Abstract
Calcification of elastin occurs in many pathological cardiovascular diseases including atherosclerosis. We have previously shown that purified elastin when subdermally implanted in rats undergoes severe calcification and aluminum chloride (AlCl3) pretreatment of elastin inhibits calcification. In the present study we investigated whether matrix metalloproteinase (MMP) binding to elastin and elastin degradation is prevented by AlCl3 pretreatment. Subdermal implantation of AlCl3-pretreated elastin showed significantly lower MMP-9 and MMP-2 activity surrounding the implant as compared to the control implants. AlCl3 pretreatment also significantly inhibited elastin implant calcification at the seven-day implant period (AlCl3-pretreated 4.07 ± 1.27, control 23.82 ± 2.24 μg/mg; p<0.0001). Moreover, elastin gel zymography studies showed that gel pretreatment with AlCl3 inhibited elastolysis by MMP-9. We also demonstrate significant suppression of MMP-2 activity in aortic wall segments of AlCl3-pretreated porcine bioprosthetic heart valve implants as compared to control valve implants in sheep mitral valve replacement studies. AlCl3 pretreatment also significantly inhibited calcification of elastin in this model. Thus, we conclude that aluminum ion binding to elastin prevents MMP-mediated elastolysis and thus prevents elastin calcification.
Pathological calcification occurs in a number of diseases including atherosclerosis, Marfan’s syndrome, congenitally bicuspid heart valves, heart valve implants, and vascular grafts. 1-4 Elastin, a major structural protein present in the extracellular matrix (ECM) of arterial walls in internal and external elastic lamellae, provides elastic recoil to the arteries. Elastin-oriented calcification is observed in many cardiovascular diseases including atherosclerosis and aortic aneurysm. 1,2,5,6 The mechanisms of elastin-oriented calcification are not completely elucidated and there are no therapies to prevent this type of calcification. We have recently developed a rat subdermal implant model to study mechanisms of calcification of purified elastin. 7 We have shown that subdermal implantation of purified elastin leads to severe elastin calcification and aluminum chloride (AlCl3) pretreatment of elastin completely inhibits elastin calcification. 7 The reason for inhibition of calcification was attributed to irreversible binding of aluminum ions to elastin. Aluminum chloride pretreatment to prevent aortic wall calcification in bioprosthetic heart valves (BPHV) is currently under clinical investigation.
Although it has previously been shown that AlCl3 pretreatment alters the structure of purified elastin and prevents aortic calcification, 7 the exact biological mechanisms for its action are not known. We wanted to study whether alterations in the elastin structure caused by aluminum ion binding leads to inhibition of matrix metalloproteinase (MMP)-elastin binding. MMP-2 and MMP-9 are known to bind insoluble elastin and this binding is considered a first step in elastin degradation. 8 In a separate study, we reported that matrix metalloproteinases such as MMP-2 and MMP-9 are activated within the elastin implant and take an active part in elastin degradation and calcification. 9 In the current study we show that AlCl3 pretreatment of elastin leads to an inhibition of MMP-mediated degradation of elastin, which in turn leads to inhibition of elastin-oriented calcification in a rat subdermal implantation model as well as in sheep mitral valve replacement studies.
Materials and Methods
Aluminum Chloride Treatment of Elastin
Elastin fibers (3 g; 0.25–10 mm; from bovine neck ligament; F65, Elastin Products Company, Inc., Owensville, MO) were treated in 100 mmol/L AlCl3 in water for 2 hours at 25°C with gentle mixing. The AlCl3-pretreated elastin was washed extensively with sterile 0.9% NaCl solution to remove unbound AlCl3. Control elastin was re-hydrated and washed repeatedly in sterile 0.9% NaCl.
