Abstract
Leukocyte adhesion to the diabetic retinal vasculature results in early blood-retinal barrier breakdown, capillary nonperfusion, and endothelial cell injury and death. Previous work has shown that intercellular adhesion molecule-1 (ICAM-1) and CD18 are required for these processes. However the relevant in vivo stimuli for ICAM-1 and CD18 expression in diabetes remain unknown. The current study investigated the causal role of endogenous vascular endothelial growth factor (VEGF) and nitric oxide in initiating these events. Diabetes was induced in Long-Evans rats with streptozotocin, resulting in a two- to threefold increase in retinal leukocyte adhesion. Confirmed diabetic animals were treated with a highly specific VEGF-neutralizing Flt-Fc construct (VEGF TrapA40). Retinal ICAM-1 mRNA levels in VEGF TrapA40-treated diabetic animals were reduced by 83.5% compared to diabetic controls (n = 5, P < 0.0001). VEGF TrapA40 also potently suppressed diabetic leukocyte adhesion in retinal arterioles (47%, n = 11, P < 0.0001), venules (36%, n = 11, P < 0.0005), and capillaries (36%, n = 11, P < 0.001). The expression of endothelial nitric oxide synthase (eNOS), a downstream mediator of VEGF activity, was increased in diabetic retina, and was potently suppressed with VEGF TrapA40 treatment (n = 8, P < 0.005). Further, VEGF TrapA40 reduced the diabetes-related nitric oxide increases in the retinae of diabetic animals. The inhibition of eNOS with N-ω-nitro-l-arginine methyl ester also potently reduced retinal leukocyte adhesion. Although neutrophil CD11a, CD11b, and CD18 levels were increased in 1-week diabetic animals, VEGF TrapA40 did not alter the expression of these integrin adhesion molecules. Taken together, these data demonstrate that VEGF induces retinal ICAM-1 and eNOS expression and initiates early diabetic retinal leukocyte adhesion in vivo. The inhibition of VEGF bioactivity may prove useful in the treatment of the early diabetic retinopathy.
The adhesion of leukocytes to the retinal vasculature is one of the earliest events in experimental diabetes. 1 Enhanced vascular permeability, endothelial cell damage, and capillary nonperfusion are some of the pathological consequences of diabetic retinal leukocyte adhesion. 1,2 When neutralizing anti-intercellular adhesion molecule-1 (ICAM-1) antibodies are administered to newly diabetic animals, the leukocyte-related pathologies are dramatically reduced. 1,2 Similarly, when the bioactivity of the ICAM-1 counterreceptor CD18 is inhibited, diabetic retinal leukocyte adhesion is potently suppressed. 3 Diabetic retinopathy in rodents recapitulates much of the pathology of human diabetic retinopathy. In both species, the diabetic retinal vasculature up-regulates ICAM-1, contains increased numbers of leukocytes, and develops blood-retinal barrier breakdown and capillary nonperfusion 1-5 Moreover, the leukocyte-related pathologies observed in early diabetes persist into established diabetes 1,4 (Joussen and colleagues, unpublished data).
The injection of vascular endothelial growth factor (VEGF) into normal nondiabetic eyes recapitulates many of the retinal vascular changes triggered by diabetes, including ICAM-1 up-regulation, leukocyte adhesion, vascular permeability, and capillary nonperfusion. 6-9 Although VEGF is expressed in the early diabetic retina, 10,11 it is not known if it triggers the retinal ICAM-1 up-regulation and leukocyte adhesion seen early in the disease. Nor is it known if VEGF alters the expression of surface integrins required for neutrophil adhesion to the diabetic retinal vasculature.
In brain endothelium, VEGF-induced ICAM-1 up-regulation is mediated by nitric oxide (NO). 12 NO, a molecule with both cytotoxic and signaling capabilities, is generated by various NO synthases (NOS). Although the endothelial isoform, eNOS, is up-regulated in diabetic neural ganglia, 13 its expression and regulation by VEGF in another neural tissue, the diabetic retina, remains unknown.
In the current studies, the role of endogenous VEGF in the induction of retinal ICAM-1 and leukocyte adhesion was studied in vivo. The regulation of retinal eNOS and NO by VEGF was also examined, as was the effect of VEGF on the expression of the neutrophil integrins CD11a, CD11b, and CD18. Overall, these experiments investigated the direct causal role of VEGF and NO in the initiation of the earliest stages of diabetic retinopathy.
Materials and Methods
Animals
Male Long-Evans rats weighting ∼200 g were used in these experiments. All protocols abided by the Association for Research in Vision and Ophthalmology (ARVO) statement on the Use of Animals in Ophthalmology and Vision Research and were approved by the Animal Care and Use Committee of the Children’s Hospital. The animals were fed standard laboratory chow and allowed free access to water in an air-conditioned room with a 12-hour light-dark cycle. Except as noted below, the animals were anesthetized with ketamine (80 mg/kg; Ketalar, Parke-Davis, Morris Plains, NJ) and xylazine (4 mg/kg; Rompun, Harver-Lockhart, Morris Plains, NJ) before all experimental manipulations.
