Abstract
The CpG island methylator phenotype (CIMP) is a newly described mechanism for carcinogenesis in colorectal carcinomas and adenomas characterized by methylation of multiple CpG islands. The causes of CIMP are unknown. We studied CIMP in hyperplastic polyps (HPs), with emphasis on patients with multiple HPs (5 to 10 HPs), large HPs (one HP >1 cm) or hyperplastic polyposis (>20 HPs). Methylation of p16, MINT1, MINT2, MINT31, and hMLH1 was analyzed by methylation-specific polymerase chain reaction in 102 HPs, 8 serrated adenomas, 19 tubular adenomas, and 9 adenocarcinomas from 17 patients, with multiple/large HPs or hyperplastic polyposis and in 16 sporadic HPs from 14 additional patients. Sporadic HPs were CIMP-negative (not methylated at any locus), but 43% of HPs from multiple/large HPs, or hyperplastic polyposis were CIMP-high (two or more methylated loci, P = 0.00001). Methylation among the four loci was correlated within HPs (odds ratio, 3.41; P = 0.002), and the methylation status of HPs within the same patient was also correlated (odds ratio, 5.92; P = 0.0001). CIMP-high HPs were present primarily in patients with a predominance of HPs in the right colon and/or serrated adenomas (P = 0.0009) and were associated with the absence of K-ras proto-oncogene mutations (odds ratio, 5.08; P = 0.03). Our findings of concordant CpG island methylation of HPs in multiple/large HPs or hyperplastic polyposis supports the concept that some patients have a hypermethylator phenotype characterized by methylation of multiple HPs and other colorectal lesions. The hypermethylator phenotype is related to patient-specific factors, such as carcinogenic exposure or genetic predisposition.
Colorectal cancer is the second most common cause of cancer deaths in the United States. Most colorectal cancers develop from adenomatous polyps, and morphological and genetic progression in an adenoma-adenocarcinoma sequence and in hereditary colorectal cancer syndromes are well described. 1-5
CpG islands are 0.5- to 2-kb regions rich in cytosine-guanine dinucleotides and are present in the 5′ region of approximately half of all human genes. 6 Methylation of cytosine within CpG islands is associated with loss of gene expression and is observed in physiological conditions, such as X chromosome inactivation 7 and aging, 8 and in neoplasia. 9 Transcriptional repression by methylation in colorectal cancers inactivates the p16 cell-cycle regulator, 10 the estrogen receptor growth suppressor, 8 the THBS1 angiogenesis inhibitor, 11 the TIMP3 metastasis suppressor, 12 the O6-methylguanine DNA methyltransferase DNA repair gene, 13 and the hMLH1 nucleotide mismatch repair gene. 14 The recently discovered CpG island methylator phenotype (CIMP) is a novel pathway characterized by methylation of multiple CpG islands in colorectal carcinomas and adenomas. 15-17 CIMP-high adenomas and carcinomas have a distinct genetic profile with frequent mutations of the K-ras gene, but lack of p53 mutations. 16 CIMP status is not correlated among multiple adenomas from the same patient. 17 The mechanism of methylation of multiple CpG islands is postulated to be either aberrant de novo methylation because of mutation in a DNA-methyltransferase or loss of protection against de novo methylation through the loss of a trans-activating factor. 18-20
Hyperplastic polyps (HPs) are usually present in the left colon, small in size, and considered to be benign in nature. However, patients with hyperplastic polyposis, characterized by the presence of numerous HPs and/or large HPs, have increased risk of colorectal cancer. 21-28 A HP-serrated adenoma-carcinoma sequence is proposed as an alternative pathway to the adenoma-carcinoma sequence. 28-31
We investigated the possibility that CpG island methylation is a major molecular defect in patients with multiple/large HPs or hyperplastic polyposis, and report a high degree of patient-specific concordant methylation in HPs and other lesions, representing a hypermethylator phenotype.
Materials and Methods
Characteristics of Patients and Specimens
The patients and specimens have been described in detail previously. 28 The patients were classified into three groups based on the number and size of HPs: multiple HPs (patients with 5 to 10 HPs), hyperplastic polyposis (patients with >20 HPs), and large HPs (patients with HP >1 cm), as described previously. 28 Predominance of HPs in the right colon and predominance of HPs in the left colorectum were defined by the location of the majority of HPs in the right colon or in the left colon and rectum, respectively. We initially studied 129 HPs from 23 patients. All histological sections had limited quantities of DNA extracted from tissue in paraffin-embedded specimens. Twenty-seven HPs, eight tubular adenomas, and three carcinomas from three patients with familial hyperplastic polyposis and three patients with hyperplastic polyposis from the previous study were excluded because of insufficient DNA for further analysis. Thus, we studied 102 HPs, 8 serrated adenomas, 19 tubular adenomas, and 9 carcinomas from 17 patients with hyperplastic polyposis. Histologically normal mucosa was available from 13 patients.
Sixteen sporadic HPs from 14 patients undergoing resection of colorectal cancer at the MD Anderson Cancer Center, Houston, TX, were also analyzed. All patients had given informed consent for the collection of specimens according to institutional guidelines.