Rat Subdermal Implantation of Elastin
Immature male Sprague-Dawley rats (27 to 35 days old, 80 to 90 g; Harlan, Indianapolis, IN) were sedated with acepromazine (0.5 mg/kg; Ayerst Laboratories, Inc., Rouse Point, NJ) and maintained on isoflurane gas (2 to 2.5%) throughout surgery. A small incision was made on the back of the rats and two subdermal pouches were created. Fifteen rats received two ∼75 mg AlCl3-pretreated elastin implants per animal, while an additional 15 rats received two control elastin implants per animal. Five rats from each group were sacrificed by CO2 asphyxiation at 1, 4, and 7 days, and elastin samples explanted. A portion of explant from three rats from each group was placed in phosphate-buffered formalin for histological analyses. One sample from each rat was frozen at −85°C for MMP extraction and zymography studies. The other sample was lyophilized and used for quantitative calcium and phosphorous determination.
Sheep Mitral Valve Replacement Samples
Paraffin blocks of stentless BPHV, derived from glutaraldehyde-crosslinked porcine aortic valves (Toronto SPV) implanted in the mitral position in sheep, were obtained from St. Jude Medical Inc., St. Paul, MN. The valves were functional for 150 days and were explanted by elective surgery. Samples included three blocks of control glutaraldehyde-crosslinked aortic wall explants and three blocks of AlCl3-pretreated aortic wall explants. The AlCl3 treatment conditions for bioprostheses were similar to our elastin samples (see above).
Immunohistochemical Staining for MMP-2 and Alizarin Red Staining for Calcium
Formalin-fixed aortic wall segments of porcine BPHV from sheep explants were embedded in paraffin. Sections (6 μm) were deparaffinized in xylene followed by a graded series of ethanol to water. Immunohistochemical staining for MMP-2 was done using rabbit polyclonal antibody for MMP-2 (Sigma, St. Louis, MO) at a 1:500 dilution in PBS. Normal rabbit immunoglobin G (Sigma) was used as negative control. Immunohistochemical staining for MMP-2 was done using the R.T.U. Vectastain Universal ABC Kit (Vector Laboratories, Burlingame, CA) and visualized using the NovaRED substrate kit for peroxidase (Vector Laboratories). Sections were counterstained in Gill’s hematoxylin and mounted. Additional sections were stained with 1% alizarin red S solution (Poly Scientific Research & Development Corp., Bay Shore, NY) for 3 minutes and counterstained with 1% light green solution for 5 to 10 seconds.
Gelatin Zymography
Elastin samples from rat subdermal explants were homogenized in extraction buffer (50 mmol/L Tris, 10 mmol/L CaCl2, 2 mol/L guanidine-HCl, 0.2% Triton X-100, pH 7.5). Homogenized explants were centrifuged 10,000 × g for 10 minutes and supernatants recovered and dialyzed for 18 hours against 50 mmol/L Tris and 0.2% Triton X-100, pH 7.5. Protein concentrations in dialysates were determined using a bicinchoninic acid protein assay kit (Sigma). Samples with equal protein concentrations (15 μg) were mixed 1:2 with zymogram sample buffer (62.5 mmol/L Tris, 25% glycerol, 4% SDS, 0.01% bromophenol blue, pH 6.8) and electrophoresed under non-reducing conditions on 10% sodium dodecyl sulfate-polyacrylamide gels containing 0.1% gelatin (BioRad, Hercules, CA). Following electrophoresis, gels were placed in renaturation buffer (2.5% Triton X-100) for 30 minutes to remove SDS and then incubated for 16 hours at 37°C in development buffer (50 mmol/L Tris, 200 mmol/L NaCl, 5 mmol/L CaCl2, 0.02% Brij-35, pH 7.5). Gels were stained with Coomassie blue R-250. Following destaining, MMPs appear as clear white bands on a blue background. Band densities were analyzed using NIH Image software.