Induction of Diabetes
After 12 hours of fasting, the animals received a single 60-mg/kg intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO) in 10 mmol/L of sodium citrate buffer, pH 4.5. Control nondiabetic animals were fasted and received citrate buffer alone. Twenty-four hours later, animals with blood glucose levels >250 mg/dl were considered diabetic. All experiments were performed 1 week after the induction of diabetes. The diabetic state was confirmed a second time before analysis.
ICAM-1 RNase Protection Assay
Retinae were gently dissected free and cut at the optic disk immediately after enucleation, and frozen in liquid nitrogen. Total RNA was isolated according to the acid guanidinium thiocyanate-phenol-chloroform extraction method. A 425-bp EcoRI/BamHI fragment of rat ICAM-1 cDNA was prepared by the reverse transcriptase-polymerase chain reaction. The polymerase chain reaction product was cloned into pBluescript II KS vector. After linearization by digestion with EcoRI, transcription was performed with T7 RNA polymerase in the presence of [32P]dUTP generating a 225-bp riboprobe. Sequencing verified the identity of the cloned cDNA. Ten μg of total cellular RNA was used for the ribonuclease protection assay. All samples were simultaneously hybridized with an 18S riboprobe (Ambion, Austin, TX) to normalize for variations in loading and recovery of RNA. Protected fragments were separated on a gel of 5% acrylamide, 8 mol/L urea, 1× Tris-borate-ethylenediaminetetraacetic acid, and quantified on a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Quantitation was completed within the linear range of signal.
VEGF TrapA40 and Interleukin (IL)-6R Trap Reagents
VEGF TrapA40 and IL-6R Trap were synthesized at Regeneron Pharmaceuticals Inc. (Tarrytown, NY). VEGF TrapA40 consisted of immunoglobulin repeats 1 to 3 of the extracellular domain of human Flt-1 fused to the Fc portion of human IgG1. The protein was expressed in Chinese hamster ovary cells and purified via protein A affinity chromatography and size exclusion chromatography. The recombinant Flt-Fc chimera was then chemically modified to improve the pharmacokinetic profile of the parent molecule, without affecting its ability to bind VEGF with high affinity (Rudge, SJ Wiegand, GD Yancopoulos, unpublished data).
In detail, Chinese hamster ovary-derived parental Flt(1-3Ig)Fc was incubated with sulfo-NHS-acetate (Pierce, Rockford, IL) in phosphate-buffered saline (PBS)/5% glycerol, pH 7.2, such that the acetylation reagent was present in 40-fold molar excess. The acetylation reaction specifically modifies the ε amino group of lysines present in the parental molecule. The mixture was placed on a rocker and incubated overnight at room temperature. The acetate-modified flt(1-3Ig)Fc termed FltFc A40, was then extensively dialyzed against PBS/5% glycerol (25-kd molecular weight cut-off (MWCO)) tubing. After dialysis, the concentration was checked spectrophotometrically (absorbance at 280 nm) and modification was assessed by isoelectric focusing analysis. With this modification the pI shifts from 9.5 for parental Flt(1-3Ig)Fc to 5.8 to 6.5 for FltFc A40. The purity of the modified recombinant protein was determined to be >95% by Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein was filter sterilized and stored in PBS, pH 7.2, containing 5% glycerol at −20°C. VEGF TrapA40 bioactivity was confirmed in endothelial cell proliferation assays before its use (data not shown). IL-6R Trap was made from the extracellular domain of human IL-6Rα (the low-affinity IL-6 receptor) fused to the Fc domain of human IgG1. IL-6R Trap binds only human IL-6 with low affinity, and not mouse or rat IL-6. IL-6R Trap was Chinese hamster ovary cell derived, purified via protein A and size exclusion chromatography, and was >95% pure on Coomassie-stained gels. VEGF TrapA40 and IL-6R Trap were dissolved in sterile Tris-BisTris-Cl-sodium acetate (TBA) buffer (15 mg/ml). On day 7 of diabetes, diabetic rats were randomized to receive a single 25 mg/kg intraperitoneal injection of either VEGF TrapA40 or IL-6R Trap.
