Abstract
Undifferentiated human melanoma cell lines produce a large chondroitin sulfate proteoglycan, different from the well-known melanoma-specific proteoglycan mel-PG (Heredia and colleagues, Arch Biochem Biophys, 333: 198–206, 1996). We have identified this proteoglycan as versican and analyzed the expression of versican in several human melanoma cell lines. Versican isoforms are expressed in undifferentiated cell lines but not in differentiated cells, and the isoform expression pattern depends on the degree of cell differentiation. The V0 and V1 isoforms are found on cells with an early degree of differentiation, whereas the V1 isoform is present in cells with an intermediate degree of differentiation. We have also characterized some functional properties of versican on human melanoma cells: the purified proteoglycan stimulates cell growth and inhibits cell adhesion when cells are grown on fibronectin or collagen type I as substrates, and thus may facilitate tumor cell detachment and proliferation. Furthermore, we have analyzed the expression of versican in human melanocytic nevi and melanoma: 10 benign melanocytic nevi, 10 dysplastic nevi, 11 primary malignant melanomas, and 8 metastatic melanomas were tested. Immunoreactivity for versican was negative in benign melanocytic nevi, weakly to strongly positive in dysplastic nevi, and intensely positive in primary malignant melanomas and metastatic melanomas. Our results indicate that versican is involved in the progression of melanomas and may be a reliable marker for clinical diagnosis.
Versican belongs to the family of large aggregating proteoglycans (PGs) that also includes the large cartilage-derived PG aggrecan, and two smaller PGs expressed in nervous tissues, namely neurocan and brevican. 1 All four of these PGs share some structural characteristics and are able to bind to hyaluronate. These PGs have three structural domains: the N-terminal region (G1 domain) consists of an immunoglobulin-like loop and two-link protein-like tandem repeats and is responsible for binding hyaluronate; the central domain carries the glycosaminoglycan side chains; and the C-terminal globular region (G3 domain) consists of two epidermal growth factor-like elements, a C-type lectin domain, and a complement regulatory protein (CRP)-like (sushi) module and may interact with simple carbohydrates and glycosaminoglycans and probably with other proteins such as tenascin-R. 2,3 The central domain of versican consists of two large subdomains, designated GAG-α and GAG-β that are encoded by two alternatively spliced exons. In mammals, versican appears as four possible spliced variants: the largest one contains both GAG-α and GAG-β and is designated V0; the other variants are V1 (contains GAG-β), V2 (contains GAG-α), and V3 (lacks any GAG subdomain). 2
Versican was first described in human fetal fibroblasts more than 10 years ago, 4 but detailed analysis of tissue expression has been performed only recently with the availability of specific antibodies. In human adults, versican has been found in loose connective tissues, often associated with the elastic fiber network, in smooth muscle, cartilage, the central and peripheral nervous system, and the three wall layers of veins and elastic arteries. 5 In normal adult skin, versican appears localized in the stratum basale of the epidermis, as well as in the papillary and reticular layers of the dermis. 6,7
Versican has been described in a number of tumor types, 8 including brain tumors such as gliomas, medulloblastomas, neurofibromas, and meningiomas. Regarding gliomas, a decrease of versican expression has been described in the extracellular matrix, whereas there is an up-regulation of versican in tumor vessels compared to normal cerebral vessels. 9 Because of its ability to interact with hyaluronate, 10 tenascin 11 and other proteins, and cytokines, 12-15 versican may contribute to the malignant properties of tumor cells. In this sense, it has been described that the versican-rich extracellular matrices exert an anti-adhesive effect on the cells, thus facilitating tumor cell migration and invasion. 16 Since versican is highly expressed in fast growing tissues and cells, it has been suggested that versican plays a role in cell proliferation, a notion supported by the finding that a miniversican construct promoted NIH3T3 fibroblasts and astrocytoma cell proliferation, probably through the epidermal growth factor-like motifs in the G3 domain. 17,18
Our group has previously reported an unidentified high-molecular weight PG in undifferentiated human melanoma cell lines. 19 In the present work, we identify this PG as versican and describe its presence in in vivo melanocytic lesions. We also analyze how the various versican isoforms relate to cell differentiation and the role of versican in the biological properties of melanoma cells.
Materials and Methods
Cell Culture and Metabolic Labeling
Human melanoma cell lines SK-mel-131 (clone 1.36-1-5), SK-mel-131 (clone 3.44), SK-mel-23, SK-mel-37, Rider, Mewo, AX3, and DX2 originally derived from human melanomas by Houghton and colleagues 20 and U251 human astrocytoma cells were obtained from Dr. F. X. Real (I.M.I.M., Barcelona, Spain). Cells were grown in a humidified atmosphere at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin (all from GibcoBRL/Life Technologies). Subconfluent cultures were grown in serum-free medium for 8 hours and labeled during 16 hours with 100 μCi/ml of carrier-free [35S]sulfate (Amersham Pharmacia Biotech) in low sulfate (0.2 mmol/L) medium, or with 20 μCi/ml l-[35S]methionine in methionine-free medium (Promix, Amersham Pharmacia Biotech). The medium was then removed, a cocktail of protease inhibitors was added [10 mmol/L ethylenediaminetetraacetic acid (Sigma) 5 mmol/L benzamidine (Sigma), 5 mmol/L N-ethylmaleimide (Sigma), 1 mg/ml pepstatin A (Sigma), 0.5 mg/ml leupeptin (Sigma), and 1 mmol/L phenylmethyl sulfonyl fluoride (Sigma)] and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 6% or a 3 to 10% gradient polyacrylamide gel, as described by Laemmli. 21 To ensure that the conditioned medium corresponding to the same number of cells was used, duplicate wells were seeded and cells were counted in a Neubauer chamber.