Calcium and Phosphorus Determination
Analyses of calcium and phosphorus content in rat subdermal explants were done following previously described procedures. 10,11 Briefly, capsule-free lyophilized elastin explants (16–23 mg) were placed in 1 ml 6 N HCl and heated at 95°C for 10 hours. Samples were evaporated under a continuous stream of nitrogen gas and residual material dissolved in 1 ml 0.01 N HCl. Content of calcium in explants was determined with an atomic absorption spectrophotometer (Perkin-Elmer Model 3030, Perkin-Elmer, Norwalk, CT). Phosphorus content was measured on the same samples using the molybdate complexation assay. 11
Elastin Zymography for MMP-9 Activity
Elastin zymography was performed following reported procedures with modification. 12,13 Purified MMP-9 (3 μg in water; Elastin Products Company, Inc.) was loaded on 10% Tris-HCl SDS-PAGE gels containing α-elastin (1.2 mg/ml; EPC). Following electrophoresis, gels were washed in renaturation buffer for 30 minutes followed by three 5-minute washes in distilled water to remove Triton X-100. One gel was placed in 100 ml of 100 mmol/L AlCl3 in water and the other gel in distilled water for 2 hours at room temperature. Unbound AlCl3 was removed by washing the gel repeatedly in distilled water. The control gel was washed similarly to the AlCl3-pretreated gel. Gels were incubated in activation buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 1 μmol/L ZnCl2, 1 mmol/L p-aminophenyl mercuric acetate (APMA), 1% Triton X-100, 0.02% NaN3, pH 7.6) for 1 hour at 37°C. Gels were washed three times for 5 minutes with activation buffer without APMA followed by incubation in this buffer for 16 hours at 37°C. Staining and analyses of gels were performed using the procedures described above for gelatin zymography.
Statistical Analysis
Data are reported as mean ± SEM. Student’s t-tests were used to determine differences between control and AlCl3-pretreated groups. The data were termed statistically significant when p<0.05.
Results
AlCl3 Pretreatment Significantly Inhibits Elastin-Oriented Calcification
Calcium and phosphorous content in control explants increased steadily during the 7-day implant period (Table 1) ▶ . Content of calcium and phosphorous in AlCl3-pretreated explants were uniformly low at 1 and 4 days and were slightly increased on day 7 (Table 1) ▶ . Elastin implants pretreated with AlCl3 had significantly lower (p<0.0001) levels of both calcium and phosphorous at days 4 and 7 as compared to control elastin samples on the same day. Ratios of calcium to phosphorus in both control and AlCl3-pretreated elastin implants varied from 0.43 to 1.39, indicating the presence of immature calcium phosphate crystallization at these early time points.
Table 1.
Elastin Calcification in Rat Subdermal Implants
| Group | Calcium (μg/mg ± SEM) | Phosphorus (μg/mg ± SEM) | Ca:P molar ratio |
|---|---|---|---|
| Control 1d | 0.77 ± 0.40 | 1.13 ± 0.16 | 0.52 |
| Control 4d | 7.52 ± 1.11 | 4.19 ± 0.42 | 1.39 |
| Control 7d | 23.82 ± 2.24 | 13.94 ± 1.48 | 1.32 |
| AlCl3 1d | 0.64 ± 0.28 | 1.13 ± 0.13 | 0.43 |
| AlCl3 4d* | 0.72 ± 0.24 | 1.19 ± 0.18 | 0.47 |
| AlCl3 7d* | 4.07 ± 1.27 | 2.96 ± 0.70 | 1.06 |
*Denotes differences (p < 0.0001) in Ca and P between AlCl3 and control groups.
