Abstract
Oxidative modification of low-density lipoprotein is thought to promote arterial lipid accumulation and atherogenesis. Previous studies reported on the presence of certain lipid or protein oxidation products in lesions, although a systematic investigation measuring several oxidation parameters and the accumulation of nonoxidized lipids and antioxidants at various stages of atherosclerosis has not been performed in the same tissue. Using the intimal lipoprotein-containing fraction of human aortic lesions, we demonstrate here that cholesterol accumulated with lesion development and that this increase was already significant at the fatty streak stage. By comparison, cholesterylesters increased significantly only in fibro-fatty and more complex lesions that also contained significantly increased amounts of cholesterylester hydro(pero)xides and 27-hydroxycholesterol. Cholesterylester hydroxides were the major lipid oxidation product detected. Despite accumulation of oxidized lipid, α-tocopherol was also present and maintained at a comparable level over the disease process. Of the oxidized protein moieties measured only o,o-dityrosine increased with disease, although chlorotyrosines were present at relatively high levels in all lesions compared to healthy vessels. Our data show that accumulation of nonoxidized lipid precedes that of oxidized lipid in human aortic lesions.
The development of atherosclerosis to clinical manifestations is a protracted process that spans several decades. It is well established that lipids, specifically cholesterol, accumulate as the disease progresses 1 and that these lipids are derived from plasma low-density lipoprotein (LDL). Aberrant uptake of LDL and its associated lipids by intimal monocytes that have infiltrated from the blood is believed to generate foam cells and thereby initiate atherosclerosis. 2 Foam cells are the hallmark of the fatty streak/early lesion from which the more advanced and complicated lesions derive. In advanced lesions, a fibrous cap is formed from collagen and a lipid-rich necrotic core. Vessel lumen narrowing because of the enlarged fibrous cap and plaque rupture lead to clinical manifestations.
The definitive mechanisms leading to the initiation and development of atherosclerosis are unknown. However, retention of LDL in the vessel wall because of interaction and complex formation with extracellular matrix components is well-documented 3 and is thought to be important. Further, a bulk of evidence suggests that oxidative modifications to LDL contribute to its retention. 4-6 Both arterial wall retention and oxidative modification of LDL are thought to contribute to foam cell formation.
In vitro studies with plasma LDL have shown that oxidative modifications proceed in different stages and involve changes to LDL’s antioxidants, lipids, and protein components. The nature and extent of these alterations vary depending on the type of oxidant involved, the oxidant to lipoprotein ratio used, and the extent to which oxidation is allowed to proceed. Originally 4 the pathway was described by the sequential depletion of endogenous antioxidants including α-tocopherol (α-TOH), followed by the formation of lipid hydroperoxides and their fragmentation to aldehydes and other reactive secondary products of lipid oxidation. The latter can modify apolipoprotein B-100 so that the altered particle becomes a ligand for the scavenger receptor, 7 and in vitro exposure of macrophages to such oxidized LDL can lead to foam cell formation. 8 More recently, it has become apparent that not all oxidants convert the lipoprotein along the above pathway. Thus, hypochlorite (a two-electron oxidant produced by myeloperoxidase) generates a high-uptake LDL by primarily oxidizing the protein component, although some consumption of α-TOH and formation of lipid hydroperoxides is always observed. 9 The fact that oxidized high-uptake LDL can be formed via different pathways raises questions of their relative importance and, hence, the extent of lipid versus protein modification during the oxidative modification of LDL in vivo.
Whether in vivo LDL lipid peroxidation proceeds beyond the depletion of α-TOH, LDL’s major antioxidant, is unclear. This is an important issue, as secondary lipid oxidation products including aldehydes are not formed to any substantial extent in the presence of the vitamin. 10 Low α-TOH to cholesterol content has been reported in advanced lesions, 11 although our earlier studies with advanced human lesions showed normal levels of α-TOH per cholesteryl ester (CE). 12,13 Expressing α-TOH per CE is more appropriate as the vitamin scavenges peroxyl radicals produced during the peroxidation of fatty acids containing bisallylic hydrogen; 14 CE represent the major source of such fatty acids in lipoproteins and lesions, whereas cholesterol does not contain bisallylic hydrogen.
The mechanisms responsible for oxidation of LDL within the vessel wall remain undefined. Several oxidants have been proposed to be involved, including macrophages, 15-lipoxygenase, myeloperoxidase and oxidants derived from its action, reactive nitrogen metabolites, NAD(P)H oxidases, and transition metals. 15 Unlike the situation with atherosclerosis in animals in which a gene knock-out approach can be used, much of the evidence supporting the involvement of the different oxidants in human atherosclerosis is derived from the detection of generic or specific oxidation products in different types of lesion materials. A problem with this approach has been that to date, the disease stage-dependent accumulation of several different lipid and protein oxidation products has not been studied systematically in the same tissue samples and together with the accumulation of nonoxidized lipids. We therefore examined various parameters that reflect the oxidation status of both lipid (CE, cholesterol) and protein residues (tyrosine (Tyr), phenylalanine (Phe), and the content of lipophilic antioxidants (α-TOH, ubiquinol-10) in the intimal lipoprotein-containing fraction of human aortic lesions spanning atherosclerotic disease development.
Materials and Methods
Materials
Cholesteryl linoleate (C18:2), cholesteryl arachidonate (together referred to as CE), and coenzyme Q9 (ubiquinone-9) were purchased from Sigma Chemical Co.(St. Louis, MO). d,l-α-TOH was purchased from Eastman-Kodak (Rochester, NY). [3H]C18:2 [cholesteryl-1,2,6,7-3H(N)], 91.5 Ci/mmol, was from Dupont-NEN (Boston, MA). (25R)-Cholest-5-en-3β-26-diol (27HC) was from Research Plus Inc. (Bayonne, NJ) and cholest-5-en-3β-ol-7-one (7KC) was from Steraloids Inc. (Wilton, NH). Organic solvents of HPLC quality were obtained from Mallinckrodt (Clayton, Australia) and Merck (EM Science, Gibbstown, NJ). Before use, all aqueous solutions were stored over Chelex-100 (Bio-Rad Laboratories, Richmond, CA) to remove contaminating transition metals. All other chemicals were of the highest available purity and nanopure water (Millipore Systems, Australia) was used throughout.
Specimens
Postmortem materials were obtained from 20- to 66-year-old patients from the NSW Institute of Forensic Medicine, Sydney, Australia, with the approval of the local Human Ethics Review Committee. Aorta were resected within 24 hours of death and washed in ice-cold 50 mmol/L phosphate buffer containing 3-aminotriazole (10 mmol/L), diethylenetriamine pentaacetic acid (0.1 mmol/L), and butylated hydroxytoluene (20 μmol/L), pH 7.4 (buffer A). Aortas were stored in buffer A at −80°C.
