Abstract
Transgenic mice with vascular endothelial growth factor (VEGF) driven by the rhodopsin promoter (rho/VEGF mice) develop neovascularization that originates from the deep capillary bed of the retina and grows into the subretinal space. In rho/VEGF mice, VEGF expression in photoreceptors begins between postnatal days 5 and 7, the period when the deep capillary bed is developing. An important question is whether or not the developmental stage of the deep capillary bed is critical for occurrence of neovascularization. Also, although rho/VEGF mice are extremely useful for the study of ocular neovascularization, there are some applications for which the early onset of VEGF expression is a disadvantage. In this study, we used the reverse tetracycline transactivator (rtTA) inducible promoter system coupled to either the rhodopsin or interphotoreceptor retinoid-binding protein (IRBP) promoter to control the time of onset of VEGF transgene expression in photoreceptors. In the absence of doxycycline, adult double-transgenic rho/rtTA-TRE/VEGF or IRBP/rtTA-TRE/VEGF mice showed little VEGF transgene expression and no phenotype. The addition of doxycycline to the drinking water resulted in prominent transgene expression and evidence of neovascularization within 3 to 4 days. Like rho/VEGF mice, the neovascularization originated from the deep capillary bed of the retina, but it was more extensive and caused outer retinal folds followed by total retinal detachment. Real-time polymerase chain reaction and enzyme-linked immunosorbent assay demonstrated that the mice with inducible expression of VEGF that developed retinal detachment had much higher ocular levels of VEGF mRNA and protein compared to rho/VEGF mice that manifest a much milder phenotype. These data demonstrate that regardless of developmental stage of the vascular bed, increased expression of VEGF in the retina is sufficient to cause neovascularization, and high levels of expression cause severe neovascularization and traction retinal detachment. Mice with inducible expression of VEGF in the retina provide a valuable new model of ocular neovascularization.
Damage to retinal capillaries resulting in retinal ischemia is a constant feature in diseases complicated by retinal neovascularization. Therefore, the demonstration that vascular endothelial cell growth factor (VEGF) is up-regulated by hypoxia 1,2 focused attention on VEGF as a candidate mediator of retinal neovascularization. Circumstantial evidence suggesting that VEGF plays a role was provided by temporal and spatial correlation of VEGF expression with retinal neovascularization in patients and animal models. 3-8 Inhibition of retinal neovascularization by several different types of VEGF antagonists has demonstrated that VEGF is a necessary stimulatory factor. 9-13 Somewhat surprisingly (because hypoxia is not known to play a role), VEGF signaling was also found to be necessary for development of choroidal neovascularization in a murine model. 12,14
Although these studies clearly implicated VEGF as a stimulatory factor for ocular neovascularization, other studies showed that in some situations expression of VEGF by itself was insufficient to induce retinal neovascularization. The retinas of patients with nonproliferative diabetic retinopathy or other retinal diseases may show elevated levels of VEGF without retinal neovascularization. 15,16 In primates, multiple intravitreous injections of VEGF result in iris neovascularization 17 and retinal vascular abnormalities including endothelial cell proliferation, 18 but do not cause definite retinal neovascularization. Similarly, intraocular implantation of pellets that provide sustained release of VEGF in primates fails to stimulate retinal neovascularization. 19 However, intraocular implantation of similar pellets that release VEGF in rabbits results in transient retinal neovascularization. 19
To resolve the issue of whether or not increased levels of VEGF are capable of stimulating retinal neovascularization, a full-length cDNA for VEGF165 was coupled to the bovine rhodopsin promoter and transgenic mice were generated that selectively express VEGF in photoreceptors. 20 In these rho/VEGF mice, VEGF expression was turned on in photoreceptors at postnatal day 5 to postnatal day 6 and throughout the next week, neovascularization sprouted from the deep capillary bed of the retina and grew into the subretinal space. This indicates that increased expression of VEGF at a time when retinal vascular development is occurring is sufficient to cause neovascularization from the deep capillary bed, but it also raises several questions. Why does the neovascularization only originate from the deep capillary bed and not from superficial capillaries or choroidal vessels? Is the time that the transgene is turned on, which is during retinal vascular development, a critical determinant of the phenotype? Would alteration of the level of VEGF expression result in alteration of the location and/or amount of neovascularization? In this study, we have addressed these questions by using retina-specific promoters combined with an inducible promoter system, the reverse tetracycline transactivator system. 21