AlCl3 Pretreatment of Elastin Leads to Lower MMP Expression in the Implant
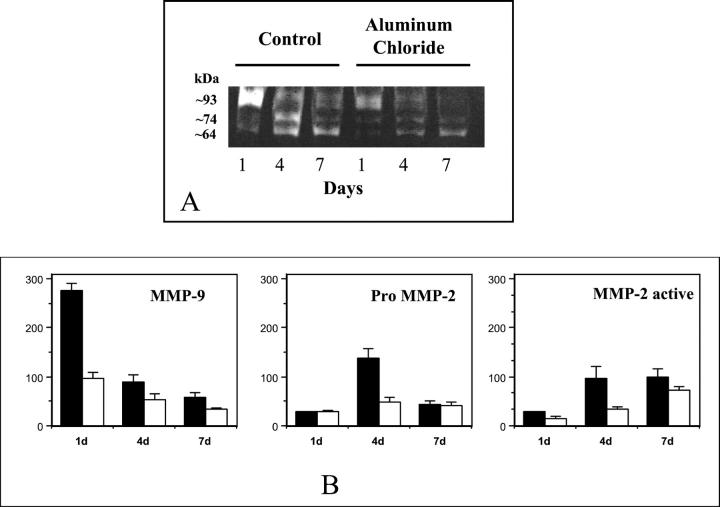
Gelatin zymography of control and AlCl3-pretreated explants revealed the presence of gelatinase positive bands at approximately 93-, 74-, and 64-kd at all of the time points examined (Figure 1) ▶ . We assigned the 93-kd band to MMP-9 and the 74- and 64-kd bands to MMP-2 in pro and activated forms, respectively. 8 Activities of MMP-9 and MMP-2 within the implant were time-dependent. The 93-kd band for MMP-9 was more prominent on day 1 and was decreased on days 4 and 7 in the control group. A significant reduction in MMP-9 activity was observed in AlCl3-pretreated implants as compared to controls at all time points (Figure 1B) ▶ . Activity of MMP-2 (74- and 64-kd bands) was much less evident on day one than MMP-9 in both control and AlCl3-pretreated elastin explants. This suggests that MMP-9 is expressed before MMP-2 in these implants (Figure 1B) ▶ . In control explants pro-MMP-2 (74-kd band) activity was highest on day 4 and then decreased on day 7. In contrast, pro-MMP-2 activity in AlCl3-pretreated implants remained low and constant (Figure 1B) ▶ . In control samples the amount of the active form of MMP-2 (64-kd band) on day 1 was low and then increased gradually on days 4 and 7, suggesting that MMP-2 is being activated with time. The active form of MMP-2 also gradually increased with time in AlCl3-pretreated samples, however, the band intensity in AlCl3-pretreated samples was lower than control samples suggesting lower activation of MMP-2 (Figure 1B) ▶ . Overall, MMP band intensities were higher in control samples, clearly showing that AlCl3 pretreatment of elastin significantly reduced MMP activity in implants.
Figure 1.
Representative zymogram of gelatinase activity of elastin explants. Bands of gelatinase activity corresponding to molecular masses of approximately 93, 74 and 64 kd (MMP-9, pro MMP-2 and active MMP-2 respectively) were detected (A). Band density analysis (B) showed higher activity of MMP-9 on day 1 and decreasing activity was observed on days 4 and 7 in both groups. Significantly lower MMP-9 activity was seen AlCl3-pretreated explants. Both proMMP-2 and active form of MMP-2 showed limited activity on day 1 with increased activity on days 4 and 7. Activity of active MMP-2 forms was greater in control than in the AlCl3-pretreated elastin explants (control, solid bars; AlCl3-pretreated, open bars).