Bisulfite Treatment of DNA and Methylation-Specific Polymerase Chain Reaction
The methylation status of p16, MINT1, MINT2, MINT31, and hMLH1 was determined by bisulfite treatment of DNA followed by methylation-specific polymerase chain reaction, as described with modification. 32 These loci were chosen based on a previous study that showed that they offered excellent discrimination for CIMP and that they were unmethylated (<10% methylation) in normal tissues. 15
In brief, 2 μg of microdissected genomic DNA was denatured with 2 mol/L NaOH at 37°C for 10 minutes, followed by incubation with 3 mol/L sodium bisulfite (pH 5.0) at 50°C for 16 hours in the dark. DNA was then purified using the DNA Cleanup Kit (Promega, Madison, WI) as recommended by the manufacturer, incubated with 3 mol/L of NaOH at room temperature for 5 minutes, precipitated with 10 mol/L of ammonium acetate and 100% ethanol, washed with 70% ethanol, and finally resuspended in 20 μl of distilled water.
The primers and polymerase chain reaction (PCR) conditions for p16 were the same as reported by Herman and colleagues. 32 The primers for MINT1 were 5′-AATTTTTTTATATATATTTTCGAAGC-3′ and 5′-AAAAACCTCAACCCCGCG-3′ for methylated alleles; and 5′-AATTTTTTTATATATATTTTTGAAGTGT-3′ and 5′-AACAAAAAACCTCAACCCCACA-3′ for unmethylated alleles. The cycling conditions for MINT1 were 95°C for 10 minutes and 37 cycles of 95°C for 30 seconds and 55°C for 45 seconds. The primers for MINT2 were 5′-TTGTTAAAGTGTTGAGTTCGTC-3′ and 5′-AATAACGACGATTCCGTACG-3′ for methylated alleles; and 5′-GATTTTGTTAAAGTGTTGAGTTTGTT-3′ and 5′-CAAAATAATAACAACAATTCCATACA-3′ for unmethylated alleles. The cycling conditions for MINT2 were 95°C for 10 minutes and 40 cycles of 95°C for 30 seconds and 60°C for 45 seconds. The primers for MINT31 were 5′-TGTTGGGGAAGTGTTTTTCGGC-3′ and 5′-CGAAAACGAAACGCCGCG-3′ for methylated alleles; and 5′-TAGATGTTGGGGAAGTGTTTTTTGGT-3′ and 5′-TAAATACCCAAAAACAAAACACCACA-3′ for unmethylated alleles. The cycling conditions for MINT31 were 95°C for 10 minutes and 38 cycles of 95°C for 30 seconds and 60°C for 45 seconds. The primers for hMLH1 were 5′-GATAGCGATTTTTAACGC-3′ and 5′-TCTATAAATTACTAAATCTCTTCG-3′ for methylated alleles; and 5′-AGAGTGGATAGTGATTTTTAATGT-3′ and 5′-ACTCTATAAATTACTAAATCTCTTCA-3′ for unmethylated alleles. The cycling conditions for hMLH1 were 95°C for 10 minutes and 40 cycles of 95°C for 30 seconds and 53°C for 45 seconds.
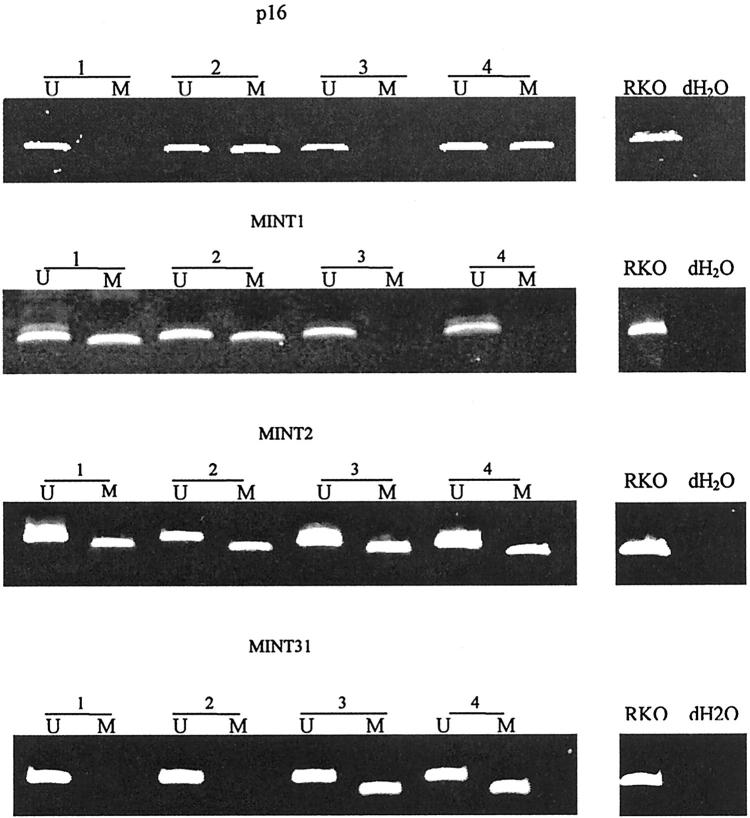
In brief, 2 μl of bisulfite-treated DNA was used as template for PCR reactions using primers specific for methylated and unmethylated alleles. RKO, a colon cancer cell line (American Type Culture Collection, Manassas, VA), was used as a positive control, and PCR reaction without DNA was used as the negative control in each batch of reaction. PCR products from methylated and unmethylated reactions were electrophoresed on 6% acrylamide gels and visualized by ethidium bromide staining (examples in Figure 1 ▶ ). For quantitation of methylated and unmethylated alleles, gel photographs were digitized using a BioRad imager (Bio-Rad, Richmond, CA) and evaluated by densitometry using the manufacturer’s software. The results were expressed as percentage of methylation by determining the density of the methylated band relative to the sum of the methylated and unmethylated bands. The loci used in this study are unmethylated (<10% methylated) in normal tissues. Therefore, any locus showing ≥10% methylation was considered positive.
Figure 1.