Lesion Classification
Lesion stages were initially classified macroscopically according to Stary and colleague’s 16 nomenclature and definition. The descending thoracic and abdominal aorta from a total of 35 patients was resected and designated into four distinct groups; initial lesions (Stary classes I-II), fatty streak lesions (II-III), fibro-fatty lesions (IV-V), and complex/ulcerated lesions (V-VI), representing the spectrum of atherosclerosis. A tissue section from each specimen was removed and fixed in 10% formalin in phosphate-buffered saline and transferred to 70% (v/v) ethanol for histology, using hematoxylin and eosin staining (Table 1) ▶ . Remaining aortas were then stored in buffer A at −80°C until used.
Table 1.
Comparison of Macroscopic and Histology Classification of Aortic Lesions
| Stary classification group | Intima, n | Age range, years | Macroscopic assessment | Histology versus macroscopic assessment (% agreement) |
|---|---|---|---|---|
| I–II | 24 | 20–64 | No/initial lesions | 88 |
| II–III | 24 | 20–66 | Fatty streak lesions | 79 |
| IV–V | 18 | 35–66 | Fibro-fatty lesions | 83 |
| V–VI | 11 | 35–66 | Ulcerated/complex lesions | 91 |
Sample Preparation
Sample preparation was essentially as described. 17 Briefly separated intima was weighed and tissue samples from the same group pooled to yield ∼10 g. Each 10-g pool represented a single sample (n = 1) for analysis. Intimas were frozen in liquid N2, pulverized, and reconstituted in 50 mL of buffer B [10 mmol/L phosphate/0.15 mol/L NaCl, pH 7.4, containing 0.3 mmol/L ethylenediaminetetraacetic acid, 0.1 mmol/L diethylenetriamine pentaacetic acid, 10 mmol/L 3-aminotriazole, 20 μmol/L butylated hydroxytoluene, 1 mmol/L phenylmethyl sulfonyl fluoride, 0.002% (w/v) elastatinal, 2 mmol/L benzamidine, 1 μmol/L d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone, 0.008% (w/v) gentamicin, 0.008% chloramphenicol, and 10 μmol/L reduced glutathione]. Ubiquinol-9 (5 μmol/L) prepared from ubiquinone-9, 18 and α-tocotrienol (1 μmol/L 12 ) were also added as internal standards for estimation of recoveries during the processing steps. The samples were gently agitated end-over-end overnight in the dark at 4°C and then centrifuged at 2750 × g for 30 minutes at 4°C in a swing-bucket centrifuge. The resulting tissue supernate (SN-1) was further ultracentrifuged at 100,000 × g for 30 minutes at 4°C, the pellet and the top lipemic layer discarded, and a tissue supernate (SN-2) collected for subsequent analyses. SN-2 contains all lesion lipoproteins as it represents the starting material for sequential floatation ultracentrifugation. 17
Aortic lesion SN-2 samples (<500 μL) were added to hexane:methanol (5:1, v/v) for extraction of CE hydro(pero)xides (CE-O(O)H), cholesterol, CE, and α-TOH and the hexane phase reconstituted in propan-2-ol and subjected to HPLC as described previously. 19,20 For oxysterol analyses aliquots (500 μL of SN-2) were extracted with chloroform/methanol (2:1, v/v) with added 19-hydroxycholesterol as an internal standard (5 μg/tube). The chloroform phase was evaporated and transferred with diethyl ether (2.5 mL) to a screw-cap tube before cold alkaline saponification. Total cholesterol was also determined in separate aliquots (50 μL) after saponification initiated by a methanolic KOH solution (20% w/v, 2.0 mL). Cholesteryl propyl ether was used as an internal standard (5 μg/tube). The tubes were flushed with argon and the contents mixed overnight at 4°C before water (2.0 mL) and hexane (2.5 ml) were added. The tubes were then mixed for 30 seconds and centrifuged at 1600 × g for 5 minutes at 10°C. Oxysterol and cholesterol in the ether/hexane phase were then determined.
To account for inadvertent oxidation occurring during aortic lesion work-up, [3H]C18:2 (50 μCi) was added to four separate aortic (∼1 g wet weight) lesions. The lesions were subjected to the tissue and analyte extraction processes described above in buffer B (5 mL). Radiolabeled C18:2 and/or C18:2-O(O)H fractions were identified by RP HPLC coupled with on-line radiometric detection.
The hydroxylation product of Tyr (3,4-dihydroxyphenylalanine; DOPA) and those of Phe (o-Tyr, m-Tyr) and o,o-dityrosine (diTyr) in aortic tissue were determined as described previously. 21 Briefly SN-2 aliquots (900 μL) were mixed with sodium deoxycholate (100 μL, 0.3% w/v) and trichloroacetic acid (100 μL, 50% w/v). The delipidated protein samples were then freeze-dried and subjected to gas phase, acid-catalyzed hydrolysis using 6 N HCl containing mercaptoacetic acid (5% v/v) and phenol (1% v/v) under anaerobic conditions. Freeze-dried hydrolysates were reconstituted in water (100 μL) and filtered (0.22 μm) before HPLC. 21
HPLC Analyses
CE-O(O)H, cholesterol, CE, and α-TOH in lesion supernates were quantified using RP HPLC coupled with UV, electrochemical, and postcolumn chemiluminescence detection as described. 19,20 For detection of radiolabeled compounds the above HPLC conditions were combined with on-line radiometric detection and a scintillant-to-flow ratio of 1.
Oxysterols were separated by normal phase HPLC using an Alltima Silica column (0.46 × 15 cm; 100 Å, 5 μm) with a 3-cm guard column (Ultremex, 3-μm particle size; Phenomenex, Thornleigh, Australia) and eluted with hexane/propan-2-ol/acetonitrile (94.8/4.6/0.6, v/v/v) at 1 mL/min. The mobile phase was sparged with helium during analysis and lipids detected at 210 and 234 nm. Total cholesterol was determined by RP HPLC using propan-2-ol:acetonitrile: water (54:44:2, v/v/v). All chromatography assignment and quantification of individual peaks was based on co-elution and area comparison with authentic standards.
DOPA, m-Tyr, o-Tyr, and diTyr in hydrolysates were isolated on a Zorbax ODS column at 1 mL/min elution and a gradient of solvent A (100 mmol/L sodium perchlorate in 10 mmol/L sodium phosphate buffer, pH 2.5) and solvent B (80% methanol in water) as described. 22 The eluant was monitored by in-series UV (280 nm) and fluorescence detection (280 nm excitation for all components and emission wavelength of 320 nm for DOPA, m-Tyr and o-Tyr and 410 nm for diTyr). The identity and quantity of representative samples were checked by electrochemical detection 23 and mass spectroscopy. 21,22 Artifactual formation of m-Tyr, o-Tyr, DOPA, and diTyr during protein hydrolysis of atherosclerotic tissue using the methodology described herein has been investigated in detail. 21,23,24 The maximum artifactual formation of the analytes determined by this method consistently ranges from 20 μmol/mol of m-Tyr, o-Tyr, and diTyr to 200 μmol/mol of DOPA per parent amino acid.
