Abstract
The pathogenesis of hepatitis C virus (HCV)-associated liver injury involves many genes from multiple pathogenic pathways. cDNA array analysis, which examines the expression of many genes simultaneously, was used to achieve new insights into HCV liver injury. Membrane-based cDNA arrays of 874 genes compared HCV-associated cirrhosis with autoimmune hepatitis-associated cirrhosis as an inflammatory and cirrhotic control, and with nondiseased liver tissue. Array analysis identified many differentially expressed genes that are important in inflammation, fibrosis, proliferation, signaling, apoptosis, and oxidative stress. Genes up-regulated in HCV-associated cirrhosis were predominantly associated with a Th1 immune response, fibrosis, cellular proliferation, and apoptosis. Novel observations of differential gene expression included increased expression of secreted apoptosis-related protein 3, a Wnt pathway gene possibly involved in cellular apoptosis. EMMPRIN (CD147) and discoidin domain receptor 1 (CD167) were also shown to be increased and are likely to play a role in liver fibrosis. Real-time quantitative reverse transcriptase-polymerase chain reaction confirmed the increased expression of 15 genes. The comparison of HCV cirrhosis with autoimmune hepatitis cirrhosis showed a marked difference in the apoptosis-associated gene profile with HCV cirrhosis characterized by increased proapoptotic gene expression whereas autoimmune hepatitis was characterized by increased expression of both antiapoptotic and proapoptotic genes. Furthermore, expressionof β-catenin and the fibrosis-associated protein EMMPRIN were localized by immunohistochemistry to the plasma membranes of hepatocytes and biliary epithelium. In conclusion, HCV-associated cirrhosis was characterized by a proinflammatory, profibrotic, and proapoptotic gene expression profile.
Investigation into the pathogenesis of hepatitis C virus (HCV)-related liver injury has focused on the virus itself, the inflammatory response, the specific immune response, and the role of the hepatic stellate cell (HSC) in fibrosis. 1,2 Hepatic inflammation in chronic HCV is characterized by a persistent intrahepatic Th1 immune response with ongoing viral replication. 3,4 Th1-associated intrahepatic cytokines such as interleukin (IL)-2, interferon-γ (IFN-γ), IL-18, and tumor necrosis factor (TNF) are up-regulated in chronic HCV infection. 3,5,6 Furthermore, chemokines and receptors that promote the accumulation of Th1 cells, such as CXC chemokine receptor (CXCR)-3, its ligands CXCL10 (IFN-γ-induced protein; IP-10), CXCL9 (IFN-γ-induced monokine; HuMIG) and CC chemokine receptor 5 and its ligands CCL5 (regulated on activation normal T-cell expressed and secreted; RANTES), and CCL3 and CCL4 (macrophage inflammatory protein-1α and -1β) are selectively increased in chronic HCV disease. 7-9 The specific immune response in HCV has been closely studied and shows diminished anti-HCV-specific CD4+ and CD8+ responses in chronic versus acute HCV infection. 1,10 Although intrahepatic HCV-specific cytotoxic T cells are readily detected in chronic HCV infection only 1 to 2% of the CD8+ hepatic infiltrate is HCV-specific. 11 Therefore, in chronic HCV infection the Th1 response is insufficient to clear the virus and is associated with a predominantly nonspecific chronic inflammatory response resulting in persistent liver injury. 1,10,11
In HCV liver injury this inflammatory response is associated in many individuals with progressive tissue fibrosis, the development of cirrhosis, and finally hepatocellular cancer (HCC). Some data suggests that certain HCV proteins when overexpressed in vivo or in vitro may be oncogenic. 12 However, it is likely that the development of HCV-associated cancer is related to the persistence of chronic unrelenting intrahepatic inflammation with an attendant proliferative response and is not directly because of HCV. 13,14 This contention is supported by the observation that HCV-associated HCC without previous cirrhosis is rare, unlike the situation in chronic HBV infection. 13 Furthermore, HCV-associated liver injury is characterized by increased hepatocyte proliferation and cell turnover. 15,16 Associated with the increased cellular proliferation there is greater expression of proapoptotic genes such as Fas in HCV-related liver injury. 17,18 Fibrosis in chronic HCV infection is characterized by HSC activation by transforming growth factor-β (TGF-β) and other inflammatory mediators with increased connective tissue growth factor (CTGF) production and attendant increased matrix turnover, collagen deposition, up-regulation of matrix metalloproteinases (MMPs), 19 and expression of the gelatinase fibroblast activation protein. 20
cDNA array analysis is a powerful descriptive method of examining the expression profile of hundreds to thousands of genes in unison. Three types of arrays are commonly available: 1) nylon membrane arrays using radiolabeled probes (32P or 33P), 2) glass slide microarrays using dual fluorophore-labeled probes, and 3) DNA chip technology with oligonucleotides fixed to a silica substrate. 21 Although both microarray technology and DNA chip technology are powerful techniques their widespread use is limited by cost, availability of equipment, and expertise. 21,22 In contrast, using cDNA arrays on nylon membranes with radiolabeled probes is a more readily accessible technology. 23-25
Two reports profile gene expression in chronic human liver disease using cDNA array analysis; our own membrane cDNA array analysis comparing primary biliary cirrhosis (PBC) to primary sclerosing cholangitis (PSC) cirrhosis 26 and a microarray comparison of chronic HCV with chronic HBV liver injury. 27 It is unclear whether the dissimilar experimental strategies and array methodologies adopted give equivalent results. The experimental strategy that we adopted utilizes cDNA array analysis as an initial approach to profile gene expression, which is followed by confirmation, in individual patient samples, of differential expression by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR). Further, differential gene expression is categorized with pathogenic processes, enabling the identification of gene groups or pathways that may be important in liver pathobiology.
The aim of this report was to use cDNA array analysis to examine intrahepatic differential gene expression in HCV-associated cirrhosis. This article describes multiple novel observations of differentially expressed genes in the linked pathogenic processes of inflammation, regeneration, apoptosis, and fibrosis. The data provides further evidence for a persistent Th1-associated inflammatory response. In addition, a striking difference was seen in the expression of apoptosis-related genes in HCV-associated cirrhosis compared to autoimmune hepatitis (AIH)-associated cirrhosis.
Materials and Methods
Tissue and RNA Isolation
Total RNA was isolated from end-stage cirrhotic HCV (n = 6; 4 males and 2 females; mean age, 50 years) and end-stage cirrhotic AIH (n = 4; 4 females; mean age, 23 years) tissue obtained from liver explants. Nondiseased tissue was obtained from transplant donor liver biopsies and from normal tissue obtained during hepatic metastasis resection (n = 8; 4 males and 4 females; mean age, 39 years). The mean age of all of the patients at time of specimen collection was 39 years. All nondiseased tissue was obtained from patients who were noncirrhotic whereas all of the cirrhotic tissue was from patients who were Child-Pugh class C. Chronic lobular inflammatory activity, piecemeal necrosis, and end-stage cirrhosis was evident in all HCV- and AIH-associated cirrhosis specimens. Tissue was obtained following institutional ethics committee approval and Australian Medical Research Council guidelines. Total RNA and poly A+ mRNA were isolated as previously described. 26
cDNA Array Analysis
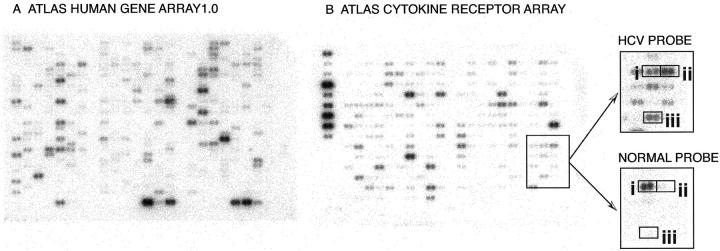
Two nylon membrane-based cDNA arrays were used: the ATLAS Human Gene Array 1.0 (588 genes and 9 housekeeping genes) and the ATLAS Cytokine/Receptor Array (268 genes and 9 housekeeping genes) (Clontech Laboratories, Inc., Palo Alto, CA) (Figure 1) ▶ . A list of all of the genes on these two arrays with summaries of gene functions is at http://www.clontech.com/atlas.
Figure 1.
Examples of cDNA arrays. ATLAS Human Gene Array 1.0 (597 genes) (A) and ATLAS Cytokine Receptor Array (277 genes) (B), both probed with 32P-dCTP-labeled HCV mRNA (pooled from four patients). The magnified portion of the ATLAS Cytokine Receptor Array compares the 32P-dCTP signal from the HCV-associated cirrhosis and normal liver probes. Differences in signal intensity could be discerned, even without ubiquitin housekeeping gene adjustment, by using hepatocyte growth factor activator (i) as a reference. This analysis produced differential expression ratios of twofold in DDR1 (CD167) (ii) and 4.6-fold in CXCR4 (iii).