Methylation analysis of CpG islands in HPs. Methylation of p16, MINT1, MINT2, and MINT31 was evaluated by methylation-specific PCR using primers for methylated (M) and unmethylated (U) alleles of bisulfite-treated DNA. Loci examined and HP numbers are indicated above each gel. DNA from RKO, a colon cancer cell line, was used as a positive control, and dH2O without DNA was used as negative control.
CIMP Status of HPs, Serrated Adenomas (SAs), Adenomas, and Carcinomas
HPs, SAs, adenomas, and carcinomas were classified as CIMP-negative if none of the loci were methylated; CIMP-low, if one locus was methylated; and CIMP-high, if two or more loci were methylated.
K-ras Mutations, Loss of Heterozygosity of Chromosome 1p, and Microsatellite Instability-High (MSI-High)
K-ras mutations, loss of heterozygosity of chromosome 1p, and MSI in HPs and SAs from patients with hyperplastic polyposis were reported previously. 28 MSI-high was defined by presence of allelic shift in comparison with control DNA in at least 30% of evaluated markers.
Statistical Analysis
The primary statistical endpoint of this study was the determination of factors associated with CIMP status of HPs. Patients with more than one HP were represented multiple times in this data set. Each HP was represented by a methylation index (number of loci methylated/number of loci evaluated). To model correctly the within-polyp and between-polyp correlation as well as simultaneously partition out the effects of the various factors considered, marginal logistic regression models for correlated binary data were used to assess associations between CIMP status and the various polyp and patient characteristics. Estimates were obtained using the generalized estimating equation approach of Zeger and Liang. 33 An appropriate correlation structure was chosen to account for possible correlations both between HPs within patients and within HPs between observations from different loci. Both patient and polyp characteristics were tested for association with methylation status. The factor locus was also included in the model to account for locus-specific methylation rates. Relationships between HPs within patients and within HPs between loci were represented as odds ratios, in which an odds ratio of greater than one suggests positive correlation in CIMP status within patients and within polyps, respectively.
Results
There were 11 men and 6 women with multiple/large HPs, or hyperplastic polyposis. The mean age was 64 ± 12 years (range, 46 to 84 years). The demographic data and characteristics of each individual patient and the number of HPs, adenomas, and carcinomas in each individual are summarized in Table 1 ▶ . The sporadic HPs were from 11 men and 3 women, with a mean age of 64 ± 11 years (range, 48 to 80 years). The mean size of the polyp in this group was 0.3 cm (range, 0.1 to 0.7 cm). There were 2 HPs from the right colon and 14 from the left colon.
Table 1.
Patient Demographics, Characteristics, and Histopathological Type of Lesions in Patients with Multiple/Large HPs, or Hyperplastic Polyposis
| Patient | Age, yrs | Sex | Hyperplastic polyps, total/analyzed | Serrated adenomas, total/analyzed | Tubular adenomas, total/analyzed | Colorectal cancer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with predominance of HPs in left colorectum without serrated adenomas | ||||||||||||
| 1 | 54 | F | 4*/4 | — | — | − | ||||||
| 2 | 62 | M | Numerous/5 | — | — | + | ||||||
| 3 | 65 | F | >100/3 | — | 5/4 | + | ||||||
| 4 | 76 | F | >50*/3 | — | 1/1 | +§¶ | ||||||
| 5 | 67 | M | Numerous/14 | — | 2/0 | + | ||||||
| 6 | 54 | M | 8/7 | — | 3/0 | + | ||||||
| 7 | 51 | F | 10/7 | — | — | + | ||||||
| 8 | 61 | M | 8/3 | — | 1/0 | + | ||||||
| Patients with predominance of HPs in right colon without serrated adenomas | ||||||||||||
| 9 | 48 | F | 36/15 | — | 9/2 | + | ||||||
| 10 | 82 | M | 6†/6 | — | 4/3 | + | ||||||
| 11 | 84 | M | 2*/2 | — | 2/2 | +¶ | ||||||
| Patients with predominance of HPs in left colorectum with serrated adenomas | ||||||||||||
| 12 | 57 | M | 22/4 | 2/2‡ | 4/1 | + | ||||||
| 13 | 55 | M | 13*/3 | 1/1‡ | 3/0 | − | ||||||
| 14 | 76 | M | 7/5 | 3/1 | 8/0 | + | ||||||
| Patients with predominance of HPs in right colon with serrated adenomas | ||||||||||||
| 15 | 78 | M | 9/8 | 4/3 | 6/2 | − | ||||||
| 16 | 46 | M | 26/10 | 1/1 | 8/2 | + | ||||||
| 17 | 68 | F | Multiple*/3 | 1/0 | 2/2 | +§¶ | ||||||
*Also had a large hyperplastic polyp.
†Four large hyperplastic polyps.
‡Admixed hyperplastic adenomatous polyp.
§Multiple, mucinous colon cancers.
¶Right-sided colon cancer.
Methylation of Sporadic HPs
None of the sporadic HPs were methylated at any of the four loci (Figure 2, A and B) ▶ and were classified as CIMP-negative.
Figure 2.