Gas Chromatography/Mass Spectroscopy (GC/MS) Determination of Chlorinated Tyrosine
3-Chlorotyrosine (ClTyr) and 3,5-dichlorotyrosine (diClTyr) were determined by stable isotopic dilution GC/MS as described. 25 Briefly, samples were dried and proteins hydrolyzed using methanesulfonic acid (4 mol/L) containing 1% (v/v) phenol (100 μL). The hydrolysate was diluted in 0.1% trifluoroacetic anhydride (200 μL) and purified using solid phase extraction. Tyr, ClTyr, and diClTyr were eluted using 80% methanol containing 0.1% trifluoroacetic anhydride (1.1 mL) and dried. Amino acids were derivatized with n-propanol/HBr (3.5 mol/L) (300 μL) and then with trifluoroacetic anhydride/ethyl acetate (1:4, v/v) (100 μL). Derivatized samples were finally diluted in ethyl acetate (300 μL) before GC/MS analysis. The initial column temperature of 65°C was maintained for 2 minutes and then increased to 150°C (30°C/minute), 170°C (4°C/minute), 200°C (5°C/minute), and to 260°C (30°C/minute). The injector, auxiliary channel, and ion source temperatures were set to 250, 280, and 150°C, respectively. Pulsed splitless injection was used with selective ion monitoring. The background levels of diClTyr and diTyr in bovine serum albumin determined by this method range from 15 to 40 μmol/mol. 25
Statistical Analysis
Kruskill-Wallis one-way analysis of variance on ranks and Mann-Whitney t-tests were used to evaluate significant differences at a P value 0.05 (two-tailed).
Results
We have determined previously the extent of lipid accumulation, lipid oxidation, and antioxidant status in advanced human atherosclerotic lesions, and have shown that α-TOH is not extensively depleted despite the co-existence of oxidized CE. 12,13 Herein we report on the characterization of antioxidants, lipid, oxidized lipid, and proteins from the intimal lipoprotein fraction of human aorta representing the earlier spectrum of the disease process.
Aortic intimal tissue was designated into four groups based on disease status according to Stary and colleagues: 16 I-II, initial lesions; II-III, fatty streaks; IV-V, fibro-fatty lesions; and V-VI, ulcerated/complex lesions (Table 1) ▶ . A total of 35 aortas were used, and all materials were obtained from patients ≥20 years old. Thereby the material in group I-II contained intima with diffuse thickening and initial lesions and is distinct from fatty streak lesions and normal artery tissue. 16 Consequently a large number (n = 24, Table 1 ▶ ) of individual tissue samples representing stage I-II were required to generate four separate pools of tissue for analysis. Macroscopic assessment enabled processing of the specimens before histology thereby averting a freeze/thaw cycle and potential generation of artifacts. Although visual assessment does not reflect intimal thickness we obtained >80% agreement (Table 1) ▶ , demonstrating that visual classification was useful and appropriate.
After low-temperature pulverization of the intima, the reconstituted tissue (SN-1) was subjected to centrifugation and removal of a lipemic layer to yield a tissue supernate designated SN-2. This supernate contains all tissue lipoproteins and thereby all analytes reported below reflect lipoprotein-associated parameters. Next we investigated the potential autoxidation of lipid (C18:2) and recovery of the internal standard, ubiquinol-9 to assess artifactual oxidation during sample work-up. Overall, tests with [3H]C18:2 (see Materials and Methods) indicated that the radiolabel eluted as unmodified C18:2 (n = 4), whereas on average, 60% (mean, n = 4) of added ubiquinol-9 was recovered as total CoQ9 in SN-2 (data not shown). The contents of lipid, both unmodified and oxidized, lipid-soluble antioxidants, and protein oxidation markers in SN-2 from the various lesions were then determined to compare relative changes in lipid, protein, and oxidation parameters in the disease process more systematically and comprehensively than reported previously.
An increase in cholesterol is a prominent early disease marker. 1 In agreement, the content of cholesterol per protein increased with increasing lesion severity, with significant changes noted in the early disease stages (Table 2) ▶ . Lipoprotein-associated CE (the sum of cholesteryl arachidonate and C18:2) also accumulated (Table 2) ▶ , although significant changes were not obtained until fibro-fatty lesions were present. The level of CE per cholesterol in lesion lipoproteins decreased in the most advanced lesions (V-VI).
Table 2.
Protein-Standardized Unmodified and Oxidized Lipid Contents in Aortic Lesions
| Lesion group, n | Protein | Chol | CE | CE-OOH | CE-O(O)H | 27HC | 7KC |
|---|---|---|---|---|---|---|---|
| I–II (4) | 1.51 (0.16) | 10.6 (1.2) | 13.1 (3.4) | ND | 0.10 (0.03) | 0.02 (0.02) | 0.03 (0.01) |
| II–III (8) | 1.43 (0.31) | 28.6* (7.1) | 34.2 (12.6) | ND | 0.18 (0.05) | 0.29 (0.12) | 0.05 (0.01) |
| IV–V (9) | 1.44 (0.13) | 79.9* (19.7) | 69.7* (18.6) | 20.5 (5.1) | 0.82* (0.30) | 0.64* (0.08) | 0.09 (0.02) |
| V–VI (7) | 1.49 (0.62) | 92.5* (29.3) | 43.6 (14.9) | 14.0 (5.9) | 1.00* (0.31) | 0.96* (0.13) | 0.14* (0.03) |
The protein, lipid, and oxidized lipid content in the lipoprotein-containing tissue supernate (SN-2) derived from the intima of aortic lesions were determined as described in Materials and Methods. Each n value represented a tissue pool (10 g) derived from up to six pieces of intima obtained from different subjects and overall a total of 35 human aortas were used. The data represent mean values (SEM) expressed as nmol/mg protein for all analytes except protein (mg/ml) and CE-OOH (pmol/mg). ND, none detected.
*P < 0.05 compared with group I–II.
In addition to increasing content of unmodified lipid, an accumulation of lipoprotein lipid oxidation products was also found with increasing disease severity. CE-containing polyunsaturated lipids are the primary targets for one-electron mediated oxidation, and oxidized CEs are found in lesions 12,13,26,27 but are essentially absent in plasma LDL. 13 CE hydroperoxides (CE-OOH) were not detected in early lesions, ie, stages I-III (Table 2) ▶ . However, they were detected in fibro-fatty and advanced lesions, albeit at much lower amounts (<2.5%) than the sum of CE hydroperoxides and hydroxides (CE-O(O)H) (Table 2) ▶ indicating that CE hydroxides (ie, CE-OH) were the major product. Similar to hydroperoxides, CE-O(O)H were significantly increased only in fibro-fatty and more complex lesions (≥stage IV) (Table 2) ▶ . We also measured 7KC as a marker of one-electron-mediated cholesterol oxidation and 27HC, an enzymatic product of the mitochondrial cytochrome P450 enzyme, sterol 27-hydroxylase. Of these, 27HC was present at higher levels than 7KC (Table 2) ▶ . Whereas 7KC content increased significantly only in the most complex lesions (group V-VI), disease stage-dependent increases in 27HC were significant in fibro-fatty lesions (Table 2) ▶ .