Retinal Leukostasis Quantitation
Deep anesthesia was induced with 50 mg/kg of sodium pentobarbital. The chest cavity was carefully opened and the left ventricle was entered with a 14-gauge perfusion cannula fixed to a vessel clamp, carefully avoiding ventricular obstruction. The right atrium was opened with a 12-gauge needle to achieve outflow. With the heart providing the motive force, 250 ml/kg of PBS was perfused to clear erythrocytes and nonsticking leukocytes. Fixation was then achieved via perfusion with 1% paraformaldehyde and 0.5% glutaraldehyde at a pressure of 100 mmHg. At this point the heart stopped. A systemic blood pressure of 120 mmHg was maintained by perfusing a total volume of 200 ml/kg for 3 minutes. The inhibition of nonspecific binding with 1% albumin in PBS (total volume 100 ml/kg) was followed by perfusion with fluorescein isothiocyanate-coupled Concanavalin A lectin (20 μg/ml in PBS, pH 7.4, total concentration 5 mg/kg body weight) (Vector Laboratories, Burlingame, CA). The latter stained adherent leukocytes and the vascular endothelium. Lectin staining was followed by 1% bovine serum albumin/PBS perfusion for 1 minute, and PBS perfusion alone for 4 minutes, to remove excess Concanavalin A (Vector Laboratories). 2
The retinae were flat-mounted in a water-based fluorescence anti-fading medium (Southern Biotechnology, Birmingham, Alabama) and imaged via fluorescence microscopy (Zeiss Axiovert, fluorescein isothiocyanate filter; Zeiss, Lakewood, NJ). Only whole retinae in which the peripheral collecting vessels of the ora serrata were visible were used for analysis. Leukocyte location was scored as being either arteriolar, venular, or capillary. The large vessels emanating from the optic nerve and their first grade branches were defined as being either arterioles or venules. Arterioles were differentiated from venules by virtue of their smaller diameter. Vessels between these major vessels have a diameter approximating the size of an adherent leukocyte. These vessels were considered as capillaries. The total number of adherent leukocytes per retina was counted.
Quantification of Retinal NO
NO was converted to nitrite and the total nitrite concentration of each retina was estimated using the Total Nitric Oxide assay (R&D Systems, Minneapolis, MN). Briefly, each retina was placed in 100 μl of 40 mmol/L Tris buffer (pH 7.8) supplemented with 3 mmol/L dithiothreitol, 1 mmol/L l-arginine, 1 mmol/L NADH, and 4 μmol/L each of FAD, FMN, and H4 biopterin (Sigma), and homogenized with mechanical homogenization. The samples were subsequently cleared by centrifugation and retinal protein levels estimated using a commercial assay (BCA kit; Bio-Rad, Hercules, CA). Equal amounts of protein per sample were ultrafiltered through a 10,000 molecular weight cutoff filter to eliminate proteins. The samples were subsequently incubated with nitric reductase to convert NO to nitrite, according to the manufacturer’s instructions, and incubated with the Griess reagent (1% sulfonamide, 0.1% naphthylethylene diamine dishydrochloride, 2.5% H3PO4) (Sigma) at room temperature for 10 minutes. Nitrite was determined at 550 nm using a microplate reader and the concentration was calculated using sodium nitrite standards.
Enzyme-Linked Immunosorbent Assay for eNOS
Each retina was placed in 100 μl of solution (4°C) consisting of 20 mmol/L imidazole hydrochloride, 100 mmol/L KCl, 1 mmol/L MgCl, 1 mmol/L EGTA, 1% Triton, 10 mmol/L NaF, 1 mmol/L sodium molybdinate, and 1 mmol/L ethylenediaminetetraacetic acid supplemented with protease inhibitors (Complete Mini; Roche, Basel, Switzerland). Samples were centrifuged for 10 minutes at 13,000 rpm. Two μl of the supernatant was used for protein determination via the mini BCA assay (Pierce Scientific, CA). A commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) was used to quantitate eNOS levels according to the manufacturer’s instructions. The reaction was stopped and the absorption measured in an ELISA reader at 450 nm. All measurements were performed in duplicate. The tissue sample concentration was calculated from a standard curve and corrected for protein concentration.
Enzyme-Linked Immunosorbent Assay for ICAM-1
Each retina was homogenized in 100 μl of solution consisting of 20 mmol/L imidazole hydrochloride, 100 mmol/L KCl, 1 mmol/L MgCl, 1 mmol/L EGTA, 1% Triton, 10 mmol/L NaF, 1 mmol/L sodium molybdinate, and 1 mmol/L ethylenediaminetetraacetic acid. The solution was supplemented with a cocktail of protease inhibitors (Complete, Roche) before use. Samples were cleared via centrifugation for 10 minutes at 13,000 rpm and assessed for protein concentration with the BCA assay (Mini BCA kit, Pierce Scientific). Flat-bottom 96-well microtiter plates (Immuno-Plate I 96-F; Nunc, Naperville, IL) were coated with 50 μl/well (1 ng/ml) of the specific rabbit anti-ICAM-1 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) in coating buffer (0.6 mol/L NaCl, 0.26 mol/L H3PO4, and 0.08 N NaOH, pH 9.6) for 16 to 24 hours at 4°C. Nonspecific sites were then blocked with 2% bovine serum albumin in PBS for 60 minutes at 37°C, followed by sample addition of a 50-μl aliquot in duplicate, and incubated for 60 minutes at 37°C. After washes, 50 μl of a biotinylated rabbit polyclonal antibody (3.5 μg/ml in PBS, pH 7.5, 0.05% Tween-20, and 2% fetal calf serum) was added and incubated for 45 minutes at 37°C. The plates were washed, streptavidin-peroxidase conjugate (1/1000, R&D Systems) was added, and the plates were incubated for 30 minutes at 37°C. The plates were again washed, the substrate TMB (3,3′,5,5′-tetramethylbenzidine; Kirkegaard & Perry, Gaithersburg, MD) was added for color development, and the reaction was quenched with 100 μl of 1 mol/L H2PO4. The plates were then read at 450 nm with an automated microplate reader.
