Abstract
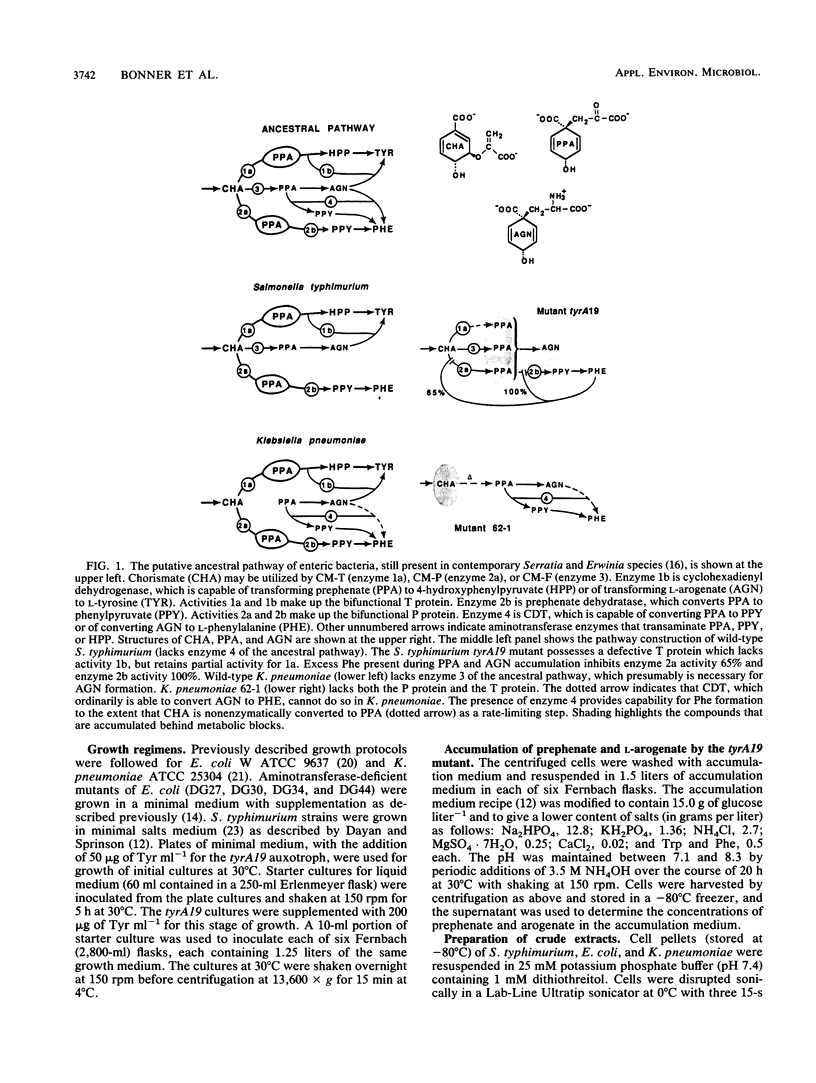
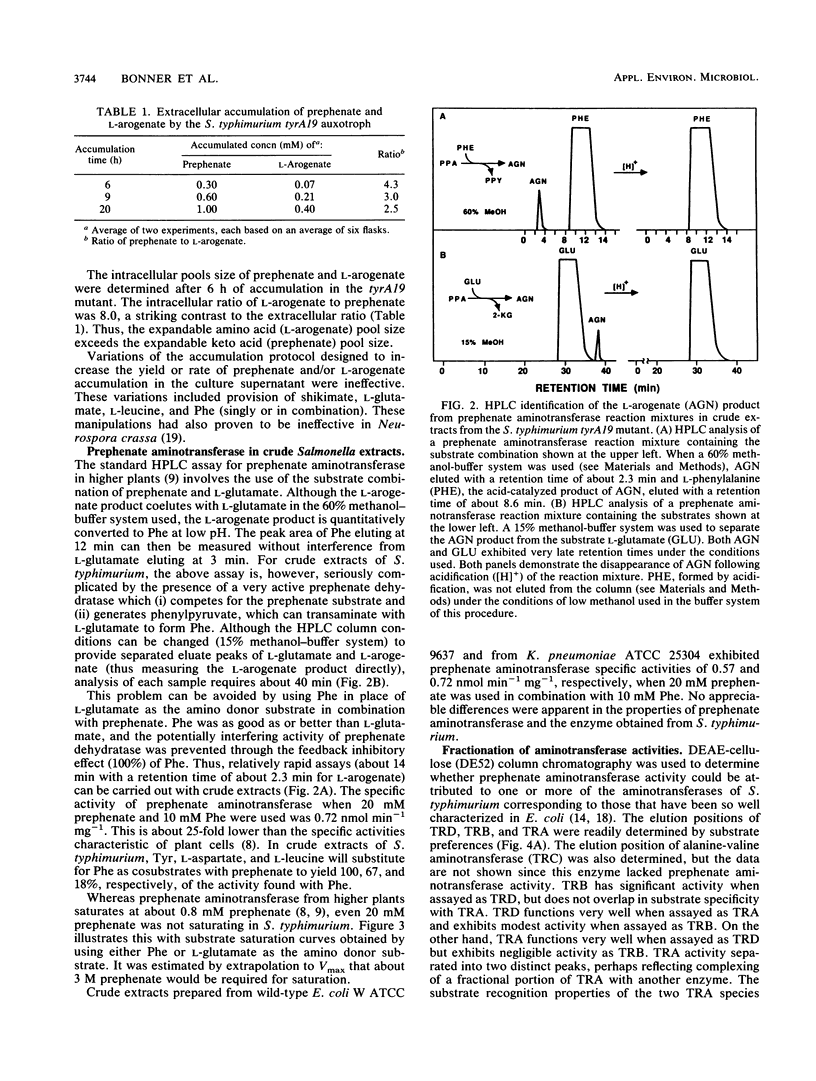
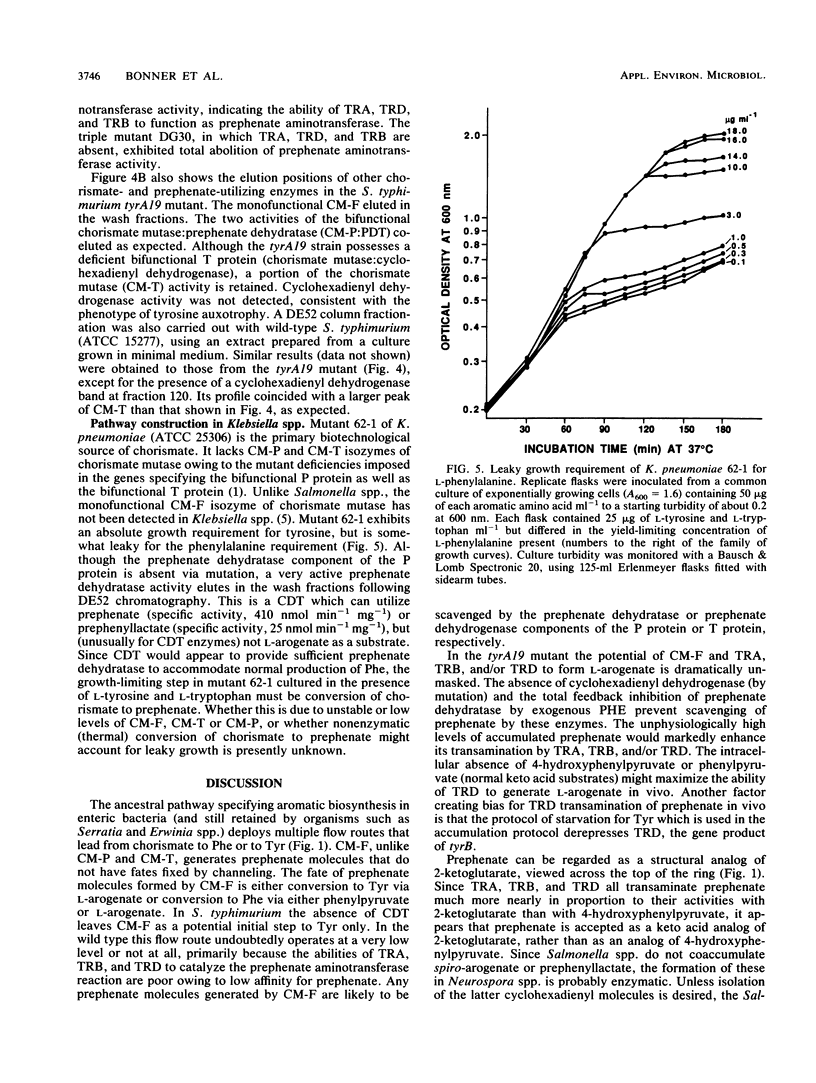
The pathway construction for biosynthesis of aromatic amino acids in Escherichia coli is atypical of the phylogenetic subdivision of gram-negative bacteria to which it belongs (R. A. Jensen, Mol. Biol. Evol. 2:92-108, 1985). Related organisms possess second pathways to phenylalanine and tyrosine which depend upon the expression of a monofunctional chorismate mutase (CM-F) and cyclohexadienyl dehydratase (CDT). Some enteric bacteria, unlike E. coli, possess either CM-F or CDT. These essentially cryptic remnants of an ancestral pathway can be a latent source of biochemical potential under certain conditions. As one example of advantageous biochemical potential, the presence of CM-F in Salmonella typhimurium increases the capacity for prephenate accumulation in a tyrA auxotroph. We report the finding that a significant fraction of the latter prephenate is transaminated to L-arogenate. The tyrA19 mutant is now the organism of choice for isolation of L-arogenate, uncomplicated by the presence of other cyclohexadienyl products coaccumulated by a Neurospora crassa mutant that had previously served as the prime biological source of L-arogenate. Prephenate aminotransferase