Materials and Methods
Generation of Double-Transgenic Mice with Doxycycline-Inducible Expression of VEGF in the Retina
Transgenic mice that carry the reverse tetracycline transactivator under control of the photoreceptor-specific promoters for rhodopsin (rho/rtTA mice, line D) or interphotoreceptor retinoid-binding protein (IRBP/rtTA mice, line K) have been generated and characterized. 22 A plasmid containing the tetracycline response element (TRE) was digested with BamHI, and the 598-bp BamHI fragment of VEGF that was previously used to generate rho/VEGF transgenic mice 20 was inserted. After transformation, a clone with correct orientation of the VEGF fragment was identified by sequencing. DNA was purified by the CsCl gradient technique and cut with XhoI and HindIII yielding a 1529-bp TRE/VEGF/SV40 poly A fusion gene. The fusion gene was purified and transgenic mice were generated as previously described. 20 Forty-eight mice were obtained and screened by polymerase chain reaction (PCR) of tail DNA using transgene-specific primers (primer 1: 5′-TCGAGTAGGCGTGTACGG-3′, primer 2: 5′-GCAGCAGCCCCCGCATCG-3′) that result in a 429-bp specific product. Tail DNA was obtained by overnight digestion of a 1- to 2-mm tail segment in 0.5 mg/ml proteinase K, 10 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, 20 mmol/L ethylenediaminetetraacetic acid, and 2% Triton X-100 at 55°C. Four founder mice were identified and mated with C57BL/6 mice. One founder failed to reproduce and therefore three TRE/VEGF transgenic lines were obtained. Mice from each of these lines were mated with rho/rtTA and IRBP/rtTA mice and double transgenics were identified by PCR of tail DNA. The TRE/VEGF transgene was identified using the primers listed above. The rho/rtTA or IRBP/rtTA transgenes were identified as previously described 22 using rtTA primer (5′-GTTTACCGATGCCCTTGGAATTGACGAGT-3′), IC40 primer (5′-GATGTGGCGAGATGCCCTTGGAATTGACGAGT-3′), and IC41 primer (5′-CAAGCAACTCCTGATGCCAAAGCCCTGCCC-3′).
Retinal Reverse Transcriptase (RT)-PCR
Adult double-transgenic mice were given drinking water containing 10 mg/ml of doxycycline and 5% sucrose. After various times, mice were sacrificed, eyes were removed, and retinas were dissected. Retinal RNA was isolated using the guanidine isothiocyanate method as described by Chomczynski and Sacchi. 23 Reverse transcription was performed with ∼0.5 μg of total RNA, reverse transcriptase (SuperScript II; Life Technologies, Inc., Gaithersburg, MD), and 5.0 μmol/L of oligo dT primer. Aliquots of the cDNAs were used for PCR amplification using primers that specifically amplify human VEGF (5′-CACCCATGGCAGAAGGAGGAG-3′ and 5′-CAAATGCTTTCTCCGCTCTGA-3′). Titrations were performed to ensure that PCR reactions were performed in the linear range of amplification. Mouse S16 ribosomal protein primers (5′-CACTGCAAACGGGGAAATGG-3′ and 5′-TGAGATGGACTGTCGGATGG-3′) were used to provide an internal control for the amount of template in the PCR reactions.
Real-Time RT-PCR
Real time PCR allows for precise quantitation of mRNA using total RNA from a single retina and obviates the need for Northern blots using pooled retinas. Retinal RNA was isolated using Trizol solution (Life Technologies, Inc.) as directed by the manufacturer. Residual DNA was removed by DNase-treatment with DNA-free (Ambion, TX). One μg of RNA was reverse-transcribed using Superscript II (Life Technologies, Inc.). Real-time quantitative PCR was performed and analyzed using the SYBR Green I format on the LightCycler rapid thermal cycler system (Roche Molecular Biochemicals, Indianapolis, IN) using a previously published procedure and primer pairs specific for human VEGF. 24 Hot Start Taq DNA polymerase and the FastStart Master SYBR Green I reaction mix (Roche Molecular Biochemicals) were used. During amplification, SYBR Green fluorescence was automatically acquired during the elongation step of each cycle by the LightCycler software. After the amplification protocol, melting curve analysis was performed. Quantification was done with the LightCycler Software by linear regression analysis using a series of specimen dilution curves and a series of standard curves generated with known amounts of cDNA (linearized plasmid containing the requisite VEGF cDNA diluted in 10-fold increments up to 1:10,000). Linear regression (log concentration versus cycle number) for each dilution set of standard and each sample had similar slopes (−1/log efficiency), comparable y intercepts, an r correlation value of −1.00, and a mean squared error lower than 0.25. Multiple runs did not differ in amplification efficiency by more than ±0.05. Through the use of appropriate external standards for quantification, suitable internal controls for assuring the integrity of the reverse transcriptase reaction, and accurate determinants for analysis it was possible to determine very precise mRNA levels.
