Abstract
Background and Objective
To determine the optimal magnetic resonance imaging (MRI) methodology to assess photodynamic therapy (PDT)-induced histopathological responses in the prostate.
Study Design/Materials and Methods
Laparotomy was performed in five healthy dogs. Cylindrical diffuser was placed in the prostates to deliver light of 50–300 J/cm at 150 mW/cm and 763 nm to activate IV-injected Tookad (1 mg/kg b.w.). Fast spin echo (FSE) T2-weighted, post-contrast-enhanced T1-(CE-T1) and diffusion weighted images (DWI) were obtained pre- and 2 days, 7 days, and 1 month post-PDT. Radiological-histopathological correlation was performed at 7 days (n = 4) and 1 month (n= 1) after PDT. A qualitative assessment of signal changes and apparent diffusion coefficient (ADC) mapping was performed.
Results
At 2 or 7 days post-PDT, there was good spatial correlation between PDT-induced hemorrhagic necrosis and unenhanced regions on CE-T1 images. There was a rapidly and persistently enhancing rim corresponding to edema and inflammation. FSE T2 and DWI showed altered signal but did not clearly define necrosis in all cases. At 1 month, it was hard to correlate MR images to histopathologic changes as they represented a mixture of necrosis and developing fibrosis, which led to a mixed signal intensity and less demarcated contrast enhancement.
Conclusions
At 7 days after PDT, gadolinium DTPA contrast-enhanced MRI is superior to DWI and T2 imaging in assessing the boundary of Tookad PDT-induced tissue necrosis in the normal canine prostate.
Keywords: MRI assessment, prostate, photodynamic therapy, PDT, Tookad
INTRODUCTION
Prostate cancer is the leading malignant disease in men and the second leading cause of cancer-related death in the western world. In the United States, approximately 232,090 new cases and 30,350 prostate cancers deaths were predicted for 2005 [1]. Early detection of prostate cancers that can be cured by local treatment will continue to rise due to the increasing use of prostate specific antigen (PSA) screening and other biomarkers of prostate cancer detection [2]. Radical prostatectomy and brachytherapy are both commonly used for the treatment of localized prostate cancer, with control rates above 80% for low-risk disease [3]. But these treatments are less effective for high-risk disease and often associated with considerable side effects and morbidity. Therefore, less invasive treatment alternatives are needed. Cryosurgical ablation, high-intensity focused ultrasound (HIFU), and radiofrequency interstitial ablation are promising minimally invasive or non-invasive alternatives for the treatment of localized prostate cancer [4]. However, the follow-up of these alternative modalities has been too short-term to determine their roles in the treatment of prostate cancer.
Photodynamic therapy (PDT) is a unique treatment modality in which a systemically or locally administered photosensitizer is activated by irradiating the lesion site with light of appropriate wavelength. PDT has been used for localized superficial or endoluminal malignant diseases [5]. Recently, its clinical application has also been explored for several solid tumors [6]. PDT seems to have great potential in the treatment of prostate cancer [7–9]. Experimental treatment of human prostate cancer was studied in Europe and North America for patients who failed conventional radiotherapy. An attempt to develop an interstitial Photofrin-PDT procedure was first made in the early 1990s [10]. Recent clinical trials of Foscan-PDT and ALA-PDT on patients who had failed radiotherapy showed a post-PDT decrease in prostate PSA levels [11,12]. The preliminary results from two ongoing clinical trials of motexafin lutetium-PDT and Tookad-PDT designed to totally ablate the entire prostate gland are also encouraging [13–15]. Further clinical investigation with corresponding long-term outcomes are required to evaluate the effectiveness of PDT for the treatment of prostate cancer.
Histopathological assessment of PDT-induced tissue lesions of the whole-mount prostatic specimen has been used widely in pre-clinical PDT studies. For prostate cancer patients, PSA in combination with biopsies might be useful for staging and prognosis of prostate cancer after treatment [16]. However, a sensitive and non-invasive assessment of prostate response to PDT would allow oncologists to evaluate the effectiveness of the treatment in the early post-treatment time periods. That would allow rapid modifications in the regimen such as repeated or extended treatment.
Several emerging techniques for monitoring acute response of prostate to PDT or other treatments have been investigated. Electrical bioimpedance spectroscopy (EBS) is a fast and relatively simple technique for the real-time characterization of the impedance spectra of tissue structure during therapy. EBS can differentiate the extra- and intra-cellular fluid volumes and therefore detect PDT-induced intracellular edema in animal tumor models [17]. Microbubble enhanced ultrasound can show early response to treatment, such as the vascular enhancement decline. Color Doppler and power transrectal ultrasound in combination with microbubble contrast can detect and quantify changes in prostate cancer vascularity [18]. Metabolic assessment of prostate cancer using positron emission tomography (PET) indicate a decrease in glucose uptake and accumulation after hormone therapy [19]. The use of PET for the follow-up of PDT-treated tumors in several pre-clinical studies has been reported [20]. Magnetic resonance imaging (MRI) can facilitate prostate delineation and tumor localization. Multi-section fast dynamic contrast agent-enhanced MRI can also provide additional information and be valuable for the evaluation of prostate damage and therefore the effectiveness of treatment [21–23]. However, the number of publications on the use of MRI for the follow-up of PDT-treated prostate is limited.
Tookad (Pd-Bacteriopheophorbide, also known as WST09; Negma-Lerads, Toussus-Le-Noble, France) is a novel intra-vascular photosensitizer. Tookad-PDT mediated interstitial ablation is a promising alternative that has been shown to induce extensive hemorrhagic necrosis in various animal models [24–26]. When activated by 763-nm laser light, it eradicates tumor by destroying its feeding vessels and blood supply. Because of the marked and rapid decrease in perfusion induced by vascular targeted Tookad-PDT [26–28], we hypothesized that PDT-induced acute changes in prostate gland could be measured accurately with post-gadolinium-enhanced MRI. Areas of necrosis might also be expected to have a higher free water content and thus diffusion-weighted MRI and T2-weighted MRI may also be of value. Our early experience of a human trial suggested that gadolinium (Gd) contrast-enhanced T1-weighted imaging could show PDT-induced necrosis. The necrosis was evident over many minutes and thus, high resolution T1 images could be obtained [29]. The aims of this pre-clinical study were to determine the optimal MRI methodology for assessing PDT-induced acute lesions and to establish histopathological correlation of the imaging results. Because of the difficulty in finding spontaneous prostate cancer-bearing animals, normal canine prostate has been used in this study due to its resemblance in size and anatomical structure to the human prostate. Our previous pre-clinical studies indicate that Tookad-PDT can induce a similar scale of acute lesion in normal prostate as well as in pre-ionizing irradiated prostate and spontaneous canine prostate cancer. The MRI data obtained from this pre-clinical study are useful for developing optimal MRI methodology for human study and immediately benefit current PDT clinical trials.