Treatment with N-ω-Nitro-l-Arginine Methyl Ester (L-NAME)
The NO synthase inhibitor L-NAME (Sigma) was dissolved in saline and filter- sterilized. A freshly prepared solution (30 mg/ml) was administered via intraperitoneal injection at a dose of 30 mg/kg every other day to diabetic and nondiabetic animals. Control animals received injections of solvent alone. Eight days after the induction of diabetes, each animal had received four treatment doses. Control and L-NAME-treated animals were analyzed for retinal leukocyte adhesion on day 8 as described above.
Flow Cytometry
The monoclonal antibodies (mAbs) used in the flow cytometry experiments were purified IgG of murine origin. The fluorescein isothiocyanate-conjugated mAbs LFA-1α chain (anti-rat CD11a), WT.5 (anti-rat CD11b), WT.3 (anti-rat CD18), and phycoerythrin-conjugated mAb OX-1 (anti-rat CD45) were all obtained from Pharmingen (San Diego, CA).
The surface expression of CD11a, CD11b, and CD18 on rat neutrophils from nondiabetic, diabetic, and diabetic VEGF TrapA40-treated animals was determined via flow cytometry as previously described. 3 Briefly, whole blood anti-coagulated with ethylenediaminetetraacetic acid (Life Technologies, Inc., Grand Island, NY) was obtained from the hearts of deeply anesthetized rats. Neutrophils were isolated from whole blood by density gradient centrifugation with NIM2 (Neutrophil Isolation Media; Cardinal Associates, Santa Fe, NM) according to the manufacturer’s instructions. Red blood cells were lysed via hypotonic lysis. The preparations contained >91% neutrophils as determined by eosin and methylene blue staining (Leukostat Staining System; Fisher Scientific, Pittsburgh, PA).
The cells were resuspended in 5% Dulbecco’s modified Eagle’s medium containing 20 μg/ml of fluorescein isothiocyanate-labeled mAb to CD11a, CD11b, and CD18 (Pharmingen), incubated for 15 minutes at 25°C, and counterstained with phycoerythrin-coupled mAb to CD45. The cells were then washed with PBS and incubated with 1 mg/ml propidium iodide (Molecular Probes, Eugene, OR) to identify dead cells. After centrifugation for 5 minutes at 500 × g, the cells were resuspended in 300 ml of PBS and surface fluorescence was analyzed with a FACScan (Becton Dickinson, San Jose, CA). Vital neutrophils were gated manually on the basis of their characteristic foreword and side light-scattering properties. The surface expression is presented as the percentage of positive neutrophils.
Statistics
All results are expressed as the mean ± SD. The data were analyzed by Whitney-Mann U-test with post hoc comparisons tested using Fisher’s protected least significant difference procedure. Differences were considered statistically significant when P values were <0.05. For the comparison of CD11a, CD11b, and CD18 expression with flow cytometry, a nonparametric one-way analysis of variance (Friedmann’s test) was used.
Results
Endogenous VEGF Increases Retinal ICAM-1 mRNA and Protein Levels
Twenty-four hours after treatment with 25 mg/kg of VEGF TrapA40, ICAM-1 mRNA levels were quantified via ribonuclease protection assay. When normalized to 18S, retinal ICAM-1 mRNA levels in diabetic animals treated with VEGF TrapA40 were 3.83 ± 2.52 (optical density units) (n = 11) versus 23.3 ± 8.84 (n = 13) in the untreated diabetic animals (Figure 1 ▶ , P < 0.0001). Compared to nondiabetic control animals, diabetic animals showed a threefold increase in ICAM-1 protein levels (0.35 ± 0.035 versus 1.007 ± 0.09 pg/mg, P < 0.0001, n = 6). Treatment with VEGF TrapA40 reduced the ICAM-1 protein to those of the nondiabetic animals (1.007 ± 0.09 to 0.42 ± 0.03 pg/mg, P < 0.0005, n = 8). There were no differences between the untreated diabetic controls and those that received IL-6 (from 1.007 ± 0.09 to 1.01 ± 0.17, n = 6). The ICAM-1 levels of isolated leukocytes from nondiabetic and diabetic animals were assessed by immunoassay and were found to lack detectable ICAM-1 expression (data not shown).
Figure 1.