Histopathological Evaluation of Retinas
Untreated double-transgenic mice and those treated with various doses of doxycycline for various time periods were sacrificed and eyes were rapidly removed and frozen in optimum cutting temperature embedding compound (OCT; Miles Diagnostics, Elkhart, IN). Frozen sections (10 μm) of eyes were histochemically stained with biotinylated Griffonia simplicifolia lectin B4 (GSA) (Vector Laboratories, Burlingame, CA) that selectively binds to vascular cells. Slides were incubated in methanol/H2O2 for 10 minutes at 4°C, washed with 0.05 mol/L Tris-buffered saline (TBS), pH 7.6, and incubated for 30 minutes in 10% normal porcine serum. Slides were incubated 2 hours at room temperature with biotinylated lectin and after rinsing with 0.05 mol/L TBS, they were incubated with avidin coupled to peroxidase (Vector Laboratories) for 45 minutes at room temperature. After being washed for 10 minutes with 0.05 mol/L TBS, slides were incubated with diaminobenzidine to give a brown reaction product. Some slides were counterstained with hematoxylin and all were mounted with Cytoseal.
Quantitation of Ocular VEGF Levels
At the time mice were sacrificed, eyes were removed, a cut was made at the limbus, the lens was carefully removed, and the remainder of each eye was snap-frozen in liquid nitrogen. Eye homogenates were prepared by Dounce homogenization followed by three freeze/thaw cycles in phosphate-buffered saline with 100 μmol/L of phenylmethyl sulfonyl fluoride. Homogenates were microfuged and the protein concentration of supernatants was measured using a Bio-Rad Protein Assay Kit (BioRad, Hercules, CA). Enzyme-linked immunosorbent assay of the samples was performed using the Quantikine VEGF assay kit (R&D Systems, Minneapolis, MN) using the manufacturer’s instructions. Serial dilutions of recombinant VEGF165 were assayed to generate a standard curve with the limit of detection at 16 pg/ml.
Results
Double-Transgenic Mice with Doxycycline-Inducible Expression of VEGF in the Retina
Three TRE/VEGF transgenic lines were obtained and mice from each of these lines were mated with rho/rtTA or IRBP/rtTA transgenic mice and double-hemizygous rho/rtTA-TRE/VEGF and IRBP/rtTA-TRE/VEGF transgenics were identified by PCR of tail DNA. At 3 weeks of age, mice were treated with 10 mg/ml of doxycycline in their drinking water. After 2 weeks, mice were sacrificed and retinal RNA was isolated. RT-PCR for human VEGF showed that both IRBP/rtTA-TRE/VEGF and rho/rtTA-TRE/VEGF double-hemizygous transgenics generated from one of the TRE/VEGF lines had minimal expression of human VEGF in the absence of doxycycline, but strong expression when the mice had been given doxycycline in their drinking water (Figure 1) ▶ . Double-hemizygous transgenics generated from the other two TRE/VEGF lines failed to show good inducible expression of VEGF; one showed low-level transgene expression and the other showed little difference in expression in the presence or absence of doxycycline (not shown).
Figure 1.
Expression of human VEGF in the retina of double-transgenic mice in the presence and absence of doxycycline. One of three lines of TRE-VEGF-transgenic mice showed good inducible expression of VEGF when mated with rho/rtTA or IRBP/rtTA mice. At postnatal day 21, each type of double transgenic (n = 3) was given normal drinking water or drinking water containing 10 mg/ml of doxycycline. After 3 days, the mice were sacrificed and retinal RNA was isolated. Using primers specific for human VEGF, RT-PCR showed little expression of VEGF mRNA in untreated double transgenics and strong expression in those treated with doxycycline. Comparable amounts of starting RNA are indicated by similarity of the bands from amplifications using primers specific for S16 ribosomal RNA.
Doxycycline Treatment Results in Neovascularization and Traction Retinal Detachment in rho/rtTA-TRE/VEGF or IRBP/rtTA-TRE/VEGF Double-Transgenic Mice
Untreated rho/rtTA-TRE/VEGF or IRBP/rtTA-TRE/VEGF mice had normal retinas with no evidence of neovascularization (Figure 2A ▶ , and Figure 3, A and B ▶ ). Mice at several ages were examined and through 5 weeks of age (Figure 3B) ▶ . GSA-stained retinas showed completely normal superficial, intermediate, and deep capillary beds and no ectopic vessels. Both types of double-transgenic mice were treated with 10 mg/ml of doxycycline in their drinking water and sacrificed between 4 and 10 days later. Four days after starting doxycycline, both rho/rtTA-TRE/VEGF and IRBP/rtTA-TRE/VEGF mice showed neovascularization originating from the deep capillary bed and extending into the photoreceptor layer. In some mice, the appearance was very similar to that seen in V6 rho/VEGF mice (Figure 2, I and J) ▶ . 20 At 5 or 6 days after initiation of doxycycline, several different phenotypes were seen including mild neovascularization (Figure 3D) ▶ such as that in V6 rho/VEGF mice, severe neovascularization with thick cords of vascular cells extending into and disrupting the photoreceptor layer (Figure 3, E and F ▶ , arrows) resulting in outer retinal folds and focal retinal detachments (asterisks), partial retinal detachments (Figure 2C) ▶ , or extensive traction retinal detachments (Figure 2; E to H ▶ , and Figure 3; G to J ▶ ). Four eyes examined at 8 to 10 days after initiation of doxycycline all had retinal detachments (Figure 2, G and H ▶ ; Figure 3, I and J ▶ ).