MATERIALS AND METHODS
Animal Model
As a pilot study, five healthy adult male Beagle dogs (Marshall Farms, North Rose, NY), 4–7 years old and body weight (b.w.) of 10–14 kg, underwent surgery, interstitial PDT procedures and MRI scans prior to and 7 days post-PDT. One animal underwent additional MRI at 2 days post-treatment and another at 1 month post-treatment (Table 1). All animal studies were approved by the institutional animal care and use committees of HealthONE Alliance and Colorado State University.
TABLE 1.
Treatment and MRI Scheme
| Dog ID
|
#1
|
#2
|
#3
|
#4
|
#5
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treated site | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right |
| Position of the cylindrical diffuser tip | Perpendicular to the urethra | Perpendicular to the urethra | Perpendicular to the urethra | Parallel to the urethra | Perpendicular to the urethra | |||||
| Drug dose (mg/kg b.w.) | 1 | |||||||||
| Light dose (J/cm) | 50 | 100 | 50 | 200 | 300 | 200 | 200 | 200 | 150 | 100 |
| MRI scans | Prior to PDT × 2 | Prior to PDT × 1 | Prior to PDT × 2 | Prior to PDT × 2 | Prior to PDT × 1 | |||||
| 7-day post-PDT × 1 | 2-day post-PDT × 1 | 7-day post-PDT × 1 | 7-day post-PDT × 1 | 7-day post-PDT × 1 | ||||||
| 7-day post-PDT × 1 | 1-month post-PDT × 1 | |||||||||
PDT Procedures
All dogs were prepared for surgery following a standard canine laparotomy procedure [24]. Both lobes received a single interstitial light treatment for radiographic and histological assessment of PDT-induced lesions. Light source was provided with a portable 763-nm diode laser (Ceralas; CeramOptec GmbH of Biolitec AG, Bonn, Germany). The laser output was directly coupled into a Y-splitter (Ocean Optics, Inc., Dunedin, FL) allowing simultaneous treatment of the bilateral lobe. The interstitial irradiation was delivered through a cylindrical diffuser fiber (1-cm active tip; CD 603-10C; CeramOptec GmbH). The diffuser fiber was placed in the middle section of prostate through the ventral surface either perpendicular or parallel to the prostatic urethra. Photosensitizing drug Tookad® (2.5 mg/ml; Negma-Lerads) was administered at a dose level of 1 mg/kg b.w. by a slow IV infusion over a period of 10 minutes. Laser light was delivered 4 minutes after the onset of drug infusion. There was approximately a 6 minutes overlap between drug infusion and light irradiation. A total light dose of 50 J/cm to 300 J/cm was delivered, over a period of 5.5 minutes to 33.3 minutes at a fixed light fluence rate of 150 mW/cm, to the left lobe or the right lobe, respectively (see Table 1). The difference in treatment protocols and light doses on each dog was to achieve different amounts of necrosis and a spread of effect to perform correlations with imaging results.
Benadryl and steroids were used to counteract the effect of Cremophor EL-P, the co-solvent of Tookad, on the blood pressure. Benadryl (IV, 0.7~1.4 mg/kg) and Dexamethasone (SQ, 2 mg per dog) were given 24 hours before and immediately prior to the photosensitizer infusion. Three dogs were placed in a metabolic cage for urine collection to monitor the urethral damage during the first 24 hours post-PDT up to 4 days post-PDT.
MRI Scan
All animals received identical MRI scans prior to and 7 days post-PDT. Three animals underwent additional pre-PDT scan, one animal additional MRI at 2 days post-treatment and another at 1 month post-treatment (Table 1). Actual MRI protocols were identical for all animals.
MR imaging was performed under general anesthesia using a 1.5 Tesla GEMS Signa HiSpeed LX 9.0 scanner (Milwaukee, WI) (see Table 1). Dogs were positioned in dorsal recumbency with pelvic limbs taped in extension. A sand bag was placed over the abdomen to minimize possible motion. Acquisition was done with the pelvis centered in a standard GE quadrature coil designed for head imaging. The following pulse sequences were performed: axial T2-weighted fast spin echo (FSE) (TR = 3,000 milliseconds, TE = 102 milliseconds, slice thickness = 3 mm, gap = 0 mm, matrix = 320 × 224, field of view = 16 cm); axial diffusion weighted image (DWI) (TR = 4,000 milliseconds, TE = 99 milliseconds, slice thickness = 3 mm, gap = 0 mm, b-value = 600 seconds/mm2 T2, matrix 128 × 128, field of view = 24 cm); spin echo T1-weighted fat suppressed (TR = 450 milliseconds, TE = 20 milliseconds, slice thickness = 3 mm, gap = 0 mm, matrix = 256 × 192, filed of view = 16 cm). The T1-weighted sequences were performed before and after gadolinium enhancement. Contrast enhancement (CE) was achieved via IV bolus injection of 0.1 mmol/kg b.w. gadolinium DTPA (Magnevist, Berlex Laboratories, Trenton, NJ). Slice prescriptions for all series were identical for direct comparison.
A qualitative assessment of signal changes occurring within the prostate was performed for the T2-weighted images, post-contrast-enhanced T1 (CE-T1)-weighted images and DWIs. For quantitative analysis, the MRI section for each of the pulse sequences that corresponded to the pathologic section with the largest area of necrosis was chosen. For the pulse sequences where a clear region of necrosis could be identified, this area was measured independently in the left and right half of the prostate. MRI assessment was performed by a radiologist experienced in prostate MRI. Apparent diffusion coefficient (ADC) maps were generated from the DW sequences using an Advantage Windows Workstation v4.0 (GE Medical Systems, Milwaukee, WI). Differences in ADC pre- and post-therapy were compared in regions corresponding to areas of PDT-induced necrosis.
Histopathologic Examination
Treated animals were euthanized using barbiturate overdose after the last MRI scan at 7 days post-PDT (n = 4) or 1 month post-PDT (n = 1). At necropsy, the prostates were removed, photographed, and fixed in 10% neutral buffered formalin for over 24 hours. For histological examination and H&E staining of the MRI-scanned prostates, the prostates were dissected and sectioned axially into 3 mm blocks corresponding to the cross sections of MRI scanning. In cases where the whole tissue block (> 25 × 25 mm) did not fit onto a single micro slide (75 × 25 mm), it was sectioned and digitally reconstructed after mounting and staining. Regions of necrosis were demarcated on the digital photo of the section which showed the maximal amount of necrosis. Each digital photo of histological slide was reviewed with the MRI images by a radiologist. Matching MRI slice were chosen based on matching the background radial architecture and the presence of any additional structures such as intraprostatic cysts on T2-weighted images with similar appearances on the histological slides.