Elastolysis by MMP-9 Is Prevented by AlCl3 Pretreatment of Elastin
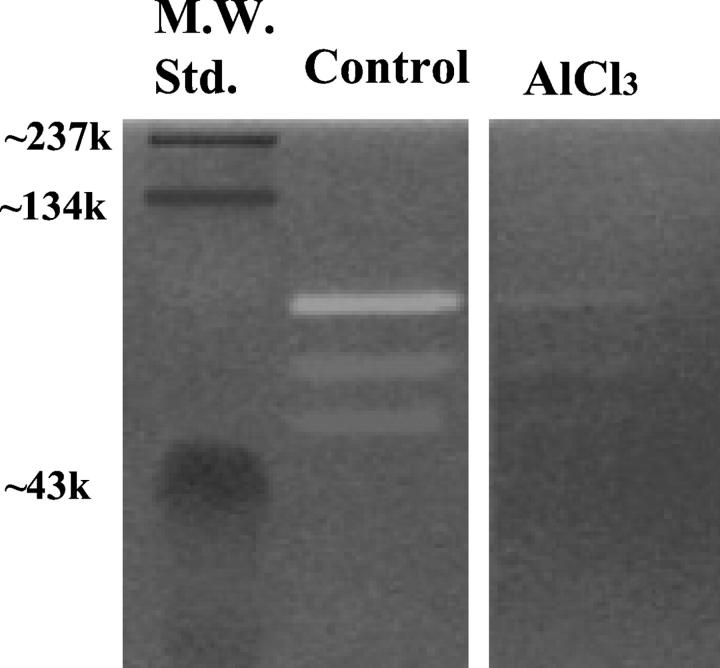
To test whether MMP-9 mediated elastolysis is prevented by AlCl3 pretreatment, we prepared elastin zymogram gels (control and AlCl3-pretreated) and looked at MMP-9 activity by applying purified MMP-9 enzyme to the gel. Analysis of MMP-9 activity in α-elastin zymogram gels revealed the presence of positive bands of MMP-9 activity in the control gel with estimated molecular masses of approximately 90, 66, and 54 kd (Figure 2) ▶ . We assume lower bands to be either degradation products of MMP-9 or impurities in commercial MMP-9 preparation. Barely visible bands were observed when elastin gels were pretreated with AlCl3; clearly showing that AlCl3 pretreatment significantly prevented MMP-9 mediated degradation of elastin (Figure 2) ▶ .
Figure 2.
Representative zymogram of MMP-9 activity in control and AlCl3-pretreated α-elastin gels. Bands of elastolytic activity of approximately 90, 66, and 54 kd were observed in the control gel. Elastolytic bands present in the AlCl3-pretreated gel were barely visible compared to bands in the control gel, suggesting inhibition of elastolysis.
MMP-2 Inhibition and Calcification in Sheep Mitral Valve Replacements
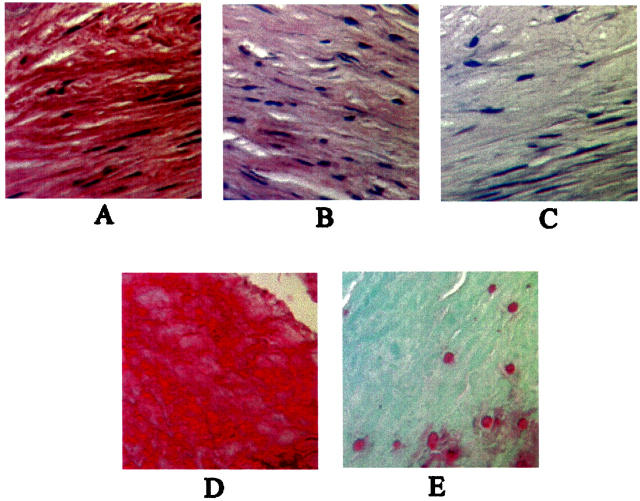
Subdermal results clearly showed lower MMP activity and inhibition of calcification in AlCl3-pretreated samples. To determine whether MMP suppression and inhibition of calcification occurs in circulatory implants, we obtained AlCl3-pretreated aortic wall explants from porcine BPHV that were implanted for 150 days in the sheep mitral valve position. We chose to look at MMP-2 activity as our subdermal studies showed that MMP-2 is expressed later in the calcification process. Control untreated aortic wall segments showed strong immunostaining for MMP-2 associated with elastin fibers in the aorta (Figure 3A) ▶ , while AlCl3-pretreated aortic wall segments showed minimal, if any, staining for MMP-2 (Figure 3B) ▶ . No staining was seen in the negative control (Figure 3C) ▶ . At this time, control porcine aortic wall segments from BPHV were severely calcified (Figure 3D) ▶ , while AlCl3 pretreatment significantly inhibited elastin-oriented calcification (Figure 3E) ▶ as shown by alizarin red staining for calcium.
Figure 3.
