Abstract
The calcium-binding protein S100A4 induces the metastatic phenotype in rodent models of breast cancer, and its expression strongly correlates with reduced survival in human breast and bladder cancer. We have established an orthotopic model of bladder cancer by injecting a cell line derived from a carcinogen-induced rat bladder tumor into the muscular wall of syngeneic rats. MYU-3L cells produce rapidly growing, invasive tumors in the bladder wall but they fail to metastasize. Transfection of MYU-3L cells with a plasmid vector directing overexpression of the S100A4 gene generates variants in which S100A4 expression is elevated by up to sevenfold in comparison with the untransfected cells. Variants overexpressing S100A4 produce primary tumors at similar frequencies and latencies to the parental cell line, a significant number of which metastasize to the para-aortic lymph nodes or lungs. Expression of S100A4 protein in the primary tumors was heterogeneous, but was stronger and more consistent in the metastases, suggesting that transfectants overexpressing S100A4 possess an enhanced ability to form metastatic lesions. We conclude that overexpression of S100A4 can induce the metastatic phenotype in this rodent model of bladder cancer. Taken together with the results from our parallel studies of human bladder cancer, these data suggest a significant role for S100A4 in bladder cancer metastasis and identify a potential new target for systemic therapy in patients with this disease.
Transitional cell carcinomas of the bladder vary greatly in their capacity to invade adjacent stroma resulting in local progression, and to disseminate widely giving rise to distant metastases. At presentation, ∼25 to 30% of bladder tumors are classified as muscle-infiltrative tumors that, by definition, have already demonstrated the ability to invade and are associated with a significant risk of metastasis (30 to 60%). Patients with these tumors have a significantly reduced 5-year survival rate (40%), often correlated to the development of metastases after the failure of conventional treatment strategies such as radical surgery or radiotherapy. Once a diagnosis of metastatic bladder cancer is made, the outlook is grave with a median survival of only 12 months for this group of patients. The molecular mechanisms that promote metastasis of transitional cell carcinoma are poorly understood.
One gene product that has been strongly associated with the metastatic phenotype is the calcium-binding protein S100A4. Expression of S100A4 is associated with metastasis and poor survival in human bladder cancer. 1 Moreover, expression of S100A4 has been correlated with poor survival and development of metastasis in other human solid carcinomas, including those of the breast, 2 colon, 3 and stomach. 4 Overexpression of S100A4 has been shown to induce the metastatic phenotype in experimental rodent models of breast cancer, 5-9 whereas down-regulation of S100A4 using anti-sense or ribozymes has been shown to reduce metastatic potential in the Lewis lung carcinoma model 10 and in a rodent model of osteosarcoma. 11
Although expression of S100A4 has been associated with the metastatic phenotype, the function of this protein and its role in the metastatic process is unclear. S100A4 is a small, 9-kd calcium-binding protein closely related to S100 protein, that has been reported to co-localize with the cytoskeletal proteins, F-actin and non-muscle myosin, 5,12,13 and another member of the S100 protein family, S100A1. 14 S100A4 has been reported to prevent the phosphorylation of non-muscle myosin by protein kinase C. 15 Therefore, it is possible that S100A4 can regulate the function of cytoskeletal proteins, or impinge on signal transduction pathways ultimately controlling cell movement or adhesion. Indeed, mouse S100A4 has been shown to increase motility when transfected into mouse mammary adenocarcinoma cells. 16 Mouse S100A4 has also been shown to induce down-regulation of E cadherin expression in mouse mammary carcinoma cells. 17 E cadherin is an important epithelial cell-cell adhesion molecule whose expression is down-regulated in many invasive solid carcinomas, including bladder cancer. 18,19 However, it is also possible that the S100A4 protein is involved in invasion; when transfected into osteosarcoma cells, an anti-S100A4 ribozyme has been shown to reduce the expression of matrix metalloproteinases and induce expression of inhibitors of these enzymes, resulting in reduced invasive potential. 11 The mechanism of activation of S100A4 gene expression is unclear, but hypomethylation of the gene has been correlated with increased expression in rodent mammary and human colon carcinoma cell lines. 20
Although expression of S100A4 is associated with metastasis and poor survival in human bladder cancer, it is not known whether S100A4 plays a direct role in the induction of the metastatic phenotype in bladder cancer. To investigate this, we have established an orthotopic model of bladder cancer using inbred rats. We now report that overexpression of S100A4 in an invasive but nonmetastatic rat bladder cancer cell line, when injected into the bladder wall, yields metastatic variants.
Materials and Methods
Animals and Cell Culture
Male Fisher 344 rats (4 weeks old) were purchased from Harlan UK Ltd. (Banbury, Oxon, UK). Experiments were approved by the Local Ethics Committee and performed under project license PPL 60/2610. All cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin in an atmosphere of 95% air, 5% CO2 at 37°C.
Transfection of MYU-3L Cells
MYU-3L cells (a generous gift from Prof. R. Oyasu, Northwestern University, Chicago, IL) were established from a tumor that developed in a heterotopically transplanted bladder treated with normal rat urine after MNU initiation. 21 These cells produce invasive but nonmetastatic tumors when injected subcutaneously into nude mice 21 or instilled into heterotopically transplanted bladders in nude mice. 22 The plasmids pSV2neo 23 and pSV2neo-S100A4 were amplified in Escherichia coli and purified using a Qiagen Endotoxin-Free Plasmid Midiprep kit (Qiagen, Crawley, W. Sussex, UK). Ten μg of supercoiled plasmid were transfected into MYU-3L cells using a calcium-phosphate mammalian transfection kit (Stratagene, Amsterdam, the Netherlands). Colonies were visible after 2 to 3 weeks. Colonies were picked using sterile cloning rings and expanded to form sublines.