RESULTS
Post-Surgical Observations
The surgical wound healed well in all treated dogs. Dogs showed no post-PDT urethral complications. None of the dogs showed urinary retention and urinary catheterization was not needed. Urinalysis of three animals showed only trace blood in the urine samples collected post-PDT indicating microhematuria (Table 2).
TABLE 2.
Urinalysis
| Dog ID | #1 | #3 | #4 | |
|---|---|---|---|---|
| Blood | Pre-PDT | Neg. | Neg. | n/a |
| 24 hours post-PDT | 3+ | n/a | n/a | |
| 4 days post-PDT | 3+ | 3+ | ||
| RBC in sediment(cells/hpf) | Pre-PDT | 0 | 0 | n/a |
| 24 hours post-PDT | Packed | n/a | n/a | |
| 4 days post-PDT | n/a | 20–50 | 50–100 |
Histopathologic Findings
At 7 days post-treatment (n = 4), the PDT-induced macroscopic lesions were characterized by acute hemorrhagic necrosis with patchy sub-capsular hyperemia and marked edema (Fig. 1). The PDT-induced prostate lesions were well delineated from the unaffected adjacent normal tissue, and the zone of necrosis increased with the increase of the delivered light dose as described in detail in previous publications [24–26,30]. Moderate inflammatory response was seen in periprostatic connective and fatty tissues. Microscopic examination showed a total destruction of glandular epithelium and connective interstitial tissues. Severe necrosis was characterized by marked hemorrhage and hemorrhagic necrotic debris was seen at the central zone of the irradiated area (solid line—Zone 1, Fig. 2A). In all cases, the left and right regions of necrosis fused to varying extent in the midline to form a single focus of necrosis (Fig. 2 through 4). Atrophic glandular tissue, patchy mononuclear cell infiltration, fibromuscular hyperplasia, neovascularization, edema, and dilatation of glandular structures were seen around the central zone (dotted line—Zone 2, Fig. 2A). There was a mixture of normal prostatic tissue and moderate mononuclear cell infiltration beyond Zone 2. Urethral mucosa and capsule were intact.
Fig. 1.
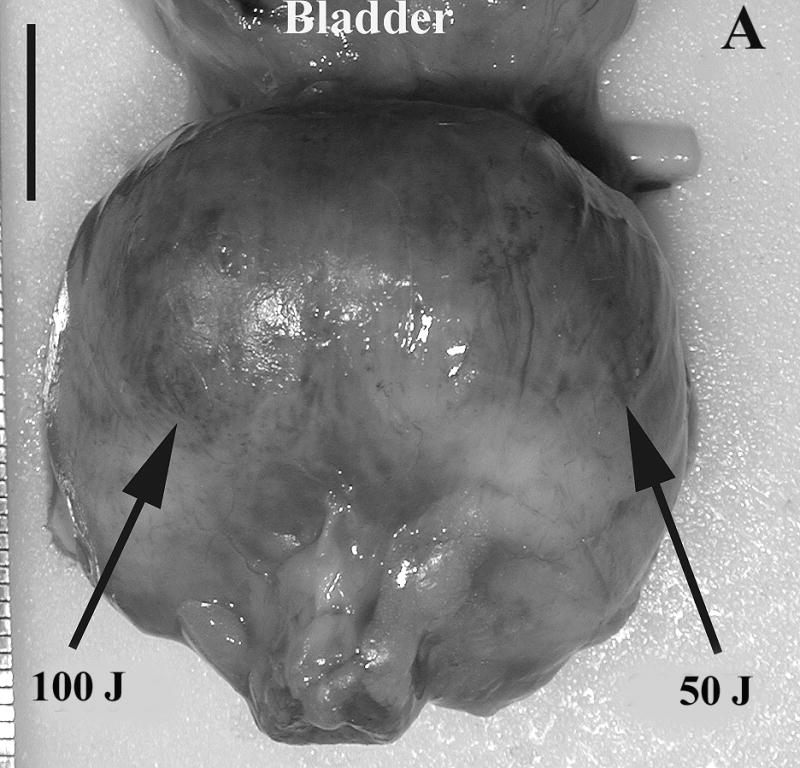
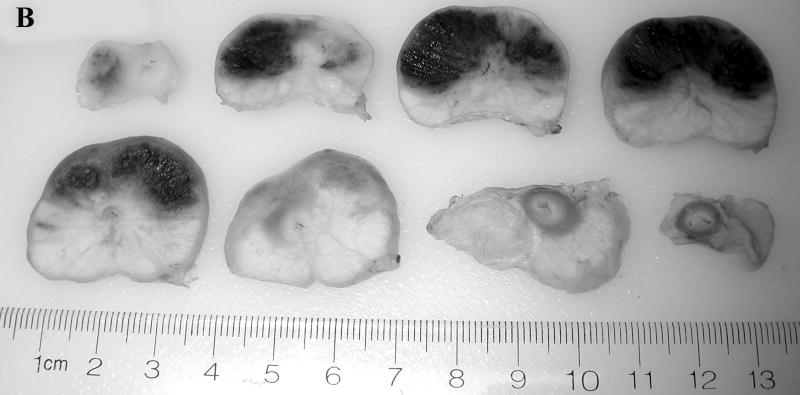
Prostate 7 days after Tookad-PDT. A: Gross view of the prostate of Dog #1 prior to formalin fixation. Patchy sub-capsular hyperemia was visible at both lobes. B: Dissected view of the same prostate after formalin fixation. Images are displayed with the dog’s anatomic left on the right side of the image. The left lobe received 50 J/cm and the right lobe 100 J/cm. Dark regions correspond to the PDT-induced hemorrhagic necrosis. (Bar = 1 cm).
Fig. 2.