Probes from end-stage cirrhotic HCV, end-stage cirrhotic AIH, and donor liver and normal liver tissues were hybridized once to the ATLAS Cytokine Array and once to the ATLAS Human Gene Array. The probe in each experiment was made by first pooling equal amounts of total RNA from four individuals in each group and then isolating polyA+ mRNA. The pooled polyA+ mRNA was subsequently labeled using the appropriate CDS primer mix (Clontech Laboratories, Palo Alto, CA) with 32P-dCTP and hybridized to the membranes as described previously. 26 The pooling of samples is a means of normalizing for individual differences in array analysis. 28 Therefore, the array results presented represent a single hybridization from pooled patient samples. Probe synthesis, subsequent hybridization, and analysis of the expression data were performed as previously described. 26 Low abundance signals were defined as signals less than twice background and represented up to 61% of the signals on the arrays used. Given the use of 32P-labeled probes to maximize sensitivity 90 signals were excluded from subsequent analysis because of blooming or membrane contamination. The housekeeping gene used for normalizing data was ubiquitin. 26
Quantitative RT-PCR
The differential expression of select genes identified after cDNA array analysis was confirmed by quantitative real-time RT-PCR. Briefly, the mRNA expression of these genes was measured separately in six individuals with HCV-associated cirrhosis and four nondiseased liver tissue samples in duplicate on two different cDNA samples as previously described. 26,29 The genes assayed were: helix-loop-helix protein 1R21, transducer of Erb B2 (TOB), CTGF, extracellular MMP inducer (EMMPRIN, CD147), stromal cell-derived factor 1 receptor (CXCR4), CDC-like kinase 2 (CLK2), discoidin domain receptor (DDR)-1, ephrin A3, secreted apoptosis related protein 3 (SARP3), secreted frizzled related protein 3 (FRITZ), β-catenin, neuromodulin (GAP43), glial cell-derived neurotrophic factor (GDNF), neogenin, and brain-derived neurotrophic factor (BDNF).
The primer sequences were: CTGF forward 5′ TCCCACCCAATTCAAAAC 3′, reverse 5′ CAAAATAGCAGGCATATTACTCG3′; TOB forward 5′ AACAAGCATCCAAAGAGAG 3′, reverse 5′ TTACAGCAGCAGAGTGACC 3′; helix-loop-helix 1R21 forward 5′ ACCCCCAAGTTCTAAGGTC 3′, reverse 5′TCGCATTGTTACAGAAAGTC 3′; CXCR4 forward 5′ TCAGTGAGGCAGATGACAG 3′ and reverse 5′ TCCCAATGTAGTAAGGCAG 3′; SARP3 forward 5′ CAGTGTGAGATGGAG-CACAG 3′, reverse 5′ GTAGAAGAAAGGGTAGTAGAGG 3′; EMMPRIN forward 5′ ACAAGATCACTGACTCTGAGGAC 3′, reverse 5′ TTC-TCAATGTGTAGCTCTGACC 3′; β-catenin forward 5′ CATTACAACTCTCCACAACC 3′, reverse 5′ CAGATAGCACCTTCAGCAC 3′; FRITZ forward 5′ CAGTAGTGGAGGTGAAGGAG 3′, reverse 5′ GAGTCCAAGATGACGAAG 3′; ubiquitin forward 5′ GTTGATCTTTGCTGGAAAAC 3′, reverse 5′ AATGCCTTCCTTGTCCTG 3′; CLK2 forward 5′ AAAGCATAAGCGACGAAG 3′, reverse 5′ TTCATAGGAATGCCGATAG 3′; DDR1 forward 5′ TTTCCCTCGATCTCGACTCC 3′, reverse 5′ GAGCCTCGACATGATCTTCAC 3′; ephrin A3 forward 5′ GGCCACGAGTACTACTACATCTCC 3′, reverse 5′ CAGCAGACGAACACCTTCATC 3′; GAP43 forward 5′ TGACGACCAAAAGATTGAAC 3′; reverse 5′ TTCTCCCTTCTTCTCCACC 3′; GDNF forward 5′ CACCAGATAAACAAATGGCAG 3′, reverse 5′ TTCATAGCCCAGACCCAAG 3′; neogenin forward 5′ CATGAGAGACTGGAGCTGAAAC 3′, reverse 5′ TGGAAAT-GATGGTGAGGG 3′; and BDNF forward 5′ TAAAGTGGGAAGAAGGAG 3′, reverse 5′ TAGGGAGA-AAGCA-GAAAC 3′.
Immunohistochemistry
Liver tissue was stored at −70°C, cut in a cryostat at −18°C and fixed in 100% acetone at −20°C. Sections were blocked with 0.5% azide, 1% bovine serum albumin, and 1% normal rabbit serum for 20 minutes before application of the monoclonal primary antibody [β-catenin C19220 (Transduction Laboratories, Lexington, KY), EMMPRIN MEM-M6/1 30 ] at 10 μg/ml for 2 hours. The secondary antibody, a 1:50 rabbit anti-mouse horseradish peroxidase (P0240; DAKO, Carpinteria, CA), was applied for 45 minutes. The peroxidase activity was visualized using NovaRED (Vector Laboratories, Burlingame, CA). Tissue was then counterstained in 0.1% Mayer’s hematoxylin (Amber Scientific, Belmont, WA) and mounted in Eukitt (O. Kindler GmBH and Co., Freiburg, Germany).
Statistical Analysis of Gene Array Data
Results are expressed as means ± SE. Statistical comparisons were performed using a nonparametric Mann-Whitney U test. Statistical analysis used Statview 4.5.1 (Abacus Concepts, Berkley, CA) and regression plots were generated in KaleideGraph 3.0 (Synergy Software, Reading, PA).
Results
Gene Profiling of HCV-Associated Cirrhosis
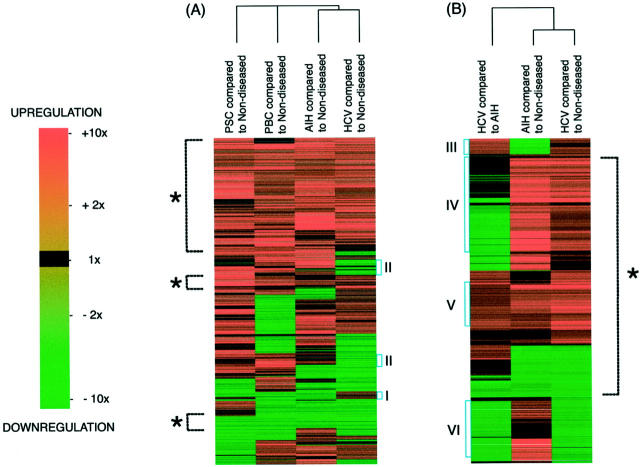
The clustering of genes based on their relative expression (Figure 2) ▶ indicated gene groups that were similarly up-regulated or down-regulated. In addition, it was a rapid screen for gene groups of interest. This clustering analysis revealed that ∼50% of genes had a similar pattern of expression in HCV-, AIH-, and PSC-associated cirrhosis and PBC compared to nondiseased tissue (Figure 2A) ▶ . Similarly, in the two hepatitic diseases HCV-associated cirrhosis and AIH-associated cirrhosis >70% of genes had similar patterns of expression compared to nondiseased tissue (Figure 2B) ▶ . Although the patterns of gene expression were similar there were differences in the degree of differential gene expression in HCV- and AIH-associated cirrhosis when compared to nondiseased tissue (Figure 2B) ▶ . All differential expression data are depicted by color intensity in the supplemental material clustering figure and the dendrogram on that figure is labeled with each gene name.
Figure 2.
Clustering of the 874 genes. Each line across all comparisons represents a single gene with up-regulation indicated in increasing red and down-regulation indicated in increasing green. Genes were clustered according to the nature and extent of their differential expression; the four comparisons of HCV cirrhosis, AIH cirrhosis, PBC cirrhosis, and PSC cirrhosis to nondiseased tissue (A) and HCV cirrhosis and AIH cirrhosis compared to each other as well as to nondiseased tissue (B). There was ∼50% similarity expression in the various forms of cirrhosis in (A) and >70% similarity in the patterns of expression in hepatitic HCV- and AIH-associated cirrhosis compared to nondiseased tissue (B, indicated in both instances by the dashed line with an asterisk). This graphical depiction of the data identifies groups of genes, such as those that appear uniquely expressed in HCV compared to other forms of cirrhosis with either increased (I) or decreased (II) expression compared to nondiseased tissue. Further, increased gene expression in hepatitic cirrhosis (B) showed genes with increased expression in HCV-associated cirrhosis compared to both nondiseased tissue and AIH-associated cirrhosis (III), both HCV- and AIH-associated cirrhosis compared to nondiseased liver tissue (IV and V), HCV-associated cirrhosis compared to AIH-associated cirrhosis in addition to both of these diseases compared to nondiseased liver tissue (V), and AIH- but not HCV-associated cirrhosis compared to nondiseased tissue (VI).