Figure 2.

Treatment with doxycycline in rho/rtTA-TRE/VEGF mice results in retinal neovascularization and traction retinal detachment. At postnatal day 21, 10 mg/ml of doxycycline was put in the drinking water of rho/rtTA-TRE/VEGF double-transgenic mice (n = 10). The top right of each panel shows the number of days after starting doxycycline. A: At postnatal day 21, the retina of a double transgenic that was not treated with doxycycline stained with GSA shows normal retinal vessels containing brown reaction product. Note the normal superficial, intermediate, and deep capillary beds and complete lack of vessels in the photoreceptor layer. B: Four days after addition of 10 mg/ml of doxycycline to the drinking water, there are new blood vessels extending from the deep capillary bed into the outer nuclear layer (arrow). The retina is stained with GSA and counterstained with hematoxylin. C: Five days after addition of 10 mg/ml of doxycycline to the drinking water, there is a peripheral retinal detachment. D: High-power view of the posterior retina of the same eye shown in C shows several new vessels extending from the deep capillary bed into the outer nuclear layer with associated folds indicating traction. E: Five days after addition of 10 mg/ml of doxycycline to the drinking water of another rho/rtTA-TRE/VEGF transgenic, there is a total retinal detachment. F: High-power view of the retina of another double transgenic 5 days after starting doxycycline shows degeneration and disorganization of the detached retina with vessels equally prominent in the outer and inner retina. G: Eight days after starting doxycycline, the retina is totally detached, but not highly elevated. H: High-power view of the retina of the eye shown in G shows a mass of vascular cells in the subretinal space. Note that in this shallowly detached retina, the retinal layers are still relatively intact, although in some areas portions of the outer retina are obscured by vascular cells. I: An adult (postnatal day 50) rho/VEGF mouse from the V6 line shows a completely attached retina. Traction retinal detachments have never been seen in these mice that express VEGF in photoreceptors. J: A high-power view of the retina of the eye shown in I demonstrates numerous blood vessels extending from the deep capillary bed through the outer nuclear layer into the subretinal space (arrows). Despite a few outer retinal folds, there is no significant traction and no retinal detachment.
Figure 3.

Treatment with doxycycline in IRBP/rtTA-TRE/VEGF mice results in retinal neovascularization and traction retinal detachment. At postnatal day 21, 10 mg/ml of doxycycline was put in the drinking water of IRBP/rtTA-TRE/VEGF double-transgenic mice (n = 10). The top right of each panel shows the age of untreated mice or the number of days after starting doxycycline. A: At postnatal day 28, the retina of a double transgenic that was not treated with doxycycline stained with GSA shows normal retinal vessels containing brown reaction product. Note the normal superficial, intermediate, and deep capillary beds and complete lack of vessels in the photoreceptor layer. B: At postnatal day 35 in an untreated double transgenic, there are normal retinal vessels and no neovascularization. C: Four days after addition of 10 mg/ml of doxycycline to the drinking water, there is neovascularization originating from the deep capillary bed of the retina and beginning to invade the photoreceptor layer. The retina is stained with GSA and counterstained with hematoxylin. D: Five days after starting doxycycline, there is a new vessel extending from the deep capillary bed into the photoreceptor layer (arrow). The section is stained with GSA and there is no counterstain. E: Another double transgenic 5 days after starting doxycycline shows folding of the outer retina associated with vascular invasion (arrows) and a focal retinal detachment (asterisk). F: Six days after initiation of doxycycline, this double transgenic shows large clumps of vascular cells disrupting the outer retina (arrow) causing folds and focal detachment (asterisk). G: Six days after addition of 10 mg/ml of doxycycline to the drinking water, this double transgenic has a total retinal detachment that is pulled up to the back of the lens similar to the pathological appearance of retinas from infants with severe stage 5 retinopathy of prematurity, although the neovascularization is not primarily along the inner surface of the retina as is true in retinopathy of prematurity. H: High-power view of the retina of the same eye shown in G demonstrates folding of the retina on itself, marked disorganization of the layers of the retina, and extensive neovascularization throughout the outer retina. I: Ten days after starting doxycycline, this double transgenic has a partial retinal detachment with prominent folding of the detached retina. J: High-power view of the retina of the eye shown in I shows new vessels throughout the entire retina with clumps of vascular cells in the subretinal space.