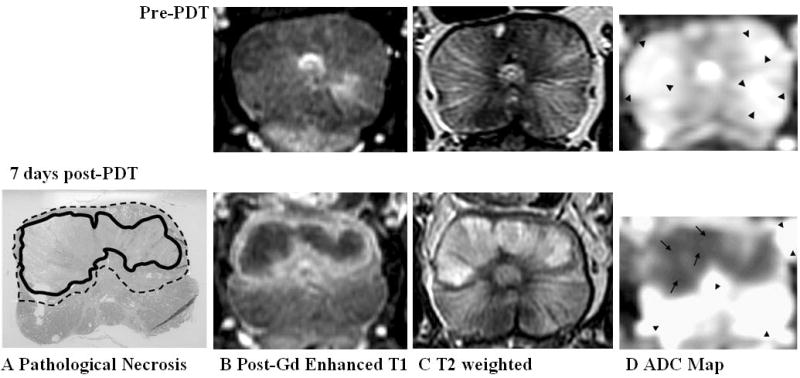
Prostate 7 days after Tookad-PDT with good necrosis delineation on all pulse sequences. Top panel: Pre-PDT of Dog #1. Bottom panel: 7 days post-PDT. Images are displayed with the dog’s anatomic left on the right side of the image. A: Pathologic slide showing region of necrosis (Zone 1—solid line) outlined in black and transition region (Zone 2—dotted line) consisting of chronic inflammation, edema, atrophic glandular tissue, and dilatation of glandular structures. B: Post-gadolinium-enhanced T1-weighted images shows Zone 1 as dark non-enhancing tissue and Zone 2 as a thin high signal intensity rim. C: On T2-weighted images, the region of necrosis shows increased signal with a dark rim corresponding to Zone 2. D: ADC map (Scale: arrow = 1,000 × 10−6 mm2/second, arrow head = 2,500 × 10−6 mm2/second) shows marked decrease in ADC in regions of necrosis (Zone 1). A transition zone (Zone 2) is visible but the lower spatial resolution of the DWI sequence limits interpretation.
Fig. 4.
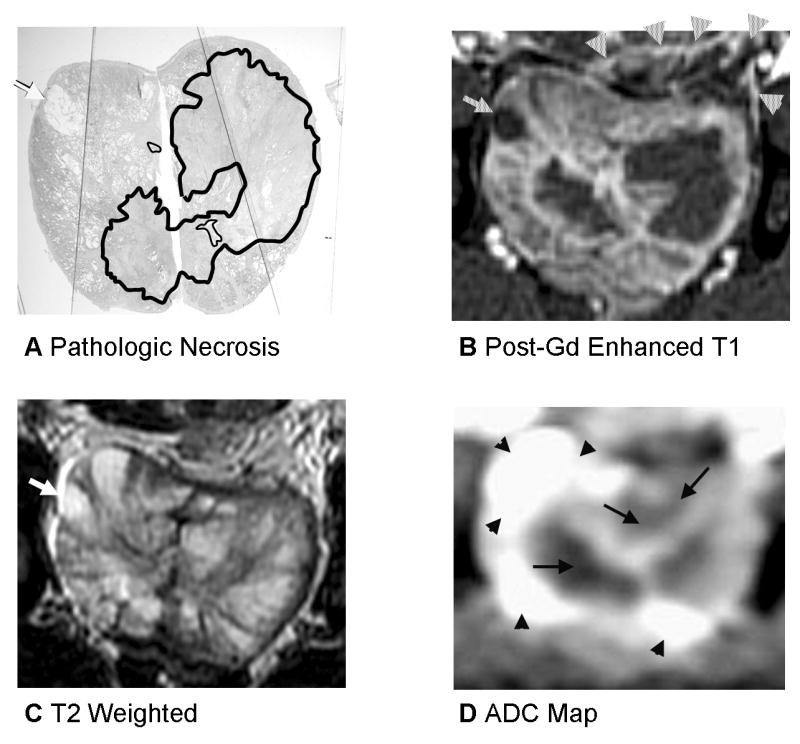
Prostate 7 days after Tookad-PDT with heterogenous signal changes on T2 and ADC and deformation from periprostatic hemorrhage. A: Pathologic slide (Dog #3, the left lobe received 300 J/cm and the right lobe 200 J/cm). Similar histopathological findings as described in the Figure 2. Images are displayed with the dog’s anatomic left on the right side of the image. B: Post-gadolinium-enhanced T1-weighted images shows necrotic region well. Cyst is demonstrated as a dark region as well (arrow) and is recognizable and was present on baseline images as well. There is compression of the prostate by periprostatic fluid and hemorrhage on the left (arrowheads). C: On T2-weighted images, the region of necrosis is not denned at all. D: ADC map shows heterogenous ADC changes on the left (same scale as the Fig. 2).
At 1 month post-treatment (n = 1), the prostate gland looked irregular and smaller. No gross lesions were seen at the periprostatic and pelvic plexus regions. The dissected sections showed partial resolution of necrosis accompanied with a small cystic area in each lobe. Microscopic changes were characterized by resolving necrosis, fibrosis, and atrophy. The dilatation of glandular structures and two small cavitations were confirmed. Some sections showed an infiltration of inflammatory cells. The PDT-induced changes were well delineated from the unaffected adjacent normal tissue. No inflammatory response was seen in periprostatic connective and fatty tissues. Urethral mucosa and capsule were intact (Fig. 5E,F).
Fig. 5.
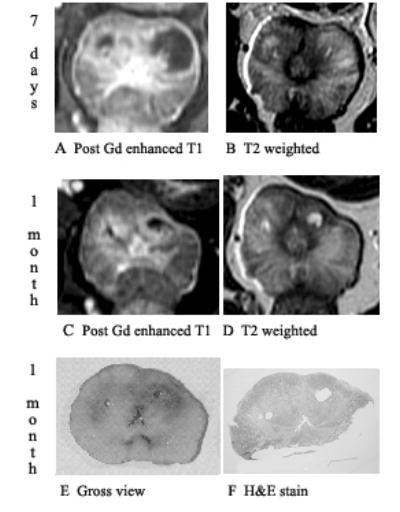
Prostate 7 days and 1 month after Tookad-PDT showing shrinkage of changes on post-gadolinium-enhanced T1-weighted images and signal changes on T2. Dog #5 (the left lobe received 150 J/cm and the right lobe 100 J/cm). Images are displayed with the dog’s anatomic left on the right side of the image. A: Post-gadolinium-enhanced T1-weighted images of 7 days post-PDT shows necrotic region well. B: On T2-weighted images of 7 days post-PDT, the margin of necrosis is poorly defined. C: Shrinkage of treatment effect on post-gadolinium-enhanced T1-weighted images at 1 month post-PDT. D: On T2-weighted images of 1 month post-PDT, cystic areas becomes very well defined. E: Gross view of whole mount. F: Pathologic slide shows two cystic areas surrounded by fibrosis corresponding to the unenhanced T1 and focal bright T2 areas.
MRI Scan
All five animals received identical MRI scans prior to and 7 days post-PDT. Three animals underwent additional pre-PDT scan, one animal additional MRI at 2 days post-treatment and another at 1 month post-treatment (see Table 1). Since pre-PDT MR images of normal prostates looked similar, only one set of pre-PDT MR images were showed in the Figure 2 as a reference (Fig. 2. Top panel).