activity was not conferred by a discrete enzyme, but rather was found to be synonymous with the combined activities of aspartate aminotransferase (aspC), aromatic aminotransferase (tyrB), and branched-chain aminotransferase (ilvE). This conclusion was confirmed by results obtained with combinations of aspC-, tyrB-, and ilvE-deficient mutations in E. coli. An example of disadvantageous biochemical potential is the presence of a cryptic CDT in Klebsiella pneumoniae, where a mutant carrying multiple enzyme blocks is the standard organism used for accumulation and isolation of chorismate.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Jensen R. A. New prospects for deducing the evolutionary history of metabolic pathways in prokaryotes: aromatic biosynthesis as a case-in-point. Orig Life Evol Biosph. 1988;18(1-2):41–57. doi: 10.1007/BF01808779. [DOI] [PubMed] [Google Scholar]
- Ahmad S., Jensen R. A. The phylogenetic origin of the bifunctional tyrosine-pathway protein in the enteric lineage of bacteria. Mol Biol Evol. 1988 May;5(3):282–297. doi: 10.1093/oxfordjournals.molbev.a040496. [DOI] [PubMed] [Google Scholar]
- Ahmad S., Jensen R. A. The prephenate dehydrogenase component of the bifunctional T-protein in enteric bacteria can utilize L-arogenate. FEBS Lett. 1987 May 25;216(1):133–139. doi: 10.1016/0014-5793(87)80771-8. [DOI] [PubMed] [Google Scholar]