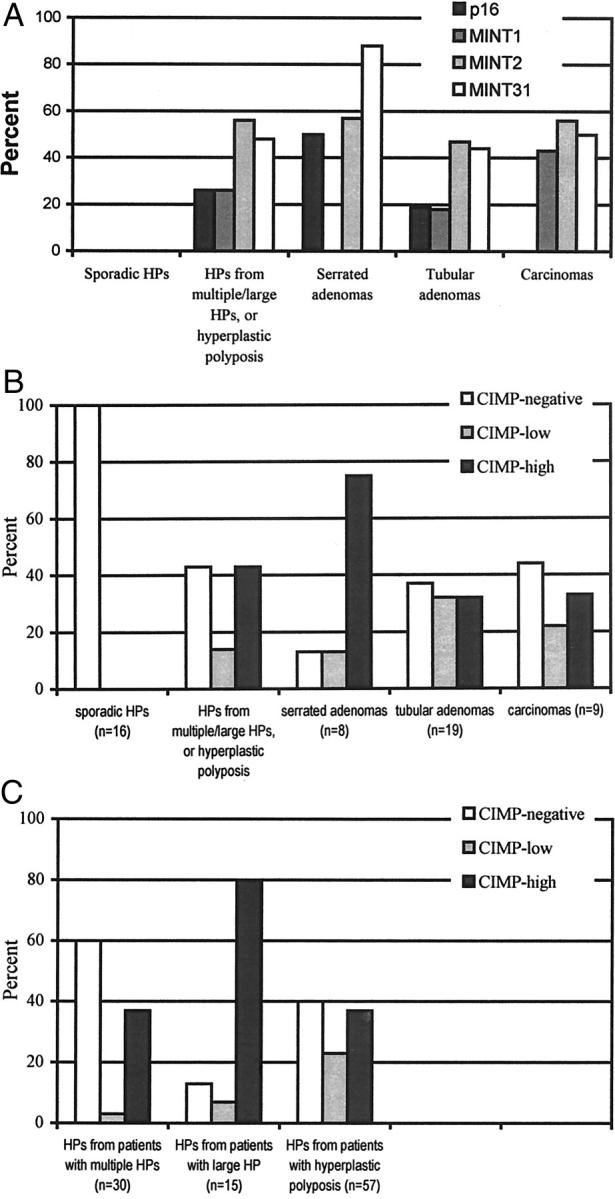
A: Prevalence of methylation at p16, MINT1, MINT2, and MINT31 in HPs, serrated adenomas, tubular adenomas, and carcinomas. B: CIMP status of sporadic HPs and of HPs, serrated adenomas, tubular adenomas, and carcinomas from patients with multiple HPs, large HPs, and hyperplastic polyposis. C: CIMP status of HPs from patients with multiple HPs, large HPs, and hyperplastic polyposis.
Methylation of Nonlesional Mucosa, Polyps, and Cancers from Patients with Multiple/Large HPs, or Hyperplastic Polyposis
Nonlesional Mucosa
The apparently normal colorectal mucosa was analyzed for methylation in 16 samples from 13 patients (Figure 3) ▶ . Only three patients had methylation (patients 4, 9, and 15) in the mucosa taken adjacent to a neoplasm: methylation of p16 was present in mucosa adjacent to a tubular adenoma in patient 15, MINT1 was methylated in mucosa adjacent to a tubular adenoma in patient 9, and p16 and MINT1 were methylated in mucosa adjacent to a carcinoma in patient 4. The methylation at the normal mucosae in these three patients was concordant with the adjacent adenoma or carcinoma. No methylation was present in seven samples from mucosa of the resection margins or in six additional samples of mucosa adjacent to a lesion. Thus, 6% (1 of 16) of normal mucosae were classified CIMP-high, 12% (2 of 16) were CIMP-low, and 81% (13 of 16) were CIMP-negative.
Figure 3.
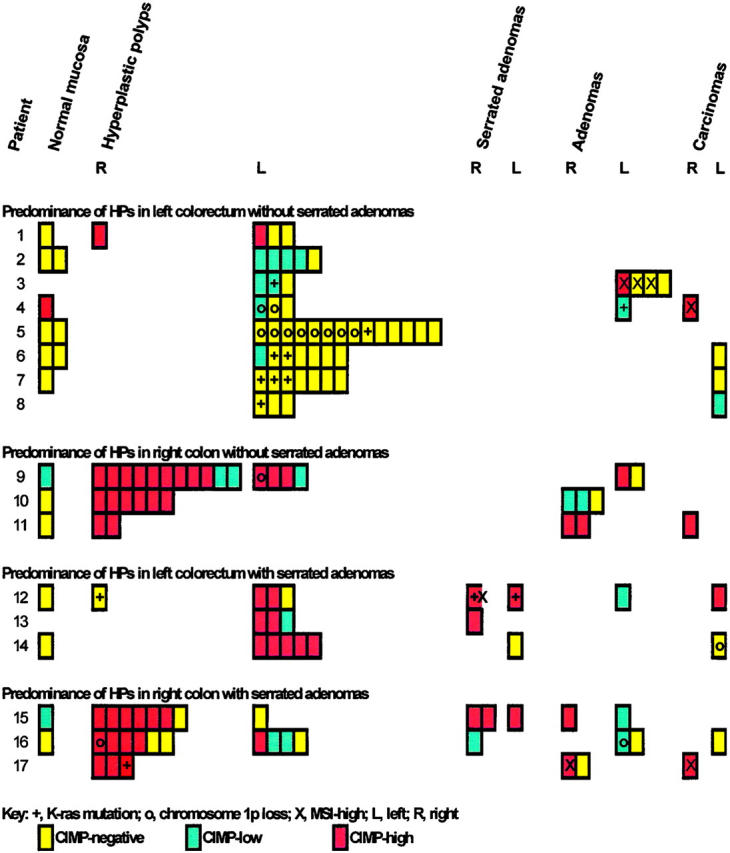
Methylation status of HPs, serrated adenomas, tubular adenomas, and carcinomas from patients with multiple HPs, large HPs, and hyperplastic polyposis.