On the T2-weighted images of 1 week post-PDT, in three of five dogs, there were focal areas of increased signal with defined margins corresponding to regions of necrosis (Zone 1) (Figs. 2C and 3C). Peri-necrotic inflammation, edema, and atrophy (Zone 2) exhibited both low signal and high signal (Figs. 2C and 3C). In two of five prostates, there was inhomogeneous T2 signal within regions of necrosis (Figs. 3C and 4C) making precise delineation of necrosis difficult. In one of five prostates, the region of necrosis was extremely poorly marginated despite clearly defined necrotic areas on pathologic examination (Fig. 4C). Thus treatment boundaries could not be completely and accurately defined by T2 signal in two of five prostates. On the T2-weighted images of 1 month post-PDT, the prostate gland was irregular and smaller compared to that of 7 days (Fig. 5B,D). The cystic areas became very well defined.
Fig. 3.
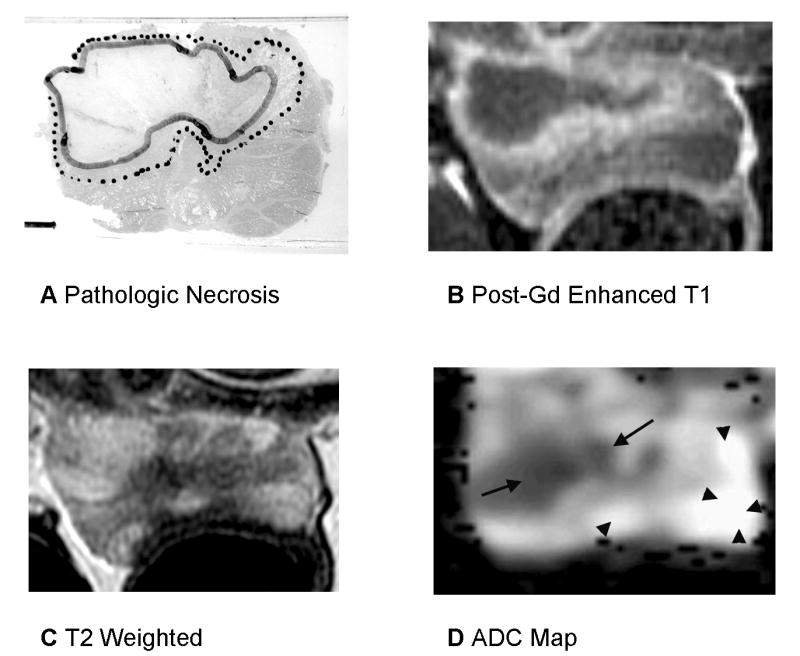
Prostate 7 days after WST-09 PDT with heterogenous signal changes on T2-weighted imaging. A: Pathologic slide (Dog #2, the left lobe received 50 J/cm and the right lobe 200 J/cm). Similar histopathological findings as described in the Figure 2. Images are displayed with the dog’s anatomic left on the right side of the image. B: Post-gadolinium-enhanced T1-weighted images shows Zone 1 as dark non-enhancing tissue and Zone T2 as a thin high signal intensity rim. There is compression of the prostate by stool in the recum possibly resulting in a smaller measured area of necrosis. C: On T2-weighted images, the region of necrosis shows increased signal with a dark rim corresponding to Zone 2 however regions of decreased signal are seen within the necrotic area on the left side. D: ADC map shows marked decrease in ADC in regions of necrosis (Zone 1) however the anterior margin on the right is irregular and does not correspond to the histologic necrosis contour (same scale as the Fig. 2).
On DWIs, there was a significant decrease in the ADC (P<0.0001) in regions of necrosis from 2,692 × 10−6 mm2/second (mean value of pre-PDT ADC, eight measurements from five animals) to 1,059 × 10−6 mm2/second (mean value of 7 days post-PDT ADC, five measurements from five animals) (Fig. 6). Treated areas could be defined with areas of necrosis clearly seen in three of five prostates however in one case (Fig. 3D), there was spatial distortion of the image from susceptibility artifact related to the use of an echo planar imaging technique for the DW imaging; in two prostates, there was poor delineation of intra versus extraprostatic treatment effect (Figs. 3D and 4D) and in one case, there was a heterogenous drop in ADC within a necrotic region making precise delineation difficult (Fig. 4D). Thus although marked ADC changes were seen in treated areas, a complete and accurate definition of treatment boundaries was not possible in two of five prostates.
Fig. 6.
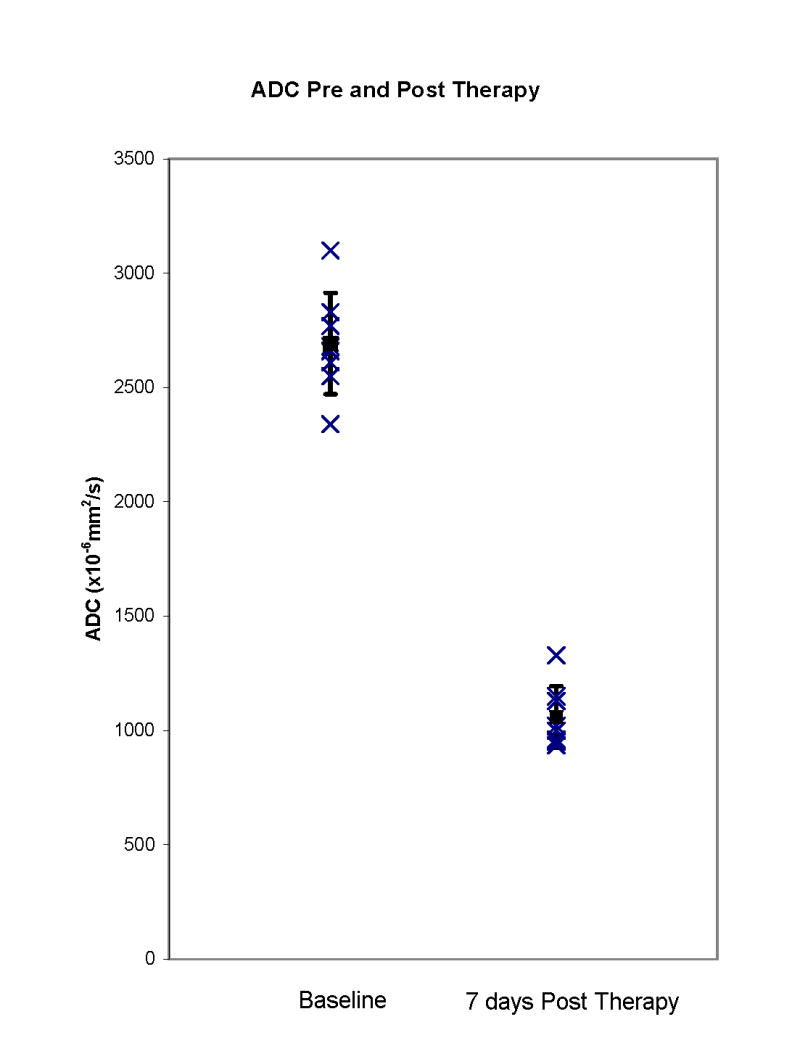
ADC in regions of necrosis pre- and post-Tookad-PDT. There is a significant decrease (P<0.001) in ADC in regions of necrosis 7 days post-PDT.