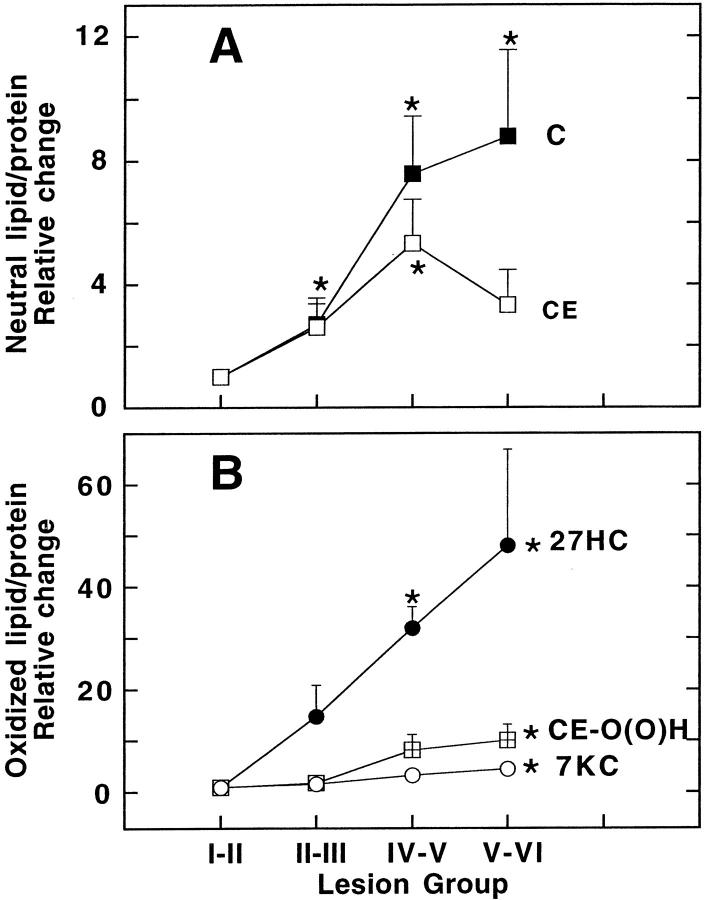
To assess the dependency of the changes in nonoxidized and oxidized lipids on lesion development, we expressed the content of each type of lipid relative to the corresponding parent molecule in the intima showing the least disease, ie, stage I-II (Figure 1) ▶ . Thus, cholesterol increased up to eightfold with lesion development (Figure 1A) ▶ . Also, the relative changes in CE were similar to cholesterol except in the most complex lesions where CE markedly decreased. Similar to unmodified lipid, lipoprotein-associated CE-O(O)H, 27HC, and 7KC increased significantly with lesion severity (Figure 1B) ▶ .
Figure 1.
Disease-stage-dependent changes in protein-standardized lipid and oxidized lipid in aortic lesions. Intimas were processed to yield a tissue supernate (SN-2) that contains all lesion lipoproteins as described in Materials and Methods. The content of both unmodified (A) and oxidized lipid (B) in SN-2 per protein (expressed as nmol/mg) was then determined by HPLC (Table 2) ▶ . Relative changes in protein-standardized cholesterol (C, ▪) and CE (□) CE-O(⊞)H (•), 27HC (○), and 7KC (μ) were expressed as a fraction of those observed in tissue containing no/initial lesions (group I-II). The data represent mean values ± SEM with asterisks indicating P < 0.05 compared with group I-II.
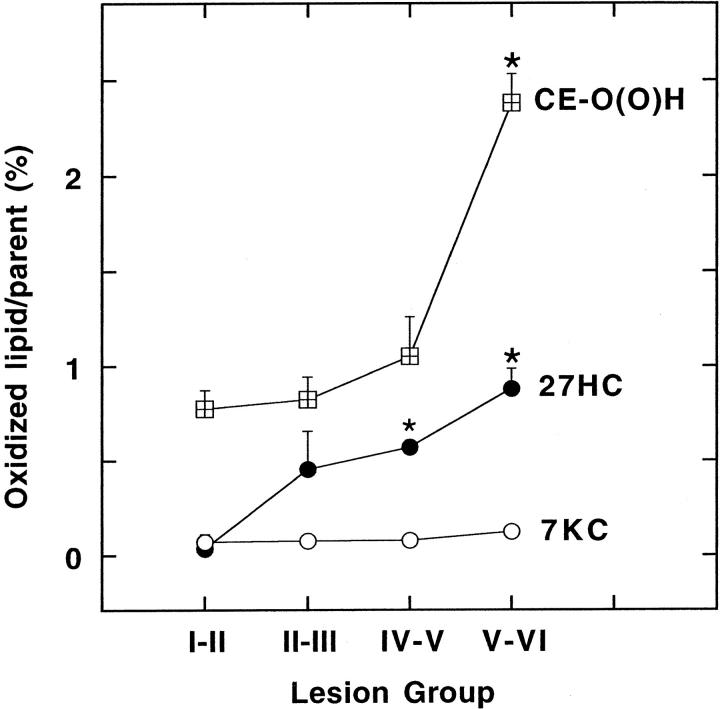
To distinguish the above changes in lipid load from the proportion of lipids oxidized, we next expressed the different oxidation products per respective parent molecule in a disease stage-dependent manner (Figure 2) ▶ . The amount of CE-O(O)H per CE increased significantly in advanced lesion stages (V-VI) and comprised 2.4% of the parent molecule (Figure 2) ▶ . Compared to CE-O(O)H however, the percentage of 27HC per parent molecule comprised 0.9% even at the most advances stages of the disease. Furthermore, the intimal content of 7K per total cholesterol did not increase significantly (Figure 2) ▶ .
Figure 2.
Content of parent molecule-standardized oxidized lipids in lesions. The extent to which individual lipids in lesion lipoproteins were oxidized was examined by measuring the content of CE-O(O)H per CE (⊞) and 27HC (•) and 7KC per total cholesterol (○) (expressed as percent of parent) in the various lesion groups. The data represent mean values ± SEM with asterisks indicating P < 0.05 compared with group I-II.
Despite the relatively large content of oxidized lipid, the lipid-soluble antioxidants α-TOH and ubiquinol-10 (measured as CoQ10) remained detectable at each disease stage (Table 3) ▶ . In fact, the intimal content of α-TOH expressed per protein was similar for most lesions except for a significantly elevated level in stages IV-V (Table 3) ▶ . In general, the α-TOH/CE ratio remained constant independent of lesion severity (Table 3) ▶ . CoQ10, the reduced form of which is an effective endogenous antioxidant for LDL lipid, 28 was present at 2 to 5 times lower concentrations than α-TOH and the levels of this antioxidant did not seem to alter with disease progression (Table 3) ▶ .
Table 3.