Quantitative analysis of the ICAM-1 mRNA-expression in the retinal tissue by RNase protection assay. Ribonuclease protection assay for ICAM-1 and 18S was performed on 1-week diabetic rat retinae (A). Diabetic animals received either 25 mg/kg of VEGF (n = 13 eyes) TrapA40 or no treatment at all (n = 11). When normalized to 18S, retinal ICAM-1 levels in the eyes of animals pretreated with VEGF TrapA40 were 83.5% lower than in the eyes of animals pretreated with IL-6R Trap control (B) (P < 0.0001). C: Quantitative analysis of the ICAM-1 protein levels in the retinal tissue by an ELISA-based technique. Diabetic animals showed a threefold increase in ICAM-1 protein levels (from 0.35 + 0.035 to 1.007 ± 0.09 pg/mg, P < 0.0001, n = 6), when compared to nondiabetic control animals. The ICAM-1 levels were reduced to the levels of the nondiabetic animals (from 1.007 ± 0.09 to 0.42 ± 0.03 pg/mg, P < 0.0005, n = 8) on treatment with VEGF TrapA40, whereas the ICAM-1 protein levels in the untreated diabetic rats were no different from those that received IL-6 (from 1.007 ± 0.09 to 1.01 ± 0.17, n = 6).
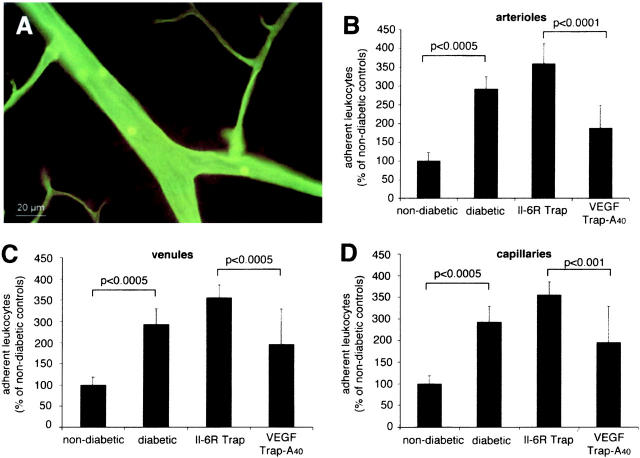
Endogenous VEGF Initiates Diabetic Retinal Leukostasis
Leukocyte adhesion was quantified using the lectin perfusion technique (Figure 2A) ▶ . 2 The total number of retinal leukocytes in the nondiabetic animals was 32.16 ± 7.16 in arterioles, 34.83 ± 6.52 in venules, and 33.66 ± 7.86 in capillaries, resulting in a lower density of leukocytes per vessel length in the capillaries. As was previously observed, 1,2 leukocyte adhesion in the diabetic retinae was increased two- to threefold as compared to the nondiabetic animals (Figure 2B) ▶ . VEGF TrapA40 significantly reduced leukocyte adhesion in the retinal arterioles (47%, n = 11, P < 0.0001), venules (36%, n = 11, P < 0.0005), and capillaries (36%, n = 11, P < 0.001) when compared to the IL-6R Trap-treated diabetic animals (Figure 2C) ▶ .
Figure 2.
Lectin staining with Concanavalin A. A: Retinal venule of a diabetic animal with adherent leukocytes after perfusion removal of nonadherent blood elements. Adherent leukocyte density after lectin staining in arterioles (B), venules (C), and capillaries (D). Compared to the diabetic animals, the retinae of nondiabetic animals showed two- to threefold more adherent leukocytes in the arterioles, venules, and capillaries, respectively (P < 0.001 per condition). When the animals were treated with VEGF TrapA40, the leukocyte counts were 47.8%, 37.0%, and 36.8% lower in the arterioles, venules, and capillaries, respectively, when compared to the control IL-6R Trap-treated animals (P < 0.001 per condition).
Endogenous VEGF Increases NO in Diabetic Retina
The total nitrite concentration was estimated from retinal tissue using a total NO assay based on the conversion of NO to nitrate and its subsequent quantification (Figure 3) ▶ . Compared to the retinae of nondiabetic animals, the retinae of diabetic animals demonstrated a 4.25-fold increase in normalized NO levels (31.69 ± 3.27 μmol/L versus 134.92 ± 7.83 μmol/L; P < 0.0001, n = 10). Treatment with VEGF TrapA40 reduced the retinal NO levels almost to nondiabetic levels (50.15 ± 4.85 μmol/L retinal weight; P > 0.05 versus nondiabetic controls, n = 8).
Figure 3.
The total nitrite concentration was estimated from retinas using the Griess reagent. There was a 4.25-fold increase in the total retinal nitrite levels (31.69 ± 3.27 μmol/L versus 134.92 ± 7.83 μmol/L; P < 0.0001, n = 10) in the diabetic animals versus the nondiabetic controls. The retinal nitrite levels in the diabetic animals treated with VEGF TrapA40 were reduced to those of the nondiabetic animals (50.15 ± 4.85 pg/mg retinal weight; P > 0.05 versus nondiabetic controls, n = 8).