Immunohistochemical staining for MMP-2 in the aortic wall segment of porcine bioprosthetic heart valve implants from sheep showed strong positive MMP-2 staining close to the elastin fibers (A). AlCl3-pretreated aortic wall segments showed minimal staining for MMP-2 (B). Negative control is shown in C. Alizarin red staining for calcium showed severe elastin-oriented calcification in control aortic wall segments (D), while calcification was significantly inhibited in AlCl3-pretreated implants (E). Magnification (A–E), × 400.
Discussion
Our results clearly demonstrate that binding of aluminum ions to elastin inhibits MMP-mediated elastolysis. Moreover, such AlCl3-pretreated elastin showed significantly lower activity of MMPs when implanted subdermally in rats or in circulatory heart valve implants in sheep, which in turn led to a significant inhibition of elastin-oriented calcification.
Aluminum Chloride Pretreatment for Inhibition of Pathological Calcification
Our interest in studying the effects of AlCl3 pretreatment were due to earlier findings that AlCl3 pretreatment inhibits BPHV calcification. 14,15 It is also one of the few pretreatments that prevent calcification of the aortic wall segment of BPHV. 16 As the aortic wall segment has elastin as its major ECM component, we initiated research in studying effects of aluminum ion pretreatment on elastin structure. We demonstrated that aluminum ions strongly associate with elastin and prevent calcification of purified elastin. 7 However, previous studies did not elucidate how this aluminum ion binding to elastin leads to prevention of calcification.
In a separate study, we recently showed that when elastin fibers were implanted subdermally in rats, MMP-2 and MMP-9 were expressed surrounding the implant and inhibition of MMP activity, by local administration of a MMP inhibitor (BB-1101), led to a significant inhibition of calcification. 9 However, in that study we did not show whether MMP binding and elastin degradation are the initial steps in elastin calcification. Based on these two earlier studies, we asked the question whether aluminum ion binding to elastin prevents MMP-mediated elastin degradation and thus prevents its calcification.
MMPs and Calcification
Matrix metalloproteinases have a specialized function in the turnover of the ECM. MMPs are precisely regulated and targeted to specific extracellular substrates by a wide variety of cells during numerous normal tissue processes, such as wound healing, bone resorption, and morphogenesis. 17 In contrast, over-production of MMPs is a hallmark of many destructive diseases, such as arthritis, chronic ulceration, cancerous tumors, atherosclerotic plaques, restenosis, and aortic aneurysms. 18-21 MMPs have also been detected in association with calcification of bioprostheses. 22 With respect to elastin-mediated calcification, both MMP-2 and MMP-9 are known to bind insoluble elastin, 8 and each have been shown to be actively involved in elastin degradation. 23 Elastin peptides generated by elastic fiber degradation are chemotactic in nature and stimulate cell proliferation, calcium ion flux modification, as well as MMP secretion. 24,25 Our results with elastin gel zymography studies for the first time show that aluminum ion binding to elastin inhibits MMP-9 mediated elastin degradation. We hypothesize that degradation of elastin is the first step in elastin calcification. It has been shown earlier that elastin peptides are prone to calcification and calcium binding to elastin peptides initiates elastin calcification. 26 Our subdermal elastin implant studies also demonstrate that when aluminum ions are bound to elastin, a significantly lower amount of MMP-2 and MMP-9 are expressed surrounding the implant. Importantly, statistically significant reduction of MMP-9 expression at day 1 and proMMP-2 expression at day 4 was seen in AlCl3-pretreated explants (Figure 2) ▶ . It is generally known that cells secrete proenzyme forms and proenzymes attach to the ECM before the activation step. 27 Aluminum ion binding to elastin may somehow block MMP-elastin binding and subsequent activation. Thus, it is possible that a significantly lower degradation of elastin would take place for AlCl3-pretreated elastin.