Variation of Phenotype with Dose of Doxycycline in IRBP/rtTA-TRE/VEGF Mice
To determine whether the severity of the phenotype in IRBP/rtTA-TRE/VEGF double transgenics could be altered by decreasing the dose of doxycycline, adult mice were divided into four groups and given a daily subcutaneous injection of 0.01, 0.05, 0.1, or 0.5 mg/g body weight. The doxycycline was delivered by injection rather than oral administration to better control the dose provided. Mice were sacrificed at 8 days after initiation of the injections. Gross pathological examination of eyes and microscopic evaluation of serial sections stained with GSA showed that the incidence of retinal detachment increased from 0 of 10 mice treated with 0.01 mg/g body weight/day to 17 of 18 mice treated with 0.5 mg/g body weight/day (Figure 4) ▶ . The remaining mouse in the 0.5 mg/g group had retinal neovascularization without retinal detachment and therefore 100% of the mice in the high-dose group had an abnormal phenotype. The percentage of mice with neovascularization and no detachment was greatest in the 0.05 mg/g group and decreased in the 0.1 mg/g and 0.5 mg/g groups in which the vast majority of mice that had neovascularization also had retinal detachment. Therefore, both the incidence of an abnormal phenotype and its severity increased as the dose of doxycycline was increased.
Figure 4.
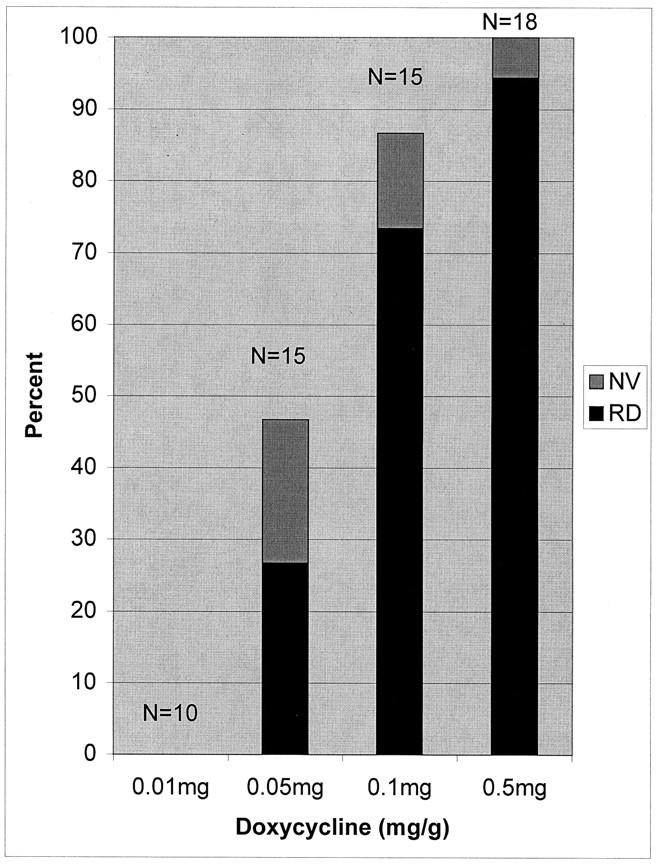
Increase in severity of phenotype with increase in dose of doxycycline in IRBP/rtTA-TRE/VEGF double-transgenic mice. At postnatal day 21, IRBP/rtTA-TRE/VEGF double-transgenic mice were given daily subcutaneous injections of 0.01, 0.05, 0.1, or 0.5 mg/g body weight of doxycycline, and after 8 days the mice were sacrificed. The eyes were frozen in OCT, sectioned, and stained with GSA. Each bar shows the percentage of mice that had neovascularization and retinal detachment (RD, black bar) and the percentage that had neovascularization without retinal detachment (NV, gray bar).
Correlation of VEGF mRNA Level and Phenotype in Double Transgenics with Inducible Expression of VEGF
Double-transgenic rho/rtTA-TRE/VEGF or IRBP/rtTA-TRE/VEGF mice were treated with daily subcutaneous injections of one of several doses of doxycycline and after 8 days the mice were sacrificed and eyes were removed. Retinal RNA was isolated from one eye of each mouse and histopathological evaluation was performed on the opposite eye. The level of retinal human VEGF mRNA assessed by real time RT-PCR in wild-type mice was undetectable. Double-transgenic mice that did not receive doxycycline or those that received 0.01 mg/g body weight/day had relative copy numbers of VEGF mRNA in the range of 1000 to 10,000 (Figure 5) ▶ . In general, mice treated with higher doses of doxycycline (0.1 and 0.5 mg/g body weight/day) had higher levels of VEGF mRNA in the retina than those treated with lower doses (0.01 or 0.05 mg/g body weight/day).
Figure 5.
