Abstract
The hypothetical multistep model for breast carcinogenesis indicates that invasive carcinoma arises via a series of intermediate hyperplastic lesions through various grades of atypia to in situ and invasive carcinoma. Non-atypical hyperplasia [hyperplasia of usual type (HUT)] is a nonobligate precursor of breast cancer. Although its further morphological subclassification is unlikely, refining is more likely to depend on defining biological markers of risk. Having assembled a cohort of benign proliferative breast lesions of known outcome, we studied the expression of estrogen receptor-α (ER-α) and Ki-67 using morphometric image analysis as well as dual-labeled immunofluorescence in HUT foci and in surrounding normal lobules of 25 patients that progressed to breast cancer and 19 controls. Those patients that progressed to breast cancer (cases) showed significantly higher ER-α [median, 57.00% of cells within individual HUT foci; interquartile range (IQ), 33.48 to 67.78] and Ki-67 (median, 3.82%; IQ, 0.85 to 11.28) expression in their HUT foci compared with controls (ER-α median, 30.27%; IQ, 19.75 to 52.50 and Ki-67 median, 0.77%; IQ, 0.0458 to 1.72, P = 0.008 and <0.001). No significant difference in expression of dual-stained cells was found between cases and controls. Although normal lobules from cases showed higher ER-α expression compared with controls, this was not statistically significant. Our data point to a previously undescribed hormone-dependent pathway in this particular group of breast neoplasms and suggest the possibility of selective hormonal therapy to suppress the proliferative potential of these benign but high-risk breast lesions. The findings of this study might have important implications for improving breast cancer screening and management strategies.
Hyperplasia of usual type (HUT) is a common lesion associated with an increased risk of subsequently developing breast cancer. 1 It encompasses a spectrum of changes ranging from minimal stratification of intraluminal cells to proliferations that fall just short of atypical ductal hyperplasia. However, not all hyperplastic proliferations are committed to the development of breast cancer. Although morphological subgroups have not been identified, there is evidence from molecular studies that HUT is heterogeneous. 2 Biological markers would be helpful to subdivide HUT and to determine the putative role of these markers in the process of mammary carcinogenesis.
Epidemiological and experimental evidence suggest that breast cancer risk is related to the duration of estrogen exposure during puberty, the early postmenopausal period, and the menopausal period. 3 Furthermore, the anti-estrogen tamoxifen decreases proliferation in breast cancer 4 although its role in preventing the disease is at dispute at present. 5 Estrogen is also associated with epithelial proliferation in noncancerous breasts during the menstrual cycle and in pregnancy. 6,7 Hankinson and colleagues 8 demonstrated a statistically significant positive association between the risk of breast cancer and circulating levels of estrogen providing a strong evidence of causal relationship between postmenopausal estrogen levels and the risk of breast cancer.
It has been suggested that estrogen receptor (ER)-α positivity in benign breast epithelium could be a risk factor for breast malignancy 9 because the presence of ER-α is thought to render cells susceptible to proliferation stimulus of estrogens. The median of the percentage of ER-α-positive cells was higher in Australian postmenopausal females when compared with the Japanese. These data are compatible with the hypothesis that expression of ERs in normal breast tissues increases the risk of breast cancer, and provides an explanation for the poor international concordance between breast cancer occurrence and estrogen production rates or blood concentrations. 10
Several other studies have shown a very tight association between Ki-67 immunoreactivity and the cell cycle, with expression beginning in the mid to late G1, rising through S phase and G2 to reach maximum in mitosis. 11,12 Clarke and colleagues 13 described almost mutual exclusion of steroid receptor expression and cell proliferation as evidenced by the lack of dual immunostaining for ER-α and Ki-67 antigen.
Previous work in this laboratory revealed dysregulation of ER-positive proliferating cells, increasing from normal lobules through hyperplasia to in situ and invasive breast cancer. 14 These findings were followed by identification of heterogeneity in ER-α and proliferation in non-atypical hyperplasia. 15 Although those two studies showed some interesting observations, no clinical outcome data were available. We have now identified a significant number of ductal proliferative lesions representing a step of progression toward the development of breast carcinomas mixed with morphologically similar lesions of age and date of biopsy matched controls. We have studied the relationship between ER-α status and cellular proliferation in this cohort of hyperplastic epithelial breast lesions with known clinical outcome in an attempt to verify the putative role of this interaction in the process of breast carcinogenesis.