- Bonner C. A., Jensen R. A. Novel features of prephenate aminotransferase from cell cultures of Nicotiana silvestris. Arch Biochem Biophys. 1985 Apr;238(1):237–246. doi: 10.1016/0003-9861(85)90161-4. [DOI] [PubMed] [Google Scholar]
- Bonner C., Jensen R. Prephenate aminotransferase. Methods Enzymol. 1987;142:479–487. doi: 10.1016/s0076-6879(87)42059-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Fischer R., Jensen R. Prephenate dehydratase (monofunctional). Methods Enzymol. 1987;142:507–512. doi: 10.1016/s0076-6879(87)42063-6. [DOI] [PubMed] [Google Scholar]
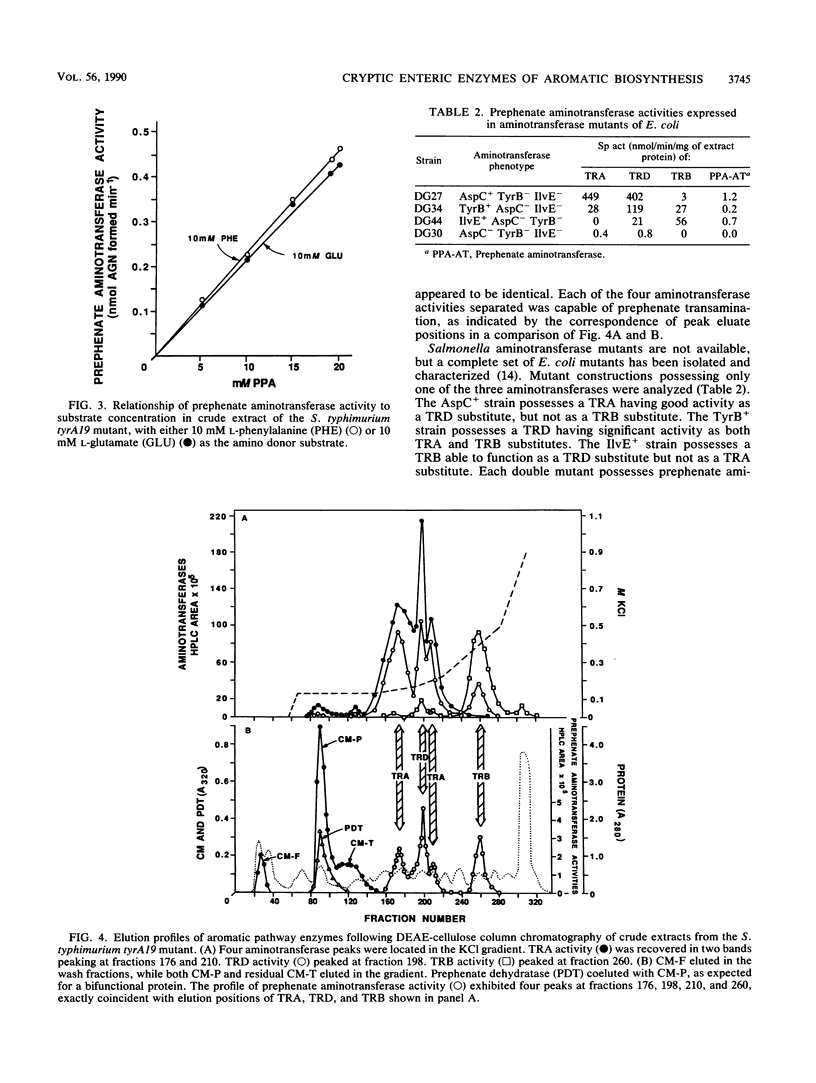
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Biochemical pathways in prokaryotes can be traced backward through evolutionary time. Mol Biol Evol. 1985 Mar;2(2):92–108. doi: 10.1093/oxfordjournals.molbev.a040338. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H. Intracellular roles of microbial aminotransferases: overlap enzymes across different biochemical pathways. Crit Rev Microbiol. 1981;8(3):229–266. doi: 10.3109/10408418109085080. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L., Shaw D. C., Gibson F. The purification and characterisation of chorismate mutase-prephenate dehydrogenase from Escherichia coli K12. Biochim Biophys Acta. 1971 Mar 23;229(3):795–804. doi: 10.1016/0005-2795(71)90298-4. [DOI] [PubMed] [Google Scholar]