Disease Stage-Associated Changes in Lipid-Soluble Antioxidant Content in Aortic Lesions
| Lesion stage | α-TOH | CoQ10 | α-TOH/CE | α-TOH/cholesterol |
|---|---|---|---|---|
| I–II | 53 (31) | 33 (15) | 4.1 (1.5) | 4.8 (2.2) |
| II–III | 142 (79) | 45 (19) | 5.3 (0.9) | 4.9 (0.8) |
| IV–V | 347 (86)* | 75 (21) | 7.3 (2.5) | 5.4 (1.6) |
| V–VI | 148 (81) | 78 (33) | 3.5 (1.5) | 2.0 (0.9) |
α-TOH and CoQ10 content in the lipoprotein-containing tissue supernate (SN-2) derived from aortic lesions were determined by HPLC as described in Materials and Methods. The data represent mean values ± (SEM) and are expressed as pmol/mg protein or mmol/mol CE or cholesterol.
*P < 0.05 compared with group I–II.
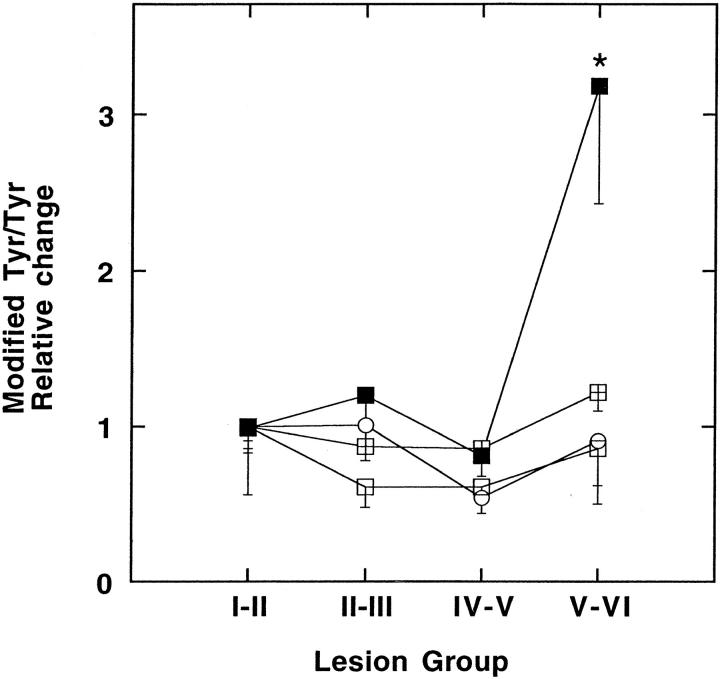
In addition to lipid, protein amino acid moieties may be susceptible to oxidative attack in the intima. Reaction of Tyr and Phe with hydroxyl radicals yields DOPA and o-Tyr, m-Tyr, and Tyr radical formation can generate diTyr. In addition, myeloperoxidase generates ClTyr, diClTyr, or diTyr and there is substantial evidence to suggest participation of myeloperoxidase during atherogenesis. 15 In contrast to nonoxidized and oxidized lipid few of the protein oxidation markers determined in this study varied significantly between lesion groups when standardized to total protein (Table 4) ▶ or to parent molecule (Figure 3) ▶ . A notable exception was diTyr that increased significantly in the most advanced lesions (Table 4 ▶ and Figure 3 ▶ ).
Table 4.
Protein-Standardized Oxidized Protein Moieties in Aortic Lesions
| Lesion stage, n | Tyr | DOPA | m-Tyr | o-Tyr | diTyr | ClTyr | diClTyr |
|---|---|---|---|---|---|---|---|
| I–II (4) | 288 (39) | 216 (58) | 37 (21) | 53 (11) | 21 (1) | 170 (30) | 110 (73) |
| II–III (8) | 426 (88) | 261 (46) | 37 (16) | 74 (16) | 34 (9) | 178 (26) | 56 (14) |
| IV–V (9) | 231 (27) | 150 (32) | 26 (8) | 45 (9) | 15 (4) | 114 (18) | 34 (6) |
| V–VI (7) | 204 (12) | 174 (18) | 25 (4) | 53 (7) | 42 (8)* | 174 (45) | 87 (53) |
Proteins in SN-2 were delipidated, hydrolyzed, and derivatized as described in Materials and Methods. Oxidized Tyr and Phe moieties were determined using HPLC with electrochemical detection (DOPA, o-Tyr, m-Tyr, and diTyr) or GC/MS (ClTyr and diClTyr). Phe:Tyr was 0.74 (0.05) and did not vary between lesion groups. The data represent mean values (SEM) in pmol/mg except Tyr (nmol/mg).
*P < 0.05 compared with group I–II.
Figure 3.
Relative changes in modified tyrosine residues in aortic intima. The type and extent to which Tyr residues were modified in intimal tissue was examined by measuring DOPA (⊞), diTyr (▪), ClTyr (○) and diClTyr (□). The content of oxidized Tyr residues, calculated per parent molecule, was expressed as a fraction of those observed in lesion group I-II. The data represent mean values ± SEM with asterisks indicating P < 0.05 compared with group I-II.
Discussion
We have investigated biochemical changes in human aortas associated with atherogenesis by analyzing lipid, protein, and antioxidant contents and by measuring the accumulation of specifically oxidized moieties in the lipoprotein fraction prepared from aortic intimal lesions from early- to end-stage disease. Consistent with the notion that lipid accumulation increases with lesion severity we observed cholesterol to accumulate with advancing disease and this increase was already significant in the earliest stages, ie, fatty streaks. By comparison, CEs were significantly increased only in fibro-fatty and more complex lesions that also contained higher concentrations of protein-standardized CE-O(O)H and the enzymatic oxysterol, 27HC. The accumulation of cholesterol and CE preceded the increases in the corresponding oxidation products 27HC and CE-O(O)H, with the latter representing the major lipid peroxidation product detected. Together, these results suggest that in human atherosclerosis significant accumulation in primary lipoprotein lipid (per)oxidation products does not precede the accumulation of nonoxidized lipid.
According to the LDL oxidation theory of atherosclerosis, 4 oxidation of LDL occurs early in the disease process and precedes foam cell accumulation and fatty streak formation. Despite the popularity of the theory, the accumulation of native and oxidized lipid and protein in relation to disease stage is rarely quantified and compared. Rather, single oxidation parameters are commonly determined at only a single disease stage or, alternatively, lesion parameters are compared to the concentration of total plasma or LDL cholesterol. We therefore compared the relative changes in protein and selected lipids that represent the major oxidizable components of plasma lipoproteins, and oxidation parameters of both in the intima of human aortic tissue displaying lesions of increasing severity. Importantly, tissue was resected within 24 hours of death. Within this time frame there are no significant changes to the biochemical composition of arterial tissue and the contents of free and esterified cholesterol, and other lipids and protein remain stable. 29 In addition, the levels of CE-O(O)H per parent detected in the most severe aortic lesions (2.4%) were less than that observed for carotid lesions (3.5%) processed freshly within 2 hours of resection. 13 Further, we compared analytes from various grades of lesion derived from the same aortic tissue sample and verified that artifactual oxidation of CE did not occur during sample processing. As CEs are more susceptible to oxidation than cholesterol, Tyr and Phe, the results obtained therefore likely represent the situation in vivo.