Hyperplastic Polyps
Forty-three percent (44 of 102) of HPs from these patients were classified as CIMP-high, 14% (14 of 102) were CIMP-low, and 43% (44 of 102) were CIMP-negative (P = 0.00001 versus sporadic HPs, and P = 0.0045 versus nonlesional mucosa; Figure 2, A and B ▶ ; Figure 3 ▶ ).
The methylation statuses of different loci in the same HP were positively correlated (odds ratio, 3.41; P = 0.002; Figure 3 ▶ ), suggesting that some HPs from patients with multiple/large HPs, or hyperplastic polyposis, have CIMP-high. 15 In addition, CIMP statuses for different HPs within the same patient were positively correlated (odds ratio, 5.92; P = 0.0001), suggesting concordance of methylation in multiple HPs within individual patients.
The methylation status of HPs was tested for associations with patient characteristics, with age, the site and size of HPs, and with other genetic alterations in HPs (Table 2) ▶ . There was no association between methylation and age. Patients with the three phenotypic groups of multiple HPs, large HPs, and hyperplastic polyposis had overlapping CIMP status in HPs from the same patient (P = 0.8; Figure 2C ▶ ). HPs from patients with large HPs were invariably methylated, but HPs from patients with hyperplastic polyposis and multiple HPs as defined in the previous study 28 were heterogeneous for methylation status. HPs were more commonly methylated in patients with SAs, predominance of HPs in right colon, or both SAs and predominance of HPs in right colon (P = 0.0009; Figure 3 ▶ and Table 2 ▶ ). Methylation was present in 22% (10 of 46) of HPs in patients with predominance of HPs in left colorectum without SAs (odds ratio, 1.00), 100% (23 of 23) of HPs from patients with predominance of HPs in right colon without SAs (odds ratio, 27.55; 95% confidence limit, 7.33 to 103.60), 83% (10 of 12) of HPs from patients with predominance of HPs in left colorectum with SAs (odds ratio, 9.55; 95% confidence limit, 3.72 to 24.53), and 76% (16 of 21) of HPs from patients with predominance of HPs in right colon with SAs (odds ratio, 4.60; 95% confidence limit, 1.54 to 13.68).
Table 2.
Patient and Polyp Characteristics in Relation to CIMP Status of Hyperplastic Polyps in Patients with Multiple/Large HPs or Hyperplastic Polyposis, Odds Ratio, and 95% Confidence Intervals (CI) from Generalized Estimating Equation Marginal Regression Models
| Factors | CIMP-negative, % (fraction) | CIMP-low, % (fraction) | CIMP-high, % (fraction) | Odds ratio (CI) | Chi-square | P value |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| With serrated adenoma | ||||||
| Predominance of HPs in right colon | 0 (0/23) | 13 (3/23) | 87 (20/23) | 27.55 (7.3, 103.6) | 16.52 | 0.009 |
| Predominance of HPs in left colorectum | 17 (2/12) | 8 (1/12) | 75 (9/12) | 9.55 (3.7, 24.5) | ||
| Without serrated adenoma | ||||||
| Predominance of HPs in right colon | 24 (5/21) | 14 (3/21) | 62 (13/21) | 4.60 (1.5, 13.7) | ||
| Predominance of HPs in left colorectum | 78 (36/46) | 17 (8/46) | 4 (2/46) | 1.00 | ||
| Age (continuous, years) | — | — | — | 1.02 (0.98, 1.8) | 1.52 | 0.22 |
| Hyperplastic polyp characteristics | ||||||
| Site | ||||||
| Left | 66 (38/58) | 16 (9/58) | 19 (11/58) | 0.39 (0.1, 1.1) | 2.63 | 0.10 |
| Right | 11 (5/44) | 14 (6/44) | 75 (33/44) | 1.00 | ||
| Size (continuous, mean± SD, mm) | 3.0± 1.5 | 3.1± 1.6 | 5.1± 4.3 | 1.04 (0.9, 1.2) | 0.70 | 0.40 |
| K-ras mutation | ||||||
| Absent | 40 (37/92) | 12 (11/92) | 48 (44/92) | 5.08 (1.5, 17.6) | 4.72 | 0.03 |
| Present | 70 (7/10) | 30 (3/10) | 0 | 1.00 | ||
| 1p loss | ||||||
| Absent | 41 (39/95) | 14 (13/95) | 45 (43/95) | 3.24 (0.7, 15.7) | 1.94 | 0.16 |
| Present | 71 (5/7) | 14 (1/7) | 14 (1/7) | 1.00 |
The HP characteristics that associated with CIMP status of HPs were absence of K-ras mutations, absence of loss of heterozygosity of chromosome 1p, and site of HP in the right colon (Figure 3 ▶ and Table 2 ▶ ). K-ras mutations were present in 0% (0 of 44) of CIMP-high HPs, 21% (3 of 14) of CIMP-low HPs, and 16% (7 of 44) of CIMP-negative HPs (odds ratio, 5.08; P = 0.03). Similarly, loss of heterozygosity of chromosome 1p was present in 2% (1 of 44) of CIMP-high HPs, 7% (1 of 14) of CIMP-low HPs, and 11% (5 of 44) of CIMP-negative HPs (not significant). HPs in the left colon and rectum were less methylated than HPs in the right colon, and larger HPs were more frequently methylated. The site and size of HPs were not independently associated with methylation status but were dependent on patient characteristics.
Serrated Adenomas
Most of the SAs were methylated: 75% (6 of 8) of SAs were CIMP-high, 12% (1 of 8) were CIMP-low, and 12% (1 of 8) were CIMP-negative (Figures 2B and 3) ▶ ▶ .