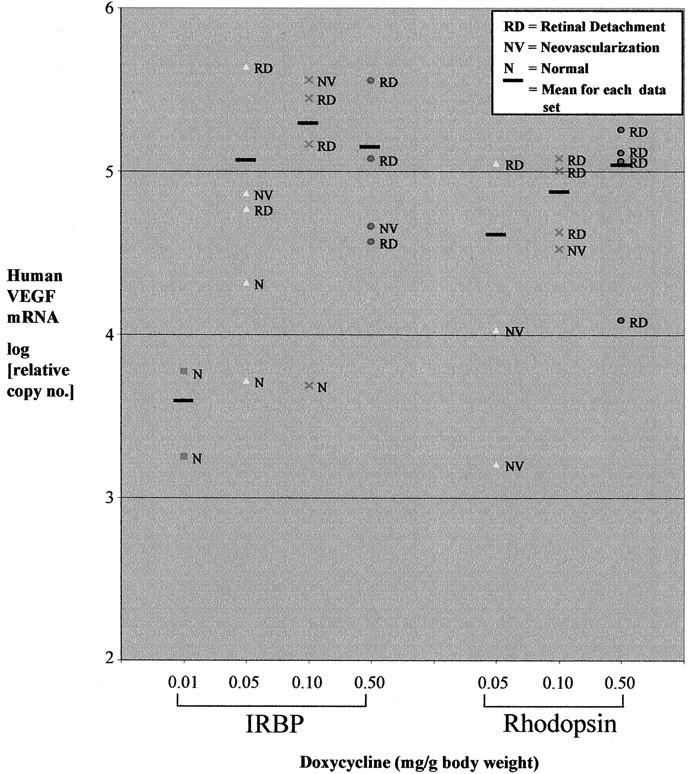
Correlation between dose of doxycycline, ocular VEGF mRNA level, and phenotype in the fellow eye of double-transgenic mice. A: Adult IRBP/rtTA-TRE/VEGF (IRBP) or rho/rtTA-TRE/VEGF (rhodopsin) double-transgenic mice were given daily subcutaneous injections of 0.01, 0.05, 0.10, or 0.5 mg/g body weight of doxycycline and sacrificed after 8 days. One eye was processed for frozen sections and the retina from the other eye was dissected and total RNA was isolated and the amount of human VEGF mRNA was quantified by real time RT-PCR. Each point shows the relative human VEGF cDNA amount reverse transcribed from 1 μg of RNA from a single mouse retina determined by linear regression through comparison of a dilution set for each sample to a dilution series generated with standard linearized plasmid VEGF cDNA. Adjacent to each point is the abbreviation for the phenotype (see key) of the fellow eye determined by microscopic analysis of serial sections stained with GSA. The horizontal line indicates the mean VEGF mRNA level for each group.
In the IRBP/rtTA-TRE/VEGF mouse group, two mice were treated with 0.01 mg/g body weight/day of doxycycline and both had low levels of retinal VEGF mRNA in one eye and a normal phenotype in the other eye. Five mice were treated with 0.05 mg/g body weight/day of doxycycline; two of these mice had low levels of retinal VEGF mRNA in one eye and a normal phenotype in the other eye and the other three had high levels of retinal VEGF mRNA in one eye and neovascularization in the other eye with two of the three having retinal detachment. Four of five mice treated with 0.10 mg/g body weight of doxycycline had a high level of VEGF mRNA in one eye and a dramatic phenotype in the other eye, whereas the other mouse had a low level of VEGF mRNA in one eye and a normal phenotype in the other eye. Four of four mice treated with 0.5 mg/g body weight/day of doxycycline had a high level of VEGF mRNA in one eye and a dramatic phenotype in the opposite eye.
Fewer rho/rtTA-TRE/VEGF mice were available and therefore none were treated with 0.01 mg/g body weight/day of doxycycline and only three doses starting with 0.05 mg/g body weight/day were examined. All of the mice showed a phenotype and although the numbers were small, as the dose of doxycycline increased, there was an increase in both average VEGF mRNA level and percentage of mice with retinal detachment. Therefore, in both types of double-transgenic mice, there was a positive correlation between dose of doxycycline, level of retinal VEGF mRNA, and severity of the phenotype. The level of retinal VEGF mRNA was assessed in six rho/VEGF mice and the mean (±SEM) relative copy number was 23,860 ± 1633.