LDL lipid accumulation is a prominent and early marker for atherosclerosis. Consistent with this, the content of cholesterol significantly and linearly increased with disease severity (Table 2) ▶ . As expected, the CE content also increased with increasing lesion severity, although the CE load per cholesterol in lesion lipoproteins tended to decrease in the most advanced lesions. This is consistent with an earlier report showing a decrease in C18:2 in fibrous plaques, 30 as CE measured herein represents the sum of C18:2 and cholesteryl arachidonate. This suggests increased hydrolysis of lipoprotein CE in advanced lesions and/or preferential loss of CE rather than cholesterol from lipoproteins.
We selected CE-O(O)H and 7KC as markers of nonenzymatic, free radical-mediated lipid (per)oxidation. The former are the major lipid oxidation products formed when lipoproteins undergo in vitro oxidation and contribute to the in vitro formation of high-uptake LDL. 7 In addition, CE-O(O)H are the major lipid oxidation products in human lesions 1,12,26,31 and hydroxylated fatty acids are associated with plaque instability. 31 Notably, CE-O(O)H and oxysterols are not found in normal iliac arteries 12,32 or in circulating plasma lipoproteins. 13 Our data show a disease stage-dependent increase in intimal CE-O(O)H (Table 2) ▶ suggesting that overall CE-O(O)H is a suitable parameter to monitor the extent of lipoprotein lipid oxidation in the vessel wall.
As lesion severity increased, the percentage of lipoprotein-associated CE present as CE-O(O)H increased to a maximum of 2.3%. Although significantly greater than any other oxidation parameter determined here, this value is at the low end of the range of values reported previously for carotid plaque samples. 12,13 The configurational isomer distribution of C18:2-OH (JM Upston, AC Terentis, R Stocker, unpublished results) indicate that particularly in the early stages of atherosclerosis, the majority of CE-O(O)H detected were formed in the presence of α-TOH. Indeed, we show herein that α-TOH did not decrease with advancing atherosclerosis, consistent with our previous data 12,13 and a separate detailed GC/MS-based study of α-TOH oxidation products in human lesion homogenates. 33 The notion that oxidation of lipoprotein lipid in the vessel wall proceeds via tocopherol-mediated peroxidation 34,35 suggests a likely mechanism for the formation of most CE-O(O)H in the presence of α-TOH in lesions.
Our observation that even advanced lesions contain normal α-TOH and at most 2.3% of its CE oxidized to CE-O(O)H has potentially important implications for several reasons. First, the overall extent of lipid oxidation is substantially less than that needed for the in vitro conversion of LDL to a high-uptake form by Cu(II). 10,36 This conclusion is consistent with the finding that the extent of oxidative change to aortic LDL, as assessed by electrophoretic mobility or other physical parameters, is less than that required for scavenger receptor-mediated uptake. 37
Second, our observation has potential implications for the formation of both minimally modified LDL and isoprostanes. Oxovaleroyl, glutaroyl, and epoxyisoprostane (the three active moieties identified in minimally modified LDL generated in vitro 38 ) and isoprostanes are secondary lipid oxidation products. The formation of isoprostanes is essentially prevented as long as α-TOH is present. 39 Consistent with this, isoprostane levels in atherosclerotic lesions are 20-fold to 300-fold lower per parent molecule than lipid hydroxides. 31 Like isoprostanes, oxovaleroyl and glutaroyl are generated from arachidonic acid-containing phospholipids, most likely via fragmentation of arachidonic acid hydroperoxides, a process that is inhibited strongly in the presence of α-TOH. 40 Indeed, the generation of fragments of polyunsaturated fatty acids is almost completely prevented during LDL oxidation as long as α-TOH remains present. 10 This indicates that the bioactive phospholipids generated during in vitro oxidation of LDL and present in minimally modified LDL may not be formed in substantial amounts in lesion lipoproteins the α-TOH content of which remains essentially intact. Arachidonic acid is also present in cellular phospholipids and detection of its secondary oxidation products in homogenates of lesion material does not, by itself, establish their presence in lipoproteins. In fact, immunohistochemical studies suggest that lesion isoprostanes are predominantly associated with foam cells. 41 Similarly, using EO6, a monoclonal antibody specific for oxovaleroyl-containing phospholipid, 42 Palinski and colleagues 43 showed predominant co-localization of this epitope with foam cells in lesions of cholesterol fed New Zealand White and LDL receptor-deficient rabbits. Additional studies are needed to fully establish the precise extent of oxidation of lesion LDL, whether the oxidized arachidonate moieties detected 41,43 represent cellular membrane lipids (eg, from apoptotic or necrotic cells) or lipids from lipoproteins taken up, and how these oxidation parameters relate to lesion CE-O(O)H.
In contrast to CE-O(O)H, the oxidation of cholesterol was primarily enzymatic. Of the analytes measured, 27HC showed the greatest and earliest relative changes with disease progression (Figure 1) ▶ , and accounted for 0.9% of total cholesterol (Figure 2) ▶ . The disease-associated increase in the cholesterol-standardized 27HC content is consistent with a previous report 44 and infers a disease-associated increase in the activity of sterol 27-hydroxylase that co-localizes with macrophages in lesions. 45 Interestingly, the 27-hydroxylase pathway has been suggested to be important in the removal of extrahepatic cholesterol. 46 As the accumulation of cholesterol preceded that of 27HC, the formation of the latter may be interpreted as a physiological (potentially anti-atherogenic) response to cholesterol load.
Similar to 27HC, the presence of increased protein-standardized CE-O(O)H in fibro-fatty lesions may reflect the increased lipid load of that lesion stage and suggests that LDL lipid oxidation follows lipid accumulation in the temporal sequence of atherogenic events. However, it is possible that in the early disease stages lipid oxidation products are effectively metabolized or removed from the artery wall, but that their formation may still contribute to promotion of atherogenesis. Indeed, one study of human fetal fatty streak lesions reported the presence of malondialdehyde- and 4-hydroxynonenal-modified lysine (measured indirectly by immunological techniques) in the absence of monocytes/macrophages. 47 These authors suggested that intimal LDL accumulation and oxidation is an early event that contributes to monocyte recruitment into the vessel wall. To more precisely implicate a causal role of lipid (per)oxidation in atherogenesis the metabolism and/or removal of oxidation products is worthy of further study.