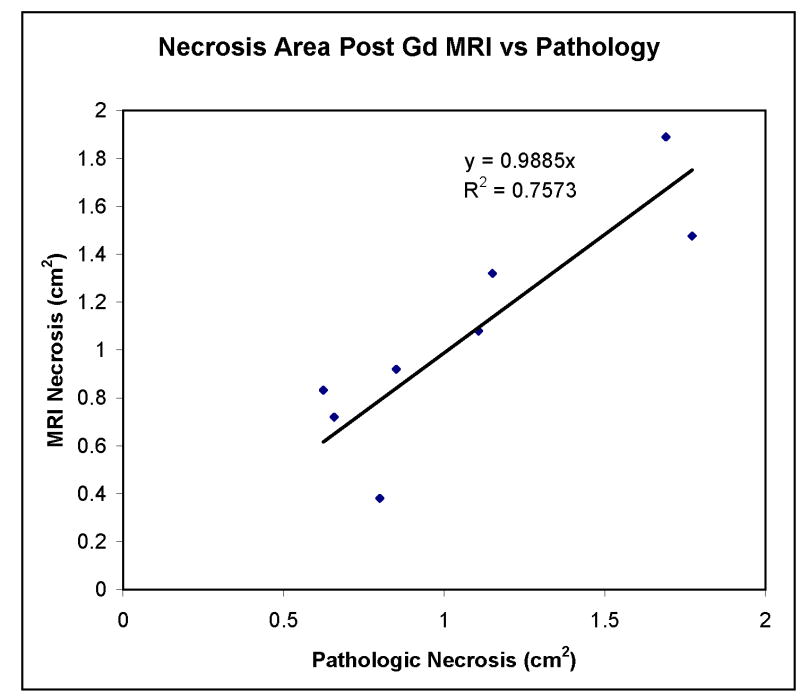
In all prostates, on post-gadolinium contrast-enhanced images of 7 days post-PDT, there were areas showing no enhancement corresponding to pathologic areas of necrosis (Zone 1) (Figs. 2B, 3B, 4B, and 5A). Margins were well defined in all cases along the entire circumference of necrosis. Outside of this zone, a second zone of increased enhancement was identified corresponding to pathologic Zone 2 changes (Figs. 2B, 3B, and 4B). Varying degrees of intra and extraprostatic faint high T1 signal were identified prior to contrast enhancement indicative of areas of hemorrhage within necrotic regions or periprostatic hemorrhage. Periprostatic enhancement related to post-surgical inflammation was also seen. There was a good correlation between areas of necrosis defined by post-contrast-enhanced MRI and pathologic section with a linear slope of 0.99 and a correlation coefficient of 0.75 (Fig. 7). There was variability in the area measurements. Some of this is likely related to distortion of the specimen from the in vivo state. The dog prostate was noted to be quite deformable in vivo. Its shape was easily altered by stool in the rectum or periprostatic hemorrhage (Fig. 3). On post-gadolinium contrast-enhanced images of 1 month post-PDT, there were areas showing no enhancement with shrinkage compared to that of 7 days (Fig. 5A,C) which corresponded to cysts and other defects in the gross specimen (Fig. 5E).
Fig. 7.
Necrosis area as measured on post-gadolinium-enhanced MRI versus histopathologic evaluation. There is a good correspondence between measured areas based on MRI and pathology in each of the eight prostate halves at 7 days post-PDT.
DISCUSSION
For many anticancer therapies, it is desirable to accurately monitor and quantify early tumor response and to determine the effectiveness of the treatment regimen. In this study, we evaluated MRI for monitoring early response of prostate gland to PDT.
Tookad-PDT produces cytotoxic reactive oxygen species which lead to vascular occlusion, stasis, and resultant necrosis [31,32]. MRI offers a variety of methods for assessing these treatment responses. For example, T2-weighted imaging is sensitive to alterations in tissue water content, DW imaging is sensitive to altered diffusion of mobile extracellular water, and contrast-enhanced imaging may be sensitive to alterations in tissue perfusion. DWI is a very sensitive technique to determine water mobility changes and is useful for an accurate assessment of acute lesion caused by chemotherapy and cryosurgery [33,34]. Our previous study demonstrated that PDT-induced acute lesions were characterized by marked hemorrhagic necrosis and severe edema. Alterations in tissue perfusion can be identified by blood perfusion CT scan [26]. These lesions also involve significant cell and/or tissue water mobility changes and might be detectable by DWI. Given the mechanism of action of PDT photosensitizers such as Tookad, one might expect alterations in all of these parameters in the treated area based on increased water content from necrosis and edema and vascular occlusion.
Post-contrast-enhanced MRI was the most representative method of delineating regions of necrosis in these canine prostates. The correlation between cross sectional necrosis area on MRI versus pathology, still have some variability, however. We noted deformability of the prostate in vivo and tissue deformation during the removal and pathologic processing of the prostate a likely contributor to this variability. This is notable in one case where periprostatic hemorrhage indented the anterior prostatic margin resulting in a smaller necrosis volume in vivo. In some cases, the whole prostate could not be placed on a single histologic slide. Large whole mount processing of the specimens may have reduced variability in measurement. The peri-necrotic changes (Zone 2) were seen as areas of increased enhancement. This may be related to increased permeability and hyperemia in the peri-necrotic regions related to the host-response. This helped in delineating the treated areas on post-gadolinium-enhanced images.
The ADC calculated from DWI was markedly lower in regions of necrosis. This is counterintuitive as one might expect cell loss and more water diffusion in these areas and thus a higher ADC. Lowering of ADC has been noted in prostate tissue immediately after cryosurgery [34]. As with Tookad-PDT, hemorrhage was also noted in treated regions after cryosurgery. The explanation for reduced ADC 1 week after Tooakd-PDT is uncertain but it is interesting to note that hemorrhagic necrosis is a feature of both forms of treatment. Plaks et al. have described a biphasic ADC response in cell models of prostate carcinoma with an initial drop within hours of Tookad-PDT followed by a subsequent rise [28]. We have not evaluated the prostate within hours of treatment in this study. Delayed ADC changes many days after treatment may be different as well as the response in canine prostate to PDT as compared to the cellular models used by Plaks et al. It is likely that the timing of post-therapy imaging will be an important factor in the usefulness of DWI in monitoring therapeutic response.