Materials and Methods
Patients
We designed a case-control study using histological specimens collected between the beginning of January 1979 and the end of January 1999 from patients in the Merseyside and Cheshire area, UK. Those comprised patients who underwent biopsy revealing benign diagnoses at three hospitals (The Royal Liverpool University Hospital, Broadgreen Hospital, and Lourdes Hospital). Included as study cases, were all patients with a benign breast lesion followed by in situ or invasive cancer of either breast at least 6 months after the benign lesion. Each study case was matched for age and date of biopsy with three controls that had histories of benign breast lesions only. When a patient had more than one benign specimen, then all specimens were examined. The study has been conducted on 674 biopsy specimens from 502 patients including 120 benign biopsies from patients who subsequently developed breast cancer and 382 controls that were not known to develop breast cancer spanning a 20-year follow-up period (Table 1) ▶ . Excluded from the study cases and controls were all patients with axillary fat, scars from previous mastectomies, breast skin- and lymph node-only specimens, in situ or invasive breast cancer before the benign diagnosis in either breast, in situ or invasive breast cancer in either breast less than 6 months after the benign breast lesion, and patients who had another cancer before breast cancer.
Table 1.
Distribution of HUT in Study Cases and in Controls
| HUT | Case, no. (%) | Controls, no. (%) | Total |
|---|---|---|---|
| Present | 38 (31.66) | 79 (20.68) | 117 |
| IHC* | 25 | 19 | 44 |
| Absent | 82 (68.34) | 303 (79.32) | 385 |
| Total | 120 (100) | 382 (100) | 502 |
*Number of patients with HUT analyzed by immunohistochemistry.
Slides of a total of 674 biopsy specimens of benign breast lesions were examined and classified into different benign categories initially by one pathologist and later reviewed jointly by the two pathologists (AMS and JPS) and a consensus reading was obtained. All diagnoses were made following the Pathology Guidelines of the UK Breast Screening Program 16 and were performed blindly without knowledge of the outcome of the cases. Furthermore, all subsequent malignant tumors were examined and classified.
Immunostaining Procedure
Slides and blocks from patients with HUT were selected and reviewed. From the 117 patients in which HUT was identified morphologically, staining was performed on 44 patients that comprised 25 that progressed to breast cancer and 19 controls (Table 2) ▶ . ER-α was detected with a mouse monoclonal anti-ER-α antibody (clone 1D5; DAKO Ltd., Ely, Cambridge, UK) and Ki-67 with a rabbit polyclonal anti-Ki-67 antibody (Novocastra, Newcastle-on-Tyne, UK). Adjacent morphologically normal lobules were also stained. Immunostaining was performed using a standard streptavidin-biotin method with previous pressure cooking for antigen unmasking. Negative controls in which the primary antibody was omitted and three positive controls of ER-positive breast carcinoma of varying staining intensities were included in each batch of immunostaining. The method was identical to that used for routine assessment of ER and Ki-67 status in which the laboratory performs well in the UK External Quality Assessment Scheme. Also stained were 21 malignant breast tumors that developed after benign diagnoses of HUT using the identical anti-ER-α monoclonal antibody and staining procedure.
Table 2.
Expression of ER-α and Ki-67 in Normal and Hyperplastic Foci in High-Risk and Normal Patients
| Total no. (foci) | Cases, no. (foci) | Controls, no. (foci) | % in cases (IQ) | % in controls (IQ) | P value* | |
|---|---|---|---|---|---|---|
| ER(N) | 43 (63) | 27 (41) | 16 (22) | 28.22 (12.18–45.98) | 20.10 (8.9–46.68) | 0.33 |
| ER(HUT) | 44 (131) | 25 (68) | 19 (63) | 57.00 (33.48–67.78) | 30.27 (19.75–52.5) | 0.008† |
| Ki-67(N) | 31 (43) | 16 (27) | 15 (16) | 0.70 (0–0.09) | 0.76 (0–4.38) | 0.35 |
| Ki-67(HUT) | 38 (87) | 16 (42) | 22 (45) | 3.82 (0.85–11.28) | 0.77 (0.0458–1.72) | <0.001† |
ER, Estrogen receptor α; N, normal lobules; IQ, interquartile range.
*Significant if P ≤ 0.05.
†Highly significant.
Dual Immunofluorescence Staining
This was performed as previously described 15 by the application of 100 μl of a mixture of both primary antibodies (diluted appropriately in 5% bovine serum albumin/Tris-buffered saline) for 80 minutes. The dilution used for the monoclonal ER antibody 1D5 (DAKO) was 1:30 and for the monoclonal rabbit anti-human Ki-67antibody (Novocastra) was 1:100. This was followed by the application of 100 μl of a mixture of both secondary antibodies diluted in 5% bovine serum albumin/Tris-buffered saline for 30 minutes. The secondary antibodies used were tetramethylrhodamine B isothiocyanate-conjugated swine anti-rabbit antibody 1:50 (DAKO) and biotinylated sheep anti-mouse antibody 1:100 (Amersham Life Sciences, UK). The slides were then incubated with fluorescein-avidin conjugate 1:100 (diluted in 5% bovine serum albumin/Tris-buffered saline) for 30 minutes to visualize the anti-mouse antibody. All incubations were at room temperature and washes in phosphate-buffered saline were performed in between. The slides were then coverslipped and mounted using an anti-fading medium containing 4′,6-diaminido-2-phenylindole (Vectashield; Vector Laboratories, UK) to stain DNA.