As subdermal implant studies do not provide blood/material interactions and to confirm that the subdermal results are relevant to the circulatory situation where blood interactions take place, we studied MMP-2 activity in samples from aortic wall of BPHV implants that were implanted in sheep in the mitral position for 150 days. An aortic wall segment of BPHV contains significant amounts of elastin and severe elastin-oriented calcification occurs in these tissues in the sheep mitral valve replacement model. 28 We show a significant reduction in MMP-2 activity in AlCl3-pretreated porcine aortic wall segments of BPHV similar to our subdermal implant study results along with a significant inhibition of calcification (Figure 3) ▶ . Thus, the sheep implant data confirms that similar mechanisms of calcification may exist in the subdermal implantation model and in the blood environment. Moreover, the circulatory explants from sheep were functional for 150 days, much more time than our 7-day subdermal implant studies. Thus, our data clearly shows that aluminum ions bind irreversibly to elastin and suppress MMP activity around the implant for an extended period. A high MMP activity has been found in many cardiovascular diseases such as atherosclerosis and aortic aneurysm as well as in BPHV and pathological calcification is also observed in these diseases. 1,2,5,6,18-21 Based on the present study and our earlier work on MMP inhibition leading to prevention of calcification, we propose that MMP-mediated ECM degradation and connective tissue damage, specifically of elastic fibers of internal and external elastic lamellae, may initiate calcification process of the ECM that has been found in these diseases.
Conclusions
In conclusion, results of the current study clearly demonstrate the strong inhibition of MMP-mediated elastolysis by aluminum ion binding to elastin. Activity of MMPs in purified elastin and aortic wall segments of BPHV were significantly reduced in samples pretreated with AlCl3, and calcification of these same tissues was prevented in comparison to the calcified control tissues. MMP-2 and MMP-9 activity was shown to be time dependent, indicating that these MMPs are controlled through different regulatory mechanisms.
Acknowledgments
We thank Linda Jenkins for her help with the histology studies.
Footnotes
Address reprint requests to Naren Vyavahare, 501–1 Rhodes Research Center, Department of Bioengineering, Clemson University, Clemson, SC 29634. E-mail: narenv@clemson.edu.
Supported by National Institutes of Health grant HL-61652 and American Heart Association Scientist Development Grant (N.R.V.).
References
- 1.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC: Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res 2001, 88:37-43 [DOI] [PubMed] [Google Scholar]
- 2.Bobryshev YV, Lord RS, Warren BA: Calcified deposit formation in intimal thickenings of the human aorta. Atherosclerosis 1995, 118:9-21 [DOI] [PubMed] [Google Scholar]
- 3.Sabet HY, Edwards WD, Tazelaar HD, Daly RC: Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc 1999, 74:14-26 [DOI] [PubMed] [Google Scholar]
- 4.Schoen FJ, Levy RJ: Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res 1999, 47:439-465 [DOI] [PubMed] [Google Scholar]
- 5.Angelini A, Basso C, Grassi G, Casarotto D, Thiene G: Surgical pathology of valve disease in the elderly. Aging (Milano) 1994, 6:225-237 [DOI] [PubMed] [Google Scholar]
- 6.David TE: Aortic valve replacement with stentless porcine bioprostheses. J Cardiovasc Surg 1998, 13:344-351 [DOI] [PubMed] [Google Scholar]
- 7.Vyavahare N, Ogle M, Schoen FJ, Levy RJ: Elastin calcification and its prevention with aluminum chloride pretreatment. Am J Pathol 1999, 155:973-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emonard H, Hornebeck W: Binding of 92 kDa and 72 kDa progelatinases to insoluble elastin modulates their proteolytic activation. Biol Chem 1997, 378:265-271 [DOI] [PubMed] [Google Scholar]