Differential Expression in HCV-Associated Cirrhosis
Of the 20 most up-regulated genes (Table 1) ▶ , the expression of CTGF, TOB, SARP3, cytokine receptor EB13, and cyclin-dependent kinase inhibitor 1C (CDKN1C) p57KIP2 were greater in HCV-associated cirrhosis compared to either nondiseased liver tissue (Table 1A) ▶ or AIH-associated cirrhosis (Table 1B) ▶ .
Table 1.
The Twenty Most Up-Regulated Genes
| Ratio of gene up-regulation by array | |
|---|---|
| (A) The twenty most up-regulated genes in HCV cirrhosis compared to nondiseased tissue | |
| Calcium/calmodulin dependant protein kinase type IV catalytic subunit | 4.6 |
| Cyclin-dependent kinase inhibitor 1C (CDKN1C) p57-KIP2 | 3.6* |
| Cellular retinoic acid-binding protein II | 5.2 |
| Connective tissue growth factor (CTGF) | 17.4* |
| Cytokine receptor EBI3 | 3.9* |
| CXCL8 (IL-8) | 4.7 |
| CXCL10 (IP-10) | 4.0 |
| Dihydropyridine-sensitive L-type calcium channel beta-3 subunit (CAB3A/CAB3B) | 3.7 |
| Insulin-like growth factor-binding protein 1 (IGFBP1) | 4.3 |
| Insulin-like growth factor-binding protein 3 (IGFBP3) | 3.7 |
| Integrin beta 1; VLA4 beta; CD29 | 5.7 |
| Leucocyte interferon-induced peptide 6–16 | 3.8 |
| Neurotrophic tyrosine kinase receptor-related 3 | 4.9 |
| Prohibitin | 4.6 |
| Proto-oncogene c-jun; transcription factor AP-1 | 6.2 |
| Secreted apoptosis related protein 3 (SARP3, sFRP5) | 5.5* |
| Stromal cell derived factor 1 (CXCL12) receptor (SDF-1 receptor; CXCR4) | 4.6 |
| Transducer of ERBB-2 (TOB) | 5.1* |
| Transforming growth factor β 3 (TGF β 3) | 4.8 |
| Wee1Hu CDK tyrosine 15-kinase; wee- 1-like protein kinase | 4.3 |
| (B) The twenty most up-regulated genes in HCV cirrhosis compared to AIH-associated cirrhosis | Ratio of gene up-regulation |
| CDC-like kinase 2 (CLK-2) | 2.4 |
| Connective tissue growth factor (CTGF) | 2.5* |
| Cyclin-dependent kinase inhibitor 1C (CDKN1C) p57-KIP2 | 2.2* |
| Cytokine receptor EBI3 | 1.8* |
| Cytotoxic ligand TRAIL receptor | 2.3 |
| Ephrin A3 | 3.4 |
| Ephrin B3 | 2.7 |
| Ephrin type A receptor 3 | 2.1 |
| Discoidin domain receptor 1 (DDR1; CD167) | 2.2 |
| Glutathione-S-transferase M1 | 2.2 |
| HLA class I C-4 antigen alpha subunit | 3.3 |
| Insulin-like growth factor II (IGF-II) | 2.3 |
| Interferon alpha 2 | 3.5 |
| Low-affinity nerve growth factor receptor (NGF receptor) | 1.8 |
| Neuromodulin (GAP43) | 2.0 |
| Prothymosin alpha | 2.0 |
| Secreted apoptosis related protein 3 (SARP3; sFRP5) | 6.0* |
| TNF-related apoptosis inducing ligand (TRAIL) | 2.1 |
| Transducer of ERBB-2 (TOB) | 3.5* |
| Tyrosine-protein kinase receptor UFO (Axl) | 1.8 |
Up-regulation in both HCV- and AIH-associated cirrhosis are marked with asterisks. Compensation for variation in reference gene signal intensity was applied.
Gene expression in HCV and AIH that was less than nondiseased liver tissue was also examined. Erb B4 receptor, stem cell factor, and CC chemokine receptor-2 were markedly down-regulated in HCV- and AIH-associated cirrhosis compared to nondiseased liver tissue (supplemental material). In HCV-associated cirrhosis bone morphogenetic protein 3, Erb B4, hepatocyte growth factor activator, dual-specificity mitogen-activated protein kinase, high-affinity IL-8 receptor A, glutathione peroxidase, and androgen receptor were all decreased greater than fivefold in HCV-associated cirrhosis compared to nondiseased tissue.
Differential expression at 1.5-fold or greater is presented in the supplemental material, categorized according to known involvement in pathogenic processes. In addition, the 20 most up-regulated and down-regulated genes in both HCV- and AIH-associated cirrhosis are listed in the supplemental material.
Differential Expression in HCV-Associated Cirrhosis Compared to Nondiseased Liver
Inflammation (Figure 3, A and B) ▶
Figure 3.
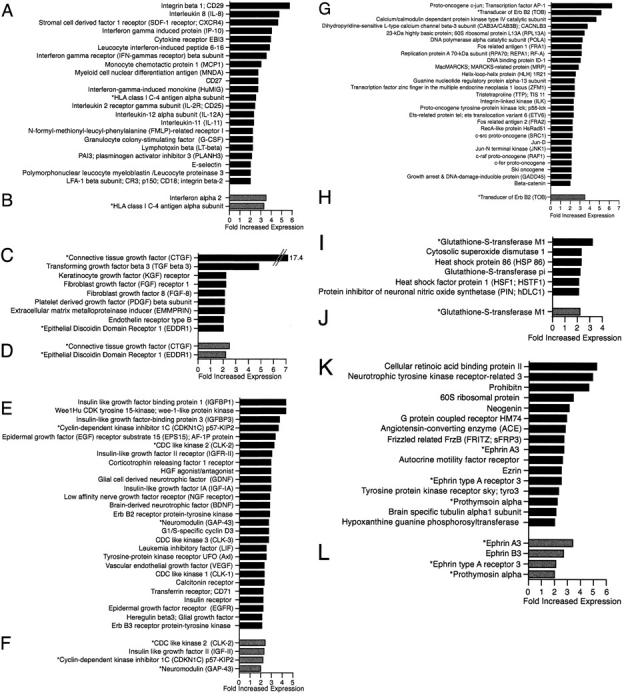
Up-regulation of inflammatory, fibrosis, proliferation, signaling, and stress response-associated genes. Genes up-regulated twofold or greater in HCV-associated cirrhosis compared to nondiseased liver tissue (A, C, E, G, I, and K) and AIH-associated cirrhosis (B, D, F, H, J, and L). Genes were categorized by association with inflammation (A and B); fibrosis (C and D); proliferation, growth, and regeneration (E and F); intracellular signaling and nuclear (G and H); stress response/oxidative stress (I and J); and uncategorized (K and L). Genes up-regulated in both comparisons are marked with asterisks.
Th1-associated molecules such as IFN-γ receptor β subunit, IL-2 receptor γ subunit, IL-12α subunit, CXCL9, CXCL10, and lymphotoxin β (Figure 3) ▶ and RANTES and macrophage inflammatory protein-1β (supplemental material) were increased in HCV-associated cirrhosis compared to nondiseased liver. No Th2-associated cytokines were up-regulated in HCV-associated cirrhosis (supplemental material, can be viewed at http://www.centenary.usyd.edu.au/research/HCVarray.html). Increased expression in HCV of CCL2 (monocyte chemoattractant protein-1) and the chemokine receptors CXCR4 and N-formyl-methionyl-leucyl-phenylalanine related receptor I was seen. Adhesion molecules such as integrin-β1 (CD29), integrin-β2 (CD18), and E-selectin (CD62E) as well as effectors of tissue damage such as polymorphonuclear leukocyte myeloblastin were up-regulated. CD29, CXCL8 (IL-8), CXCR4, CXCL10, cytokine receptor EB13, leukocyte IFN-induced peptide 6-16, IFN-γ receptor β subunit, and CCL2 were up-regulated greater than threefold in end-stage cirrhotic HCV tissue compared to nondiseased liver.
Fibrosis (Figure 3, C and D) ▶
A number of growth factors and growth factor receptors implicated in fibrosis, such as fibroblast growth factor-8, platelet-derived growth factor β subunit, keratinocyte growth factor receptor, and fibroblast growth factor-1 receptor, were increased in HCV-associated cirrhosis. Fibrosis mediators such as CTGF, TGF-β3, endothelin receptor type B, EMMPRIN, and DDR1 were also up-regulated. CTGF and TGF-β3 showed greater than threefold increased expression in HCV.