Purification of the High-Molecular Weight PG
Conditioned medium (6.8 I)from U251 astrocytoma cells were concentrated fivefold using a tangential flux system with a 100-kd cutoff (MiniUltrasette, Filtron). The concentrated fraction was purified by ion-exchange chromatography on DEAE-Sephacel (10 × 1 cm, Pharmacia) equilibrated with a buffer containing 0.2 mol/L NaCl, 50 mmol/L Tris-HCl, pH 7.2. Elution was performed with a linear salt gradient up to 2 mol/L NaCl at 0.5 ml/min flux rate. Fractions eluting at 0.98 to 1.28 mol/L NaCl were pooled, concentrated by ultrafiltration (Amicon Inc., Beverly, MA), and dialyzed against the same buffer.
Partially purified PGs were applied to a MonoQ HR5/5 (1 ml, Amersham Pharmacia Biotech) equilibrated with a buffer containing 0.2 mol/L NaCl, 50 mmol/L Tris-HCl, pH 7.2. Elution was performed with a linear salt gradient up to 2 mol/L NaCl at 0.5 ml/min flux rate. One-ml fractions were collected and analyzed for radioactivity, for protein concentration (BioRad Protein Assay; BioRad Laboratories GmbH, Heidelberg, Germany) and for the presence of hexuronic acid, using the carbazole method of Bitter and Muir. 22 A final purification step was achieved by gel chromatography on a Superdex 200 column (FPLC system, Amersham Pharmacia Biotech) equilibrated with 50 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.2, and eluted with the same buffer at 0.3 ml/min flux rate.
Enzymatic Digestions and Electrophoretic Analysis
Enzymatic digestions were performed at 37°C for 16 hours with chondroitinase ABC (50 mU/ml in 33 mmol/L sodium acetate, 33 mmol/L Tris-HCl ,pH 8.0; Seikagaku, Tokyo, Japan) or heparitinase (10 mU/ml in 10 mmol/L calcium acetate, 100 mmol/L sodium acetate, pH 7.0; Seikagaku). The incubations were terminated by boiling the samples for 5 minutes. Samples were analyzed in a 6% or a 3 to 10% polyacrylamide gradient gel.
Generation of Antibodies
A sample of purified high-molecular weight PG (50 to 100 μg measured as hexuronic acid) in phosphate-buffered saline was mixed with an equal volume of Freund’s complete adjuvant (Serva GmbH, Heidelberg, Germany), injected to rabbits according to standard immunization protocols and boosted every 4 to 6 weeks. Test bleeds were made 10 to 14 days after each boost and analyzed by immunoprecipitation or Western blot. The antiserum was purified from undesirable antibodies using affinity columns made by coupling the 0.2 mol/L fraction (non-PG) from a DEAE-Sephacel column to CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech). The antibody recognized the protein core of versican, but it was unable to detect the whole PG form in Western blot as well as in immunocytochemistry/immunohistochemistry.
Western Blot Analysis
Aliquotes of the conditioned media were digested with chondroitinase ABC as described above and analyzed in a 3 to 10% SDS-PAGE under reducing conditions. After electrophoresis, proteins were transferred onto Immobilon-P membranes (Millipore). The blot was placed in a blocking solution consisting of 5% Blotto in Tris-buffered saline and incubated for 2 hours at 4°C. The membranes were incubated with the polyclonal anti-high molecular weight PG antibodies (1/1000) or anti-GAG-α or anti-GAG-β antibodies (1:100; kindly provided by Dr. D. R. Zimmermann, University of Zürich, Zürich, Switzerland) in 5% Blotto in Tris-buffered saline for 16 hours, washed, and visualized by chemiluminescence (ECL System, Amersham).
Proliferation and Adhesion Assays
Cells were seeded in 96-well plates until 50% confluence and incubated in RPMI 1640 10% fetal calf serum for 48 hours. The medium was replaced by serum-free RPMI 1640 in the presence of different concentrations of the PG preparation and incubated overnight. Cell proliferation was determined by an enzyme-linked immunosorbent assay (Roche Molecular Biochemicals) based on the incorporation of 5-bromo-2′-deoxyuridine (BrdU) into the DNA of proliferating cells, followed by an anti-BrdU antibody conjugated to peroxidase, addition of tetramethylbenzidine (TMB) as substrate, and quantification by monitoring the absorbance at 450 nm.
For cell adhesion assays, Dynatech 96-well plates were coated with fibronectin (5 μg/ml) or collagen I (3 μg/ml) for 16 hours at 4°C followed by incubation with a solution of 0.1% bovine serum albumin in phosphate-buffered saline (PBS) for 1 hour. Negative controls were coated only with the same concentration of bovine serum albumin. Cells were seeded in these plates at a density of 4 × 10 4 cells/well in the presence of the PG preparation and incubated for 2 hours at 37°C. The medium was removed, wells were rinsed twice with PBS in the presence of Ca2+ and Mg2+, and the adhered cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature. The wells were rinsed and incubated with 0.1% violet crystal for 20 minutes. After rinsing, a solution of 0.1 mol/L HCl was added and the absorbance at 630 nm was determined.
Immunocytochemistry
Cells were grown in coverslips, rinsed with PBS, and fixed with 4% paraformaldehyde for 20 minutes at room temperature. After rinsing four times with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 10 minutes and nonspecific binding sites were blocked for 20 minutes with 1% bovine serum albumin/0.02% goat serum. Cells were then incubated overnight with the anti-versican antibody (1:10) at 4°C. Subsequently, cells were washed four times with PBS and incubated with an anti-rabbit-FITC antibody (Roche). Cultures were studied with a Nikon Eclipse E800 epifluorescence microscope and photographed with an integrated camera system.
Immunohistochemistry
A total of 39 melanocytic lesions were studied, which corresponded to 10 benign melanocytic nevi, 10 dysplastic nevi, 11 primary malignant melanomas, and 8 metastatic malignant melanomas. Slides and paraffin blocks were selected from the archives of the Pathology Department at the Hospital del Mar-IMIM-UAB.