Assessment of Immunostaining
To maximize consistency of scoring, only nuclei showing moderate or strong staining were regarded as positive. Ductal proliferations were assessed for the percentage of ER-α(+) and Ki-67(+) cells within lesions using a Leica KS-300 image analysis system. Each focus of HUT was identified within both cases and controls according to the criteria of the UK Breast Screening Program. 16 Every image was automatically digitized before analysis using a custom-designed program that detected the nuclear diaminobenzidine reaction product as well as unstained nuclei. Before counting, each field was masked to remove from the analysis all elements (eg, adjacent normal tissues) that were not components of the HUT. The area of the resulting unmasked field was calculated automatically so that all numerical counts were thereafter generated with respect to unit area of HUT foci. The analysis yielded two sets of data that included both stained and unstained cells for each selected focus. All epithelial cells present within every focus and all HUT foci for each patient were examined. The percentage of positive cells was calculated as a proportion of total number of cells present in each HUT focus. The percentage of ER(+) and Ki-67(+) cells was then averaged for each patient. Stromal cells, myoepithelial cells, and macrophages remaining within the unmasked fields were not included in this analysis. Contiguous ER staining was also assessed as defined by the criterion of 10 or more ER(+) cells in contact with each other. ER positivity was defined using a 10% cutoff conventionally used as the median value of its expression in each patient. 17 As internal controls for each patient, identical criteria were applied to the adjacent but microscopically normal nonatrophic lobules to assess whether dysregulation of both markers occurred in normal lobules.
Assessment of Fluorochrome-Labeled Staining
Each field was examined under high power for the red (tetramethylrhodamine B isothiocyanate), green (fluorescein), and blue (4′,6-diaminido-2-phenylindole) fluorochromes, using appropriate filters (Zeiss filters 15, 10, and 2, respectively) to assess the presence or absence of dual-labeled cells. The percentage of dual-expressing cells was calculated in relation to total cell number within hyperplastic foci and adjacent normal lobules.
Statistics
Possible association between each benign lesion, including HUT and subsequent malignant transformation, was measured by Pearson’s chi-square test (without continuity correction) and calculated with Minitab for Windows, version 12. The relative risk and its 95% confidence interval (CI) for this lesion and for the ER-rich and proliferative HUT foci as well as the odds ratio were calculated with the StatCalc program within EpiInfo version 6 18 using a 10% cutoff point for ER positivity. 17 Foci positive for Ki-67 included all those containing figures more than zero. For all calculations, the median values of expression were used for both the HUT foci and for normal lobules. The data from immunohistochemistry and immunofluorescence were analyzed by the nonparametric two-sided Mann-Whitney test, Spearman’s rank-correlation coefficient (rs), and multiple logistic regression analysis (taking a predicted probability of P = 0.5 as cutoff point in classification tables) using SPSS for Windows (version 10). For comparing ER status in HUT and subsequent malignant tumors, a paired t-test (confirmed by a paired Wilcoxon test) was used.
Results
In this series of 502 cases comprising 117 patients containing non-atypical epithelial proliferations, HUT was found more frequently in those patients who subsequently developed breast cancer when compared with controls (Table 1 ▶ ; Pearson’s chi-square test, P = 0.01). The relative risk and the odds ratio of developing breast cancer after a benign biopsy that included HUT were respectively 1.53 (CI, 1.10 to 2.13) and 1.78 (CI, 1.10 to 2.88). The mean age of the patients analyzed immunohistochemically was 50.66 years. The mean age for the cases that progressed to breast cancer was 52.53 years (range, 35.52 to 69.77 years) whereas that of the controls was 48.05 years (range, 38.60 to 59.42 years). The average number of cells examined in each focus of HUT was 546.23 (range, 66 to 3974 cells).
ER-α Expression in Normal Lobules
A total of 63 morphologically normal lobules with premenopausal appearance from 43 patients examined for ER-α positivity comprised 41 from patients who subsequently developed breast cancer and 22 from matched controls. The mean number of normal lobules examined for each patient was 1.68 (range, 1 to 5 lobules). The majority of cells were unstained for ER-α, but with a few interspersed ER-α(+) cells (Figure 1A) ▶ . The median percentage of ER-α(+) cells was 28.22 [interquartile range (IQ): 12.18 to 45.98] in the cases that progressed to breast cancer and 20.1 (IQ, 8.9 to 46.68) in the controls. This difference was not statistically significant (Mann-Whitney, P = 0.35).