Consistent with our findings, the content of the free radical-derived, cholesterol-standardized 7KC, was similar in human fatty streak and advanced lesions, 48 yet homogenate of endarterectomy plaque contains more 7KC than that of normal arteries. 12,32 A possible explanation for this difference is that we analyzed the lipoprotein fraction rather than the entire intima. Indeed, in pilot studies with the first tissue supernate (SN-1, containing SN-2 plus the lipemic layer; see Materials and Methods) we observed oxysterols and cholesterol-standardized 7KC to increase with increasing disease severity (data not shown). A lower 7KC and 27HC content in the SN-2 fraction compared to SN-1 is consistent with oxysterols being concentrated in the core of lesions, rather than in lipoproteins or the fibrous cap, as has been reported for 27HC (referred to as “26-OH-CHOL” by Garcia-Cruset and colleagues 49 ).
Several groups have demonstrated the presence of oxidized protein moieties in atherosclerosis including ClTyr, 50 diTyr, 21,51 DOPA, m-Tyr, and o-Tyr. 21 However, of the protein oxidation products measured herein only diTyr increased significantly from early to late disease stage (Table 4 ▶ , Figure 3 ▶ ). These data are consistent with increased diTyr levels found in aortic lesion-derived LDL compared to plasma LDL. 51
Chlorinated Tyr was determined as both mono- and dichloro forms. ClTyr was present in higher amounts than diClTyr (Table 4) ▶ , with levels similar to those previously determined for lesion LDL (∼300 μmol/mol Tyr). 50 Interestingly, the diClTyr to ClTyr ratio in lesion lipoproteins was high (Table 4) ▶ compared to that found for reactions of HOCl with free and peptide-bound Tyr. 24 In contrast, analysis of SN-2 after removal of all lipoproteins showed comparatively fewer products and lower ratios of diClTyr to ClTyr (data not shown). A high ratio may be obtained under conditions of high local oxidant production (AJ Kettle, unpublished results), and our data supports the previous suggestion by Heinecke 52 that Tyr chlorination via myeloperoxidase activity is relatively specific for lesion lipoproteins. This is also consistent with the observation that myeloperoxidase binds to LDL. 53
Excepting diTyr, no other Tyr modification showed a clear disease stage dependency and elevated levels of diTyr were detected only in the most advanced lesion stages. diTyr may be produced via myeloperoxidase activity and its increase in complex lesions is consistent with a recent study showing increased numbers of myeloperoxidase-expressing macrophages in advanced, but not early, atherogenesis. 54 However an increase in the content of Tyr modification, particularly diTyr, appeared to be present in even early lesions compared to those values derived for normal iliac tissue, 21 normal aorta, 51 and normal LDL 55 (Table 5) ▶ . Thus, our results do not exclude the possibility that specific Tyr modifications may be early disease events and that such oxidation could contribute to the initiation of atherogenesis.
Table 5.
Comparison of Modified Tyrosine Levels in Atherosclerosis and Normal Tissue
| DOPA | diTyr | ClTyr | |
|---|---|---|---|
| Tissue/fluid | |||
| Plasma LDL | 630* | 2* | 10† |
| Normal iliac artery | ∼500‡ | ∼30‡ | |
| Normal aorta | ∼30§ | 80† | |
| Initial aortic lesions (I–II) | 730¶ | 70¶ | 200‡ |
| Carotid and advanced aortic lesions | 890¶, ∼1000‡ | ∼200‡, 230¶ | 178¶, 420† |
In conclusion, our data show that in human aortic lesions the accumulation of nonoxidized lipid precedes the accumulation of primary lipid (per)oxidation products that coexist with and are likely formed in the presence of α-TOH. These data show that vitamin E does not generally become depleted and suggest, although do not prove, that appreciable lipoprotein lipid (per)oxidation represents a response to rather than a cause for lesion development.
Acknowledgments
We thank Prof. J. Hilton of the NSW Institute of Forensic Medicine, Australia, for the human aorta lesions; Drs. Andrew Terentis and Paul Witting for helpful discussions; Emilia Ip for technical assistance; and Dr. Shanlin Fu for contributions to the protein oxidation analyses.
Footnotes
Address reprint requests to Prof. R. Stocker, Heart Research Institute, 145 Missenden Rd., Camperdown, NSW, 2050, Australia. E-mail: r.stocker@hri.org.au.
Supported by the National Heart Foundation, the National Health and Medical Research Council of Australia, and Blackmores Ltd.
References
- 1.Smith EB, Evans PH, Downham MD: Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res 1967, 7:171-186 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown MS: The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem 1977, 46:897-930 [DOI] [PubMed] [Google Scholar]
- 3.Frank JS, Fogelman AM: Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultra-rapid freezing and freeze-etching. J Lipid Res 1989, 30:967-978 [PubMed] [Google Scholar]
- 4.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989, 320:915-924 [DOI] [PubMed] [Google Scholar]
- 5.Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801-809 [DOI] [PubMed] [Google Scholar]
- 6.Diaz MN, Frei B, Vita JA, Keaney JF, Jr: Antioxidants and atherosclerotic heart disease. N Engl J Med 1997, 337:408-416 [DOI] [PubMed] [Google Scholar]
- 7.Steinbrecher UP, Lougheed M, Kwan W-C, Dirks M: Recognition of oxidized low density lipoprotein by the scavenger receptor of macrophages results from derivatization of apolipoprotein B by products of fatty acid peroxidation. J Biol Chem 1989, 264:15216-15223 [PubMed] [Google Scholar]
- 8.Henriksen T, Mahoney EM, Steinberg D: Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci USA 1981, 78:6499-6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazell LJ, Stocker R: Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages. Biochem J 1993, 290:165-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esterbauer H, Jürgens G, Quehenberger O, Koller E: Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res 1987, 28:495-509 [PubMed] [Google Scholar]
- 11.Carpenter KL, Cheeseman KH, van der Veen C, Taylor SE, Walker MK, Mitchinson MJ: Depletion of alpha-tocopherol in human atherosclerotic lesions. Free Radic Res 1995, 23:549-558 [DOI] [PubMed] [Google Scholar]
- 12.Suarna C, Dean RT, May J, Stocker R: Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of α-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol 1995, 15:1616-1624 [DOI] [PubMed] [Google Scholar]
- 13.Niu X, Zammit V, Upston JM, Dean RT, Stocker R: Co-existence of oxidized lipids and α-tocopherol in all lipoprotein fractions isolated from advanced human atherosclerotic plaques. Arterioscl Thromb Vasc Biol 1999, 19:1708-1718 [DOI] [PubMed] [Google Scholar]
- 14.Burton GW, Ingold KU: Vitamin E: application of the principles of physical organic chemistry to the exploration of its structure and function. Acc Chem Res 1986, 19:194-201 [Google Scholar]
- 15.Heinecke JW: Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis 1998, 141:1-15 [DOI] [PubMed] [Google Scholar]
- 16.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull WJ, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW: A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscl Thromb Vasc Biol 1995, 15:1512-1531 [DOI] [PubMed] [Google Scholar]