Ocular VEGF Protein Levels in rho/VEGF Mice and Mice with Inducible Expression of VEGF
Hemizygous rho/VEGF mice (n = 5 mice, 10 eyes) were sacrificed and eye homogenates were prepared at postnatal day 21, a time when VEGF mRNA levels are at steady-state and there is extensive retinal neovascularization. 20,25 Measurement of ocular VEGF protein levels in the retina in all 10 rho/VEGF mice was below the level of detection of the assay (16 pg/ml, Figure 6 ▶ ). At postnatal day 21, double-transgenic rho/rtTA-TRE/VEGF (n = 10) and IRBP/rtTA-TRE/VEGF mice (n = 10) were given daily subcutaneous injections of 0.5 mg/g body weight of doxycycline and after 3 days the mice were sacrificed and eye homogenates were prepared. The mean ocular VEGF level was 189.3 pg/μg of protein in rho/rtTA-TRE/VEGF mice and 823.5 pg/μg protein in IRBP/rtTA-TRE/VEGF, both significantly greater than levels in V6 rho/VEGF transgenic mice.
Figure 6.

Ocular VEGF levels in three transgenic mouse models. Mice from the V6 line of rho/VEGF transgenic mice (V6) were sacrificed at postnatal day 21 and eye homogenates were prepared. At postnatal day 21 double-transgenic rho/rtTA-TRE/VEGF (Opsin) and IRBP/rtTA-TRE/VEGF mice (IRBP) were treated with 0.5 mg/g body weight of doxycycline by subcutaneous injection for 3 days and then sacrificed. Eye homogenates were prepared, protein levels were measured, and then VEGF levels were measured by enzyme-linked immunosorbent assay as described in Materials and Methods. Bars represent the mean (± SEM) level of VEGF per μg total protein calculated from eye homogenates in each group.
Discussion
Increased expression of VEGF in photoreceptors beginning at postnatal day 5 to postnatal day 6 in rho/VEGF mice results in neovascularization that originates from the deep capillary bed of the retina and grows into the subretinal space mimicking many aspects of choroidal neovascularization. 20 This demonstrates that in neonatal mice, increased expression of VEGF in the retina is sufficient to cause neovascularization. In this study, we have extended those findings by using an inducible promoter system to demonstrate that increased expression of VEGF is also sufficient to cause neovascularization in the retina of adult mice. We have recently initiated doxycycline in adult mice ranging from 1 to 6 months of age and like rho/VEGF mice, the origin of the neovascularization at all ages was from the deep capillary bed of the retina. This suggests that the differential sensitivity of the deep capillary bed to increased expression of VEGF is not related to developmental stage, because that sensitivity is also present in adult mice.
Although the origin of neovascularization in mice with inducible expression of VEGF is the same as that in rho/VEGF mice, the resulting phenotype is more severe. Three to 4 days after initiation of doxycycline, particularly in mice treated with 0.05 to 0.1 mg/g body weight, the neovascularization is similar to that seen in rho/VEGF mice. By 5 to 6 days after onset of doxycycline, many mice treated with low doses and almost all mice treated with high doses showed cords of vascular cells invading the outer nuclear layer that became very thick and exerted traction that caused outer retinal folds, focal detachments, and then total detachments. During the advanced stages, neovascularization spreads throughout the subretinal space and all parts of the retina resulting in disorganized retina and large masses of vascular cells. One difference between rho/VEGF and rho/rtTA-TRE/VEGF and IRBP/rtTA-TRE/VEGF mice that is likely to contribute to the difference in phenotype is that the double transgenics, when treated with appropriate doses of doxycycline, express much higher levels of VEGF.
In addition to demonstrating that increased expression of VEGF is sufficient to cause retinal neovascularization in adult mice, rho/rtTA-TRE/VEGF and IRBP/rtTA-TRE/VEGF mice provide a new animal model that is complementary to rho/VEGF mice. The severe phenotype of the inducible transgenics provides a more stringent test of efficacy for anti-angiogenic agents. We have identified treatments that completely inhibit neovascularization in rho/VEGF transgenics. 13,26 By extending the range of disease severity for testing, the inducible transgenics provide a possible means of distinguishing between treatments that have maximum efficacy in the models with a less severe phenotype. Also, the inducible transgenics provide another outcome variable that may simplify testing. If treatments are effective, they should prevent retinal detachments, which can be rapidly detected. Alteration of the incidence of retinal detachment at a particular dose of doxycycline may provide a rapid assay for anti-angiogenic efficacy that obviates the need for time consuming image analysis. Finally, the inducible transgenics offer more flexibility in experimental design because the time of onset and the severity of disease can be regulated via the time of initiation of doxycycline treatment and the dose administered. Some experiments that are not possible with rho/VEGF mice can be done with the inducible transgenics. For instance, the long latency of recombinant adenoassociated virus (AAV) vectors makes it impossible to use AAV to express genes in the eye before the onset of neovascularization in rho/VEGF mice to determine whether expression of those genes prevents neovascularization. In the inducible transgenics, it is possible to inject expression constructs packaged in AAV and wait as long as necessary before turning on expression of VEGF.
Therefore, mice with inducible expression of VEGF in photoreceptors provide a valuable new animal model that will help to explore the pathogenesis of ocular neovascularization and identify new ways to treat this blinding complication of ocular diseases.