Sections (5-μm thick) were obtained from the paraffin blocks. After blocking the nonspecific unions with 20% goat serum in PBS for 30 minutes, they were incubated with the antibody against versican, at 1:50 dilution at 4°C overnight. After washing several times, the sections were incubated with a secondary antibody, biotin-labeled anti-rabbit goat IgG (Vector Laboratories), at a 1:300 dilution for 1 hour at room temperature. Avidin-biotin and 3- amino-9-ethylcarbazole (AEC) were used as the detection system. Negative controls were performed by using preimmune rabbit serum. The slides were counterstained with hematoxylin.
The sections were examined double blindly by two researchers (CB, MAU), who evaluated the percentage and the intensity of versican-positive melanocytic cells. The percentage was evaluated according to the following scale: 0: absence of expression; 1+, ≤5% positive cells; 2+, 6 to 25% positive cells; 3+, 26 to 50% melanocytic cells; 4+, 51 to 75% positive cells; 5+, 76 to 100% positive cells. Versican expression was graded as null, mild, moderate, or intense.
Statistical analyses were performed using the Kruskal-Wallis one-way analysis of variance test.
Results
Identification of Versican in Human Melanoma Cell Lines
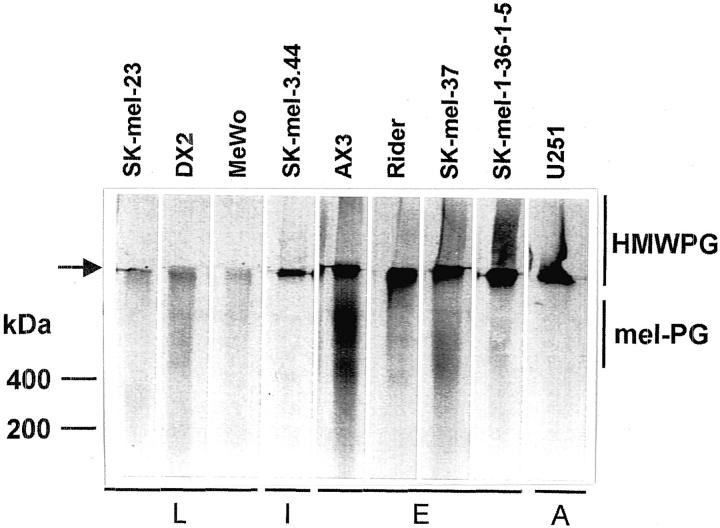
Subconfluent cultures of a number of human melanoma and astrocytoma cells were metabolically labeled with [35S]sulfate and the macromolecules present in the conditioned medium analyzed by SDS-PAGE on a 3 to 10% gel (Figure 1) ▶ . The analyzed cell lines showed a different band pattern: some cell lines (AX3, SK-mel-37, Rider, SK-mel-1.36-1-5, and SK-mel-3.44) produced a 35S-labeled component of very high molecular mass, remaining in the stacking gel (labeled HMWPG in Figure 1 ▶ ), with a similar electrophoretic mobility to the high-molecular weight PG present in U251 astrocytoma cells. Some of these melanoma cell lines also presented a second PG band located in the running gel above the 200-kd marker (AX3, SK-mel-37, and, with less intensity, Rider, and SK-mel-1.36-1-5). This band has been previously identified as the melanoma-specific PG, mel-PG, by immunological methods. 19 All these bands were subsequently identified as chondroitin/dermatan sulfate PGs by digestion with chondroitinase ABC, chondroitinase AC, and heparitinase (not shown). A second group of cell lines produced very small amounts of sulfate-labeled bands (SK-mel-23, DX2, and MeWo).
Figure 1.
PG production by human melanoma and astrocytoma cell lines. Subconfluent human melanoma cell lines and the U251 astrocytoma cell line were metabolically labeled with [35S]sulfate as described in Materials and Methods. The conditioned medium corresponding to the same number of cells was analyzed by SDS-PAGE in a 3 to 10% gel followed by fluorography. L, late degree of differentiation; I, intermediate degree of differentiation; E, early degree of differentiation; A, astrocytoma. The position of the high-molecular weight PG (HMWPG) and mel-PG are indicated. The top of the running gel is shown by an arrow.
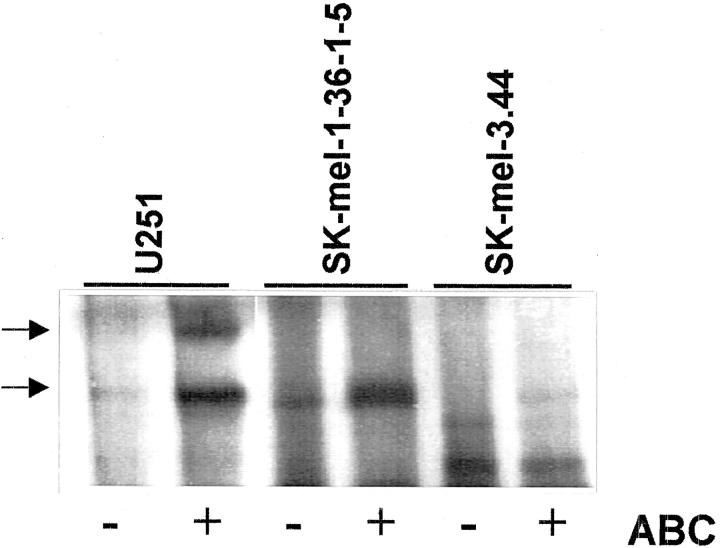
After chondroitinase ABC digestion of [35S]methionine-labeled conditioned media, a protein core composed of two bands of ∼350 and 400 kd was detected by SDS-PAGE (Figure 2) ▶ . This double band was clearly visible in U251 astrocytoma cells, whereas only the lower band is clearly visible in SK-mel-1.36-1-5 and SK-mel-3.44 cells.
Figure 2.
Detection of the protein cores from the large molecular weight PG from human melanoma and astrocytoma cell lines. Conditioned media from SK-mel-1.36-1-5 and SK-mel-3.44 melanoma cells and U251 astrocytoma cells labeled with [35S]methionine were treated with chondroitinase ABC and analyzed by SDS-PAGE in a 6% gel and fluorography. The bands observed after chondroitinase ABC treatment are shown by an arrow.