Northern Blotting
Cells were grown until 50 to 70% confluent and total RNA was isolated using an RNeasy kit (Qiagen). RNAs were separated by electrophoresis through agarose gels containing formaldehyde, transferred to nitrocellulose filters (Hybond N+, Amersham, Little Chalfont, Bucks, UK), and incubated with a rat S100A4 cDNA under hybridizing conditions as described previously. 5 After autoradiography, the filter was stripped according to the manufacturer’s protocol and reprobed with a constitutively expressed cDNA (rat glyceraldehyde-3-phosphate dehydrogenase). The levels of S100A4 mRNA were estimated from microdensitometry scans of the autoradiographs and normalized to the level of constitutively expressed glyceraldehyde-3-phosphate dehydrogenase mRNA. The levels are expressed relative to the parental MYU-3L cell line.
Tumorigenicity Studies and Metastatic Assays
Orthtopic implantation of cells into the bladder wall was performed essentially as described by Dinney and colleagues. 24 Cells were given fresh medium 24 hours before harvesting with trypsin-ethylenediaminetetraacetic acid. Harvested cells were washed twice with 10 ml of phosphate-buffered saline (PBS), and resuspended in PBS at a concentration of 2 × 10 7 cells/ml. Rats were anesthetized with isoflurane and placed in the supine position. A lower midline abdominal incision was made, and the bladder was exteriorized. Tumor cells were injected into the wall of the bladder (the injection volume ranged from 0.05 to 0.1 ml). A well-localized bleb was the sign of a technically satisfactory injection. The abdominal wall was sutured and the rats were given a single dose of postoperative analgesic (5 mg/kg carprofen, Rimadyl; Pfizer, Sandwich, UK). Rats were fed with a commercial pelleted diet (R & M no. 3, SDS Ltd., Whitham, UK) and tap water ad libitum. Rats were monitored on a daily basis for adverse effects and signs of tumor development. Tumors were detected by the presence of hematuria and palpation. The animals were culled and a full necropsy performed when any of the following were evident: rapid loss of body weight of 10% maintained for 72 hours, immobile/lethargic behavior, lack of response to gentle stimuli, tented skin tone, labored respiration, or unconsciousness. At necropsy, primary tumors in the bladder, lungs, liver, lymph nodes, gonads, kidneys, spleen, and any other tissues of abnormal appearance were fixed in formalin, processed for histology, and embedded in paraffin wax. The carcasses were X-rayed to screen for potential bony metastases. Sections of the primary tumor and at least five sections of all other tissues from different levels of the tissue block were stained with hematoxylin and eosin to screen for potential metastases.
Immunocytochemistry
Sections were rehydrated through a series of graded alcohols and incubated with hydrogen peroxide in methanol to remove endogenous peroxidase activity. After incubation with 10% (v/v) normal swine serum in PBS/1% (w/v) bovine serum albumin for 30 minutes, sections were incubated with a rabbit polyclonal antibody to human recombinant S100A4 (DAKO, Ely, UK). This antibody has been shown to be specific for S100A4 by Western blotting and gives the same results as a polyclonal antibody to rat S100A4 that cross-reacts with human S100A4 on both Western blots and immunocytochemistry. 2 Incubation of antiserum was performed at a dilution of 1 in 400 in PBS/1% bovine serum albumin overnight at ambient temperature. Bound antibody was detected using biotin-conjugated swine anti-rabbit immunoglobulins and the streptABC kit (DAKO) and Sigma fast diaminobenzidine tablets (Sigma, Poole, UK). Ten fields from each slide were examined independently by two observers for the number of carcinoma cells showing positive staining and intensity of immunostaining (negative, 0; weak, 1; or strong, 2), and scored out of 20 using the formula: staining index = staining intensity × % of positive carcinoma cells/10. In a small number of cases, in which the staining index scored by the two observers differed by more than 5, the section was rescored.
Growth Assays
Population doubling times were measured by plating 5 × 10 3 cells in 96-well plates in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin, and counting six replicate wells every 24 hours by the (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide; thiazolyl blue) (MTT) assay. The natural logarithm of absorbance at 540 nm was plotted as a function of time and the doubling time was calculated using the following formula: number of doublings per hour = lny2 − lny1/ln2/x2 − x1, where x1, y1, and x2, y2 were two points on the steepest part of the plot.
The response to transforming growth factor (TGF)-β was measured by plating 5 × 10 3 cells in 96-well plates in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin, and either no TGF-β (control), 0.5, 1.0, or 5.0 μg/ml recombinant TGF-β (R and D Systems, Abingdon, Oxon, UK). After 4 days, the cell numbers in six replicate wells were compared using the MTT assay.
Motility and Invasion Assays
1 × 10 4 cells in RPMI 1640 medium were added to a prewetted Matrigel cell culture insert (Becton Dickinson, Oxford, UK). Serum-free medium (800 μl) was added to the corresponding well of a companion plate. After 24 hours, the noninvading cells were removed from the upper surface of the membrane by scrubbing with a prewetted cotton bud. The membrane was fixed in ice-cold methanol for 1 hour and stained with Mayer’s hematoxylin. The membrane was cut out and mounted in DPX. Twenty fields were counted at ×200 magnification. Experiments were performed in triplicate. A similar procedure was used for motility assays, except inserts containing 8-μm diameter polyethylene terephthalate filters (Becton Dickinson) were used instead of Matrigel, and RPMI 1640 medium supplemented with 10% fetal bovine serum was placed in both chambers.