Proliferation, Growth, and Regeneration (Figure 3, E and F) ▶
Many genes of the insulin growth axis, including insulin receptor, insulin-like growth factor (IGF) IA, IGF-II receptor, IGF-binding protein 1 (IGFBP-1), and IGFBP-3, were increased in HCV-associated cirrhosis. The cell cycle-specific genes G1/S cyclin D3 and cell division cycle-like kinases (CLK)-1, -2, and -3 were also up-regulated. In addition to genes of the insulin axis a number of other trophic factors, hepatocyte growth factor agonist/antagonist, vascular endothelial growth factor, GDNF, low-affinity nerve growth factor receptor, neuromodulin (GAP43), granulocyte colony-stimulating factor, epidermal growth factor receptor, Erb B2 receptor, Erb B3 receptor, and glial growth factor were increased in HCV-associated cirrhosis. IGFBP-1, Wee1Hu cyclin-dependent kinase, tyrosine 15-kinase, IGFBP-3, CDKN1C, epidermal growth factor receptor substrate 15, and IGF-II receptor were increased threefold or greater in HCV-associated cirrhosis.
Intracellular Signaling and Nuclear (Figure 3, G and H) ▶
Many genes involved in signaling pathways had increased expression in HCV. Genes of the mitogen-activated protein kinase pathway (eg, JNK1) and Erb B pathway genes (eg, TOB) were up-regulated. Additionally, multiple transcription factors such helix-loop-helix 1R21 and zinc finger in the multiple endocrine neoplasia-1 loci were increased in HCV-associated cirrhosis.
Stress Response/Oxidative Stress (Figure 3, I and J) ▶
The stress response was characterized by increased expression of heat shock proteins (HSP-86 and heat shock factor-1) and genes involved in the oxidative stress response such as cytosolic superoxide dismutase 1 and glutathione-S-transferase isoforms M1 and pi.
Uncategorized Genes (Figure 3, K and L) ▶
Many genes have poorly characterized or multiple functions and were not categorized with the above pathogenic processes. Interesting differential expression included ezrin, prothymosin α, ephrin A3, and ephrin type A receptor 3, which had increased expression in HCV-associated cirrhosis.
Apoptosis (Figure 4) ▶
Figure 4.
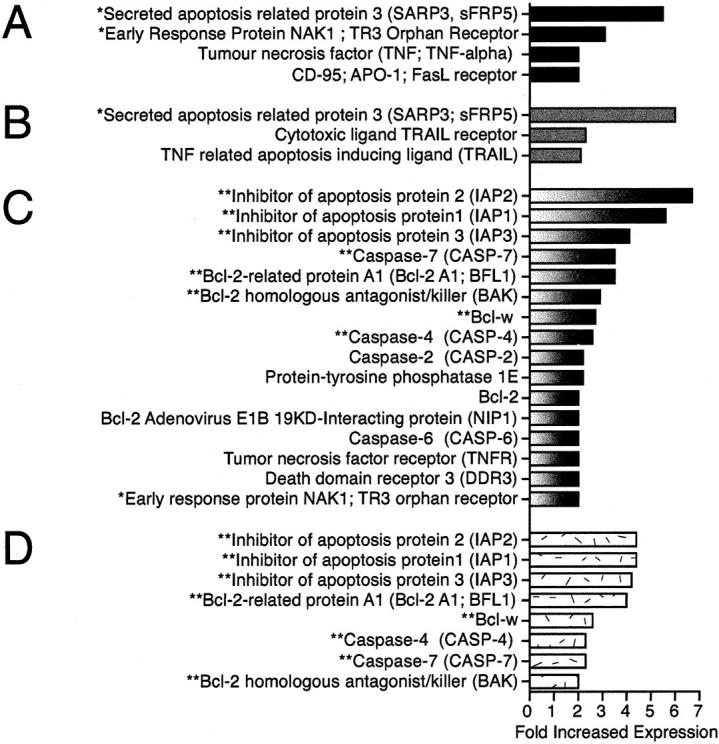
Up-regulation of apoptosis-associated genes in HCV and AIH cirrhosis. Apoptosis-associated gene up-regulation of twofold or greater in HCV-associated cirrhosis compared to nondiseased liver tissue (A) or AIH-associated cirrhosis (B) and AIH-associated cirrhosis compared to nondiseased liver tissue (C) or HCV-associated cirrhosis (D). Genes in common between comparisons are marked with asterisks.
Multiple proapoptotic molecules including Fas receptor (CD95) and TNF showed increased expression in HCV cirrhosis. The proapoptotic molecule’s early response protein NAK1, TR3 orphan receptor, and SARP3 were up-regulated greater than threefold in HCV cirrhosis compared to both nondiseased tissue and AIH-associated cirrhosis. In contrast to SARP3, the anti-apoptotic SARP1 was not differentially expressed (data not shown).
A principal feature of the cDNA array analysis was the many novel observations of differential gene expression. The mammalian homologs of Drosophila genes, β-catenin and two secreted frizzled related proteins (sFRP), SARP3 (sFRP5) and FRITZ (sFRP3), were increased in HCV-associated cirrhosis. Six oncogenes (c-jun, p56-lck, c-src, c-raf, c-fer, and ski) were up-regulated in HCV cirrhosis compared to nondiseased tissue. Furthermore, the neural molecules neurotrophic tyrosine kinase-related receptor 3, neogenin, BDNF, GDNF, low-affinity nerve growth factor receptor, high-affinity nerve growth factor receptor, neuromodulin, glial growth factor, brain-specific tubulin α1 subunit, and substance K were increased in HCV-associated cirrhosis.
Differential Expression in HCV-Associated Cirrhosis Compared to AIH-Associated Cirrhosis
The comparison between HCV- and AIH-associated cirrhosis helped to identify genes that may be preferentially involved in the pathogenesis of HCV-associated cirrhosis (Figure 3; B, D, F, H, J, and L ▶ ). Of relevance to inflammation, IFN-α2 precursor expression was greater in HCV- than AIH-associated cirrhosis. In both HCV- and AIH-associated cirrhosis up-regulation of Th1 genes was observed. However, there was also increased expression of Th2-associated IL-5, IL-11, and IL-13 in AIH-associated cirrhosis but not in HCV-associated cirrhosis (supplemental material, can be viewed at http://www.centenary.usyd.edu.au/research/HCVarray.html). IGF-II, IGF-II receptor, cell division cycle-like kinase 2, CDKN1C, neuromodulin, and the fibrosis-associated molecules CTGF and DDR1 were increased in HCV-associated cirrhosis compared to AIH-associated cirrhosis. The cytoplasmic signaling/nuclear-associated gene data highlighted some of the differences between HCV- and AIH-associated cirrhosis. TOB expression was greater in HCV- than AIH-associated cirrhosis. Differential expression was also seen in the stress-related genes, with glutathione-S-transferase M1 increased in HCV compared to AIH. Notable among the uncategorized genes was the increased expression of ephrin A3, ephrin type A receptor 3, and ephrin B3 in HCV-associated cirrhosis compared to AIH-associated cirrhosis.
A striking difference was seen in apoptosis-associated gene expression (Figure 4, C and D) ▶ . The proapoptotic SARP3 was clearly increased in HCV compared to AIH. In contrast, a number of anti-apoptosis-associated genes were up-regulated in AIH but not in HCV-associated cirrhosis compared to nondiseased tissue. These included inhibitor of apoptosis protein (IAP)-1, IAP-2, and IAP-3; and the four anti-apoptotic Bcl-2-related proteins Bcl-2, Bcl-w, Bcl-2 A1, and Bcl-2 adenovirus E1B 19-kd interacting protein.
Differential Expression in Hepatitic and Biliary Cirrhosis
The gene expression data on HCV-associated cirrhosis and AIH-associated cirrhosis from this study were examined alongside previous expression data on PBC- and PSC-associated cirrhosis. 26 This analysis identified genes that were differentially expressed in all four forms of cirrhosis compared to nondiseased tissue (Figure 5) ▶ . Consistently down-regulated genes in all forms of cirrhosis were acyl-CoA-binding protein, paired box protein PAX-5, and dual-specificity mitogen-activated protein kinase kinase 1. Hepatitic cirrhosis (HCV- and AIH-associated cirrhosis) but not biliary disease (PSC-associated cirrhosis and PBC) exhibited increased expression of prothymosin α, heat shock protein 27, glutathione-S-transferase pi, and oncostatin M (supplemental material, can be viewed at http://www.centenary.usyd.edu.au/research/HCVarray.html). Similarly, biliary, but not hepatitic, cirrhosis exhibited increased expression of IFN-γ receptor β subunit, IL-1α, and follicle-stimulating hormone receptor (supplemental material).
Figure 5.