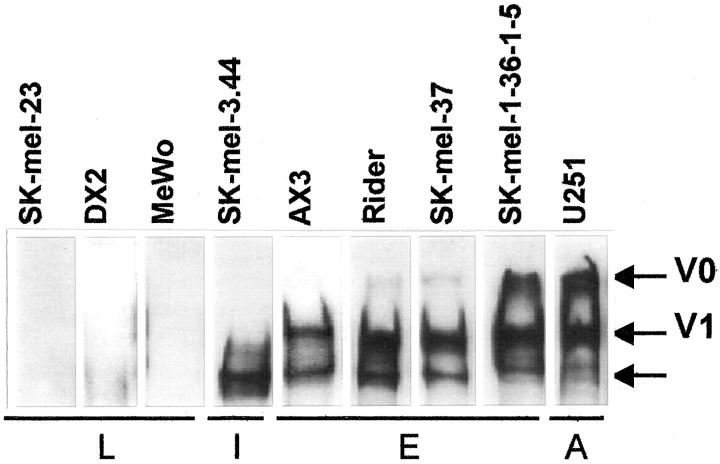
The double-band pattern in U251 astrocytoma cells has been identified in the related U251MG human glioma cell line as the V0 and V1 isoforms of the large aggregating chondroitin sulfate PG, versican, by Dours-Zimmermann and Zimmermann. 2 Thus, to confirm the identity of the large melanoma PG, versican was purified from U251 astrocytoma cell-conditioned media and antibodies were raised in rabbits against this PG. Using this antibody (Figure 3) ▶ , the V0 and V1 isoforms of versican were detected in U251 astrocytoma cells, as expected. In our panel of melanoma cell lines, all of the undifferentiated cells expressed the high-molecular weight isoforms V0 and V1 in variable amounts, whereas the differentiated cell lines SK-mel-23, MeWo, and DX2 cell lines did not express any versican isoform. A third band with a variable intensity appeared in all of the undifferentiated cell lines (Figure 3 ▶ , arrow).
Figure 3.
Identification of versican isoforms in human melanoma cell lines. Conditioned media from human melanoma cell lines were treated with chondroitinase ABC and analyzed by Western blot with the antibody raised against the high-molecular weight PG as described in Materials and Methods. L, late degree of differentiation; I, intermediate degree of differentiation; E, early degree of differentiation; A, astrocytoma. The arrow marks a putative proteolytic-derived form of versican.
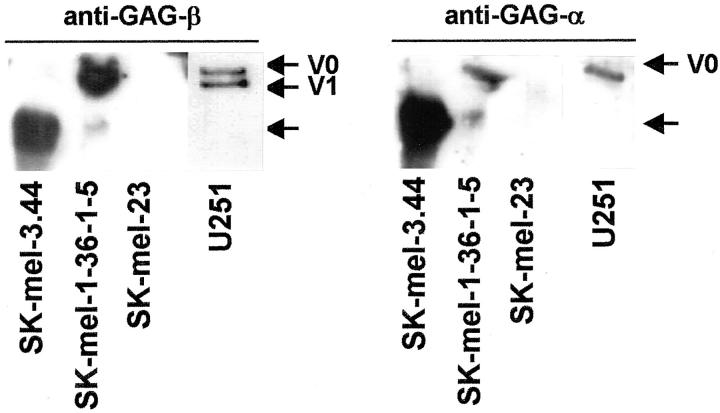
The specificity of the antibody preparation was confirmed by comparing its reactivity with the monoclonal antibodies against the GAG-α and GAG-β subdomains of versican kindly provided by Dr. D. R. Zimmermann (University of Zürich, Zürich, Switzerland). As observed in Figure 4 ▶ , the anti-GAG-β antibody was able to detect V0 and V1 isoforms in U251 astrocytoma cells, V0 and V1 as the main isoforms produced by SK-mel-1.36-5 cells, and lack of any versican isoforms in differentiated SK-mel-23 melanoma cells. As expected, the anti-GAG-α antibody specifically recognized the V0 isoform. The band highly expressed in SK-mel-3.44 cell line is probably a proteolytic product of V0, since it is detected by both the anti-GAG-α and anti-GAG-β antibodies, and it corresponds to the unidentified band in Figure 3 ▶ . On the other hand, the antibody raised by us was also reactive against recombinant versican (provided by Dr. D. R. Zimmermann; not shown).
Figure 4.
Immunodetection of versican isoforms in human melanoma and astrocytoma cell lines. Western blot of conditioned media from human SK-mel-1.36-1-5, SK-mel-3.44, and SK-mel-23 human melanoma cell lines and U251 astrocytoma cells proved with the anti-GAG-β antibody (left) and the anti-GAG-α antibody (right). The arrow marks a proteolytic-derived form of versican.
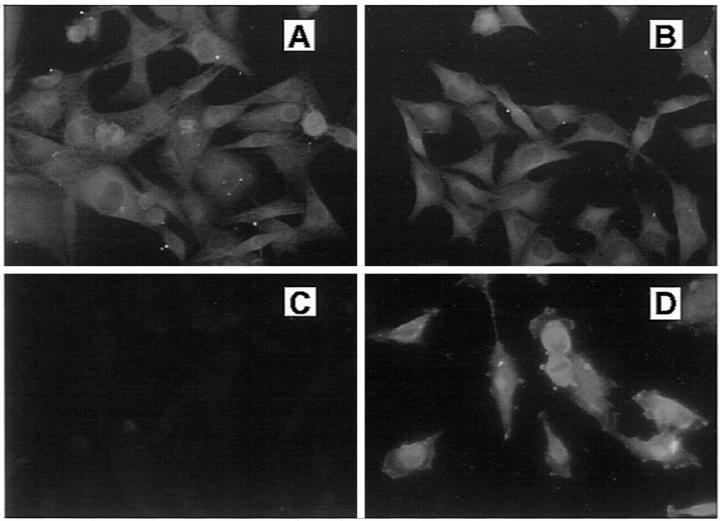
The presence or absence of versican in cell cultures was confirmed by immunocytochemistry using the antibody against purified versican. As shown in Figure 5 ▶ , in permeabilized SK-mel-1.36-1-5 and SK-mel-3.44 undifferentiated melanoma cells and U251 astrocytoma cells, versican staining was observed in the cytoplasm as a diffuse, network-like pattern, and in perinuclear vesicles. Conversely, no staining was observed in SK-mel-23-differentiated melanoma cells, indicating the absence of the PG.