Figure 1.
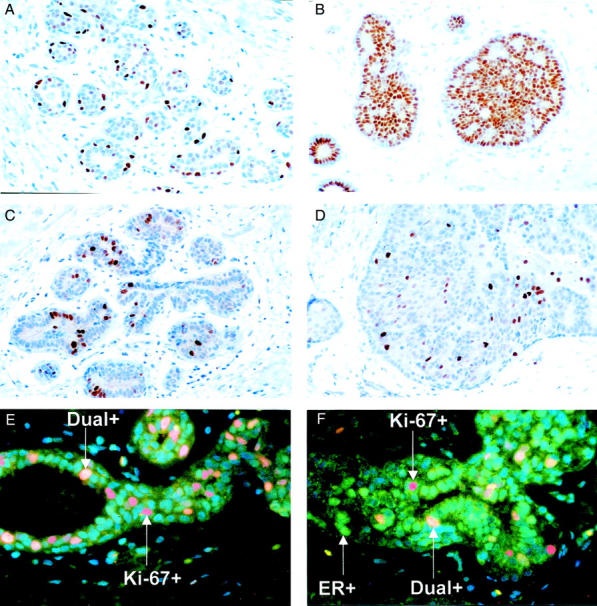
A: ER-α expression. A normal breast lobule showing ER-α(+) cells surrounded by many ER-α(−) cells. B: ER-α expression. HUT biopsy from a case that subsequently developed breast cancer showing large numbers of ER-α(+) cells. C: Ki-67 expression. A normal breast lobule from a patient that progressed to breast cancer showing some Ki-67(+) cells among a majority of nonproliferating cells. D: Ki-67 expression. HUT focus from a case that subsequently developed breast cancer showing high proliferation rate. E: Indirect immunofluorescence for ER-α (green), Ki-67 (red), and dual-labeled cells (yellow). A normal breast lobule. F: Indirect immunofluorescence for ER-α (green), Ki-67 (red), and dual-labeled cells (yellow). HUT focus. Original magnifications: ×25 (A, B, C, and D); ×40 (E and F).
Percentage and Distribution of ER-α(+) Cells in HUT
The mean number of HUT foci examined for each patient was 2.98 (range, 1 to 9 foci). Examination of 131 foci of HUT from 44 patients confirmed the expression of ER-α(+) cells in HUT foci from patients who subsequently developed breast cancer (Figure 1B) ▶ to be higher (median, 57.0%; IQ, 33.48 to 67.78) when compared with those from controls (median, 30.27%; IQ, 19.75 to 52.50). This was highly significant (Mann-Whitney, P = 0.008; Table 2 ▶ and Figure 2A ▶ ). Some HUT foci exhibited contiguous ER-α(+) staining of 10 or more ER+ in contact to each other. Contiguous ER staining correlated significantly with the percentage of ER-expressing cells irrespective of whether the specimen was a case or a control (rs = 0.44,P < 0.001). Using a 30% cutoff for ER-α positivity, most HUT foci (85.7%) showed contiguous staining. With a 60% cutoff for ER positivity, all hyperplastic foci showed contiguous staining.
Figure 2.
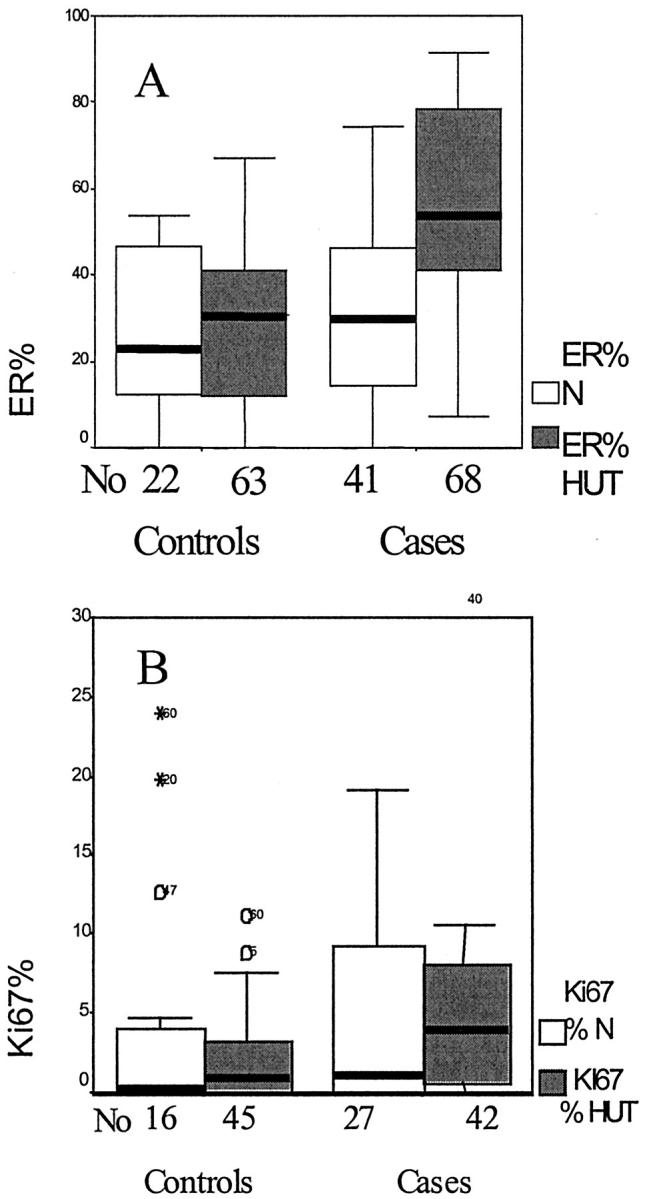
Boxplot graphs, plotted for the median ER-α (A) and Ki-67 (B) percentage in normal lobules and hyperplastic foci. The boxes contain the values between the 25th and 75th percentiles, the lines across the boxes represent the medians, the whiskers extend to the highest and lowest values excluding outliers. Open circles and asterisks identify outliers and extreme values. No, the number of foci examined; N, normal lobules.