Endogenous VEGF Increases eNOS Levels in Diabetic Retina
The enzyme eNOS was quantified from retinal protein extracts using a sensitive ELISA (Figure 4) ▶ . Compared to the retinae of nondiabetic animals, the retinae of diabetic animals demonstrated a 1.6-fold increase in normalized eNOS levels (1.23 ± 0.11 pg/mg versus 2.03 ± 0.22 pg/mg; P < 0.005, n = 10). Pretreatment with VEGF TrapA40 reduced the retinal eNOS levels to nondiabetic levels (1.20 ± 0.08 pg/mg retinal weight, P > 0.05 versus nondiabetic controls, n = 8). The eNOS levels in diabetic animals treated with IL-6R Trap did not differ significantly from the untreated diabetic animals (n = 6, P > 0.05).
Figure 4.
Retinal eNOS levels in nondiabetic, untreated diabetic, and VEGF TrapA40- and IL-6R Trap-treated diabetic animals. The retinae of nondiabetic animals contained 1.23 ± 0.11 pg/mg retinal weight eNOS compared to 2.03 ± 0.22 pg/mg in the diabetic animals. Treatment with 25 mg/kg of VEGF TrapA40 reduced eNOS levels to nondiabetic levels (1.2 ± 0.08 pg/mg). The retinal eNOS levels in the VEGF TrapA40-treated animals were significantly different from the untreated and IL-6R Trap-treated diabetic animals (P < 0.005 for each).
Inhibition of NO Decreases Leukostasis in the Diabetic Retina
Leukocyte adhesion was quantified using the lectin perfusion technique 2 (Figure 5) ▶ . Systemical treatment with the NO-synthase inhibitor L-NAME significantly reduced leukocyte adhesion in the retinal arterioles (56%, n = 10, P < 0.0001), venules (68%, n = 10, P < 0.0001), and capillaries (52%, n = 10, P < 0.0001) when compared to nontreated diabetic animals. Nondiabetic animals treated with L-NAME showed a trend toward a reduction in leukostasis in the retinal arterioles (11%, n = 11, P > 0.05), venules (27%, n = 11, P < 0.0005), and capillaries (18%, n = 11, P < 0.001) when compared to untreated nondiabetic animals.
Figure 5.

Leukocyte adhesion was determined in diabetic rats after systemic treatment with L-NAME, a NO-synthase inhibitor. L-NAME significantly reduced leukocyte adhesion in the retinal arterioles (56%, n = 10, P < 0.0001), venules (68%, n = 10, P < 0.0001), and capillaries (52%, n = 10, P < 0.0001) when compared to nontreated diabetic animals. The treatment of nondiabetic animals with L-NAME also reduced leukostasis when compared to untreated nondiabetic animals.
Endogenous VEGF Does Not Induce LFA-1 (CD11a/CD18) or Mac-1 (CD11b/CD18) Expression on Diabetic Neutrophils
The expression of surface integrins CD11a, CD11b, and CD18 was measured on neutrophils isolated from nondiabetic rats, diabetic rats, and diabetic rats treated with VEGF TrapA40. In agreement with previously published results, 3 neutrophil CD11a, CD11b, and CD18 surface expression levels were 2.1-fold (n = 3, P < 0.05), 2.6-fold (n = 3, P < 0.05), and 2.7-fold (n = 3, P < 0.05) greater in leukocytes from 1-week diabetic animals versus nondiabetic controls (Table 1) ▶ . Treatment with 25 mg/kg VEGF TrapA40 did not alter neutrophil surface integrin expression under the conditions tested (P > 0.05 for all).
Table 1.
Flow Cytometric Analysis of Integrin Molecule Expression on Neutrophils
| Nondiabetic | Diabetic | Diabetic + VEGF TrapA40 | |
|---|---|---|---|
| CD11a | 9.0 ± 9.8 | 21.3 ± 19.2 | 21.3 ± 14.5 |
| CD11b | 11.6 ± 6.1 | 30.0 ± 9.6 | 30.3 ± 8.0 |
| CD18 | 12.6 ± 4.0 | 34.3 ± 17.9 | 50.3 ± 15.2 |
Percentage of positive neutrophils (mean ± SD).
Discussion
Central to the pathology of early diabetic retinopathy are the increases in retinal ICAM-1 and neutrophil surface integrins. These molecules mediate leukocyte adhesion, a process that leads to blood-retinal barrier breakdown, capillary nonperfusion, and endothelial cell injury and death. To date, the molecular steps linking the diabetic state to retinal ICAM-1 and neutrophil integrin expression have remained unknown. In this study, endogenous VEGF is shown to be responsible, in part, for the up-regulation of ICAM-1 in the early diabetic retina. In addition, retinal eNOS, a downstream effector of VEGF bioactivity, is shown to be increased in the diabetic retina and to be induced by VEGF. Although the proximal stimuli for VEGF expression in early diabetes remain unknown, hyperglycemia per se can induce VEGF expression in retinal cells in vitro. 14 Notably, under the conditions tested, endogenous VEGF did not alter CD11a, CD11b, or CD18 surface expression levels on diabetic neutrophils.