Figure 5.
Immunocytochemistry of versican in human melanoma and astrocytoma cells. Subconfluent SK-mel-1.36-1-5 (A), SK-mel-3.44 (B), and SK-mel-23 (C) melanoma cells, and U251 astrocytoma cells (D) were grown in coverslips and fixed with 4% paraformaldehyde. After permeabilization, cells were incubated with the anti-versican antibody (1:10) and with an anti-rabbit-FITC. Cultures were viewed with a Nikon Eclipse E800 epifluorescence microscope and photographed with an integrated camera system.
Functional Properties of Versican on Human Melanoma Cells
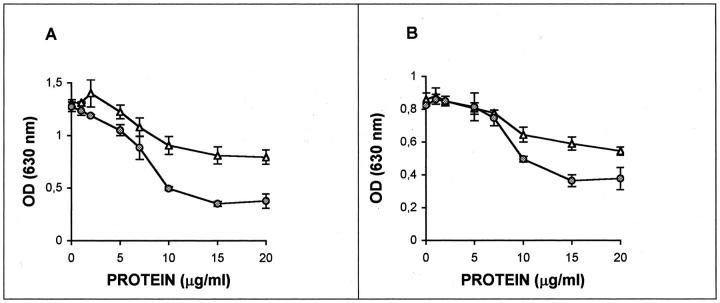
The effect of purified versican from U251 astrocytoma cell-derived conditioned medium on cell adhesion was assessed by plating SK-mel-1.36-1-5 cells on fibronectin or collagen I in the presence of different amounts of versican. As shown in Figure 6 ▶ , SK-mel-1.36-1-5 melanoma cells adhere readily to both substrates and versican is able to inhibit the adhesion to fibronectin and, less efficiently, to collagen I. When versican was treated with chondroitinase ABC to degrade the chondroitin sulfate chains, the inhibition produced by versican was reduced, especially when cells were grown on fibronectin.
Figure 6.
Versican decreases adhesion of melanoma cells on fibronectin and collagen. SK-mel-1.36-1-5 melanoma cells were seeded in Dynatech 96 well-plates coated with 5 μg/ml fibronectin (A) or 3 μg/ml collagen I (B). Negative controls were coated with the same concentration of bovine serum albumin. Cells were seeded at a density of 4 × 10 4 cells/well in the presence of purified versican and incubated for 2 hours at 37°C. The medium was removed and the adhered cells were fixed with 4% paraformaldehyde and incubated with 0.1% violet crystal for 20 minutes. After rinsing, a solution of 0.1 mol/L HCl was added and the absorbance at 630 nm was determined. Filled circle, nondigested versican; open triangle, chondroitinase ABC-digested versican. All of the values are the mean of three determinations ± SEM.
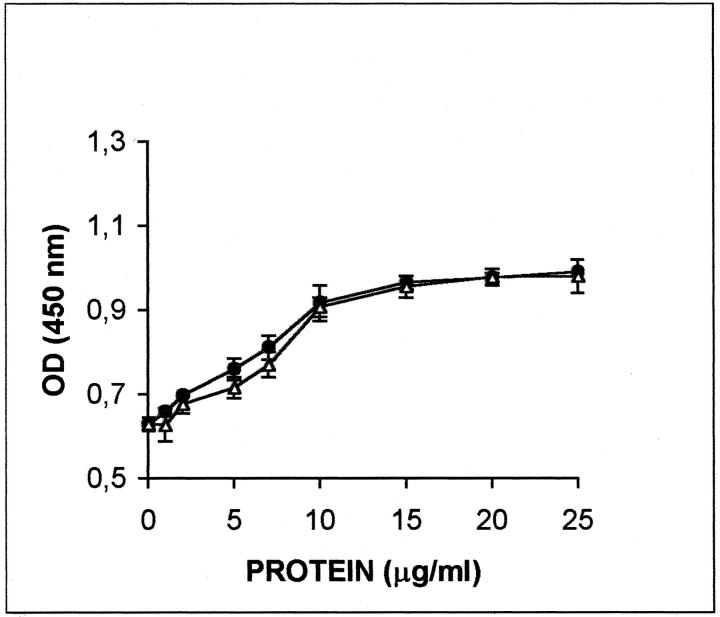
Versican was also able to induce cell proliferation in a concentration-dependent manner when added to SK-mel-1.36-1-5 cells (Figure 7) ▶ . In this case, the effect seems to be because of the protein core of versican, since the chondroitinase ABC-treated versican had the same effect than the whole PG.
Figure 7.
Versican increases cell proliferation of melanoma cells. SK-mel-1.36-1-5 melanoma cells were seeded in 96-well tissue culture plates in the presence of the indicated concentrations of purified versican. Each cell line was seeded at a concentration of 5000 cells/well. Cell proliferation was evaluated by determining the BrdU incorporation using a kit from Boehringer Mannheim. Filled circles, nondigested versican; open triangles, chondroitinase ABC-digested versican. All of the values are the mean of three determinations ± SEM.
Versican Expression in Human Melanocytic Lesions
The study of melanocytic lesions showed expression of versican in dysplastic nevi, primary malignant melanomas, and metastatic malignant melanomas. Benign melanocytic nevi did not show versican expression. Statistically significant differences between the percentages of positive melanocytic cells (P < 0.00015) and the intensity of versican expression (P < 0.0032) were found among benign melanocytic nevi, dysplastic nevi, and malignant melanomas (primary and metastatic). There were no statistically significant differences between primary malignant melanomas and metastatic malignant melanomas (Table 1 ▶ and Figure 8 ▶ ).
