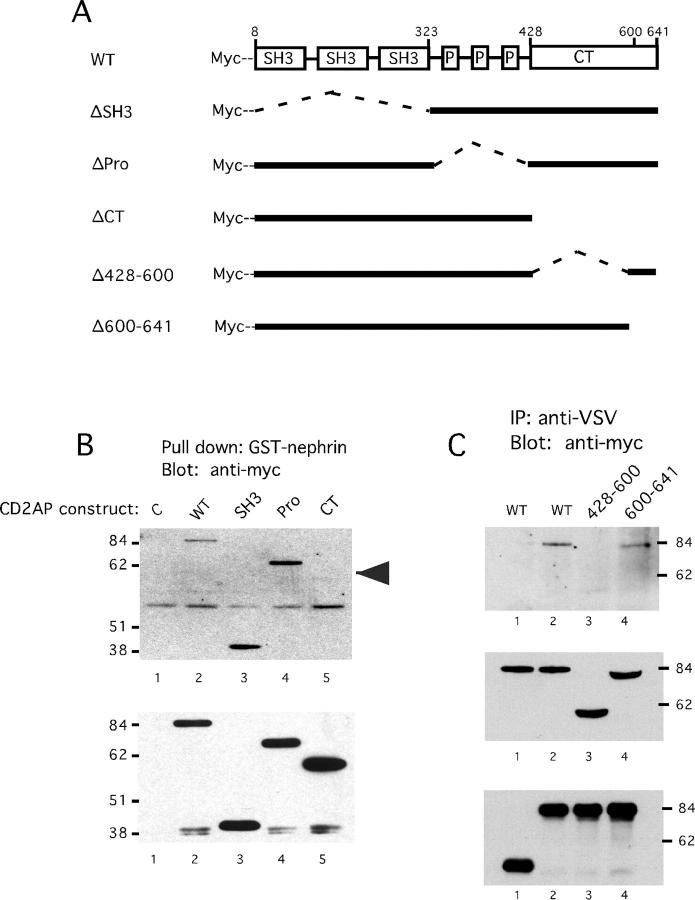
We next focused on identifying the domain of CD2AP that binds to nephrin. CD2AP contains multiple functional domains. Three mutated constructs were generated to determine which domain of CD2AP binds to nephrin. The ΔSH3 construct lacks the three SH3 domains, the ΔPro construct lacks the proline-rich domain in the center of the molecule, and the ΔCT construct lacks the C-terminal 213 residues (Figure 4A) ▶ . All of the constructs contain a myc-epitope at the N terminus to facilitate detection.
Figure 4.
Mapping the binding site for nephrin in CD2AP. A: Schematic diagram of CD2AP deletion mutants. ΔSH3 lacks residues 8–323 which contain the three SH3 domains. ΔPro lacks residues 324–427 containing the proline-rich domain. ΔCT lacks residues 428–636 encoding the C-terminal portion. Δ428–600 lacks residues 428–600 and Δ601–641 lacks residues 601–641. B: The C-terminal domain of CD2AP binds to nephrin. Wild-type or indicated CD2AP constructs were expressed in HeLa cells using the vaccinia-T7 expression. Cell lysates were incubated with GST-nephrin immobilized on agarose beads. Bound proteins were analyzed by immunoblotting with anti-myc. Uninfected HeLa cell lysate was used as control (lane 1). Whole cell lysates were also probed with anti-Myc to confirm equivalent CD2AP expression levels (lower panel). C: Residues 428–600 mediate binding of CD2AP to nephrin. Wild-type CD2AP, Æ428–600, Æ600–641 were co-expressed in HeLa cells with the VSV G/nephrin fusion protein (lanes 2–4). As a negative control, wild-type CD2AP was co-expressed with a truncated form of VSV G lacking the cytoplasmic domain of nephrin (lane 1). Immunoprecipitates were prepared with anti-VSV G antibody and analyzed by immunoblotting using anti-Myc to detect CD2AP. The same blot was reprobed using anti-VSV antisera to confirm expression levels of the VSV G/nephrin fusion protein (bottom). Whole cell lysates were also probed for CD2AP expression using Myc antibody (middle).