Results
Transfection of MYU-3L Cells
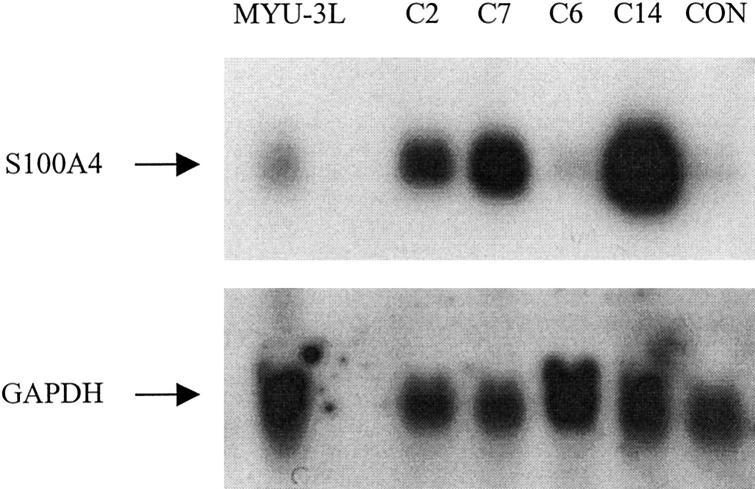
MYU-3L cells were separately transfected with pSV2neo-S100A4 and pSV2neo plasmids. Geneticin-resistant colonies were visible after 7 to 10 days. After 3 weeks of selection in medium containing geneticin, 14 colonies of cells transfected with pSV2neo-S100A4 were ring-cloned and expanded into cell lines. These were termed “MYU-C1 to -C14.” In the pSV2neo transfection experiment, all of the colonies were pooled; this pool was termed “MYU-plasmid.” The relative level of expression of S100A4 mRNA in the clones transfected with S100A4 to the parental cell line varied from 1.2 to a maximum of 6.8 (Figure 1 ▶ and Table 1 ▶ ). The parental MYU-3L cells were predominantly elongated in morphology (Figure 2A) ▶ and cells cultured from a primary tumor produced by these cells (MYU-P) were even more elongated (Figure 2C) ▶ . In contrast, the morphology of the S100A4-overexpressing cells contained a much higher proportion of epithelioid-like cells with highly ruffled plasma membranes and blebs (Figure 2B) ▶ .
Figure 1.
Expression of S100A4 mRNA by normal and transfected cell lines. Ten μg of total RNA were subjected to electrophoresis in gels containing formaldehyde, blotted onto nitrocellulose filters, and hybridized with radiolabeled cDNAs corresponding to rat S100A4 (top) or rat glyceraldehyde-3-phosphate dehydrogenase (bottom). The different lanes were hybridized with RNA from MYU-3L parental cells, S100A4-transfected cells (MYU-C2, C7, C6, and C14), or control (CON) MYU-3L cells transfected with pSV2neo (MYU-plasmid cells).
Table 1.
Expression of S100A4 by Parental MYU-3L Cells and Transfected Cell Lines in Vitro and in Vivo
| Cells* | S100A4 mRNA* | Immunocytochemical score for S100A4 protein in primary tumors† |
|---|---|---|
| MYU-3L | 1 | 1.7 ± 0.4 |
| MYU-C6 | 1.2 | 1.4 ± 0.6 |
| MYU-C10 | 1.4 | ND |
| MYU-C8 | 2.2 | ND |
| MYU-C2 | 3.4 | 6.7 ± 1.1 |
| MYU-C7 | 4.5 | 6.9 ± 0.7 |
| MYU-C14 | 6.8 | 9.0 ± 1.4 |
*Ratio of S100A4 mRNA to that in untransfected MYU-3L cells obtained after normalization for different GAPDH mRNA levels (Materials and Methods).
†Ten fields from each slide were examined independently by two observers for the number of carcinoma cells showing positive staining and intensity of immunostaining (negative, 0; weak, 1, or strong, 2), and scored out of 20 using the formula: immunocytochemical score = staining intensity × % of positive carcinoma cells/10.
Figure 2.
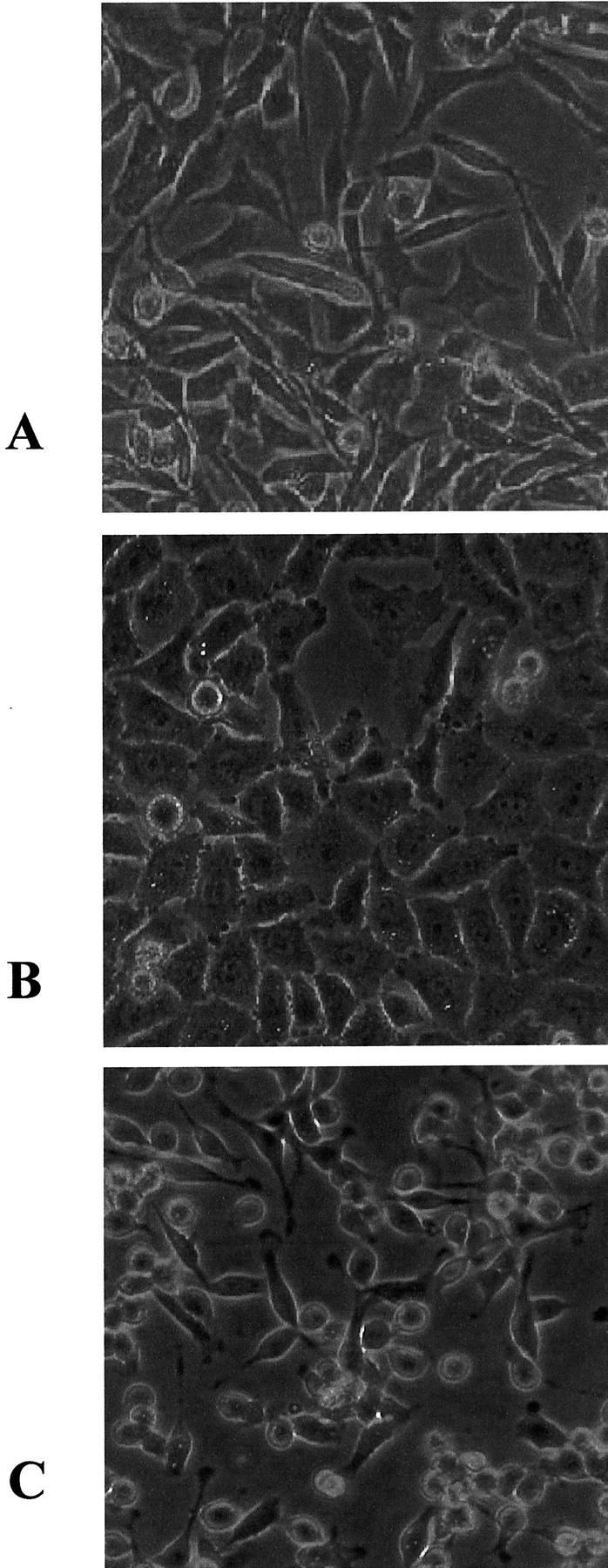
Morphology of MYU-3L and derivative cell lines. A: Parental MYU-3L cells. B: MYU-3L cells transfected with pSV2neo-S100A4 (MYU-C14). C: MYU-P cells from a primary tumor produced by MYU-3L cells. Original magnifications, ×100.