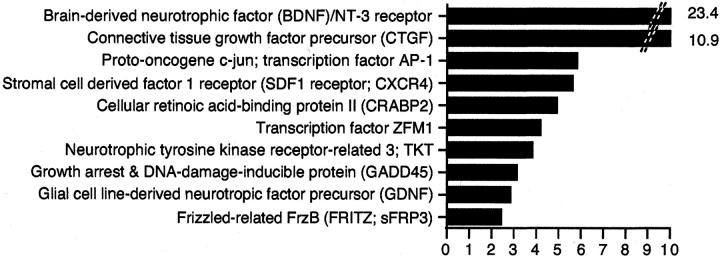
The 10 most differentially expressed genes in human cirrhosis. Data from the current study in hepatitic cirrhosis were combined with our previously published expression data on PBC and PSC cirrhosis 26 compared to nondiseased liver tissue. The genes were ranked according to their mean expression in HCV, AIH, and PSC cirrhosis and PBC compared to nondiseased tissue.
Statistical Analysis of Array Reproducibility
Correlation coefficients following regression analysis for the three comparisons for the ATLAS Human Gene Array and the ATLAS Cytokine Receptor Array, respectively, were: HCV compared to normal R 2 = 0.78 and 0.70, HCV compared to donor liver R 2 = 0.81 and 0.90, and HCV compared to AIH R 2 = 0.93 and 0.98, with P < 0.001 for all three comparisons.
The two methods of determining differential gene expression were compared by subtracting, for each gene, the ratio determined by regression analysis from the ratio determined by adjustment for housekeeping gene expression (see methods). The three comparisons, of HCV to normal, donor, and AIH, had a total of 2359 determinations of differential gene expression and the mean difference between the two methods was 0.48 ± 0.015. Additionally, the donor and normal populations were compared to determine their similarity. These two populations were compared by subtracting the ratio determined in HCV compared to normal tissue from the ratio in HCV compared to donor tissue, for each gene. The 1572 duplicate comparisons showed a mean difference of only 0.008 ± 0.026.
There were 190 genes common to both cDNA arrays. With the four probes on the two arrays there was a strong correlation of the 760 duplicate signals (R 2 = 0.76, P < 0.001). Similarly the 760 duplicate signals were compared by subtracting the regression ratio determined in HCV- or AIH-associated cirrhosis compared to nondiseased tissue. The 760 duplicate signals showed a mean difference of only 0.11 ± 0.017.
Although the data are expressed numerically and the above analyses convey its precision and reproducibility, the great strength of this technique is identification of differentially expressed genes rather than quantitation of individual gene expression. To quantify and confirm increased individual gene expression, techniques such as Northern blot analysis or quantitative RT-PCR are required. 23,24
Quantitation of Differentially Expressed mRNAs
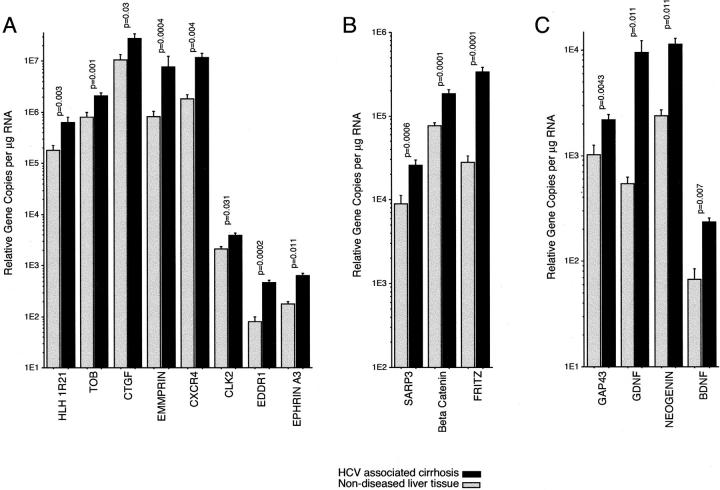
Increased expression of 15 selected genes was shown by real-time RT-PCR analysis of individual patient specimens including all of the cirrhosis specimens analyzed on the cDNA arrays (Figure 6) ▶ . Quantitation was done twice on each of two separate cDNA syntheses. CTGF was the most abundantly expressed gene with 4.3 × 10 7 ± 1.1 × 10 7 relative copies per μg RNA in HCV-associated cirrhosis. Conversely, BDNF was the least abundant transcript quantified with 2.3 × 10 2 ± 0.7 × 10 2 relative copies per μg RNA in nondiseased tissue. Ubiquitin levels were similar in all samples.
Figure 6.
Quantitation of differential expression by RT-PCR. Quantitative real-time RT-PCR data (mean and SE) on mRNA from HCV-associated cirrhosis (n = 6) and nondiseased (n = 4) liver. The depicted differential expression data are in three groups: A, extracellular matrix metalloproteinase inducer (EMMPRIN), CTGF, helix-loop-helix 1R21, TOB, CXCR4, cell division cycle-like kinase 2 (CLK2), discoidin domain receptor 1 (DDR1), and ephrin A3; B, Drosophila homologs including SARP3 and FRITZ; and C, neural-associated genes including BDNF, GDNF, and neuromodulin (GAP43).
Cellular Localization of Differentially Expressed Proteins
β-Catenin and EMMPRIN were chosen for protein localization by immunohistochemistry because these genes are important in Wnt pathway signaling and fibrosis, respectively. All hepatocytes and interlobular bile ducts were immunopositive for EMMPRIN and β-catenin in the nondiseased liver specimens examined. The localization of β-catenin and EMMPRIN was predominantly to the plasma membranes of hepatocytes and biliary epithelium. However, immunopositivity was greater in cirrhotic liver (Figure 7) ▶ . Increased EMMPRIN immunopositivity in cirrhosis was in the hepatocyte plasma membrane (Figure 7, B and C) ▶ and in proliferating bile duct structures of the fibrosis stroma (Figure 7, D and E) ▶ . Increased β-catenin immunopositivity in cirrhosis seemed to be confined to proliferating bile duct structures in the fibrous septa (Figure 7, H and J) ▶ . Similar patterns of immunostaining for both β-catenin and EMMPRIN occurred in HCV, AIH, and hepatitis B-associated cirrhosis and PBC (data not shown).
Figure 7.
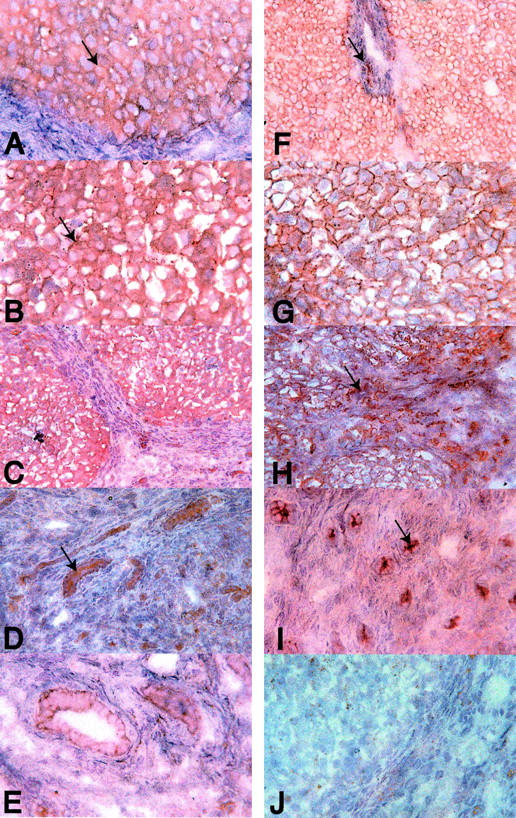
Cellular localization of β-catenin and EMMPRIN (CD147). Immunostaining of nondiseased (A and F) and HCV (B, C, E, G, and H)- and AIH (D and I)-associated cirrhosis liver specimens are shown. The immunostaining on frozen sections of EMMPRIN (A to E) and β-catenin (F to I) shows localization of these antigens predominantly to the plasma membranes of hepatocytes and biliary epithelial cells. EMMPRIN and β-catenin immunopositivity of biliary epithelium was greatest at the luminal aspect of the plasma membrane (D and I, arrows). The increased EMMPRIN immunostaining was seen in the plasma membranes of hepatocytes in cirrhosis (A and B, arrows). Increased β-catenin immunostaining in cirrhosis was confined to the proliferating bile duct structures within the fibrous septa (F and H, arrows). J: The isotype control on HCV cirrhosis. Immunoperoxidase stain using the NovaRED substrate and hematoxylin counterstain. Original magnifications: ×50 (C and H); ×100 (A, B, D, G, I, and J); and ×400 (E).