Margins of necrosis were not always easy to define as the lowering of ADC was not limited to the prostate along some margins. ADC lowering was inhomogeneous in other regions making it difficult to assess boundaries. DWI used in this experiment was based on an echo planar imaging pulse sequence which can deliver images which suffer from spatial distortion from susceptibility artifact. This will be most severe if there is air in the rectum as was the case in one of the treated animals. Alternatively spin echo-based pulse sequences that perform DWI are described [35], but not implemented fully on commercial systems. For these reasons, DWI was not the best sequence for assessing PDT effect although it showed promise regarding specificity to alterations in mobile tissue water. Spatial distortion was a limitation of the DWI method used in this study. This spatial distortion is related to the echo planar imaging (EPI) methodology employed to generate the MRI images in most commercial DWI pulse sequences. It is possible that the quality of DW imaging could be improved. For example, spatial distortion could be reduced by use of parallel imaging techniques and higher bandwidths that would reduce the length of the EPI readout [36]. Newer pulse sequence not yet commercially available for use in the prostate that use spin echo readouts would also be promising alternative methods to generating DWI images in the prostate and may provide better results in the future [37].
Finally, T2-weighted imaging alone exhibited variable signal changes. In some but not all cases, necrosis exhibited elevated signal intensity while the periphery of the treated region had a dark rim. These variable findings suggested that T2-weighted imaging was not well suited for assessment of treatment response.
We also noticed that there was a reduction in size of lesions between 2 days post-and 7 days post-therapy (n = 1, data not shown) suggesting MR imaging at 2 days may be more sensitive for detecting post-PDT treatment effect. At 1 month, the histopathologic changes included fibrosis of the necrotic tissue, atrophy, and small areas of inflammation. It was hard to correlate MR images to these changes because the progressive healing led to a more non-uniform mixture of signal intensity and demarcations in contrast enhancement. MR imaging at 1 month might not provide a clear understanding of treatment effects because multiple findings which may be manifestations of treatment or healing were taking place.
CONCLUSION
Post-gadolinium contrast-enhanced MRI is well suited to assessing regions of necrosis within the prostate after Tookad-PDT. At 7 days after PDT, gadolinium DTPA contrast-enhanced MRI is superior to DWI and T2 imaging in assessing the boundary of Tookad-PDT-induced tissue necrosis in the normal canine prostate model. Margins of pathology could not be denned as well with DWI but still has promise for assessing effects of PDT-induced necrosis on water diffusibility.
Acknowledgments
Authors are grateful to David Luck, Melinda Wilhelm, Don Maul, Jill Beckers, and Elisa French for their technical assistance.
Footnotes
Contract grant sponsor: Negma-Lerads and STEBA BIOTECH, France; Contract grant sponsor: NIH; Contract grant number: PO1-CA43892 (PI: FWH).
F.W.H. has disclosed a potential financial conflict of interest with this study.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Troyer DA, Mubiru J, Leach RJ, Naylor SL. Promise and challenge: Markers of prostate cancer detection, diagnosis and prognosis. Dis Markers. 2004;20:117–128. doi: 10.1155/2004/509276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quaranta BP, Marks LB, Anscher MS. Comparing radical prostatectomy and brachytherapy for localized prostate cancer. Oncology (Williston Park) 2004;18:1289–1302. [PubMed] [Google Scholar]
- 4.Beerlage HP. Alternative therapies for localized prostate cancer. Curr Urol Rep. 2003;4:216–220. doi: 10.1007/s11934-003-0072-5. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treatment. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muschter R. Photodynamic therapy: A new approach to prostate cancer. Curr Urol Rep. 2003;4:221–228. doi: 10.1007/s11934-003-0073-4. [DOI] [PubMed] [Google Scholar]
- 8.Martin NE, Hahn SM. Interstitial photodynamic therapy for prostate cancer: A developing modality. Photodiag Photodyn Therapy. 2004;1:123–136. doi: 10.1016/S1572-1000(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 9.Pinthus JH, Bogaards A, Weersink R, Wilson BC, Trachtenberg J. Photodynamic therapy for urological malignancies: Past to current approaches. J Urol. 2006;175:1201–1207. doi: 10.1016/S0022-5347(05)00701-9. [DOI] [PubMed] [Google Scholar]
- 10.Whitehurst C, Pantelides ML, Moore JV, Blacklock NJ. Optimization of multifiber light delivery for the photodynamic therapy of localized prostate cancer. Photochem Photobiol. 1993;58:589–593. doi: 10.1111/j.1751-1097.1993.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 11.Nathan TR, Whitelaw DE, Chang SC, Lees WR, Ripley PM, Payne H, Jones L, Parkinson MC, Emberton M, Gillams AR, Mundy AR, Bown SG. Photodynamic therapy for prostate cancer recurrence after radiotherapy: A Phase I study. J Urol. 2002;168:1427–1432. doi: 10.1016/S0022-5347(05)64466-7. [DOI] [PubMed] [Google Scholar]
- 12.Zaak D, Sroka R, Höppner M, Khoder W, Reich O, Tritschler S, Muschter R, Knüchel R, Hofstetter A. Photodynamic therapy by means of 5-ALA induced PPIX in human prostate cancer–Preliminary results. Med Laser Appl. 2003;18:91–95. [Google Scholar]
- 13.Stripp DCH, Mick R, Zhu TC, Whittington R, Smith D, Dimofte A, Finlay J, Miles J, Busch TM, Shin1 D, Kachur A, Tochner ZA, Malkowicz SB, Glatstein E, Hahn SM. Phase I trial of motexafin lutetium-mediated interstitial photodynamic therapy in patients with locally recurrent prostate cancer. Proc SPIE. 2004;5315:88–99. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.230. [DOI] [PubMed] [Google Scholar]
- 14.Gertner MR, Bogaards A, Weersink RA, McCluskey SA, Haider MA, Yue CKK, Savard J, Simpson S, Brun PH, Cohen P, Scherz A, Salomon Y, Aprikian AG, Elhilali MM, Wilson BC, Trachtenberg J. Initial results of a phase I/II trial of WST09-mediated photodynamic therapy (WST09-PDT) for recurrent prostate cancer following failed external beam radiation therapy (EBRT) Eur Urol Suppl. 2004;3:212. [Google Scholar]