An Orthotopic Model of Bladder Cancer: Incidence and Pathology of Tumors Produced by MYU-3L Cells
Injection of MYU-3L cells into the bladder produced palpable tumors in 15 of 15 (100%) rats with a mean latent period of 23 days. Hematuria was noted in all of these rats; the mean time to first detectable hematuria did not differ significantly from the latent period (Table 2) ▶ . In two rats, the presence of hematuria was intermittent. At necropsy, all of the rats were found to have large primary tumors in the bladder of size 9.4 ± 1.8 cm 3 with necrotic centers. Twelve of 15 tumors were confined to the bladder, 1 tumor had invaded the ureter, and 2 tumors had shed cells into the peritoneal cavity with multiple small seeds of <3 mm 3 present on the abdominal wall and omentum. In one of these rats, a seed was evident on one lobe of the liver; histological examination showed that this seed had invaded into the liver from the periphery. Bilateral hydronephrosis was found in four rats, and unilateral hydronephrosis in a further three rats. In two rats, the tumors had adhered to the suture site. On histological examination, all of the tumors were found to consist of undifferentiated spindle cells with highly pleiomorphic nuclei. Local invasion of muscle and/or fatty tissue was evident in all of the tumors. No metastatic lesions were detected in serial sections of the lungs, draining lymph nodes, or other tissues in any animals. On X-ray of the carcasses, no bone metastases were found (Table 2) ▶ .
Table 2.
Incidence and Pathology of Tumors Produced by Orthotopic Injection of MYU-3L and Transfected Cells
| Cells* | Tumor incidence† | Time blood first detected in urine, range (days) | Latent period, range (days) | Duration of experiment, range (days) | Incidence of metastasis‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls and cells with less than three-fold induction of S100A4 mRNA | ||||||||||
| MYU-3L (parent line) | 15 /15 | 20 (14–29) | 22 (14–34) | 42 (31–70) | 0 /15 | |||||
| MYU-P§ | 9 /10 | 11 (5–22) | 13 (9–18) | 41 (28–58) | 0 /9 | |||||
| MYU-plasmid¶ | 12 /12 | 25 (19–33) | 23 (20–25) | 43 (36–49) | 0 /12 | |||||
| MYU-C6 | 8 /8 | ND∥ | 18 (11–24) | 49 (39–57) | 0 /8 | |||||
| MYU-C2 | 6 /6 | 17 (17–18) | 19 (15–24) | 36 (28–47) | 0 /6 | |||||
| Totals | 50 /51 | 16 (5–33) | 20 (9–34) | 42 (28–70) | 0 /50 | |||||
| Cells with at least 4-fold induction of S100A4 mRNA: | ||||||||||
| MYU-C7 | 8 /8 | 25 (19–33) | 22 (15–25) | 44 (28–59) | 2 /8 | |||||
| MYU-C14 | 9 /9 | 25 (12–35) | 17 (12–22) | 39 (31–51) | 2 /9 | |||||
| Totals | 17 /17 | 25 (12–35) | 19 (12–25) | 41 (28–59) | 4 /17** | |||||
*One to 2 × 106 cells injected into the bladder wall.
†No. of animals with tumors/no. of animals injected.
‡No. of animals with metastases/no. of animals with tumors.
§MYU-3L cells cultured from a nonmetastatic primary tumor and reinjected into a further group of animals.
¶MYU-3L cells transfected with pSV2neo plasmid vector alone.
∥ND, not determined.
**Significantly more metastases than controls and cells with less than three-fold induction of S100A4 mRNA (P < 0.05; Fisher’s exact test).
Cells were cultured from a primary tumor produced by the parental MYU-3L cells. These cells were more spindle-shaped in morphology than MYU-3L cells before passage in vivo (Figure 2C) ▶ . These cells produced tumors in 9 of 10 (90%) rats with a shorter latent period and hematuria was detected earlier than in rats injected with the parental cell line (P < 0.05; Mann-Whitney U test). However, consistent with the parental line, no metastases were detected in any organ examined (Table 2) ▶ .
Incidence and Pathology of Tumors Produced by MYU-3L Cells Transfected with S100A4 DNA
Cells transfected with pSV2neo plasmid (MYU-plasmid) and two sublines of cells transfected with pSV2neo-S100A4 but with less than fourfold overexpression of S100A4 mRNA (MYU-C6 and -C2) produced tumors in 100% of rats with similar latent periods to the parental cell line. Again, no metastases were detected in any organ examined (Table 2) ▶ .