Tubular Adenomas and Carcinomas
The CIMP status of adenomas and carcinomas was more heterogeneous (Figures 2B and 3) ▶ ▶ . CIMP-high was present in 32% (6 of 19) of adenomas, CIMP-low in 32% (6 of 19), and CIMP-negative in 37% (7 of 19). CIMP-high was present in 44% (4 of 9) of carcinomas, CIMP-low in 11% (1 of 9), and CIMP-negative in 44% (4 of 9) (Figures 2B and 3) ▶ ▶ . All three right-sided colonic carcinomas were CIMP-high (patients 4, 11, and 17) as contrasted with one of six left-sided colorectal carcinomas (patient 12, P = 0.05). K-ras mutation was present in 5% (1 of 19) adenomas and in none of nine carcinomas.
MSI-High and Methylation of hMLH1
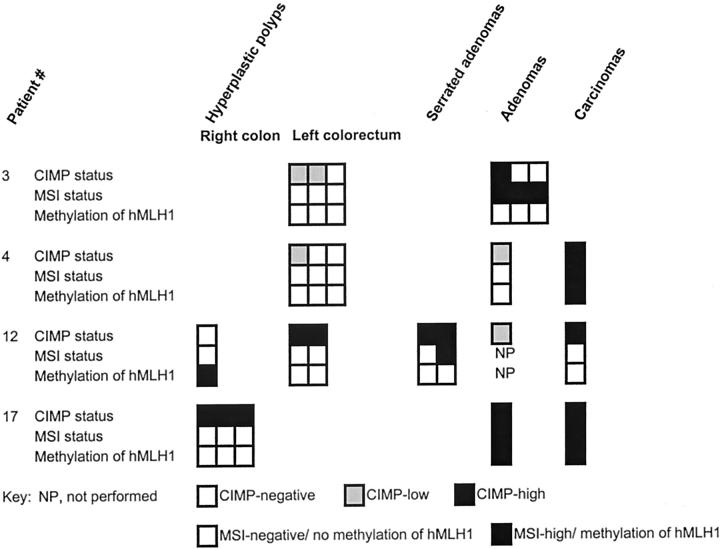
All HPs from patients with multiple/large HPs or hyperplastic polyposis were MSI-negative, but MSI-high was present in one SA, four tubular adenomas, and two carcinomas from four patients (patients 3, 4, 12, and 17; Figure 3 ▶ ). CIMP, MSI-high, and methylation of hMLH1 status were compared in multiple lesions from the four patients with MSI-high SA, tubular adenomas, or colorectal carcinomas (Figure 4) ▶ . Concordant methylation of hMLH1, CIMP-high, and MSI-high was present in an adenoma and carcinoma from one patient, and a carcinoma from another patient (patients 4 and 17), but methylation of hMLH1 was not present in all other MSI-negative HPs, SAs, adenomas, or carcinomas. Methylation of hMLH1 was not present in one CIMP-high, MSI-high SA from one patient (patient 12); and one CIMP-high and two CIMP-negative, MSI-high adenomas from another patient (patient 3).
Figure 4.
CIMP, MSI-high, and hMLH1 methylation status of HPs, serrated adenomas, tubular adenomas, and carcinomas from patients with MSI-high lesions. Concordant methylation of hMLH1, CIMP-high, and MSI-high was present in an adenoma and carcinoma from one patient and a carcinoma from another patient, but methylation of hMLH1 was not present in all other MSI-negative HPs, serrated adenomas, tubular adenomas, or carcinomas.
Discussion
We studied CpG island methylation in HPs and other colorectal lesions from patients with multiple/large HPs, or hyperplastic polyposis, and in sporadic HPs from patients with colorectal carcinomas. Normal mucosa from the same patient and a group of sporadic HPs were used as controls. Methylation was present only in the mucosa dissected adjacent to a neoplasm that could have resulted from a field defect. No methylation was present in sporadic HPs nor the normal mucosa from the resection margins. CIMP-high was present in 43% of HPs from patients with multiple/large HPs, or hyperplastic polyposis, a percentage that is similar to the prevalence of CIMP-high in sporadic colorectal carcinomas and adenomas. 15-17
A high degree of concordance was observed among HPs within the same patient with multiple/large HPs, or hyperplastic polyposis, in our study. In contrast, CIMP status was not correlated among sporadic adenomas within the same patient in another study. 17 These findings suggest that some of the patients with multiple/large HPs, or hyperplastic polyposis, may have a hypermethylator phenotype either because of genetic predisposition to develop methylated HPs and related lesions, or because of an environmental exposure that results in development of multiple methylated HPs. Effects of carcinogen exposures on CpG island methylation of estrogen receptor in lung carcinomas have previously been described. 34 This, together with the fact that most patients in the present study had no family history of colonic neoplasms, tends to favor the latter hypothesis.
Methylation was more common in patients with predominance of HPs in the right colon and/or SAs. We have previously described differences in topographic expression of p21Waf1/Cip1 cyclin-dependent kinase inhibitor and Ki-67 proliferation marker in right- and left-sided HPs from these patients. 28
The genetic alterations in sporadic HPs differ from genetic alterations in HPs from patients with multiple HPs, large HPs, or hyperplastic polyposis. Sporadic HPs have frequent K-ras mutations and loss of chromosome 1p, 35-39 but lack CpG island methylation. In contrast, HPs from patients with multiple/large HPs, or hyperplastic polyposis, have infrequent K-ras mutations, and loss of chromosome 1p in a small percentage of HPs, 28 but frequent CpG island methylation. Furthermore, K-ras mutation or loss of chromosome 1p was predominantly present in HPs from patients with predominance of HPs in the left colorectum without serrated adenomas, a set of patients who lack CIMP-high HPs or carcinomas. These results suggest that either K-ras mutations and loss of chromosome 1p are a late event or two alternative sets of early genetic events occurring in HPs, one characterized by CpG island methylation and the other by K-ras mutations or loss of chromosome 1p. The latter is further corroborated by lack of K-ras mutations in adenomas and carcinomas in these patients.