- Koch G. L., Shaw D. C., Gibson F. Tyrosine biosynthesis in Aerobacter aerogenes. Purification and properties of chorismate mutase-prephenate dehydrogenase. Biochim Biophys Acta. 1970 Sep 16;212(3):375–386. doi: 10.1016/0005-2744(70)90243-3. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Demerec M., Eisenstark A. Genetic analysis of aromatic mutants of Salmonella typhimurium. Genetics. 1967 Jun;56(2):341–351. doi: 10.1093/genetics/56.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Zamir L. O., Jung E., Jensen R. A. Co-accumulation of prephenate, L-arogenate, and spiro-arogenate in a mutant of Neurospora. J Biol Chem. 1983 May 25;258(10):6492–6496. [PubMed] [Google Scholar]
- Zamir L. O., Tiberio R., Devor K. A., Sauriol F., Ahmad S., Jensen R. A. Structure of D-prephenyllactate. A carboxycyclohexadienyl metabolite from Neurospora crassa. J Biol Chem. 1988 Nov 25;263(33):17284–17290. [PubMed] [Google Scholar]
- Zamir L. O., Tiberio R., Fiske M., Berry A., Jensen R. A. Enzymatic and nonenzymatic dehydration reactions of L-arogenate. Biochemistry. 1985 Mar 26;24(7):1607–1612. doi: 10.1021/bi00328a006. [DOI] [PubMed] [Google Scholar]