Two cell lines transfected with pSV2neo-S100A4 with at least fourfold elevated expression of S100A4 mRNA (MYU-C7 and -C14) also produced tumors in 100% of rats with similar latent periods and time to first hematuria as seen in the parental cell line (Table 2) ▶ . The tumors produced by these cell lines stained more strongly and consistently with antiserum to S100A4 than primary tumors produced by the parental, untransfected cells and by the control MYU-plasmid cells (Table 1 ▶ and Figure 3, a and b ▶ ). The relative staining index for S100A4 protein in the tumors was similar to that of the mRNA in the cell lines in vitro (Table 1) ▶ . Rats injected with these two sublines, which expressed the highest levels of S100A4 mRNA relative to the parental cell line, developed metastatic lesions. In two rats, metastatic lesions were detected in the para-aortic lymph nodes, and in another two rats, metastatic lesions were detected in the lungs (Table 2) ▶ . The lung lesions were micrometastases but were multifocal, with tumor cells being present in both blood vessels and lymphatics. In the largest micrometastases, the tumor emboli were beginning to extravasate and infiltrate into the surrounding lung tissue (Figure 3c) ▶ . The lymph node metastases were 245 and 172 mm 3 in size, and had replaced the majority of the lymph node (Figure 3d) ▶ . Expression of S100AL protein was stronger and more consistent in the metastases than in the corresponding primary tumors (Table 3) ▶ .
Figure 3.

Immunocytochemistry of transfected cell tumors stained with antiserum to S100A4. a: MYU-plasmid primary tumor showing virtually no staining. b: MYU-C14 primary tumor showing heterogeneous strong staining. c: Lung metastasis from the MYU-C14 primary tumor showing uniformly strong staining. Note that the tumor cells have breached the basement membrane and are extravasating into the surrounding lung tissue. d: Lymph node metastasis from an MYU-C14 primary tumor showing heterogeneous strong staining. Original magnifications, ×40.
Table 3.
Comparison of S100A4 Expression in Primary Tumors and Metastases
| Cells | Immunocytochemical score of S100A4 expression in primary* | Immunocytochemical score of S100A4 expression in metastasis* |
|---|---|---|
| MYU-C14 | 14.4 ± 2.2 | 18.8 ± 0.5 (lung met) |
| MYU-C7 | 7.8 ± 1.2 | 17.2 ± 0.5 (lymph node met) |
*Ten fields from each slide were examined independently by two observers for the number of carcinoma cells showing positive staining and intensity of immunostaining (negative, 0; weak, 1; or strong 2), and scored out of 20 using the formula: immunocytochemical score = staining intensity × % of positive carcinoma cells/10.
In Vitro Properties of MYU-3L Cells Transfected with S100A4 DNA
The parental MYU-3L cells, control transfected cells, and transfected cells overexpressing S100A4 had very similar growth rates in vitro in medium containing 10% fetal bovine serum with doubling times of ∼40 hours (Figure 4 ▶ , top). Malignant solid tumors often become refractory to the anti-mitogen TGF-β. Therefore, we investigated the effect of TGF-β on the growth rates of selected cell lines (Figure 4 ▶ , bottom). All of the cell lines were significantly inhibited by 5 μg/ml of TGF-β (P < 0.05, t-test), and all of the transfected cell lines were significantly inhibited, although to a lesser extent, by 0.5 μg/ml of TGF-β (P < 0.05, t-test). In fact, the metastatic cell lines overexpressing the highest levels of S100A4 showed the greatest sensitivity to growth inhibition by 5 μg/ml of TGF-β (Figure 4 ▶ , bottom).
Figure 4.
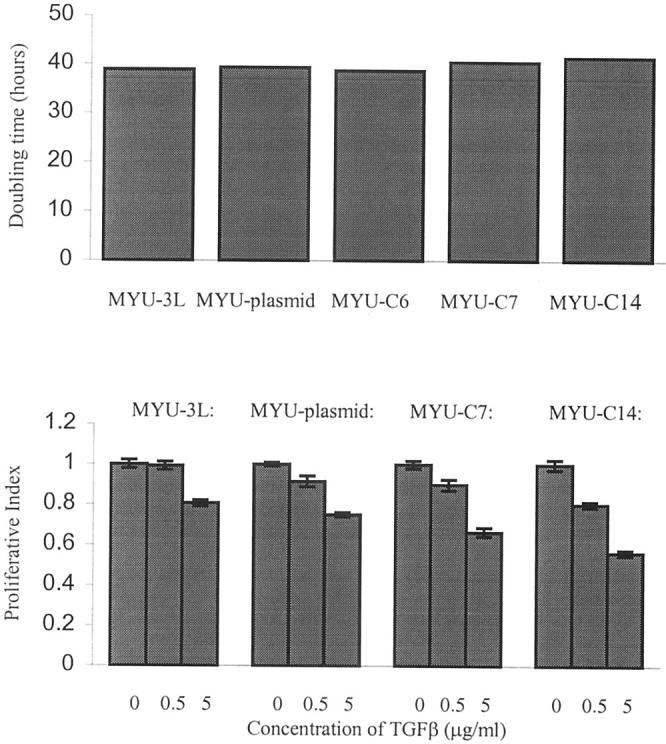
In vitro growth properties of MYU-3L and transfected cell lines. Top: Doubling times. Bottom: Responses to various concentrations of TGF-β. The data were generated using the MTT assay, as described in Materials and Methods. Proliferative index (PI) is defined as absorbance at 540 nm in the presence of TGF-β/absorbance at 540 nm of control (no TGF-β) × 100 ± SEM.