Table 1.
Evaluation of Versican Expression and Number of Positive Cells for Each Type of Melanocytic Lesion
| Lesion | No. of cases | Versican expression | % of Positive cells | Significance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Mild | Moderate | Intense | Significance | 0 | 5% | 6–25% | 26–50% | 51–75% | 76–100% | |||
| BMN | 10 | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | ||
| DN | 10 | 1 | 3 | 3 | 4 | 0 | 0 | 2 | 4 | 1 | 3 | ||
| PMM | 11 | 0 | 1 | 4 | 6 | 0 | 0 | 0 | 1 | 1 | 9 | ||
| MMM | 8 | 0 | 2 | 5 | 1 | P < 0.0032 | 0 | 0 | 0 | 0 | 0 | 8 | P < 0.00015 |
BMN, benign melanocytic nevi; DN, dysplastic nevi; PMM, primary malignant melanoma; MMM, metastatic melanoma.
Figure 8.
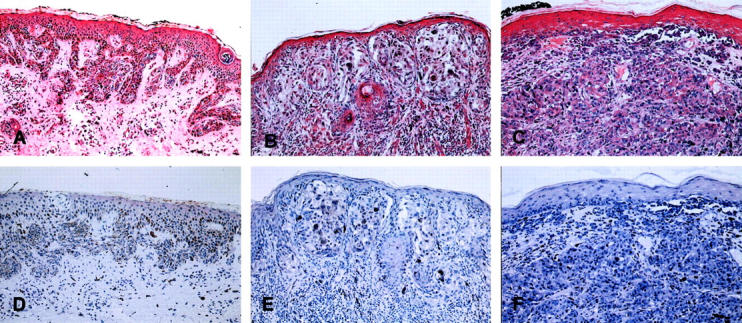
Versican expression in human melanocytic lesions. Dysplastic nevi (A and D), primary malignant melanomas (B and E), and metastatic malignant melanomas (C and F) were processed as described in Materials and Methods. Original magnifications: ×40 (versican, A–C; negative controls, D–F).
Discussion
Undifferentiated human melanoma cells produce, besides the well-recognized melanoma-specific PG, mel-PG, another high-molecular mass chondroitin sulfate PG. Our previous work showed that this PG is not recognized by the monoclonal antibody B5 raised against mel-PG and that it can be separated from mel-PG by gel chromatography in dissociative conditions. Both evidences indicate that this PG is not related to mel-PG. 19 The work presented here identifies this high-molecular weight PG as versican and establishes a close relationship between versican expression and cell differentiation in human melanoma in vitro and in vivo.
As shown in metabolic labeling experiments with [35S]sulfate and [35S]methionine, the high-molecular weight PG produced by undifferentiated human melanoma cells was similar to that produced by other cells of neuroectodermal origin, as the U251 human astrocytoma cell line. The PG produced by U251 astrocytoma cells was identified as versican by Dours-Zimmermann and Zimmermann, 2 suggesting that the melanoma PG could also be versican. By using antibodies raised against purified versican from U251 astrocytoma cells as well as antibodies directed specifically against the recombinant GAG-α and GAG-β versican subdomains, we have clearly demonstrated that versican is the main extracellular PG synthesized by undifferentiated melanoma cell lines and present in primary and metastatic malignant melanomas.
To analyze the relationship between versican expression and cell differentiation, we have used several human melanoma cell lines characterized by their morphology (epithelioid/spindle), profile of differentiation antigens, and tyrosinase activity and pigmentation. 20 By using these criteria, these cell lines have been characterized as having an early, intermediate, or late degree of differentiation. These three subsets of melanoma cell lines correspond to the features of normal melanocytes at the early, intermediate, or mature phases in melanocyte differentiation, as they express the same set of differentiation antigens. 23 Thus, there is a good relationship between the stage of melanocyte differentiation and the differentiation degree in cultured melanoma cell lines. We have shown that cell lines with an early or intermediate degree of differentiation (SK-mel-1.36-1-5, SK-mel-37, Rider, AX3, or SK-mel-3.44) secrete versican in the conditioned medium, whereas differentiated cell lines such as SK-mel-23, MeWo, or DX-2 do not produce any versican, thus linking versican production and differentiation degree. This is not a totally unexpected finding, since there are other indications in the literature that correlate both phenomena. Thus, versican is down-regulated in culture conditions promoting keratinocyte differentiation but is up-regulated in keratinocytes grown in proliferation-promoting conditions. 6 In vivo, versican is abundant throughout the entire dermis in early fetal human skin but disappears progressively from the lower half of the dermis during the fetal period 7 and normal adult human skin, where it is present in very restricted areas. 5,6
From our study, there is also a close relationship between cell differentiation degree and versican isoform pattern, since differentiated cell lines do not produce any isoform, whereas undifferentiated cell lines produce the V0 and V1 isoforms in variable amounts and cell lines with an intermediate degree of differentiation seem to produce mainly the V1 isoform. Versican isoforms differ in the size of the GAG subdomain and, subsequently, in the number of GAG chains and the existing distance between G1 and G3 subdomains. These differences should affect the ability of versican to interact with its ligands and, most probably, its physiological properties. Although there are no definitive studies on the possible differential biological role of the isoforms, it has been suggested that the size of the GAG domain could modulate the axonal growth inhibitory capacity of versican in the nervous system. 24
The presence in the Western blots of an immunoreactive band below the V1 isoform probably corresponds to a proteolytic product of V0, since it is recognized by both the anti-GAG-α and anti-GAG-β antibodies. The intensity of this band as well as that of the V0 isoform varied depending on the experiment, supporting this interpretation. The in vivo proteolytic processing of versican has been recently described in human aorta 25 and it has been suggested as having physiological relevance. Further studies are needed to analyze whether this is the case for malignant melanoma.