CIMP-high colorectal carcinomas and adenomas have frequent methylation of the p16 gene and mutations of the K-ras gene, but lack p53 gene mutation. 16,40 HPs with CIMP-high from the present study had frequent methylation of the p16 gene, but lacked K-ras mutations and p53 overexpression of the type seen with p53 gene mutation. 28 These data provide additional evidence that progression of colorectal carcinogenesis in patients with hyperplastic polyposis is distinct from sporadic CIMP-negative and CIMP-high carcinomas. There is evidence for two CIMP-positive pathways of colorectal carcinogenesis: one characterized by methylation of O6-methylguanine DNA methyltransferase DNA repair gene is associated with MSI-low, and G to A K-ras and p53 gene mutations; 13,41,42 and an another characterized by CIMP-high, paucity of K-ras gene mutation, and with or without MSI-high dependent on methylation status of hMLH1. 14-17,41
MSI-high SA, tubular adenomas, or carcinomas were present in four patients in our study. Concordant methylation of hMLH1, CIMP-high, and MSI-high was present in one tubular adenoma and two carcinomas from two patients. Another patient had MSI-high, CIMP-high SA without methylation of hMLH1. All of these three patients had CIMP-high HPs without MSI. In contrast, one patient had MSI-high in three adenomas without CIMP-high in the majority of adenomas or HPs. There was no MSI-positive, CIMP-high HPs in our study. This data suggest that methylation of hMLH1 marks a genetic and histological progression in CIMP-high lesions that develop MSI-high and undergo adenomatous or carcinomatous transformation in a few patients: CIMP-high tubular adenomas and carcinomas develop in patients with CIMP-high HPs because of methylation of hMLH1. This corroborates loss of expression of hMLH1 in MSI-high dysplastic and malignant foci in hyperplastic polyposis noted by Jass and colleagues. 31
Thus, some patients with multiple/large HPs, or hyperplastic polyposis, have concordant methylation of multiple CpG islands in HPs. The patient factors that we determined to be important for the development of heavy methylation were the presence of SA(s) and the predominance of HPs in right colon. The role of HPP1 gene methylation in the development of HPs corroborates our findings. 43 A CpG island methylation-independent pathway to hyperplastic polyposis also exists, as documented by four patients with loss of chromosome 1p or K-ras mutations in the majority of their HPs. Neoplastic progression because of microsatellite instability is also present in multiple/large HPs, or hyperplastic polyposis. 28-31
A high degree of concordance of methylation is observed among HPs in multiple/large HPs or hyperplastic polyposis within the same patient, supporting the existence of a hypermethylator phenotype. Patients with a hypermethylator phenotype will be invaluable in studying and understanding the causes of aberrant methylation in cancer.
Footnotes
Address reprint requests to Asif Rashid M.D., Ph.D., Department of Pathology, Box 85, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030-4095. E-mail: arashid@mdanderson.org.
References
- 1.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 2.Reale MA, Fearon ER: Gene defects in colorectal tumorigenesis. Young GP Rozen P Levin B eds. Prevention and Early Detection of Colorectal Cancer. 1996, :pp 63-86 WB Saunders Company, Ltd., London [Google Scholar]
- 3.Fearon ER, Dang CV: Cancer genetics: tumor suppressor meets oncogene. Curr Biol 1999, 9:R62-R65 [DOI] [PubMed] [Google Scholar]
- 4.Perucho M: Microsatellite instability: the mutator that mutates the other mutator. Nat Med 1996, 2:630-631 [DOI] [PubMed] [Google Scholar]
- 5.Chung DC: The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 2000, 119:854-865 [DOI] [PubMed] [Google Scholar]
- 6.Bird AP: CpG-rich islands and the function of DNA methylation. Nature 1986, 321:209-213 [DOI] [PubMed] [Google Scholar]
- 7.Latham KEX: Chromosome imprinting and inactivation in the early mammalian embryo. Trends Genet 1996, 112:134-138 [DOI] [PubMed] [Google Scholar]
- 8.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Sidransky D, Baylin SB: Methylation of the estrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet 1994, 7:536-540 [DOI] [PubMed] [Google Scholar]
- 9.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JPJ: Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998, 72:141-196 [PubMed] [Google Scholar]
- 10.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JPJ, Davidson NE, Sidransky D, Baylin SB: Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res 1995, 55:4525-4530 [PubMed] [Google Scholar]
- 11.Ahuja N, Mohan AL, Li Q, Stolker JM, Herman JG, Hamilton SR, Baylin SB, Issa JP: Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res 1997, 57:3370-3374 [PubMed] [Google Scholar]
- 12.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB: Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 1999, 21:103-107 [DOI] [PubMed] [Google Scholar]
- 13.Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG: Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 1999, 59:793-797 [PubMed] [Google Scholar]
- 14.Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R: Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 1997, 57:808-811 [PubMed] [Google Scholar]