When comparing ER-α expression in normal lobules and HUT foci, the percentage of positively stained cells was higher in HUT foci than in the surrounding normal lobules for those cases that progressed to cancer and for the controls. The difference was higher in biopsies from the cases that subsequently progressed (P = 0.02) although that of the controls was not statistically significant (P = 0.21; Table 3 ▶ ).
Table 3.
The Relation between ER and Ki-67 Expression in Normal and Hyperplastic Foci in Each Group
| Normal | HUT, % (range) | P value | |
|---|---|---|---|
| Cases | |||
| ER% (IQ) | 28.22 (12.18–45.98) | 57.00 (33.48–67.78) | 0.022* |
| Ki-67% (IQ) | 0.70 (0–0.09) | 3.82 (0.85–11.28) | 0.004† |
| Controls | |||
| ER% (IQ) | 20.12 (8.9–46.68) | 30.27 (19.75–52.5) | 0.207 |
| Ki-67% (IQ) | 0.76 (0–4.38) | 0.77 (0.04583–1.72) | 0.920 |
IQ, Interquartile range.
*Significant.
†Highly significant.
ER-α Expression in Carcinomas
Of the 21 breast carcinomas that were available within the archive for analysis, (Table 4) ▶ 11 were invasive ductal carcinomas (NST), 18 (85.7%) were ER-α(+) and 3 (14.3%) were ER-α(−). All three ER-α(−) tumors occurred in patients who had previously demonstrated ER-α positivity in their HUT foci. A significant correlation was found between mean ER-α expression in HUT and that of cancer (rs = 0.459, P = 0.036). A paired t-test (confirmed by a Wilcoxon test) showed that the mean shift between benign and malignant values is significantly different from zero (P = 0.047). The mean proportion of the ER-α(+) cells for the cancer that subsequently developed from HUT was 68.3%.
Table 4.
ER-α Expression in HUT and the Subsequent Breast Cancer
| Case no. | Mean ER % in HUT | Mean ER % in carcinoma | Type of carcinoma (grade) | Interval (months) |
|---|---|---|---|---|
| 1 | 57.00 | 80 | NST (III) | 47.7 |
| 2 | 50.88 | 95 | NST (III) | 144.4 |
| 3 | 35.87 | 90 | NST (I) | 31.5 |
| 4 | 27.34 | 0 | NA | 176.9 |
| 5 | 62.60 | 50 | DCIS (high) | 107.7 |
| 6 | 53.41 | 70 | Invasive lobular (II) | 51.9 |
| 7 | 61.93 | 95 | NST(II) | 58.9 |
| 8 | 29.73 | 0 | NST (III) | 41.7 |
| 9 | 48.19 | 70 | NST (II) | 19.9 |
| 10 | 68.25 | 60 | Paget’s, DCIS (high) | 23.4 |
| 11 | 79.91 | 85 | Mucinous (I) | 38.1 |
| 12 | 74.99 | 95 | DCIS (low) | 90.9 |
| 13 | 9.67 | 75 | DCIS (high) | 69.7 |
| 14 | 67.31 | 85 | Mucinous (I) | 9.2 |
| 15 | 40.74 | 50 | Mucinous (I) | 6.3 |
| 16 | 71.27 | 80 | DCIS (low) | 51.1 |
| 17 | 80.53 | 95 | NST (II) | 19.6 |
| 18 | 68.49 | 95 | NST (II) | 132.8 |
| 19 | 9.84 | 70 | NST (II) | 116.9 |
| 20 | 64.32 | 95 | NST (III) | 202.2 |
| 21 | 67.00 | 0 | NST (III) | 153.3 |
NST, invasive ductal carcinoma of no specific type; DCIS, ductal carcinoma in situ; NA, not applicable.