The presence of versican has probably a functional role in the biology of melanoma cells. Our results show that versican is an anti-adhesive substrate for melanoma cells growing in extracellular matrix components such as fibronectin and collagen I. This effect may contribute to the higher mobility of undifferentiated melanoma cells and their higher ability to migrate and develop metastasis. Besides decreasing cell adhesion, versican is also able to increase the proliferation rate of melanoma cells, thus increasing the growing potential of the tumor. In vitro studies have also suggested a growth-promoting role for versican. For example, it has been described that inhibition of versican biosynthesis by anti-sense RNA expression suppressed the malignant phenotype in MG63 osteosarcoma cells. 26 An increase in cell proliferation has been described in fibroblasts transfected with a minigene containing the G1 or G3 terminal domains of versican. 17,18
All these data reinforce the growing evidence that versican plays an active role in tumor cell biology. A marked production of chondroitin sulfate PG is a well-recognized phenomenon in a variety of malignant tumors. 27 In some cases, versican has been identified as the PG species in brain tumors, 9 histiocytoma, 8 breast cancer, 28-30 prostate cancer, 31,32 and some nonepithelial neoplasms. 33 Furthermore, in melanomas, the increase in versican is accompanied by the presence of high amounts of hyaluronate, which has been recognized in the literature for many years. 34,35 Versican is able to bind hyaluronate through its hyaluronic-binding domain near its N-terminal region and the expanded and water-enriched environment provided by these versican/hyaluronate aggregates most probably deforms the normally compact architecture of the extracellular matrix and facilitates cell movement and growth. 36
Our data also support a role for the protein core as well for the GAG chains of versican. Thus, cell proliferation appears to be driven mostly by the protein core since there is no difference between the chondroitinase ABC-treated and the whole molecule, supporting the results described in fibroblasts transfected with the versican minigene, where the growth-promoting effect has been attributed to the protein core, in particular to the epidermal growth factor-like motifs present in the G3 domain 17,18 as well as to the G1 domain. 37 On the other hand, the anti-adhesive effect seems to be because of the protein core as well as to the presence of the GAG chains, since chondroitinase ABC-digestion reduces but does not abolish this effect.
Finally, the study of versican in melanocytic lesions has shown that this PG could serve as a good marker for malignant melanoma of primary as well as metastatic origin. There is a close relationship between malignancy of the lesion and intensity and percentage of versican-positive cells. The differences in expression observed among the three groups of melanocytic lesions studied (benign melanocytic nevi, dysplastic nevi, metastatic melanoma) would be in keeping with the results obtained in previous studies on markers of cellular proliferation, which would support the concept of dysplastic nevi as a precursor lesion of melanoma. 38-41
In conclusion, our results lend support to the hypothesis that versican expressed by the most undifferentiated melanomas may facilitate tumor cell detachment and promote cell growth, thus contributing to the aggressive biological behavior of undifferentiated melanomas, and could be a reliable marker for clinical diagnosis.
Acknowledgments
We thank Dr. F. X. Real (I.M.I.M., Barcelona, Spain) for providing the cell lines used in this work, Dr. D. R. Zimmermann (University of Zürich, Switzerland) for the antibodies against GAG-α and GAG-β, Dr. A. Ariza (Hospital Germans Trias i Pujol, Badalona, Spain) for critical reading of the manuscript, and Ms. Anna Vilalta for her excellent technical assistance.
Footnotes
Address reprint requests to Anna Bassols, Ph.D., Departament de Bioquímica i Biologia Molecular, Facultat de Veterinària, Universitat Autònoma de Barcelona, 01893 Bellaterra, Spain. E-mail: anna.bassols@uab.es.
Supported by grants 00/1016 from the Fondo de Investigaciones Sanitarias (F. I. S.) and 1999SGR/0100 from the Generalitat de Catalunya. M. T. was partially supported by a fellowship from the Agencia Española de Cooperacíon Intenationcional.
References
- 1.Iozzo RV: Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem 1998, 67:609-652 [DOI] [PubMed] [Google Scholar]
- 2.Dours-Zimmermann MT, Zimmermann DR: A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem 1994, 269:32992-32998 [PubMed] [Google Scholar]
- 3.Zimmermann DR: Versican. Iozzo RV eds. Proteoglycans: Structure, Biology and Molecular Interactions. 2000, :pp 327-341 Marcel Dekker, Inc., New York [Google Scholar]
- 4.Zimmermann DR, Ruoslahti E: Multiple domains of the large fibroblast proteoglycan, versican. EMBO J 1989, 8:2975-2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heitz PU, Zimmermann DR: Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem 1996, 44:303-312 [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann DR, Dours-Zimmermann MT, Schubert M, Bruckner-Tuderman L: Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J Cell Biol 1994, 124:817-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorrell JM, Carrino DA, Baber MA, Caplan AI: Versican in human fetal skin development. Anat Embryol 1999, 199:45-56 [DOI] [PubMed] [Google Scholar]
- 8.Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegard D, Kimata K: 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res 1996, 56:3902-3908 [PubMed] [Google Scholar]
- 9.Paulus W, Baur I, Dours-Zimmermann MT, Zimmermann DR: Differential expression of versican isoforms in brain tumors. J Neuropathol Exp Neurol 1996, 55:528-533 [DOI] [PubMed] [Google Scholar]
- 10.LeBaron RG, Zimmermann DR, Ruoslahti E: Hyaluronate binding properties of versican. J Biol Chem 1992, 267:10003-10010 [PubMed] [Google Scholar]