Each of the CD2AP constructs was co-expressed with the VSV G/nephrin fusion protein in HeLa cells. However, the analysis of CD2AP binding to nephrin was complicated by the similar electrophoretic mobilities of two of the mutated CD2AP constructs to immunoglobulin heavy and light chains. We therefore tested whether CD2AP in a cell lysate could bind to a nephrin fusion protein composed of glutathione S-transferase fused to the cytoplasmic domain of nephrin. The use of GST as a fusion partner allowed us to isolate the nephrin fusion protein without using an antibody.
Cell lysates were prepared from HeLa cells overexpressing wild-type CD2AP. Purified GST-nephrin was added to the lysate and precipitated using glutathione agarose beads. GST-nephrin readily co-precipitated CD2AP demonstrating that CD2AP can bind to nephrin in vitro (Figure 4B ▶ , lane 2). This was specific to nephrin as GST by itself was unable to co-precipitate CD2AP (Figure 4B ▶ , lane 1).
The ability of each of the three mutated CD2AP constructs to bind nephrin was then tested. Cell lysates prepared from cells overexpressing each of the mutated constructs was incubated with purified GST-nephrin. Both the ΔSH3 and the ΔPro construct were easily co-precipitated by GST-nephrin (Figure 4B ▶ , lanes 3 and 4). However, no association was detected with the ΔCT construct. The C-terminal 213 residues of CD2AP were therefore required for nephrin binding.
A coiled-coiled domain is present within the C-terminal 213 residues of CD2AP at the extreme C terminus. To test whether the coiled-coiled domain is responsible for binding to nephrin, we generated two additional constructs. Δ600–641 truncates the portion of the cytoplasmic domain containing the coiled-coiled domain while Δ428–600 deletes sequences preceding the coiled-coiled domain (Figure 4A) ▶ . The Δ600–641 construct was still able to co-precipitate with VSV G-nephrin (Figure 4C ▶ , lane 4) demonstrating that the coil-coil domain of CD2AP is not involved in nephrin binding. As expected, the Δ428–600 construct was unable to bind to nephrin (Figure 4C ▶ , lane 3). This region has no recognizable structural domains, suggesting that a novel domain in CD2AP mediates its interaction with nephrin.
Discussion
We have provided further evidence that CD2AP is involved in the podocyte slit diaphragm. First, by immunoelectron microscopy we showed that CD2AP localizes in podocytes in a region close to or at the slit diaphragm. Second, using an immortalized podocyte cell line, we co-precipitated nephrin, the major component of the slit diaphragm with CD2AP. Third, we mapped the interaction between the two proteins to validate the specificity of the interaction. Overall, these data establish for the first time that CD2AP is a component of the slit diaphragm.
Discovered over 30 years ago, there is still little known about the slit diaphragm. To date, only four molecules have been identified to be components of the slit diaphragm: nephrin, 7-9 ZO-1, 5 P-cadherin, 14 and FAT. 15 CD2AP can now be added to this list. Consistent with the idea that the slit diaphragm is an adhesion structure, all of these molecules appear to be involved in cell-cell adhesions. Nephrin, a transmembrane protein, mediates the interaction between the adjacent podocyte foot processes. P-cadherin is found in adherens junctions, while ZO-1 is found in both adherens junctions and tight junctions. 16,17 FAT is a novel member of the cadherin family of receptors containing 34 cadherin repeats. 15
Nephrin, a member of the immunoglobulin superfamily, was originally identified because it is mutated in the disease congenital nephrotic syndrome of the Finnish type (CNF). 10 Nephrin is expressed almost exclusively in glomerular podocytes. Several studies using immunoelectron microscopy demonstrate that nephrin localizes to the slit diaphragm. 7-9
Because CD2AP knockout mice develop nephrotic syndrome shortly after birth, it was logical to test whether there might be a relationship between nephrin and CD2AP. We had shown previously that nephrin and CD2AP could interact when both proteins were overexpressed in HeLa cells or when both proteins were co-expressed using the yeast two-hybrid system. 3 But these data did not prove that nephrin and CD2AP interact in vivo. Here we have extended these previous findings and show now that these complexes exist when both proteins are expressed at endogenous levels in a podocyte cell line. In addition, we were able to demonstrate that CD2AP/nephrin complexes form in vitro.