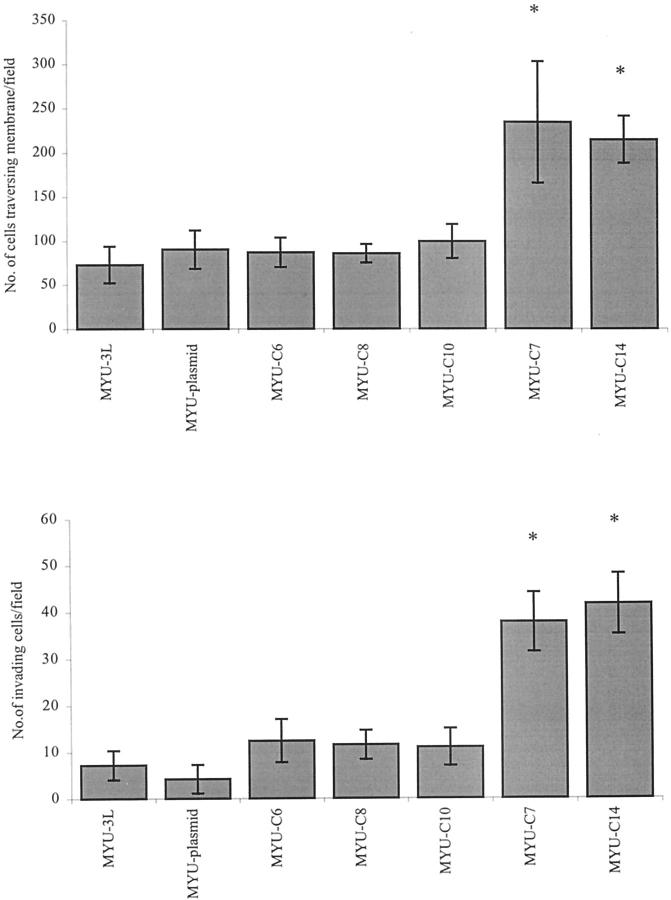
Finally, we measured the ability of the transfected cells to invade Matrigel and traverse a polyethylene terephthalate filter. The transfected cells overexpressing S100A4 by at least fourfold were significantly more able to traverse a polyethylene terephthalate filter than the parental cells, control cells transfected with plasmid alone, and transfected cells overexpressing S100A4 by less than threefold (Figure 5 ▶ , top). Similarly, the cells overexpressing S100A4 by at least fourfold were significantly more invasive through Matrigel (Figure 5 ▶ , bottom).
Figure 5.
In vitro migratory and invasive properties of MYU-3L and transfected cell lines. Top: Migration through a polyethylene terephthalate filter. Bottom: Invasion through Matrigel. Scale bars, SEM. *, Significantly greater than MYU-3L cells or MYU-plasmid cells (P < 0.05, t-test).
Discussion
In this report, we have validated an orthotopic model of invasive bladder cancer, and have shown that overexpression of the calcium-binding protein S100A4 can induce a low frequency of metastatic variants. An orthotopic model in immunocompetent animals has advantages over models of bladder cancer that either use immunodeficient rodents and/or assay for metastasis by injecting tumor cells into subcutaneous sites. In an orthotopic, immunocompetent model, the influence of the immune system, anatomical and paracrine factors of the tissue of origin, which may profoundly influence the process of metastasis, are appropriately modeled. In our study, the technique of injection into the bladder wall was chosen because intravesical instillation into the bladder can result in a low frequency of tumor takes. When injected into the bladder wall of immunocompetent rats, the MYU-3L cell line reproducibly produces invasive tumors that are nonetheless stably nonmetastatic, even after passage in vivo. These phenotypes are consistent with its behavior when injected subcutaneously into nude mice 21 or when instilled into rat bladders heterotopically transplanted into nude mice. 22 Moreover, transfection of nonspecific DNA such as a drug-resistance plasmid, does not induce the metastatic phenotype per se, as in certain other experimental systems. 25,26 Induction of the metastatic phenotype in this system is related to the level of overexpression of S100A4. The two clones of cells expressing the highest levels of S100A4 mRNA in vitro and protein in tumors in vivo produced metastatic lesions, whereas clones of cells stably transfected with the same plasmid that express levels of S100A4 mRNA induced less than fourfold relative to the parental cell line did not yield any metastatic tumors.
The relative level of S100A4 mRNA in vitro in the various transfected cell lines is reflected by and large by the S100A4 protein expression in the primary tumors in vivo, at least as determined by immunocytochemistry. However, the metastatic lesions express S100A4 more strongly and consistently than the primary tumors from which they derive. It is apparent that expression of S100A4 in the primary tumors is heterogeneous, and that cells overexpressing S100A4 may have a selective advantage for metastasis. A similar observation has been reported in human colorectal tumors, where expression of S100A4 was reported to be higher in liver metastases than in the primary tumors from the same patient. 5 Expression of S100A4 has been shown to induce the metastatic phenotype in previously nonmetastatic rat mammary epithelial cell lines after injection into the mammary fat pad 5,8,9 and in previously nonmetastatic transgenic mouse models of mammary tumorigenesis expressing MMTV-neu 6 or of the GRS/A strain. 7 The present data demonstrate that S100A4 may directly confer metastatic potential in a rodent model of bladder cancer. Therefore, overexpression of S100A4 may represent a common mechanism that favors the metastatic phenotype in multiple solid cancers. However, because the relative frequency of metastasis is quite low from apparently clonal cell lines, it is probable that other genes, in addition to S100A4, have to be selected for to induce metastasis, even to lymph nodes and lungs, in this experimental system.