- 11.Aspberg A, Miura R, Bourdoulos S, Shimonaka M, Heinegard D, Schachner M, Ruoslahti E, Yamaguchi Y: The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci USA 1997, 94:10116-10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M: Versican interacts with chemokines and modulates cellular responses. J Biol Chem 2001, 276:5228-5234 [DOI] [PubMed] [Google Scholar]
- 13.Olin AI, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A: The proteoglycans aggrecan and versican form networks with fibulin-2 through their lectin binding domain. J Biol Chem 2001, 276:1253-1261 [DOI] [PubMed] [Google Scholar]
- 14.Kawashima H, Hirose M, Hirose J, Nagabuko D, Plaas AH, Miyasaka M: Binding of a large chondroitin/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin and CD44. J Biol Chem 2000, 275:35448-35456 [DOI] [PubMed] [Google Scholar]
- 15.Zou K, Muramatsu H, Ikematsu S, Sakuma S, Salama RH, Shinomura T, Kimata K, Muramatsu T: A heparin-binding growth factor, midkine, binds to a chondroitin sulfate proteoglycan, PG-M/versican. Eur J Biochem 2000, 267:4046-4053 [DOI] [PubMed] [Google Scholar]
- 16.Yamagata M, Suzuki S, Akiyama SK, Yamada KM, Kimata K: Regulation of cell-substrate adhesion by proteoglycans immobilized on extracellular substrates. J Biol Chem 1989, 264:8012-8018 [PubMed] [Google Scholar]
- 17.Zhang Y, Cao L, Yang BL, Yang BB: The G3 domain of versican enhances cell proliferation via the epidermal growth factor-like motifs. J Biol Chem 1998, 273:21342-21351 [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Zhang Y, Cao L, Chen L, Lee V, Zheng PS, Kiani C, Adams ME, Ang LC, Paiwand F, Yang BB: Identification of the motif in versican G3 domain that plays a dominant-negative effect on astrocytoma cell proliferation through inhibiting versican secretion and binding. J Biol Chem 2001, 276:14178-14186 [DOI] [PubMed] [Google Scholar]
- 19.Heredia A, Villena J, Romaris M, Molist A, Bassols A: Transforming growth factor β1 increases the synthesis and shedding of the melanoma-specific proteoglycan in human melanoma cells. Arch Biochem Biophys 1996, 333:198-206 [DOI] [PubMed] [Google Scholar]
- 20.Houghton AN, Real FX, Davis LJ, Cordon-Cardo C, Old LJ: Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med 1987, 164:812-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 22.Bitter T, Muir HM: A modified uronic acid carbazole reaction. Anal Biochem 1962, 4:330-334 [DOI] [PubMed] [Google Scholar]
- 23.Houghton AN, Eisinger M, Albino AP, Cairncross JG, Old LJ: Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med 1982, 156:1755-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR: Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci 2000, 113:807-816 [DOI] [PubMed] [Google Scholar]
- 25.Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW: Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem 2001, 276:13372-13378 [DOI] [PubMed] [Google Scholar]
- 26.Yamagata M, Kimata K: Repression of a malignant cell-substratum adhesion phenotype by inhibiting the production of the antiadhesive proteoglycan, PG-M/versican. J Cell Sci 1994, 107:2581-2590 [DOI] [PubMed] [Google Scholar]
- 27.Sobue M, Takeuchi J, Yoshida K, Akao S, Fukatsu T, Nagasaka T, Nakashima N: Isolation and characterization of proteoglycans from human nonepithelial tumors. Cancer Res 1987, 47:160-168 [PubMed] [Google Scholar]
- 28.Alini M, Losa GA: Partial characterization of proteoglycans isolated from neoplastic and nonneoplastic human breast tissues. Cancer Res 1991, 51:1443-1447 [PubMed] [Google Scholar]
- 29.Nara Y, Kato Y, Torii Y, Tsuji Y, Nakagaki S, Goto S, Isobe H, Nakashima N, Takeuchi J: Immunohistochemical localization of extracellular matrix components in human breast tumours with special reference to PG-M/versican. Histochem J 1997, 29:21-30 [DOI] [PubMed] [Google Scholar]
- 30.Brown LF, Guidi AJ, Schnitt SJ, Van de Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF: Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res 1999, 5:1041-1056 [PubMed] [Google Scholar]
- 31.Ricciardelli C, Mayne K, Sykes PJ, Raymond WA, McCaul K, Marshall VR, Horsfall DJ: Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin Cancer Res 1998, 4:963-971 [PubMed] [Google Scholar]
- 32.Sakko AJ, Ricciardelli C, Mayne K, Tilley WD, Lebaron RG, Horsfall DJ: Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res 2001, 61:926-930 [PubMed] [Google Scholar]
- 33.Ohiwa N, Fukata S, Fukatsu T, Nagasaka T, Nara Y, Lai S, Nakashima N, Takeuchi J: Immunohistochemical localization of proteoglycans in non-epithelial tumor tissues. Connect Tissue 1991, 22:11-19 [Google Scholar]
- 34.Knudson W, Biswas C, Toole BP: Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci USA 1984, 81:6767-6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turley EA, Tretiak M: Glycosaminoglycan production by murine melanoma variants in vivo and in vitro. Cancer Res 1985, 45:5098-5105 [PubMed] [Google Scholar]
- 36.Knudson W: Tumor-associated hyaluronan: providing an extracellular matrix that facilitates invasion. Am J Pathol 1996, 148:1721-1726 [PMC free article] [PubMed] [Google Scholar]
- 37.Yang BL, Zhang Y, Cao L, Yang BB: Cell adhesion and proliferation mediated through the G1 domain of versican. J Cell Biochem 1999, 72:210-220 [DOI] [PubMed] [Google Scholar]
- 38.Barnhill RL: Current status of the dysplastic melanocytic nevus. J Cutan Pathol 1991, 18:147-159 [DOI] [PubMed] [Google Scholar]
- 39.Fogt F, Vortmeyer AO, Tahana SR: Nucleolar organizer regions (AgNOR) and Ki-67 immunoreactivity in cutaneous melanocytic lesions. Am J Dermatopathol 1995, 17:12-17 [DOI] [PubMed] [Google Scholar]
- 40.Takahashi H, Strutton GM, Parsons PG: Determination of proliferating fractions in malignant melanomas by anti-PCNA/cyclin monoclonal antibody. Histopathology 1991, 18:221-227 [DOI] [PubMed] [Google Scholar]
- 41.Korabiowska M, Brinck U, Middel P, Brinkmann U, Berger H, Radzun HJ, Ruschenburg I, Droese M: Proliferative activity in the progression of pigmented skin lesions, diagnostic and prognostic significance. Anticancer Res 2000, 20:1781-1786 [PubMed] [Google Scholar]