- 15.Weersink RA, Bogaards A, Gertner M, Davidson SR, Zhang K, Netchev G, Trachtenberg J, Wilson BC. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: Clinical experience and practicalities. J Photochem Photobiol B. 2005;79:211–222. doi: 10.1016/j.jphotobiol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Sumi S, Arai K, Yoshida K. Separation methods applicable to prostate cancer diagnosis and monitoring therapy. J Chromatogr B Biomed Sci Appl. 2001;764:445–455. doi: 10.1016/s0378-4347(01)00245-6. [DOI] [PubMed] [Google Scholar]
- 17.Gersing E, Kelleher DK, Vaupel P. Tumour tissue monitoring during photodynamic and hyperthermic treatment using bioimpedance spectroscopy. Physiol Meas. 2003;24:625–637. doi: 10.1088/0967-3334/24/2/370. [DOI] [PubMed] [Google Scholar]
- 18.Eckersley RJ, Sedelaar JP, Blomley MJ, Wijkstra H, deSouza NM, Cosgrove DO, de la Rosette JJ. Quantitative microbubble enhanced transrectral ultrasound as a tool for monitoring hormonal treatment of prostate carcinoma. Prostate. 2002;51:256–267. doi: 10.1002/pros.10065. [DOI] [PubMed] [Google Scholar]
- 19.Oyama N, Akino H, Suzuki Y, Kanamaru H, Ishida H, Tanase K, Sadato N, Yonekura Y, Okada K. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Berard V, Lecomte R, van Lier JE. Positron emission tomography imaging of tumor response after photodynamic therapy. J Environ Pathol Toxicol Oncol. 2006;25:239–250. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 21.Larson BT, Collins JM, Huidobro C, Corica A, Vallejo S, Bostwick DG. Gadolinium-enhanced MRI in the evaluation of minimally invasive treatments of the prostate: Correlation with histopathologic findings. Urology. 2003;62:900–904. doi: 10.1016/s0090-4295(03)00586-7. [DOI] [PubMed] [Google Scholar]
- 22.Pucar D, Shukla-Dave A, Hricak H, Moskowitz CS, Kuroiwa K, Olgac S, Ebora LE, Scardino PT, Koutcher JA, Zakian KL. Prostate cancer: Correlation of MR imaging and MR spectroscopy with pathologic findings after radiation therapy-initial experience. Radiology. 2005;236:545–553. doi: 10.1148/radiol.2362040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taussky D, Austen L, Toi A, Yeung I, Williams T, Pearson S, McLean M, Pond G, Crook J. Sequential evaluation of prostate edema after permanent seed prostate brachytherapy using CT-MRI fusion. Int J Radiat Oncol Biol Phys. 2005;62:974–980. doi: 10.1016/j.ijrobp.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Huang Z, Luck D, Beckers J, Brun P, Wilson BC, Scherz A, Salomon Y, Hetzel FW. Preclinical studies in normal canine prostate of a novel palladium-bacteriopheophorhide (WST09) photosensitzer for photodynamic therapy of prostate. Photochem Photobiol. 2002;76:438–445. doi: 10.1562/0031-8655(2002)076<0438:PSINCP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Chen Q, Trncic N, LaRue SM, Brun P, Wilson BC, Hetzel FW. Effects of Pd-bacteriopheophorbide (TOOKAD) mediated photodynamic therapy on canine prostate pre-treated with ionizing radiation. Rad Res. 2004;161:723–731. doi: 10.1667/rr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z, Chen Q, Luck D, Beckers J, Wilson BC, Trncic N, LaRue SM, Blanc D, Hetzel FW. Studies of a vascular-acting photosensitizer, Pd-bacteriopheophorbide (Tookad), in normal canine prostate and spontaneous canine prostate cancer. Lasers Surg Med. 2005;36:390–397. doi: 10.1002/lsm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rück A, Böhmler A, Steiner R. PDT with TOOKAD® studied in the chorioallantoic membrane of fertilized eggs. Photodiag Photodyn Therapy. 2005;2:79–90. doi: 10.1016/S1572-1000(05)00006-2. [DOI] [PubMed] [Google Scholar]
- 28.Plaks V, Koudinova N, Nevo U, Pinthus JH, Kanety H, Eshhar Z, Ramon J, Scherz A, Neeman M, Salomon Y. Photodynamic therapy of established prostatic adenocarcinoma with TOOKAD: A biphasic apparent diffusion coefficient change as potential early MRI response marker. Neoplasia. 2004;6:224–233. doi: 10.1593/neo.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haider M, Trachtenberg J, Wilson BC, Huang Z, Kraft S, El Hilali M. MRI Assessment of photodynamic therapy of the prostate: Initial experience in a Phase I/II trial. http://rsna2003.rsna.org/rsna2003/VBK/conference/event_display.cfm?em_id=3103633.
- 30.Huang Z, Chen Q, Luck D, Beckers J, Blanc D, Hetzel FW. Tookad-mediated photodynamic effects on the prostate and its adjacent tissues—In vivo study in canine models. Proc of SPIE. 2005;5689:106–111. [Google Scholar]
- 31.Vakrat-Haglili Y, Weiner L, Brumfeld V, Brandis A, Salomon Y, McLlroy B, Wilson BC, Pawlak A, Rozanowska M, Sarna T, Scherz A. The Microenvironment effect on the generation of reactive oxygen species by Pd-bacteriopheophorbide. J Am Chem Soc. 2005;127:6487–6497. doi: 10.1021/ja046210j. [DOI] [PubMed] [Google Scholar]
- 32.Borle F, Radu A, Monnier P, van den Bergh H, Wagnieres G. Evaluation of the photosensitizer Tookad for photodynamic therapy on the Syrian golden hamster cheek pouch model: Light dose, drug dose and drug–light interval effects. Photochem Photobiol. 2003;78:377–383. doi: 10.1562/0031-8655(2003)078<0377:eotptf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Jennings D, Hatton BN, Guo J, Galons JP, Trouard TP, Raghunand N, Marshall J, Gillies RJ. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI. Neoplasia. 2002;4:255–262. doi: 10.1038/sj.neo.7900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butts K, Daniel BL, Chen L, Bouley DM, Wansapura J, Maier SE, Dumoulin C, Watkins R. Diffusion-weighted MRI after cryosurgery of the canine prostate. J Magn Reson Imaging. 2003;17:131–135. doi: 10.1002/jmri.10227. [DOI] [PubMed] [Google Scholar]
- 35.Chan I, Wells W, 3rd, Mulkern RV, Haker S, Zhang J, Zou KH, Maier SE, Tempany CM. Detection of prostate cancer by integration of line-scan diffusion, T2-mapping and T2-weighted magnetic resonance imaging; a multichannel statistical classifier. Med Phys. 2003;30:2390–2398. doi: 10.1118/1.1593633. [DOI] [PubMed] [Google Scholar]
- 36.Taouli B, Martin AJ, Qayyum A, Merriman RB, Vigneron D, Yeh BM, Coakley FV. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: Preliminary experience in healthy volunteers. Am J Roentgenol. 2004;183:677–680. doi: 10.2214/ajr.183.3.1830677. [DOI] [PubMed] [Google Scholar]
- 37.Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002;47:42–52. doi: 10.1002/mrm.10014. [DOI] [PubMed] [Google Scholar]