The major rate limiting steps in the development of metastases in many tumor types are thought to be entry into the circulation and establishment of macroscopic secondary tumors once extravasation has occurred. 27,28 In the current model of bladder cancer, S100A4 is able to induce the formation of macroscopic metastases in lymph nodes and micrometastases in the lungs, the largest of which have breached the basement membrane and are beginning to extravasate. These metastatic lesions have never been observed in serial sections of the same tissues in control rats, even after reinjection of cells from the parental nonmetastatic tumors. Therefore, it would seem that S100A4 is able to promote entry into, or survival in, the circulation, and the development of macroscopic secondary tumors in lymph nodes. It is not known whether S100A4 is sufficient to give rise to macroscopic secondary tumors in the lungs in this system because the large size of the primary tumors necessitated culling of the animals. However, in a transgenic mouse model of breast cancer, S100A4 is able to promote the formation of macroscopic lung metastases. 6 MMTV-neu transgenic mice develop mammary tumors including carcinoma in situ, some of which give rise to micrometastases in the lung confined within small pulmonary blood vessels surrounded by intact vascular basement membranes. When these transgenic mice were mated with S100A4-expressing transgenic mice, macroscopic metastatic lesions developed in the lungs of the bi-transgenic mice; these lesions constituted up to 24% of the area of the lung sections.
It is unlikely that S100A4 can directly promote proliferation of tumor cells at the primary or secondary sites. In our bladder cancer model, cells overexpressing S100A4 do not have an enhanced proliferation rate in vitro. Moreover, like the parental nonmetastatic cell line, they are sensitive to growth inhibition by the anti-mitogen TGF-β. Rather than affecting growth rates per se, it is more likely that S100A4 can alter the properties of adhesion, migration, and invasion. Increases in these latter properties are highly likely given that S100A4 can associate with the cellular cytoskeleton. 5 Clones of transfected MYU-3L cells overexpressing S100A4 by at least fourfold have enhanced abilities to migrate and invade Matrigel. Moreover, these cells possess a large number of plasma membrane protrusions (lamellipodium extensions) that are characteristic of motile cells. These phenotypes are consistent with previous reports in other cellular systems showing that S100A4-transfected mammary tumor cells have enhanced motility, 8,16 osteosarcoma cells transfected with an anti-S100A4 ribozyme have reduced invasive potential, 11 and Lewis lung carcinoma cells transfected with anti-sense S100A4 have reduced cell motility and invasive potential. 10 It has also been reported that S100A4 can regulate the expression of the epithelial cell-cell adhesion molecule E cadherin. 17 However, there is no evidence that differential expression of S100A4 can affect adhesion to plastic substrata, at least in mouse mammary cell lines (R. Jenkinson and P. S. Rudland, unpublished observations). It is interesting to note that another cytoskeletal-associated protein, RhoC, has also been shown directly to induce metastasis in a mouse model of melanoma. 29 Rho proteins can regulate actomyosin-based contractility and adhesion turnover. Like S100A4, this protein can also enhance invasive and migratory properties, but does not alter growth rate. 29 Taken together, these results strongly suggest that regulation of the cytoskeleton is a fundamental aspect in the biology of metastasis.
The present data, along with our findings that S100A4 is significantly associated with the development of metastases and poor survival in human bladder cancer, suggest that this protein plays an important role in the metastasis of transitional cell carcinoma. The challenges now are to determine whether deregulation of S100A4 is a significant step in the metastasis of other solid cancers, to determine the function of the protein, and evaluate the potential of S100A4 as a target for systemic adjuvant therapy in cancers of the bladder and breast.
Acknowledgments
We thank Prof. R. Oyasu (Northwestern University, Chicago, IL) for the MYU-3L cell line; and Dr. C. P. Dinney and Prof. I. J. Fidler (MD Anderson Cancer Center, Houston, TX) for instruction in the technique of orthotopic bladder implantation.
Footnotes
Address reprint requests to Dr. B. R. Davies, Department of Surgery, School of Surgical and Reproductive Sciences, The Medical School, University of Newcastle-upon-Tyne, Newcastle NE2 4HH, UK. E-mail b.r.davies@ncl.ac.uk.
Supported by grant SP2509 from the Cancer Research Campaign, United Kingdom.
JKMs current address is Department of Surgery (Division of Urology), University of Leicester, Leicester LE1 7RH, UK.
References
- 1.Davies BR, O’Donnell M, Durkan G, Barraclough R, Rudland PS, Neal DE, Mellon JK: Expression of S100A4 is associated with metastasis and poor survival in human bladder cancer. J Pathol (in press) [DOI] [PubMed]
- 2.Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JHR, Robertson L, Barraclough R: Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res 2000, 60:1595-1603 [PubMed] [Google Scholar]
- 3.Takenaga K, Nakanishi H, Wada K, Suzuki M, Matsuzaki O, Matsuura A, Endo H: Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res 1997, 3:2309-2316 [PubMed] [Google Scholar]
- 4.Yonmura Y, Endou Y, Kimura K, Fushida S, Bandou E, Taniguchi K, Kinoshita K, Ninomiya I, Sugiyama K, Heizmann CW, Schafer BW, Sasaki T: Inverse expression of S100A4 and E cadherin is associated with metastatic potential in gastric cancer. Clin Cancer Res 2000, 6:4234-4242 [PubMed] [Google Scholar]