The CD2AP binding domain of nephrin we have identified suggests that CD2AP may play a role in a subset of patients with CNF. Most cases of congenital nephrotic syndrome are caused by a mutation in the nephrin gene which completely abolishes expression. 18 However, a smaller group of patients have a nephrin mutation (Finn minor), which generates a stop codon within the cytoplasmic domain of nephrin resulting in a protein lacking the C-terminal 132 residues of nephrin. 18 As this domain is required for binding to CD2AP, loss of this CD2AP binding may account for the phenotype of these patients. It will be interesting to determine whether a subset of patients with CNF express smaller truncations of the nephrin cytoplasmic domain.
While the exact role of CD2AP in adhesive contacts is unknown, we favor the idea that CD2AP links surface receptors with the cytoskeleton. We originally identified CD2AP as a molecule involved in the specialized adhesive complex termed the immunological synapse. 19 Now we have shown that it localizes to another specialized contact known as the slit diaphragm. This suggests that CD2AP may play a role in providing structural integrity to these adhesion complexes. Understanding CD2AP function will hopefully lead to further insights into the structure of the slit diaphragm and to a better understanding of epithelial surfaces.
Acknowledgments
We thank Marilyn Levy for performing the immunoelectron microscopy, Amy Holdorf, Gopa Green, Coky Nguyen, and Emil Unanue for critical review of the manuscript, Hui Wu for excellent technical assistance and Karl Tryggvason, Larry Holtzman, and Amin Arnout for antibodies to nephrin.
Footnotes
Address reprint requests to Andrey S. Shaw, Department of Pathology and Immunology, Washington University School of Medicine, 660 South Euclid, Box 8118, St. Louis, MO. E-mail: shaw@pathbox.wustl.edu.
Supported by grants from the National Institutes of Health to A. S. and J. M.
References
- 1.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS: A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 1998, 94:667-677 [DOI] [PubMed] [Google Scholar]
- 2.Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner JH: CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol 2000, 279:F785-F792 [DOI] [PubMed] [Google Scholar]
- 3.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312-315 [DOI] [PubMed] [Google Scholar]
- 4.Karnovsky MJ, Ainsworth SK: The structural basis of glomerular filtration. Adv Nephrol Necker Hosp 1972, 2:35-60 [PubMed] [Google Scholar]
- 5.Schnabel E, Anderson JM, Farquhar MG: The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 1990, 111:1255-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodewald R, Karnovsky MJ: Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 1974, 60:423-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 1999, 96:7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holthofer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999, 155:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzman LB, St. John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int 1999, 56:1481-1491 [DOI] [PubMed] [Google Scholar]
- 10.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein, nephrin, is mutated in congenital nephrotic syndrome. Mol Cell 1998, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 11.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997, 236:248-258 [DOI] [PubMed] [Google Scholar]
- 12.Fuerst TR, Niles EG, Studier FW, Moss B: Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA 1986, 83:8122-8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan KL, Dixon JE: Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem 1991, 192:262-267 [DOI] [PubMed] [Google Scholar]
- 14.Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 2000, 11:1-8 [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, Ohshiro K, Kawachi H, Okada H, Suzuki H, Kihara I, Yamamoto T: FAT is a component of glomerular slit diaphragms. Kidney Int 2001, 59:1003-1012 [DOI] [PubMed] [Google Scholar]
- 16.Mitic LL, Anderson JM: Molecular architecture of tight junctions. Annu Rev Physiol 1998, 60:121-142 [DOI] [PubMed] [Google Scholar]
- 17.Tsukita S, Nagafuchi A, Yonemura S: Molecular linkage between cadherins and actin filaments in cell-cell adherens junctions. Curr Opin Cell Biol 1992, 4:834-839 [DOI] [PubMed] [Google Scholar]
- 18.Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestila M, Tryggvason K: Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet 1999, 64:51-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML: The immunological synapse: a molecular machine controlling T cell activation. Science 1999, 285:221-227 [DOI] [PubMed] [Google Scholar]