Ki-67 Expression in Normal Lobules
The percentage of Ki-67(+) cells was similar (median, 0.7%) in the morphologically normal lobules within the cases and controls (Table 3 ▶ and Figure 1C ▶ ).
Percentage of Ki-67(+) Cells in HUT Foci
Eighty-seven HUT foci were examined for Ki-67 expression by morphometric analysis in both groups of patients. The percentage of proliferating cells was significantly higher (median, 3.82%; IQ, 0.85 to 11.28) in the cases (Figure 1D) ▶ compared with controls (median, 0.77%; IQ, 0.0458 to 1.72). However, the proliferation data for the HUT foci of the controls were almost identical to the data obtained from normal lobules. The high expression of Ki-67 in HUT foci in cases that progressed to cancer when compared with the controls was statistically highly significant (Mann-Whitney, P < 0.001; Table 2 ▶ and Figure 2B ▶ ). Ki-67(+) cells were mainly peripheral and discrete.
The median percentage of Ki-67(+) cells in HUT and normal foci from the cases that progressed to cancer and their controls was compared. Significantly higher Ki-67 expression was found in HUT that progressed to cancer when compared with its surrounding lobules (P = 0.004). However, controls showed no significant difference (P = 0.92; Table 3 ▶ ).
Relation of ER and Ki-67 Expression in HUT Foci to Breast Cancer Risk
The odds ratio of ER expression (using a 10% cutoff) in HUT foci for those cases that developed breast cancer versus the controls was 3.06 (CI, 0.49 to 18.88). Expression of Ki-67 in HUT, for all positive values, for those cases that progressed versus the controls yielded an odds ratio of 1.62 (CI, 0.33 to 7.78). The age-adjusted odds ratio for ER was 2.28 (CI, 0.727 to 7.16) whereas that for Ki-67 was 1.47 (CI, 0.458 to 4.769). However, neither was statistically significant. Logistic regression analysis, using both markers in a predictive model for developing breast cancer, identified the overall correct classification rate of both ER and Ki-67 in HUT to be 67.4% with a positive predictive value of 72.2% and negative predictive value of 64.3%.
Dual-Expression Cells in Normal and Hyperplastic Foci
Cells co-expressing ER-α and Ki-67 were found in 5.1% and 13.5% of normal lobules and HUT foci, respectively (Figure 1, E and F) ▶ . Of the latter, 60% were cases that progressed whereas 40% were controls. The median expression of dual-stained cells in HUT foci was 0.0955% epithelial cells in the cases that progressed to cancer and 0.0527% epithelial cells in the controls. This difference was not statistically significant (Mann-Whitney test, P = 0.9). For normal lobules, the median percentage of dual-expressing cells was 0.0460% epithelial cells in those cases that progressed to cancer and 0.010% epithelial cells in the controls. This also was not significant (P = 0.56). Even the presence or absence of dual staining was not statistically different between the two groups.
Discussion
The relationship between proliferative epithelial lesions and increased likelihood of breast carcinoma development was first identified by Page and colleagues 19 followed by a larger follow-up study confirming this association. 20 In the present study, a relative risk of 1.53 (CI, 1.1 to 2.1) was identified after a diagnosis of HUT. Although this present series includes examples of different benign breast lesions, expression of two biological markers within the subset of HUT was studied for the following reasons: first, HUT has been previously shown to be consistently associated with an increased risk of subsequently developing breast cancer. In this present series, the level of breast cancer risk (relative risk, 1.53; CI, 1.1 to 2.1) was in accordance with previous studies that demonstrated that HUT carries an increased risk of 1.5 to 2 times that of the general population of subsequently developing breast cancer. 20-23 Second, this series includes an adequate number of patients containing HUT that could be used for statistical analysis. Third, although the number of previous studies of invasive cancer and of ductal carcinoma in situ is large, relatively few studies have examined benign hyperplastic lesions, particularly those with known clinical outcome. Because the study is retrospective and the HUT lesions are small, the study has been constrained by the availability of archival tissues for immunohistochemical staining.
Evidence from recent studies indicates that HUT is a heterogeneous entity containing subgroups identified according to the criterion of ER-α(+) proliferating cells. 15 Although, the level of risk after a benign biopsy containing HUT can be determined in a population-based group, we are still unable to predict it on individual basis, because morphological high-risk subtypes of HUT have not been identified. Further progress is likely to depend on delineating the molecular events that define these lesions. In an elegant study, Gobbi and colleagues 24 showed an association between breast cancer risk and the expression pattern of transforming growth factor-β-RII in HUT.