- 5.Davies BR, Davies MPA, Gibbs FEM, Barraclough R, Rudland PS: Induction of the metastatic phenotype by transfection of a benign rat mammary epithelial cell line with the gene for p9Ka, a rat calcium-binding protein, but not with the oncogene EJ-ras-1. Oncogene 1993, 8:999-1008 [PubMed] [Google Scholar]
- 6.Davies MPA, Rudland PS, Robertson L, Parry E, Jolicoeur P, Barraclough R: Expression of the calcium-binding protein S100A4 in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene 1996, 13:1631-1637 [PubMed] [Google Scholar]
- 7.Ambartsumian N, Grigorian M, Larsen F, Karlstrom O, Sidenius N, Rygaard J, Georgiev G, Lukanidin E: Metastasis of mammary carcinomas in GRS/A hybrid mice transgenic for the mts1 gene. Oncogene 1996, 13:1621-1630 [PubMed] [Google Scholar]
- 8.Grigorian M, Ambartsumian N, Lykkesfeldt AE, Bastholm L, Elling F, Georgiev G, Lukanidin E: Effect of mts1 (S100A4) expression on the progression of human breast cancer cells. Int J Cancer 1996, 67:831-841 [DOI] [PubMed] [Google Scholar]
- 9.Lloyd BH, Platt-Higgins A, Rudland PS, Barraclough R: Human S100A4 induces the metastatic phenotype upon benign tumour cells. Oncogene 1998, 17:465-473 [DOI] [PubMed] [Google Scholar]
- 10.Takenaga K, Nakamura Y, Sakiyama S: Expression of antisense RNA to S100A4 gene encoding an S100-related calcium-binding protein suppresses metastatic potential of high metastatic Lewis lung carcinoma cells. Oncogene 1997, 14:331-337 [DOI] [PubMed] [Google Scholar]
- 11.Bjornland K, Winberg JO, Odegaard OT, Hovig E, Loennechen T, Aasen AO, Fodstad O, Maelandsmo GM: S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of metalloproteinases in osteosarcoma cells transfected with an anti-S100A4 ribozyme. Cancer Res 1999, 59:4702-4708 [PubMed] [Google Scholar]
- 12.Gibbs FEM, Wilkinson MC, Rudland PS, Barraclough R: Interactions in vitro of p9ka, the rat S100-related, metastasis-inducing, calcium-binding protein. J Biol Chem 1994, 269:18992-18999 [PubMed] [Google Scholar]
- 13.Kriajevska M, Cardenas MN, Grigorian MS, Ambartsumain NS, Georgiev GP, Lukanidin EM: Non-muscle myosin heavy chain as a possible target for protein encoded by metastasis-related mts-1 gene. J Biol Chem 1994, 269:19679-19682 [PubMed] [Google Scholar]
- 14.Wang G, Rudland PS, White MHC, Barraclough R: Interaction in vivo and in vitro of the metastasis-inducing S100 protein S100A4 (p9Ka) with S100A1. J Biol Chem 2000, 275:11141-11146 [DOI] [PubMed] [Google Scholar]
- 15.Kriajevska M, Tarabykina S, Bronstein I, Maitland N, Lomonosov M, Hansen K, Georgiev G, Lukanidin E: Metastsis-associated mts-1 (S100A4) protein modulates protein kinase C phosphorylation of the heavy chain of non-muscle myosin. J Biol Chem 1998, 273:9852-9856 [DOI] [PubMed] [Google Scholar]
- 16.Ford HL, Salim MM, Chakravarty R, Aluiddin V, Zain SB: Expression of mts-1, a metastasis-associated gene, increases motility but not invasion of a nonmetastatic mouse mammary adenocarcinoma cell line. Oncogene 1995, 11:2067-2075 [PubMed] [Google Scholar]
- 17.Keirsebilck A, Bonne S, Bruyneel E, Vermassen P, Lukanidin E, Mareel M, van Roy F: E cadherin and metastasin (mts-1/S100A4) expression levels are inversely regulated in two tumour cell families. Cancer Res 1998, 58:4587-4591 [PubMed] [Google Scholar]
- 18.Bringuier PP, Umbas R, Schaafsma HE, Karthaus HFM, Debruyne FMJ, Schalken JA: Decreased E cadherin immunoreactivity correlates with poor survival in patients with bladder tumours. Cancer Res 1993, 53:3241-3245 [PubMed] [Google Scholar]
- 19.Syrigos KN, Krausz T, Waxman J, Pandha H, Rowlinson-Busza G, Verne J, Epenetos AA, Pignatelli M: E cadherin expression in bladder cancer using formalin-fixed, paraffin-embedded tissues: correlation with histopathological grade, tumour stage and survival. Int J Cancer 1995, 64:367-370 [DOI] [PubMed] [Google Scholar]
- 20.Tulchinsky E, Kramerov H, Ford HL, Reshetnyak E, Lukanidin E, Zain S: Characterisation of a positive regulatory element in the mts-1 gene. Oncogene 1993, 8:79-86 [PubMed] [Google Scholar]
- 21.Kawamata H, Kameyama S, Nan L, Kawai K, Oyasu R: Effect of epidermal growth factor and transforming growth factor β1 on growth and invasive potentials of newly established rat bladder cancer cell lines. Int J Cancer 1993, 55:968-973 [DOI] [PubMed] [Google Scholar]
- 22.Kameyama S, Kawamata H, Kawai K, Oyasu R: A new in vivo model for studying invasion and metastasis of rat and human bladder carcinomas. Carcinogenesis 1993, 14:1531-1535 [DOI] [PubMed] [Google Scholar]
- 23.Southern PJ, Berg P: Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoters. J Mol Appl Genet 1982, 1:327-341 [PubMed] [Google Scholar]
- 24.Dinney CPN, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ: Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-α administration. Cancer Res 1998, 58:808-814 [PubMed] [Google Scholar]
- 25.Verelle P, Lescaut V, Poupon MF, Hillova J: DAN transfection affects the metastatic capacity of tumour cells. Anticancer Res 1987, 7:181-186 [PubMed] [Google Scholar]
- 26.Kerbel RS, Waghorne C, Man HS, Elliot B, Breitman HL: Alterations of the tumorigenic and metastatic properties of neoplastic cells is associated with the process of calcium phosphate-mediated DNA transfection. Proc Natl Acad Sci USA 1987, 84:1263-1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koop S, MacDonald IC, Luzzi K, Schmidt EE, Morris VL, Grattan M, Khokha R, Chambers AF, Groom AC: Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res 1995, 55:2520-2523 [PubMed] [Google Scholar]
- 28.Chambers AF, Naumov GN, Vantyghem SA, Tuck AB: Molecular biology of breast cancer metastasis: clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Treat 2000, 2:400-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark EA, Golub TR, Lander ES, Hynes RO: Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000, 406:532-535 [DOI] [PubMed] [Google Scholar]