There is evidence to causally link VEGF to inflammation. We previously demonstrated that exogenous VEGF causes leukocyte adhesion in the retinal vasculature. 9 We have also shown that VEGF up-regulation in diabetes correlates with the increased surface integrin expression and integrin-mediated adhesion. Specifically, we showed that the β2-integrin heterodimers LFA-1 (CD11a/CD18) and Mac-1 (CD11a/CD18) are expressed at higher levels on diabetic rat neutrophils. The increased leukocyte adhesion was blocked in vivo and in vitro with neutralizing antibodies to CD11b, CD18, and ICAM-1. Skin- 7 and tumor-derived 15 VEGF has also been shown to trigger leukocyte adhesion in experimental models. In the eye, Flt-1-based VEGF inhibition was demonstrated to inhibit inflammation-related choroidal neovascularization. 16 In the latter study, inflammation, in addition to neovascularization, was reduced after the systemic administration of a soluble Flt-1-based reagent. Similar anti-inflammatory effects after VEGF inhibition were described in a model of rheumatoid arthritis. 17 The results of the current study, which show a reduction in inflammation after VEGF inhibition, are consistent with these published reports. Taken together, the evidence suggests that VEGF-induced inflammation might not be limited to the eye, but rather represents a common mechanism in disease.
In the current study, we choose to study neutrophil surface integrin expression, because neutrophils represent the majority of leukocytes in the diabetic retinae of rats 18 and humans. 19 However, we cannot exclude the possibility that monocytes are adhering to the vascular endothelium. In our earlier studies, we have shown that the adherent population is positive for the integrin CD18, a molecule that is present on neutrophils and monocytes. The enhanced neutrophil integrin expression that characterizes diabetes was not affected by the inhibition of VEGF in the current study. This finding is in general agreement with recent data showing that VEGF inhibition does not alter neutrophil CD11b and CD18 expression induced by conditioned tumor medium. 20 Nevertheless, retinal VEGF may still serve to attract leukocytes to the retinal vasculature. Monocytes, a leukocyte class operative in human diabetic retinopathy, 4,5 migrate in response to VEGF. 21 Moreover, via their own VEGF, leukocytes may serve to increase the local concentration of VEGF when they adhere to endothelium. By example, VEGF has been documented in neutrophils, 22 monocytes, 23 eosinophils, 24 lymphocytes, 25 and platelets. 26
The current data also show that endogenous VEGF is a stimulus for retinal eNOS expression. The serine/threonine protein kinase AKT/PKB was recently shown to be activated by VEGF and to mediate the induction of eNOS, a cascade leading to increased NO production, and in turn, ICAM-1 up-regulation. 12 Based on these data, we hypothesized that the inhibition of endogenous VEGF would lead to the down-regulation of retinal ICAM-1 through a reduction in eNOS expression. The results obtained in this study are consistent with this hypothesis. Retinal nitrite levels were up-regulated in the diabetic animals and were normalized via the inhibition of endogenous VEGF. Moreover, the direct inhibition of eNOS via the systemic administration of L-NAME reduced diabetic leukocyte adhesion. Diabetes-related increases in retinal NO have been described before. 27 However, the literature concerning the effect on NO on the expression of inflammatory molecules is controversial and the effects might be tissue-dependent. 12,28,29 A down-regulation of ICAM-1 expression via NO has been shown in two models of ischemia-reperfusion. 28,29 Our data, however, are in agreement with those of Radisavljevi and colleagues 12 demonstrating the action of VEGF and NO on ICAM-1 up-regulation.
Taken together, the current data causally link VEGF with the inflammatory events that characterize the earliest stages of diabetic retinopathy. As such, VEGF may serve an important potential therapeutic target for the prevention and treatment of early diabetic retinopathy.
Acknowledgments
We thank Jonathan Walsh for his assistance with the flow cytometry experiments.
Footnotes
Address reprint requests to Anthony P. Adamis, M.D., Massachusetts Eye and Ear Infirmary, 243 Charles St., Boston, MA 02114. E-mail: tony@adamis@meei.harvard.edu.
Supported by The Roberta W. Siegel Fund (to A. P. A.), the Juvenile Diabetes Foundation (to A. M. J. and A. P. A.), the National Eye Institute (grants R01 EY12611 and EY11627 to A. P. A.), the Deutsche Forschungsgemeinschaft (DFG Jo324/2-1 to A. M. J.), the Ernst und Berta Grimmke Stiftung Düsseldorf (to A. M. J.), and a Massachusetts Lions Eye Research Fund grant (to A. P. A.).