Through a nested case-control study, we have now confirmed a striking increase in both ER-α and Ki-67 expression in proliferative foci from those patients who progressed to breast cancer. In the present series, the odds ratio of developing breast cancer after a diagnosis of HUT in otherwise benign breast biopsies was 1.78 (CI, 1.10 to 2.88). The odds ratio of developing breast cancer in association with ER-positive HUT was increased to 3.06 (CI, 0.49 to 18.88). However, this was not statistically significant. When ER and Ki-67 are considered together in a logistic regression model, the overall predictive value of both markers for correctly classifying the cases that progressed to breast cancer and the controls was 67.4%. Because age, follow-up period, and geographical factors were each controlled in this study, these findings reflect a genuine increase in the expression of ER-α and Ki-67 in a high-risk subset of non-atypical lesions. Although the morphological appearance of HUT from cases and controls were histologically identical, they showed differences in hormone receptor and proliferation marker expression, which might have contributed to different biological behavior. All three ER-α(−) tumors were found in patients who had previously demonstrated ER-α positivity in their HUT foci. With respect to individual patients, expression of ER in normal lobules and in HUT foci is likely to be influenced by a number of different variable factors that include age, family history, time in the menstrual cycle, and menopausal status. Although these latter pieces of information are unavailable in this present series, and would be valuable to include in any prospective study, there are no published data to suggest that ER expression in HUT is influenced by the phase of the cycle or menopausal status.
The process of ER activation stimulates DNA synthesis, cell division, and the production of biologically active proteins that include pS2, transforming growth factor-α, and epidermal growth factor that influence cell growth and differentiation. Exposure to estrogen may contribute to mammary carcinogenesis by stimulating proliferation of a clone of precancerous cells or by increasing the chance of spontaneous mutations. Alternatively, estrogen could decrease cell-cycle transit time so that a spontaneous mutation becomes fixed before repair. An additional probability is that estrogen may have a direct genotoxic effect. 25
Our findings support the hypothesis that hormonal stimuli that induce growth and differentiation in the normal breast also contribute to the development of mammary malignancy and that the initial steps along the mammary carcinogenic pathway are estrogen-dependent. This mechanism might apply to ER-α(+) as well as ER-α(−) cancers. Increased expression of ER-α in premalignant lesions of those patients who developed breast cancer may lead to increased sensitivity of the target tissue to the effect of circulating estrogens that, in turn, could stimulate proliferation of the hyperplastic mammary epithelial cells. The increasing number of mitotic events would thus provide opportunities for genetic instability and initiation of malignancy during cell division.
In conclusion, we have demonstrated the existence of a positive association between ER-α and cellular proliferation in a subset of ductal hyperplasia that definitely progressed to breast cancer. According to our results, the increased expression of both ER-α and Ki-67, although not necessarily their simultaneous dual expression by individual cells, might define a subset of hyperplastic lesions with an increased risk of subsequent breast cancer development. This group could be selected for prophylactic anti-estrogen therapy to diminish the proliferative activity of those benign but high-risk lesions. Recently, Visscher and colleagues 26 showed that the anti-estrogen, tamoxifen, selectively inhibits the appearance or growth of preinvasive lesions in a xenograft model of early human breast cancer. These findings could have important implications for pathological diagnosis and assessment of breast cancer risk.
Acknowledgments
We thank Mrs. Christine Jarvis for technical assistance, Dr. Phil Moore for managing the database of the original case-control study, Dr. E. M. I. Williams and the staff at the Cancer Registry for providing and validating the follow-up data, Mr. Alan Williams for photographic assistance, and Mrs. Jill Gosney for secretarial support.
Footnotes
Address reprint requests to Professor Christopher S. Foster, Department of Pathology, Duncan Building, Daulby St., Liverpool, L69 3GA, United Kingdom. E-mail: csfoster@liv.ac.uk.
Support for this study was provided by the Research and Development Office of the Royal Liverpool and Broadgreen University Hospitals (NHS) Trust.