Discussion
Intrahepatic cDNA array analysis is a novel approach to examine gene expression in cirrhosis. Using cDNA array analysis we identified many differences at the mRNA level in the molecular pathways of HCV-associated cirrhosis compared to nondiseased liver tissue and AIH-associated cirrhosis. In HCV-associated cirrhosis increased expression of genes associated with a dominant Th1 immune response and chronic inflammation was seen. Furthermore, in HCV-associated cirrhosis compared to AIH-associated cirrhosis a striking difference in the profiles of apoptosis-associated genes was observed. SARP3, a proapoptotic molecule not previously studied in cirrhosis, was significantly increased in HCV-associated cirrhosis. In contrast, many anti-apoptotic molecules were up-regulated in AIH-associated cirrhosis. Concordant with the inflammation and apoptosis gene profiles, trophic growth factors such as IGF-II and IGF-II receptor were increased in HCV- compared to AIH-associated cirrhosis. Hence, cDNA array analysis showed differential gene expression patterns consistent with chronic inflammation and greater hepatocellular apoptosis and hepatocellular proliferation in HCV- than in AIH-associated cirrhosis. cDNA array analysis generated many novel observations of gene up-regulation, including increased expression of genes of the Wnt pathway and many neural genes. We observed qualitative agreement between the cDNA array and real-time RT-PCR methods of examining differential expression. Additionally, the expression data from the arrays concurred with many published studies of intrahepatic gene expression.
The inflammatory response detected by our gene array analysis concords with previous data showing that an intrahepatic Th1-like immune response persists in chronic HCV infection. 2-5,10 The expression levels of many Th1 immune response-associated genes but no Th2-associated genes were increased in HCV-associated cirrhosis. The inflammatory gene profile in HCV-associated cirrhosis differed from the mixed Th1- and Th2-associated gene expression observed in AIH (supplemental material, can be viewed at http://www.centenary.usyd.edu.au/research/HCVarray.html) and PSC 26 -associated cirrhosis and PBC. 26 The observed increases in CXCL9, CXCL10, and CXCR4 expression concord with previous studies of these chemokines in HCV infection. 8,9,31,32 Several nonspecific inflammatory mediators such as N-formyl-methionyl-leucyl-phenylalanine-related receptor 1, myeloblastin, and CXCL8 were also increased. The roles of inflammation-associated genes such as cytokine receptor EBI3, leukocyte IFN-induced peptide 6-16, IL-11, and the T-cell activation molecule CD27 have not been previously studied in HCV liver injury. Some molecules that were increased in HCV-associated cirrhosis are inflammatory cell effectors of tissue damage. For example, polymorphonuclear leukocyte myeloblastin, which degrades fibronectin, laminin, vitronectin, and collagen type IV as well as activating TNF and TGF-β and increasing CXCL8 expression, exhibited up-regulation. Given that these inflammation-associated genes are likely to have restricted expression to minor cell populations the increases in expression in those cell types may be considerable. The inflammatory gene profile in HCV-associated cirrhosis is consistent with a dominant Th1 immune response combined with a significant nonspecific inflammatory response and expression of effectors of tissue damage. Reasons for the failure of this inflammatory response to eliminate the virus remain unclear.
The fibrotic response was well characterized by the gene array analysis. Previously documented mediators of fibrosis such as CTGF, TGF-β, fibroblast growth factors, and integrins were shown to be up-regulated. 33-35 The increased expression of DDR1 in HCV-related liver injury is novel. DDR1, which triggers the release of MMP-1 in response to collagen binding. DDR2, another discoidin domain receptor, is expressed by activated HSCs, mediating their proliferation and MMP-2 release. 36 The fibrosis gene expression profiles in HCV, AIH, and PSC cirrhosis and PBC seem to be similar. We confirmed increased EMMPRIN expression in HCV cirrhosis, which is also seen in PBC, 26 by immunostaining and RT-PCR. The immunostaining showed that EMMPRIN in human cirrhosis is predominantly expressed by hepatocytes. EMMPRIN has been shown to be expressed by tumor and epithelial cells and interacts with fibroblasts resulting in increased expression of MMP-1, MMP-2 (collagenase), and MMP-3 (stromelysin-1). 37-41
The increased expression of CTGF, an important fibrosis mediator, in PBC and in HCV-, AIH-, and PSC-associated cirrhosis concords with recent studies of chronic HCV infection and experimental, CCl4-induced, cirrhosis. 19 CTGF is mitogenic and induces extracellular matrix production by fibroblasts. 42 CTGF expression increases in response to TGF-β but is not induced by platelet-derived growth factor, epidermal growth factor, or basic fibroblast growth factor. 43 The principle source of CTGF in the cirrhotic human liver is HSCs of the fibrous septa and portal tracts. 19
Cellular proliferation and regeneration are hallmarks of cirrhosis and many intrahepatic regeneration and growth-associated genes were shown to be increased in HCV-associated cirrhosis. Cell cycle-specific cyclins were up-regulated, possibly as a consequence of increased proliferation stimulated by growth factors. Increased growth factor expression included epidermal growth factor and keratinocyte growth factor. Vascular endothelial growth factor, which is associated with angiogenesis, fibrosis, and tumorigenesis was shown to be increased in HCV-associated cirrhosis, concordant with previous studies. 44 Moreover, many oncogenes and components of the insulin axis were up-regulated in HCV cirrhosis. The potent mitogenic activity of IGFs is evident in studies of malignancy. 45 An additional activity of IGFs is their apparent involvement in the initiation of HSC proliferation during hepatic fibrogenesis. 46,47 The overall increased expression of genes involved in growth and regeneration is consistent with active cellular proliferation in end-stage HCV cirrhosis. 48 Triggers of proliferation in individual cell types, especially the hepatocyte and HSC, require further investigation.
Apoptosis is an important pathogenic process in liver diseases. Viral hepatitis, and HCV infection in particular, involves increased hepatic apoptosis. Both known and novel observations of differential expression in molecules associated with apoptosis were seen in HCV-associated cirrhosis. These novel observations included increased expression, seen by RT-PCR, of SARP3, FRITZ, Wnt-5a, and β-catenin. SARP3 49 has a cysteine-rich frizzled-like domain and has not been studied in liver disease. Based on sequence homology, SARP3 and its relative FRITZ 50,51 are likely to be proapoptotic. 52 Frizzled proteins are Wnt receptors and signal transduction through β-catenin is thought to be responsible for modulating several fundamental cellular growth and differentiation signals. 53,54 Both FRITZ and SARP3 are thought to interfere with Wnt signaling and therefore influence not only apoptosis but also cell growth and polarity. 55,56 The increased β-catenin expression in HCV-associated cirrhosis suggests that there are modulators of Wnt signaling in this disease in addition to SARP3 and FRITZ. The immunostaining of β-catenin was generally consistent with previous observations on hepatocellular-associated HBV and HCV cirrhosis. 57 Importantly, we examined expression additionally in AIH cirrhosis and PBC. The previous work 57 reported immunoreactivity of β-catenin restricted to the lateral surface of hepatocyte membranes but we also observed intense expression on the luminal aspect of biliary epithelium. Up-regulation of Wnt pathway-associated genes was first noted in PBC. 26 Many more Wnt pathway-associated genes were observed to be increased in PBC- compared to HCV-associated cirrhosis. 26 However, SARP3 was not increased in PBC-, AIH-, or PSC-associated cirrhosis.
The above discussion has primarily focused on differential gene expression in HCV cirrhosis compared with nondiseased liver tissue. Such differential expression may be generally involved in cirrhosis or inflammation rather than be specific for HCV disease. Therefore, HCV cirrhosis was compared to another hepatitic cirrhosis, AIH. Although the inflammation and fibrosis gene profiles were generally similar it was of interest that IFN-α and MHC class I genes were more up-regulated in HCV-associated cirrhosis perhaps reflecting a direct viral effect. In addition, Th2-related gene expression was seen in AIH-associated cirrhosis but not in HCV-associated cirrhosis. A major difference was noted in apoptotic gene expression in both HCV and AIH cirrhosis. In HCV-associated cirrhosis the expression levels of proapoptotic genes such as Fas, TNF-α, and SARP3 were greater than in AIH-associated cirrhosis. In contrast, the recognized inhibitors of apoptosis, IAP-1, IAP-2, and IAP-3; and four anti-apoptotic Bcl-2-associated genes, Bcl-2, Bcl-w, Bcl-2-related protein A1, and adenovirus E1B 19-kd interacting protein were increased in AIH-associated cirrhosis. These differences in the expression of apoptosis-related genes may at least partially explain the observed increase in cellular apoptosis in HCV. 58,59 The increased expression in HCV-associated cirrhosis of a number of trophic factors such as IGF-II and cell cycle-specific genes compared to AIH-associated cirrhosis is consistent with greater cell turnover in HCV-associated cirrhosis. 60,61 Increased expression of genes such as the ephrins, ephrin receptors, vascular endothelial growth factor, Erb B2, and EMMPRIN possibly reflect increased cell turnover and associated remodeling that in turn predisposes to malignancy. 48 Genes such as Erb B2 62,63 and vascular endothelial growth factor 64,65 have well-defined roles in tumor progression. Thus, the data presented here may help explain the increased malignant potential of HCV liver injury compared to other chronic inflammatory liver conditions such as AIH.