- 17.Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, Steinbrecher UP, Heinecke JW: Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem 1997, 272:1433-1436 [DOI] [PubMed] [Google Scholar]
- 18.Lang JK, Gohil K, Packer L: Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem 1986, 157:106-116 [DOI] [PubMed] [Google Scholar]
- 19.Sattler W, Mohr D, Stocker R: Rapid isolation of lipoproteins and assessment of their peroxidation by HPLC postcolumn chemiluminescence. Methods Enzymol 1994, 233:469-489 [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Brodsky MH, Baker JC, Ames BN: Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem 1987, 160:7-13 [DOI] [PubMed] [Google Scholar]
- 21.Fu S, Davies MJ, Stocker R, Dean RT: Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J 1998, 333:519-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gieseg SP, Simpson JA, Charlton TS, Duncan MW, Dean RT: Protein-bound 3,4-dihydroxyphenylalanine is a major reductant formed during hydroxyl radical damage to proteins. Biochemistry 1993, 32:4780-4786 [DOI] [PubMed] [Google Scholar]
- 23.Davies MJ, Fu S, Wang H, Dean RT: Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med 1999, 27:1151-1163 [DOI] [PubMed] [Google Scholar]
- 24.Fu S, Wang H, Davies M, Dean R: Reactions of hypochlorous acid with tyrosine and peptidyl-tyrosyl residues give dichlorinated and aldehydic products in addition to 3-chlorotyrosine. J Biol Chem 2000, 275:10851-10858 [DOI] [PubMed] [Google Scholar]
- 25.Chapman ALP, Senthilmohan R, Winterbourn CC, Kettle AJ: Comparison of mono and dichlorinated tyrosines with carbonyls for detection of hypochlorous acid-modified proteins. Arch Biochem Biophys 2000, 377:95-100 [DOI] [PubMed] [Google Scholar]
- 26.Carpenter KLH, Taylor SE, Ballantine JA, Fussell B, Halliwell B, Mitchinson MJ: Lipids and oxidised lipids in human atheroma and normal aorta. Biochim Biophys Acta 1993, 1167:121-130 [DOI] [PubMed] [Google Scholar]
- 27.Harland WA, Gilbert JD, Brooks CJW: Lipids of human atheroma. VIII. Oxidised derivatives of cholesteryl linoleate. Biochim Biophys Acta 1973, 316:378-385 [PubMed] [Google Scholar]
- 28.Stocker R, Bowry VW, Frei B: Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does α-tocopherol. Proc Natl Acad Sci USA 1991, 88:1646-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ylä-Herttuala S, Nikkari T: Effect of post-mortem time on the biochemical composition of coronary arteries. Atherosclerosis 1985, 56:1-10 [DOI] [PubMed] [Google Scholar]
- 30.Smith EB: The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res 1974, 12:1-49 [DOI] [PubMed] [Google Scholar]
- 31.Mallat Z, Nakamura T, Ohan J, Leseche G, Tedgui A, Maclouf J, Murphy RC: The relationship of hydroxyeicosatetraenoic acids and F2-isoprostanes to plaque instability in human carotid atherosclerosis. J Clin Invest 1999, 103:421-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AJ, Leong S, Dean RT, Jessup W: 7-Hydroperoxycholesterol and its products in oxidized low density lipoprotein and human atherosclerotic plaque. J Lipid Res 1997, 38:1730-1745 [PubMed] [Google Scholar]
- 33.Terentis AC, Thomas SR, Burr JA, Liebler DC, Stocker R: Vitamin E oxidation in human atherosclerotic lesions. Circ Res 2002, in press [DOI] [PubMed]
- 34.Bowry VW, Stocker R: Tocopherol-mediated peroxidation. The pro-oxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc 1993, 115:6029-6044 [Google Scholar]
- 35.Upston JM, Terentis AC, Stocker R: Tocopherol-mediated peroxidation (TMP) of lipoproteins: implications for vitamin E as a potential antiatherogenic supplement. FASEB J 1999, 13:977-994 [DOI] [PubMed] [Google Scholar]
- 36.Kritharides L, Jessup W, Gifford J, Dean RT: A method for defining the stages of LDL oxidation by the separation of cholesterol and cholesteryl ester-oxidation products by HPLC. Anal Biochem 1993, 213:79-89 [DOI] [PubMed] [Google Scholar]
- 37.Steinbrecher UP, Lougheed M: Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler Thromb 1992, 12:608-625 [DOI] [PubMed] [Google Scholar]
- 38.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA: Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 1997, 272:13597-13607 [DOI] [PubMed] [Google Scholar]
- 39.Lynch SM, Morrow JD, Roberts LJ, II, Frei B: Formation of noncyclooxygenase-derived prostanoids (F2-isoprostanes) in plasma and low-density lipoprotein exposed to oxidative stress in vitro. J Clin Invest 1994, 93:998-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider C, Tallman KA, Porter NA, Brash AR: Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem 2001, 276:20831-20838 [DOI] [PubMed] [Google Scholar]
- 41.Pratico D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Maclouf J, Violi F, FitzGerald GA: Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest 1997, 100:2028-2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL: Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest 1999, 103:117-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL: Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest 1996, 98:800-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown AJ, Jessup W: Oxysterols and atherosclerosis. Atherosclerosis 1999, 142:1-28 [DOI] [PubMed] [Google Scholar]
- 45.Crisby M, Nilsson J, Kostulas V, Bjorkhem I, Diczfalusy U: Localization of sterol 27-hydroxylase immuno-reactivity in human atherosclerotic plaques. Biochim Biophys Acta 1997, 1344:278-285 [DOI] [PubMed] [Google Scholar]
- 46.Lund E, Andersson O, Zhang J, Babiker A, Ahlborg G, Diczfalusy U, Einarsson K, Sjovall J, Bjorkhem I: Importance of a novel oxidative mechanism for elimination of intracellular cholesterol in humans. Arterioscler Thromb Vasc Biol 1996, 16:208-212 [DOI] [PubMed] [Google Scholar]
- 47.Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W: Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest 1997, 100:2680-2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Cruset S, Carpenter KLH, Guardiola F, Stein BK, Mitchinson MJ: Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic Res 2001, 35:31-41 [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Cruset S, Carpenter KL, Guardiola F, Mitchinson MJ: Oxysterols in cap and core of human advanced atherosclerotic lesions. Free Radic Res 1999, 30:341-350 [DOI] [PubMed] [Google Scholar]
- 50.Hazen SL, Heinecke JW: 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest 1997, 99:2075-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leeuwenburgh C, Rasmussen JE, Hsu FF, Mueller DM, Pennathur S, Heinecke JW: Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem 1997, 272:3520-3526 [DOI] [PubMed] [Google Scholar]
- 52.Heinecke JW: Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr Opin Lipidol 1997, 8:268-274 [DOI] [PubMed] [Google Scholar]
- 53.Carr AC, Myzak MC, Stocker R, McCall MR, Frei B: Myeloperoxidase binds to low-density lipoprotein: potential implications for atherosclerosis. FEBS Lett 2000, 487:176-180 [DOI] [PubMed] [Google Scholar]
- 54.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P: Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 2001, 158:879-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu S, Dean R, Southan M, Truscott R: The hydroxyl radical in lens nuclear cataractogenesis. J Biol Chem 1998, 273:28603-28609 [DOI] [PubMed] [Google Scholar]