- 9.Vyavahare N, Jones PL, Tallapragada S, Levy RJ: Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol 2000, 157:885-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoen FJ, Hirsch D, Bianco RW, Levy RJ: Onset and progression of calcification in porcine aortic bioprosthetic valves implanted as orthotopic mitral valve replacements in juvenile sheep. J Thorac Cardiovasc Surg 1994, 108:880-887 [PubMed] [Google Scholar]
- 11.Chen P, Toribara T, Warner H: Microdetermination of phosphorus. Anal Chem 1956, 28:1756-1758 [Google Scholar]
- 12.Katsuda S, Okada Y, Imai K, Nakanishi I: Matrix metalloproteinase-9 (92-kd gelatinase/type IV collagenase equals gelatinase B) can degrade arterial elastin. Am J Pathol 1994, 145:1208-1218 [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL: Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem 1985, 260:2493-2500 [PubMed] [Google Scholar]
- 14.Webb CL, Flowers WE, Boyd J, Rosenthal EL, Schoen FJ, Levy RJ: Al+++ binding studies and metallic cation effects on bioprosthetic heart valve calcification in the rat subdermal model. ASAIO Trans 1990, 36:56-59 [DOI] [PubMed] [Google Scholar]
- 15.Webb CL, Schoen FJ, Flowers WE, Alfrey AC, Horton C, Levy RJ: Inhibition of mineralization of glutaraldehyde-pretreated bovine pericardium by AlCl3. Mechanisms and comparisons with FeCl3, LaCl3, and Ga(NO3)3 in rat subdermal model studies. Am J Pathol 1991, 138:971-981 [PMC free article] [PubMed] [Google Scholar]
- 16.Levy RJ, Qu X, Underwood T, Trachy J, Schoen FJ: Calcification of valved aortic allografts in rats: effects of age, crosslinking, and inhibitors. J Biomed Mater Res 1995, 29:217-226 [DOI] [PubMed] [Google Scholar]
- 17.Johnson LL, Dyer R, Hupe DJ: Matrix metalloproteinases. Curr Opin Chem Biol 1998, 2:466-471 [DOI] [PubMed] [Google Scholar]
- 18.Sinha S, Frishman WH: Matrix metalloproteinases and abdominal aortic aneurysms: a potential therapeutic target. J Clin Pharmacol 1998, 38:1077-1088 [PubMed] [Google Scholar]
- 19.Stetler-Stevenson WG, Yu AE: Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol 2001, 11:143-153 [DOI] [PubMed] [Google Scholar]
- 20.Galis ZS, Sukhova GK, Lark MW, Libby P: Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994, 94:2493-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikkari ST, Geary RL, Hatsukami T, Ferguson M, Forough R, Alpers CE, Clowes AW: Expression of collagen, interstitial collagenase, and tissue inhibitor of metalloproteinases-1 in restenosis after carotid endarterectomy. Am J Pathol 1996, 148:777-783 [PMC free article] [PubMed] [Google Scholar]
- 22.Simionescu A, Simionescu D, Deac R: Biochemical pathways of tissue degeneration in bioprosthetic cardiac valves. The role of matrix metalloproteinases. ASAIO J 1996, 42:M561-M567 [DOI] [PubMed] [Google Scholar]
- 23.Mecham RP, Broekelmann TJ, Fliszar CJ, Shapiro SD, Welgus HG, Senior RM: Elastin degradation by matrix metalloproteinases: cleavage site specificity and mechanisms of elastolysis. J Biol Chem 1997, 272:18071-18076 [DOI] [PubMed] [Google Scholar]
- 24.Brassart B, Randoux A, Hornebeck W, Emonard H: Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin Exp Metastasis 1998, 16:489-500 [DOI] [PubMed] [Google Scholar]
- 25.Jacob MP, Fulop T, Foris G, Robert L: Effect of elastin peptides on ion fluxes in mononuclear cells, fibroblasts, and smooth muscle cells. Proc Natl Acad Sci USA 1987, 84:995-999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urry DW: Neutral sites for calcium ion binding to elastin and collagen: a charge neutralization theory for calcification and its relationship to atherosclerosis. Proc Natl Acad Sci USA 1971, 68:810-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stetler-Stevenson WG: Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 1999, 103:1237-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herijgers P, Ozaki S, Verbeken E, Van Lommel A, Racz R, Zietkiewicz M, Perek B, Flameng W: Calcification characteristics of porcine stentless valves in juvenile sheep. Eur J Cardiothorac Surg 1999, 15:134-142 [DOI] [PubMed] [Google Scholar]