Hepatocytes are the major contributant to preparations of whole liver mRNA and our immunostaining data on EMMPRIN and β-catenin clearly indicates that hepatocytes are a major source of these proteins in the liver. However, differential gene expression in other cell types such as HSCs, endothelial cells, Kupffer cells, T cells, and macrophages may be responsible for some of our observations. Analysis of mRNA from isolated subpopulations of cells may be informative. However, such studies require careful interpretation because isolation methods may activate cells, especially when cell culture is involved, and removes cellular and extracellular matrix interactions that are present in the solid organ. Studies such as this represent a previously unparalleled means of profiling genes in the hepatic transcriptome. However, the intrahepatic gene profile presented in this and other studies represents only a small fraction of the liver transcriptome. Therefore, many potentially important genes that are differentially expressed may not have been identified in this study.
The overall interpretation of our gene array data differs from conclusions drawn using gene microarrays to explore the pathogenesis of chronic hepatitis virus infection. 27 Honda and colleagues 27 concluded that chronic HCV infection is associated with a predominant anti-inflammatory, proproliferative, anti-apoptotic intrahepatic gene profile. However, a careful examination of their data indicates a proinflammatory, proapoptotic, proproliferative expression profile in concordance with our data. Increased expression of ADAM-9, IL-15 receptor, IL-2 receptor (CD25), CD69, CD44, IFN-γ inducible protein, monokine induced by γ IFN, MHC class I, IL-1 receptor, CD53, CD58 (LFA-3), CD79, and GM-CSF was present in chronic HCV but not commented on. 27 The up-regulation of these genes indicates persisting chronic immune activation in the liver, ie, a proinflammatory not an anti-apoptotic gene profile as claimed. Their microarray data also shows up-regulation in chronic HCV infection of proapoptotic genes, including caspase 10, TNF receptor-associated factor-2 (TRAF-2), TNF receptor-associated factor-6 (TRAF-6), and TNF receptor-associated protein-1 (TRAP-1). Thus we believe that our data concord with those of Honda and colleagues. 27 Both studies reveal up-regulation of proinflammatory, proproliferative, and proapoptotic genes in HCV cirrhosis. We speculate that such a gene profile reflects the underlying basis of the propensity of HCV-associated cirrhosis to develop HCC. Such a hypothesis requires further study at the cellular and subcellular level.
Acknowledgments
We thank the Wellcome Foundation and the Clive and Vera Ramaciotti Foundation for supporting the purchase of a model 7700 Sequence Detector; and Hannes Stockinger and Vaclav Horejsi of Vienna for providing the antibody to EMMPRIN.
Footnotes
Address reprint requests to Professor G. W. McCaughan, Centenary Institute of Cancer Medicine and Cell Biology, Locked Bag No.6, Newtown, NSW, 2042, Australia. E-mail: g.mccaughan@centenary.usyd.edu.au.
Supported by the National Institutes of Health (grant RFA/DK/98.017) and the National Health and Medical Research Council of Australia. N. A. S. holds a Sir Gustav Nossal Scholarship.
References
- 1.Cerny A, Chisari FV: Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 1999, 30:595-601 [DOI] [PubMed] [Google Scholar]
- 2.McCaughan GW, Gorrell MD, Bishop GA, Abbott CA, Shackel NA, McGuinness PH, Levy MT, Sharland AF, Bowen DG, Yu D, Slaitini L, Church WB, Napoli J: Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev 2000, 174:172-191 [DOI] [PubMed] [Google Scholar]
- 3.Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW: Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 1996, 24:759-765 [DOI] [PubMed] [Google Scholar]
- 4.McCaughan GW, Napoli J, McGuinness PH, Bishop GA: T1 vs T2 cytokine response in chronic HCV: implications for mechanisms of liver injury. Viral Hepatitis Rev 1997, 3:129-142 [Google Scholar]
- 5.Bertoletti A, D’Elios MM, Boni C, De Carli M, Zignego AL, Durazzo M, Missale G, Penna A, Fiaccadori F, Del Prete G, Ferrari C: Different cytokine profiles of intrahepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology 1997, 112:193-199 [DOI] [PubMed] [Google Scholar]
- 6.McGuinness PH, Painter D, Davies S, McCaughan GW: Increases in intrahepatic CD68 positive cells, MAC387 positive cells and pro-inflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut 2000, 46:260-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR: The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 1998, 101:746-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields PL, Morland CM, Salmon M, Qin SX, Hubscher SG, Adams DH: Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol 1999, 163:6236-6243 [PubMed] [Google Scholar]
- 9.Mihm S, Patzwahl R, Meier V, Ramadori G: Enhanced expression of IFN-gamma regulated genes in patients with chronic hepatitis C infection—detection by suppression subtractive hybridization. Hepatology 1999, 30:A1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR: Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 1999, 117:933-941 [DOI] [PubMed] [Google Scholar]
- 11.He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM, Greenberg HB: Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA 1999, 96:5692-5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K: The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 1998, 4:1065-1067 [DOI] [PubMed] [Google Scholar]
- 13.Colombo M: The role of hepatitis C virus in hepatocellular carcinoma. Recent Results Cancer Res 1998, 154:337-344 [DOI] [PubMed] [Google Scholar]
- 14.Moore MA, Tsuda H: Chronically elevated proliferation as a risk factor for neoplasia. Eur J Cancer Prev 1998, 7:353-385 [DOI] [PubMed] [Google Scholar]
- 15.Farinati F, Cardin R, D’Errico A, De Maria N, Naccarato R, Cecchetto A, Grigioni W: Hepatocyte proliferative activity in chronic liver damage as assessed by the monoclonal antibody MIB1 Ki67 in archival material: the role of etiology, disease activity, iron, and lipid peroxidation. Hepatology 1996, 23:1468-1475 [DOI] [PubMed] [Google Scholar]
- 16.Miura N, Horikawa I, Nishimoto A, Ohmura H, Ito H, Hirohashi S, Shay JW, Oshimura M: Progressive telomere shortening and telomerase reactivation during hepatocellular carcinogenesis. Cancer Genet Cytogenet 1997, 93:56-62 [DOI] [PubMed] [Google Scholar]
- 17.Iio S, Hayashi N, Mita E, Ueda K, Mochizuki K, Hiramatsu N, Kanto T, Sasaki Y, Kasahara A, Hori M: Serum levels of soluble Fas antigen in chronic hepatitis C patients. J Hepatol 1998, 29:517-523 [DOI] [PubMed] [Google Scholar]
- 18.Hayashi N, Mita E: Fas system and apoptosis in viral hepatitis. J Gastroenterol Hepatol 1997, 12:S223-S226 [DOI] [PubMed] [Google Scholar]
- 19.Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, Gauthier JM, Ba N, Sobesky R, Ratziu V, Bedossa P: Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology 1999, 30:968-976 [DOI] [PubMed] [Google Scholar]
- 20.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Mueller E, Rettig WJ, Gorrell MD: Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology 1999, 29:1768-1778 [DOI] [PubMed] [Google Scholar]
- 21.Khan J, Bittner ML, Chen Y, Meltzer PS, Trent JM: DNA microarray technology: the anticipated impact on the study of human disease. Biochim Biophys Acta 1999, 25:M17-M28 [DOI] [PubMed] [Google Scholar]
- 22.Bowtell DD: Options available—from start to finish—for obtaining expression data by microarray. Nat Genet 1999, 21:25-32 [DOI] [PubMed] [Google Scholar]
- 23.Sehgal A, Boynton AL, Young RF, Vermeulen SS, Yonemura KS, Kohler EP, Aldape HC, Simrell CR, Murphy GP: Application of the differential hybridization of Atlas human expression arrays technique in the identification of differentially expressed genes in human glioblastoma multiforme tumor tissue. J Surg Oncol 1998, 67:234-241 [DOI] [PubMed] [Google Scholar]
- 24.Shim C, Zhang W, Rhee CH, Lee JH: Profiling of differentially expressed genes in human primary cervical cancer by complementary DNA expression array. Clin Cancer Res 1998, 4:3045-3050 [PubMed] [Google Scholar]