J. P. Sloane is now deceased.
References
- 1.Page DL, Dupont WD: Anatomic markers of human premalignancy and risk of breast cancer. Cancer 1990, 66:1326-1335 [DOI] [PubMed] [Google Scholar]
- 2.Lakhani SR, Slack DN, Hamoudi RA, Collins N, Stratton MR, Sloane JP: Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab Invest 1996, 74:129-135 [PubMed] [Google Scholar]
- 3.Russo J, Russo IH: Biological and molecular bases of mammary carcinogenesis. Lab Invest 1987, 57:112-137 [PubMed] [Google Scholar]
- 4.Johnston SR, MacLennan KA, Sacks NP, Salter J, Smith IE, Dowsett M: Modulation of Bcl-2 and Ki-67 expression in oestrogen receptor-positive human breast cancer by tamoxifen. Eur J Cancer 1994, 11:1663-1669 [DOI] [PubMed] [Google Scholar]
- 5.Pritchard KI: Is tamoxifen effective in prevention of breast cancer? Lancet 1998, 352:80-81 [DOI] [PubMed] [Google Scholar]
- 6.Williams G, Anderson E, Howell A, Watson R, Coyne J, Roberts SA, Potten CS: Oral contraceptive (OCP) use increases proliferation and decreases oestrogen receptor content of epithelial cells in the normal human breast. Int J Cancer 1991, 48:206-210 [DOI] [PubMed] [Google Scholar]
- 7.Battersby S, Robertson BJ, Anderson TJ, King RJ, McPherson K: Influence of menstrual cycle, parity and oral contraceptive use on steroid hormone receptors in normal breast. Br J Cancer 1992, 65:601-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE: Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 1998, 90:1292-1299 [DOI] [PubMed] [Google Scholar]
- 9.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ: Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst 1998, 90:37-42 [DOI] [PubMed] [Google Scholar]
- 10.Lawson JS, Field AS, Champion S, Tran D, Ishikura H, Trichopoulos D: Low oestrogen receptor alpha expression in normal breast tissue underlies low breast cancer incidence in Japan. Lancet 1999, 354:1787-1788 [DOI] [PubMed] [Google Scholar]
- 11.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H: Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984, 133:1710-1715 [PubMed] [Google Scholar]
- 12.Sawhney N, Hall PA: Ki-67—structure, function, and new antibodies. J Pathol 1992, 168:161-162 [DOI] [PubMed] [Google Scholar]
- 13.Clarke RB, Howell A, Potten CS, Anderson E: Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 1997, 57:4987-4991 [PubMed] [Google Scholar]
- 14.Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP: Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 1999, 155:1811-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal M, Davies MP, Shoker BS, Jarvis C, Ross Sibson D, Sloane JP: Subgroups of non-atypical hyperplasia of breast defined by proliferation of oestrogen receptor-positive cells. J Pathol 2001, 193:333-338 [DOI] [PubMed] [Google Scholar]
- 16.National Coordinating Group for Breast Screening Pathology: Pathology Reporting in Breast Cancer Screening. NHSBSP publication no. 3, 1997
- 17.Sannino P, Shousha S: Demonstration of oestrogen receptors in paraffin wax sections of breast carcinoma using the monoclonal antibody 1D5 and microwave oven processing. J Clin Pathol 1994, 47:90-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH, Dicker RC, Sullivan K, Fagan RF, Arner TG: Epi Info, Version 6: a word processing database and statistics program for epidemiology on microcomputers. 1994:pp 185-190 Centers for Disease Control and Prevention, Atlanta
- 19.Page DL, Vander Zwaag R, Rogers LW, Williams LT, Walker WE, Hartmann WH: Relation between component parts of fibrocystic disease complex and breast cancer. J Natl Cancer Inst 1978, 61:1055-1063 [PubMed] [Google Scholar]
- 20.Dupont WD, Page DL: Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985, 312:146-151 [DOI] [PubMed] [Google Scholar]
- 21.London SJ, Connolly JL, Schnitt SJ, Colditz GA: A prospective study of benign breast disease and the risk of breast cancer. JAMA 1992, 267:941-944 [PubMed] [Google Scholar]
- 22.McDivitt RW, Stevens JA, Lee NC, Wingo PA, Rubin GL, Gersell D: Histologic types of benign breast disease and the risk for breast cancer. The Cancer and Steroid Hormone Study Group. Cancer 1992, 69:1408-1414 [DOI] [PubMed] [Google Scholar]
- 23.Marshall LM, Hunter DJ, Connolly JL, Schnitt SJ, Byrne C, London SJ, Colditz GA: Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev 1997, 6:297-301 [PubMed] [Google Scholar]
- 24.Gobbi H, Dupont WD, Simpson JF, Plummer WD, Schuyler PA, Olson SJ, Arteaga CL, Page DL: Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J Natl Cancer Inst 1999, 91:2096-2101 [DOI] [PubMed] [Google Scholar]
- 25.Cohen SM, Ellwein LB: Cell proliferation in carcinogenesis. Science 1990, 249:1007-1011 [DOI] [PubMed] [Google Scholar]
- 26.Visscher DW, Nanjia-Makker P, Heppner G, Shekhar PV: Tamoxifen suppresses histologic progression to atypia and DCIS in MCFIOAT xenografts, a model of early human breast cancer. Breast Cancer Res Treat 2001, 65:41-47 [DOI] [PubMed] [Google Scholar]