Footnotes
Address reprint requests to Peter A. Campochiaro, M.D., Maumenee 719, The Johns Hopkins University School of Medicine, 600 N. Wolfe St., Baltimore, MD 21287-9277. E-mail: pcampo@jhmi.edu.
Supported by the National Eye Institute (grants EY05951, EY12609, EY00444, EY0331, and K08 EY13420 to P. G.); Research to Prevent Blindness (core grant P30EY1765), a Lew R. Wasserman Merit Award (to P. A. C.), a Career Development Award (to D. J. Z.), and an unrestricted grant; the Foundation Fighting Blindness, the Ruth and Milton Steinbach Foundation, the Juvenile Diabetes Research Foundation (to P. G.), Michael Panitch, and Dr. and Mrs. William Lake. P. A. C. is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience. D. B. is the Dolly Green Professor of Ophthalmology at UCLA.
References
- 1.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843-845 [DOI] [PubMed] [Google Scholar]
- 2.Plate KH, Breier G, Welch HA, Risau W: Vascular endothelial growth factor is a potential tumor angiogenesis factor in human gliomas in vivo. Nature 1992, 359:845-848 [DOI] [PubMed] [Google Scholar]
- 3.Adamis AP, Miller JW, Bernal M-T, D’Amico DJ, Folkman J, Yeo T-K, Yeo K-T: Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994, 118:445-450 [DOI] [PubMed] [Google Scholar]
- 4.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen MS, Aiello LM, Ferrara N, King GL: Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994, 331:1480-1487 [DOI] [PubMed] [Google Scholar]
- 5.Malecaze F, Clamens S, Simorre-Pinatel V, Mathis A, Chollet P, Favard C, Bayard F, Plouet J: Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol 1994, 112:1476-1482 [DOI] [PubMed] [Google Scholar]
- 6.Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E: Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest 1995, 72:638-645 [PubMed] [Google Scholar]
- 7.Miller JW, Adamis AP, Shima DT, D’Amore PA, Moulton RS, O’Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, Yeo T-K, Yeo K-T: Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol 1994, 145:574-584 [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH: Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 1995, 92:905-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LEH: Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995, 92:10457-10461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LES: Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci USA 1996, 93:4851-4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D’Amore PA, Miller JW: Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization. Arch Ophthalmol 1996, 114:66-71 [DOI] [PubMed] [Google Scholar]
- 12.Seo M-S, Kwak N, Ozaki H, Yamada H, Okamoto N, Fabbro D, Hofmann F, Wood JM, Campochiaro PA: Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol 1999, 154:1743-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozaki H, Seo M-S, Ozaki K, Yamada H, Yamada E, Hofmann F, Wood J, Campochiaro PA: Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol 2000, 156:679-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwak N, Okamoto N, Wood JM, Campochiaro PA: VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci 2000, 41:3158-3164 [PubMed] [Google Scholar]
- 15.Lutty GA, McLeod SD, Merges C, Diggs A, Plouet J: Localization of vascular endothelial growth factor in human retina and choroid. Arch Opthalmol 1996, 114:971-977 [DOI] [PubMed] [Google Scholar]
- 16.Vinores SA, Youssri AI, Luna JD, Chen Y-S, Bhargave S, Vinores MA, Schoenfeld C-L, Peng B, Chan C-C, LaRochelle W, Green WR, Campochiaro PA: Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathology 1997, 12:99-109 [PubMed] [Google Scholar]
- 17.Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP: Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol 1996, 114:964-970 [DOI] [PubMed] [Google Scholar]
- 18.Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP: Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 1996, 103:1820-1828 [DOI] [PubMed] [Google Scholar]
- 19.Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K: Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res 1997, 64:505-517 [DOI] [PubMed] [Google Scholar]
- 20.Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, Zack DJ, Campochiaro PA: Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997, 151:281-291 [PMC free article] [PubMed] [Google Scholar]
- 21.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H: Transcriptional activation by tetracyclines in mammalian cells. Science 1995, 268:1766-1769 [DOI] [PubMed] [Google Scholar]
- 22.Chang MA, Horner JW, Conklin BR, DePinho RA, Bok D, Zack DJ: Tetracycline-inducible system for photoreceptor-specific gene expression. Invest Ophthalmol Vis Sci 2000, 41:4281-4287 [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 24.Simpson DA, Feeney S, Boyle C, Stitt AW: Retinal VEGF mRNA measured by SYBR Green I fluorescence: a versatile approach to quantitative PCR. Molec Vision 2000, 6:178-183 [PubMed] [Google Scholar]
- 25.Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA: Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmol Vis Sci 1998, 39:180-188 [PubMed] [Google Scholar]
- 26.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA: Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol 2001, 188:253-263 [DOI] [PubMed] [Google Scholar]