References
- 1.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP: Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA 1999, 96:10836-10841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP: Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol 2001, 158:147-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, Luscinskas FW, Adamis AP: Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci 2000, 41:1153-1158 [PubMed] [Google Scholar]
- 4.Schröder S, Palinski W, Schmid-Schönbein GW: Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 1991, 139:81-100 [PMC free article] [PubMed] [Google Scholar]
- 5.McLeod DS, Lefer DJ, Merges C, Lutty GA: Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol 1995, 147:642-653 [PMC free article] [PubMed] [Google Scholar]
- 6.Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP: Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 1996, 103:1820-1828 [DOI] [PubMed] [Google Scholar]
- 7.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukamura D, Monsky D, Claffey KP, Jain RK: Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998, 111:1-6 [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Perez V, Ma N, Miyamoto K, Peng HB, Liao JK, Adamis AP: VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci 1999, 40:1808-1812 [PubMed] [Google Scholar]
- 9.Miyamoto K, Khosrof S, Bursell S-E, Moromizato Y, Aiello LP, Ogura Y, Adamis AP: Vascular endothelial growth factor-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 2000, 156:1733-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata T, Nakagawa K, Khalil A, Ishibashi T, Inomata H, Sueishi K: The relation between expression of vascular endothelial growth factor and breakdown of the blood retinal barrier in diabetic rat retinas. Lab Invest 1996, 74:819-825 [PubMed] [Google Scholar]
- 11.Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW: Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 1997, 38:36-47 [PubMed] [Google Scholar]
- 12.Radisavljevi Z, Avraham H, Avraham S: Vascular endothelial growth factor up-regulates ICAM-1 expression via the phosphatidylinositol 3 OH-kinase/AKT/nitric oxide pathway and modulates migration of brain microvascular endothelial cells. J Biol Chem 2000, 275:20770-20774 [DOI] [PubMed] [Google Scholar]
- 13.Zochodne DW, Verge VM, Cheng C, Hoke A, Jolley C, Thomsen K, Rubin I, Lauritzen M: Nitric oxide synthase activity and expression in experimental diabetic neuropathy. J Neuropathol Exp Neurol 2000, 59:798-807 [DOI] [PubMed] [Google Scholar]
- 14.Sone H, Kawakami Y, Okuda Y, Kondo S, Hanatani M, Suzuki H, Yamashita K: Vascular endothelial growth factor is induced by long-term high glucose concentration and up-regulated by acute glucose deprivation in cultured bovine retinal pigmented epithelial cells. Biochem Biophys Res Commun 1996, 221:193-198 [DOI] [PubMed] [Google Scholar]
- 15.Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK: During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med 1996, 2:992-997 [DOI] [PubMed] [Google Scholar]
- 16.Honda M, Sakamoto T, Ishibashi T, Inomata H, Ueno H: Experimental subretinal neovascularization is inhibited by adenovirus-mediated soluble VEGF/flt-1 receptor gene transfection: a role of VEGF and possible treatment for SRN in age-related macular degeneration. Gene Ther 2000, 7:978-985 [DOI] [PubMed] [Google Scholar]
- 17.Miolata J, Maciewiez R, Kendrew J, Feldmann M, Paleolog E: Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest 2000, 80:1195-1205 [DOI] [PubMed] [Google Scholar]
- 18.Schroder S, Palinski W, Schmid-Schonbein GW: Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol 1991, 139:81-100 [PMC free article] [PubMed] [Google Scholar]
- 19.McLeod DS, Lefer DJ, Merges C, Lutty GA: Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol 1995, 147:642-653 [PMC free article] [PubMed] [Google Scholar]
- 20.Wu QD, Wang JH, Bouchier-Hayes D, Redmond HP: Neutrophil-induced transmigration of tumour cells treated with tumour-conditioned medium is facilitated by granulocyte-macrophage colony-stimulating factor. Eur J Surg 2000, 166:361-366 [DOI] [PubMed] [Google Scholar]
- 21.Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D: Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 1990, 172:1535-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J: Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 1997, 41:4153-4161 [PubMed] [Google Scholar]
- 23.Iijim K, Yoshikawa N, Connolly DT, Nakamura H: Human mesangial cells and peripheral blood mononuclear cells produce vascular permeability factor. Kidney Int 1993, 44:959-966 [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi T, Weller PF: Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 1997, 17:70-77 [DOI] [PubMed] [Google Scholar]
- 25.Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, Peoples GE, Klagsbrun M: Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res 1995, 55:4140-4145 [PubMed] [Google Scholar]
- 26.Mohle R, Green D, Moore MA, Nachman RL, Rafii S: Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 1997, 94:663-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmo A, Cunha-Vas JG, Carvalho AP, Lopes MC: Nitric oxide synthase activity in retinas from non-insulin-dependent diabetic Goto-Kakizaki rats: correlation with blood-retinal barrier permeability. Nitric Oxide 2000, 4:590-596 [DOI] [PubMed] [Google Scholar]
- 28.Lindemann S, Sharafi M, Spieker M, Buerke M, Fisch A, Grosser T, Veit K, Gierer C, Ibe W, Meyer J, Darius H: NO reduces PMN adhesion to human vascular endothelial cells due to downregulation of ICAM-1 mRNA and surface expression. Thromb Res 2000, 97:113-123 [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Xu B, Hock CE, Nagele R, Sun FF, Wong PY: NO modulates P-selectin and ICAM-1 mRNA and hemodynamic alterations in hepatic I/R. Am J Physiol 1998, 275:H2191-H2198 [DOI] [PubMed] [Google Scholar]