- 15.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JPJ: CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA 1999, 96:8681-8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyota M, Ohe-Toyota M, Ahuja N, Issa JPJ: Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA 2000, 97:710-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid A, Shen L, Morris JS, Issa JPJ, Hamilton SR: CpG island methylation in colorectal adenomas. Am J Pathol 2001, 159:1129-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mummaneni P, Yates P, Simpson J, Rose J, Turker MS: The primary function of a redundant Sp1 binding site in the mouse aprt gene promoter is to block epigenetic gene inactivation. Nucleic Acids Res 1998, 26:5163-5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen ZJ, Pikaard CS: Epigenetic silencing of RNA polymerase I transcription: a role for DNA methylation and histone modification in nucleolar dominance. Genes Dev 1997, 11:2124-2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macleod D, Charlton J, Mullins J, Bird AP: Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev 1994, 8:2282-2292 [DOI] [PubMed] [Google Scholar]
- 21.Goldman H, Ming S, Hickock DF: Nature and significance of hyperplastic polyps of the human colon. Arch Pathol 1970, 89:349-354 [PubMed] [Google Scholar]
- 22.Estrada RG, Spjut HJ: Hyperplastic polyps of the large bowel. Am J Surg Pathol 1980, 4:127-133 [DOI] [PubMed] [Google Scholar]
- 23.Williams GT: Metaplastic (hyperplastic) polyps of the large bowel: benign neoplasms after all? Gut 1997, 40:691-692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCann BG: A case of metaplastic polyposis of the colon associated with focal adenomatous change and metachronous adenocarcinomas. Histopathology 1998, 13:700-702 [DOI] [PubMed] [Google Scholar]
- 25.Cooke SAR: Polyposis coli: the clinical spectrum in adults. S Afr Med J 1978, 53:454-457 [PubMed] [Google Scholar]
- 26.Cohen SM, Brown L, Janower ML, McCready FJ: Multiple metaplastic (hyperplastic) polyposis of the colon. Gastrointest Radiol 1981, 6:333-335 [DOI] [PubMed] [Google Scholar]
- 27.Teoh HH, Delahunt B, Isbister WH: Dysplastic and malignant areas in hyperplastic polyps of the large intestine. Pathology 1989, 21:138-142 [DOI] [PubMed] [Google Scholar]
- 28.Rashid A, Houlihan PS, Booker S, Petersen GM, Giardiello FM, Hamilton SR: Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology 2000, 119:323-332 [DOI] [PubMed] [Google Scholar]
- 29.Jass JR, Cottier DS, Pokos V, Parry S, Winship IM: Mixed epithelial polyps in association with hereditary non-polyposis colorectal cancer providing an alternative pathway of cancer histogenesis. Pathology 1997, 29:28-33 [DOI] [PubMed] [Google Scholar]
- 30.Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, Watanabe H: DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 1999, 52:5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jass JR, Iino H, Ruszkiewicz A, Painter D, Solomon MJ, Koorey DJ, Cohn D, Furlong KL, Walsh MD, Palazzo J, Edmonston TB, Fishel R, Young J, Leggett BA: Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut 2000, 47:43-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB: Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996, 93:9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42:121-130 [PubMed] [Google Scholar]
- 34.Issa JP, Baylin SB, Belinsky SA: Methylation of the estrogen receptor CpG island in lung tumors is related to the specific type of carcinogen exposure. Cancer Res 1996, 56:3655-3658 [PubMed] [Google Scholar]
- 35.Jen J, Powell SM, Papadopoulos N, Smith KJ, Hamilton SR, Vogelstein B, Kinzler KW: Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994, 54:5523-5526 [PubMed] [Google Scholar]
- 36.Otori K, Oda Y, Sugiyama K, Hasebe T, Mukai K, Fujii T, Tajiri H, Yoshida S, Fukushima S, Esumi H: High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut 1997, 40:660-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nucci MR, Robinson CR, Longo P, Campbell P, Hamilton SR: Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol 1997, 28:1396-1407 [DOI] [PubMed] [Google Scholar]
- 38.Bardi G, Johansson B, Pandis N, Bak-Jensen E, Orndal C, Heim S, Mandahl N, Andren-Sandberg A, Mitelman F: Cytogenetic aberrations in colorectal adenocarcinomas and their correlation with clinicopathologic features. Cancer 1993, 71:306-314 [DOI] [PubMed] [Google Scholar]
- 39.Lothe RA, Peltomaki P, Meling GI, Aaltonen LA, Nystrom-Lahti M, Pylkkanen L, Heimdal K, Andersen TI, Moller P, Rognum TO: Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993, 53:5849-5852 [PubMed] [Google Scholar]
- 40.Guan RJ, Fu Y, Holt PR, Pardee AB: Association of K-ras mutations with p16 methylation in human colon cancer. Gastroenterology 1999, 116:1063-1071 [DOI] [PubMed] [Google Scholar]
- 41.Whitehall VLJ, Walsh MD, Young J, Leggett BA, Jass JR: Methylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instability. Cancer Res 2001, 61:827-830 [PubMed] [Google Scholar]
- 42.Esteller M, Risques R-A, Toyota M, Capella G, Moreno V, Peinado MA, Baylin SB, Herman JG: Promoter hypermethylation of the DNA repair gene O6-methylguanine-DNA methyltransferase is associated with G: C to A:T transition mutations in p53 in primary colorectal tumorigenesis. Cancer Res 2001, 61:4689-4692 [PubMed] [Google Scholar]
- 43.Young J, Biden KG, Simms LA, Huggard P, Karamatic R, Eyre HJ, Sutherland GR, Herath N, Barker M, Anderson GJ, Fitzpatrick DR, Ramm GA, Jass JR, Leggett BA: HPP1: a transmembrane protein-encoding gene commonly methylated in colorectal polyps and cancers. Proc Natl Acad Sci USA 2000, 98:265-270 [DOI] [PMC free article] [PubMed] [Google Scholar]