- 25.Rhee CH, Hess K, Jabbur J, Ruiz M, Yang Y, Chen S, Chenchik A, Fuller GN, Zhang W: cDNA expression array reveals heterogeneous gene expression profiles in three glioblastoma cell lines. Oncogene 1999, 18:2711-2717 [DOI] [PubMed] [Google Scholar]
- 26.Shackel N, McGuinness P, Abbott C, Gorrell M, McCaughan G: Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut 2001, 49:565-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda M, Kaneko S, Kawai H, Yukihiro S, Kobayashi K: Differential gene expression between chronic hepatitis B and C hepatic lesions. Gastroenterology 2001, 120:955-966 [DOI] [PubMed] [Google Scholar]
- 28.Cristofalo VJ: A DNA chip off the aging block. Nat Med 2000, 6:507. [DOI] [PubMed] [Google Scholar]
- 29.Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Van der Putten K, McCaughan G, Eris JM, Bishop GA: Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunol Cell Biol 2001, 79:213-221 [DOI] [PubMed] [Google Scholar]
- 30.Koch C, Staffler G, Huttinger R, Hilgert I, Prager E, Cerny J, Steinlein P, Majdic O, Horejsi V, Stockinger H: T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int Immunol 1999, 11:777-786 [DOI] [PubMed] [Google Scholar]
- 31.Shimoda K, Begum NA, Shibuta K, Mori M, Bonkovsky HL, Banner BF, Barnard GF: Interleukin-8 and hIRH (SDF1-alpha/PBSF) mRNA expression and histological activity index in patients with chronic hepatitis C. Hepatology 1998, 28:108-115 [DOI] [PubMed] [Google Scholar]
- 32.Mitra P, Shibuta K, Mathai J, Shimoda K, Banner BF, Mori M, Barnard GF: CXCR4 mRNA expression in colon, esophageal and gastric cancers and hepatitis C infected liver. Int J Oncol 1999, 14:917-925 [DOI] [PubMed] [Google Scholar]
- 33.Alcolado R, Arthur MJP, Iredale JP: Pathogenesis of liver fibrosis. Clin Sci 1997, 92:103-112 [DOI] [PubMed] [Google Scholar]
- 34.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA: The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol 1999, 30:77-87 [DOI] [PubMed] [Google Scholar]
- 35.Levy MT, Trojanowski M, Reuben A: The effect of oncostatin M on collagen production by human hepatic stellate cells in culture. J Hepatol 1999, 32:218-226 [DOI] [PubMed] [Google Scholar]
- 36.Olaso E, Eng F, Friedman S: The discoidin domain receptor 2 (DDR2) is induced during stellate cell activation, mediates cell growth and matrix metalloproteinase 2 expression, and is regulated by extracellular matrix. Hepatology 1999, 30:A1009 [Google Scholar]
- 37.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K: The human tumor cell-derived collagenase stimulatory factor (EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 1995, 55:434-439 [PubMed] [Google Scholar]
- 38.DeCastro R, Zhang Y, Guo H, Kataoka H, Gordon MK, Bp T, Biswas G: Human keratinocytes express EMMPRIN, an extracellular matrix metalloproteinase inducer. J Invest Dermatol 1996, 106:1260-1265 [DOI] [PubMed] [Google Scholar]
- 39.Guo H, Zucker S, Gordon MK, Toole BP, Biswas C: Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem 1997, 272:24-27 [PubMed] [Google Scholar]
- 40.Lim M, Martinez T, Jablons D, Cameron R, Guo H, Toole B, Li JD, Basbaum C: Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett 1998, 441:88-92 [DOI] [PubMed] [Google Scholar]
- 41.Hlavcák P, Sedláková O, Sedlák J, Hunáková L, Duraj J, Sulíková M, Bízik J, Castronovo V, Chorváth B: Cell surface immunophenotype and gelatinase activity of the human breast carcinoma cell line (MCF-7/6) with functionally defective E-cadherin. Neoplasma 1999, 46:12-16 [PubMed] [Google Scholar]
- 42.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR: Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 1996, 107:404-411 [DOI] [PubMed] [Google Scholar]
- 43.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K: Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol 1999, 181:153-159 [DOI] [PubMed] [Google Scholar]
- 44.Chow NH, Hsu PI, Lin XZ, Yang HB, Chan SH, Cheng KS, Huang SM, Su IJ: Expression of vascular endothelial growth factor in normal liver and hepatocellular carcinoma: an immunohistochemical study. Hum Pathol 1997, 28:698-703 [DOI] [PubMed] [Google Scholar]
- 45.Gressner AM, Lahme B, Brenzel A: Molecular dissection of the mitogenic effect of hepatocytes on cultured hepatic stellate cells. Hepatology 1995, 22:1507-1518 [PubMed] [Google Scholar]
- 46.Scharf JG, Knittel T, Dombrowski F, Müller L, Saile B, Braulke T, Hartmann H, Ramadori G: Characterization of the IGF axis components in isolated rat hepatic stellate cells. Hepatology 1998, 27:1275-1284 [DOI] [PubMed] [Google Scholar]
- 47.Gentilini A, Feliers D, Pinzani M, Woodruff K, Abboud S: Characterization and regulation of insulin-like growth factor binding proteins in human hepatic stellate cells. J Cell Physiol 1998, 174:240-250 [DOI] [PubMed] [Google Scholar]
- 48.Rogler CE, Chisari FV: Cellular and molecular mechanisms of hepatocarcinogenesis. Semin Liver Dis 1992, 12:265-278 [DOI] [PubMed] [Google Scholar]
- 49.Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, Tomei LD, Umansky SR: SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci USA 1997, 94:13636-13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Krinks M, Moos MJ: Frzb-1, an antagonist of Wnt-1 and Wnt-8, does not block signaling by Wnts-3A, -5A, or -11. Biochem Biophys Res Commun 1997, 236:502-504 [DOI] [PubMed] [Google Scholar]
- 51.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM: Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 1997, 88:747-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z, Wang J, Han X, Zhou J, Linder S: Up-regulation of human secreted frizzled homolog in apoptosis and its down-regulation in breast tumors. Int J Cancer 1998, 78:95-99 [DOI] [PubMed] [Google Scholar]
- 53.Dale TC: Signal transduction by the Wnt family of ligands. Biochem J 1998, 329:209-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhl M, Wedlich D: Wnt signalling goes nuclear. Bioessays 1997, 19:101-104 [DOI] [PubMed] [Google Scholar]
- 55.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J: A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA 1997, 94:2859-2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang JT, Esumi N, Moore K, Li Y, Zhang S, Chew C, Goodman B, Rattner A, Moody S, Stetten G, Campochiaro PA, Zack DJ: Cloning and characterization of a secreted frizzled-related protein that is expressed by the retinal pigment epithelium. Hum Mol Genet 1999, 8:575-583 [DOI] [PubMed] [Google Scholar]
- 57.Ihara A, Koizumi H, Hasizume R, Uchiloshi T: Expression of epithelial cadherin and alpha- and beta-catenins in nontumoral livers and hepatocellular carcinomas. Hepatology 1996, 23:1441-1447 [DOI] [PubMed] [Google Scholar]
- 58.Roberts JM, Searle JW, Cooksley WG: Histological patterns of prolonged hepatitis C infection. Gastroenterol Jpn 1993, 28(Suppl 5):37-41 [DOI] [PubMed] [Google Scholar]
- 59.Nasir A, Arora HS, Kaiser HE: Apoptosis and pathogenesis of viral hepatitis C—an update. In Vivo 2000, 14:297-300 [PubMed] [Google Scholar]
- 60.Sohda T, Oka Y, Iwata K, Gunn J, Kamimura S, Shijo H, Okumura M, Yun K: Co-localisation of insulin-like growth factor II and the proliferation marker MIB1 in hepatocellular carcinoma cells. J Clin Pathol 1997, 50:135-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim KW, Bae SK, Lee OH, Bae MH, Lee MJ, Park BC: Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res 1998, 58:348-351 [PubMed] [Google Scholar]
- 62.Carver RS, Sliwkowski MX, Sitaric S, Russell WE: Insulin regulates heregulin binding and ErbB3 expression in rat hepatocytes. J Biol Chem 1996, 271:13491-13496 [DOI] [PubMed] [Google Scholar]
- 63.Collier JD, Guo K, Mathew J, May FE, Bennett MK, Corbett IP, Bassendine MF, Burt AD: c-erbB-2 oncogene expression in hepatocellular carcinoma and cholangiocarcinoma. J Hepatol 1992, 14:377-380 [DOI] [PubMed] [Google Scholar]
- 64.Yoshiji H, Kuriyama S, Yoshii J, Yamazaki M, Kikukawa M, Tsujinoue H, Nakatani T, Fukui H: Vascular endothelial growth factor tightly regulates in vivo development of murine hepatocellular carcinoma cells. Hepatology 1998, 28:1489-1496 [DOI] [PubMed] [Google Scholar]
- 65.Li XM, Tang ZY, Zhou G, Lui YK, Ye SL: Significance of vascular endothelial growth factor mRNA expression in invasion and metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res 1998, 17:13-17 [PubMed] [Google Scholar]