Abstract
Phospholipase A2 (PLA2) enzymes release arachidonic acid from cellular phospholipids in a variety of mammalian tissues, including prostate. Group IIa secretory PLA2 (sPLA2) can generate arachidonate from cellular phospholipids. We examined the group IIa sPLA2 expression in benign prostatic tissues, prostatic intraepithelial neoplasia (PIN), and adenocarcinoma to determine whether sPLA2 expression is altered in the carcinogenesis of human prostatic cancer. Thirty-three of 74 total cases (45%) of benign prostatic tissue showed positive immunohistochemical staining for group IIA sPLA2, whereas 63 of 69 total cases (91%) of high-grade PINs and 70 of 78 total cases (90%) of adenocarcinomas gave positive results. Four of 10 cases of low-grade PIN showed positive immunoreactivity for sPLA2. The number of cells staining for sPLA2 was significantly less in benign epithelium (4%) and low-grade PIN (4%) compared to high-grade PIN (40%) or adenocarcinoma (38%) (P < 0.001). There was no significant difference between high-grade PIN and adenocarcinoma in the number of cells staining positively for sPLA2. The intensity of sPLA2 immunoreactivity was also different among benign prostatic tissue, low-grade PIN, high-grade PIN, and prostatic adenocarcinoma specimens. The malignant cells demonstrated more intense immunohistochemical staining (moderate to strong staining in 81% and 69% cases for high-grade PIN and adenocarcinoma, respectively) than benign glands (moderate staining in 11% of cases). No strong staining was observed in benign glands or low-grade PIN. Our data are consistent with the contention that group IIA sPLA2 expression is elevated in neoplastic prostatic tissue and support the hypothesis that dysregulation of sPLA2 may play a role in prostatic carcinogenesis.
In mammalian cells, phospholipases A2 (PLA2s) are enzymes that release free fatty acids through catalysis of membrane phospholipids at the Sn-2 position. The resulting product, arachidonic acid, is metabolized to produce prostaglandins and leukotrienes that mediate a diverse array of biological activities including inflammation, mitogenesis, and tumor cell invasion. Several reports have implicated arachidonic acid and its metabolites as factors regulating cellular proliferation and apoptosis. 1-3 Arachidonic acid has also been implicated in the pathway of tumor necrosis factor- and Fas-induced apoptosis in various cell lines. 4-6
Several types of PLA2s have been identified. 7,8 Seven low molecular weight (14 kd) sPLA2s (including group IB, IIA, IID, IIE, III, V, and X) are known to be present in both the intracellular compartment and extracellular milieu. The 40-kd form is a calcium-independent enzyme, which is referred to as iPLA2. Cytosolic PLA2 (cPLA2) is a high molecular weight (85 kd) form found predominantly within the cytosol of cells. cPLA2 activity is regulated by intracellular Ca2+ concentrations 8,9 and shows characteristic preference for hydrolyzing arachidonic acid at the sn-2 position. In contrast, sPLA2s have a broad substrate preference. 8
Reports have been published linking arachidonic acid and its metabolites with prostatic malignancy. 10-15 Because phospholipase activity is required for phospholipid metabolism and subsequent generation of arachidonic acid, aberrant expression and function of sPLA2 may play a role in prostatic carcinogenesis. However, little is known about sPLA2 expression in human prostatic tissues. In this study, we determined the level of group IIA sPLA2 expression in specimens of human prostatic adenocarcinoma, its precursor lesion [(high-grade prostatic intraepithelial neoplasia PIN)], low-grade PIN, and benign prostatic tissue.
Materials and Methods
Tissue Samples
Seventy-eight cases of radical retropubic prostatectomy and bilateral lymphadenectomy between 1990 and 1994 were obtained from the surgical pathology files of Indiana University Medical Center. Patients ranged in age from 51 to 78 years (mean, 63 years). Grading of the primary tumor from radical prostatectomy specimens was performed according to the Gleason system. 16 The Gleason grade ranged from 4 to 10. Pathological stage was performed according to the 1997 TNM (tumor, lymph nodes, and metastasis) system. Pathological stages were T2a (n = 11 patients), T2b (n = 35), T3a (n = 26), and T3b (n = 6). Six (8%) patients had lymph node metastasis at the time of surgery.
Generation of Rabbit Polyclonal Antibody to sPLA-IIA
Rabbit polyclonal antibody specific to sPLA2-IIA was generated by immunizing rabbits with purified, recombinant human sPLA2-IIA protein. The antisera were affinity-purified. The specificity of the purified IgG antibody was confirmed by staining Chinese hamster ovary (CHO) cells that stably expressed sPLA2-IIA. CHO cells expressing sPLA2-X or sPLA-V did not stain with this antibody (Eli Lilly and Company, Indianapolis, IN).
Immunohistochemical Studies
Serial 5-μm-thick sections of formalin-fixed slices of radical prostatectomy specimens were used for the studies. Tissue blocks that contained the maximum amount of tumor and highest Gleason grade were selected. One representative slide from each case was analyzed and we recognized the limitation of sample variation. Slides were deparaffinized in xylene twice for 5 minutes and rehydrated through graded ethanols to distilled water. Endogenous peroxidase activity was inactivated by incubation in 3% H2O2 for 15 minutes. The nonspecific binding sites were blocked by incubating with 10% normal horse serum in phosphate-buffered saline (PBS) (0.01 mol/L phosphate, ph7.4, 0.137 mol/L NaCl). Tissue sections were then incubated with the polyclonal rabbit antibody against human sPLA2 (1:76,000 dilution) for 60 minutes at room temperature. After washing with PBS, biotinylated goat anti-rabbit IgG was applied for 30 minutes. Additional washing was followed by incubation with peroxidase-labeled streptavidin for 30 minutes. Immunoreactivity was visualized by incubation of sections with diaminobenzidine in the presence of hydrogen peroxide. Sections were counterstained with light hematoxylin and mounted with a coverslip. All of the procedures were performed at room temperature. No enzymatic pretreatment was required for antigen retrieval. Positive and negative controls were run in parallel with each series and appropriate results were obtained.
The extent and intensity of staining were evaluated in benign epithelium, low-grade PIN, high-grade PIN, and adenocarcinoma from the same slide for each case. Microscopic fields with the highest degree of immunoreactivity were chosen for analysis. At least 1000 cells were analyzed in each case. The percentage of cells exhibiting staining in each case was evaluated semiquantitatively on a 5% incremental scale ranging from 0 to 95%. A numeric intensity score between 0 and 3 was assigned to each case on a scale from 0 to 3 (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining).
Statistical Analysis
The mean percentage of immunoreactive cells in benign epithelium, low-grade PIN, high-grade PIN, and adenocarcinoma were compared using one-way analysis of variance with a random subject effect to correlate the within-subject measurements. The intensity of staining in benign epithelium, low-grade PIN, high-grade PIN, and adenocarcinoma were compared using Cochran-Mantel-Haenszel tests for correlated ordered categorical outcomes. Pairwise comparisons between the tissue types were made if the analysis of variance revealed significant treatment effects. A P value <0.05 was considered significant, and all P values were two-sided.
Results
Immunoreactive group IIA sPLA2 was evident with an exclusive cytoplasmic staining pattern in cells (Figure 1) ▶ . No immunoreactivity was seen in the stromal cells. sPLA2 immunoreactivity was found in 70 cases (90%) of cancer, 63 cases (91%) of high-grade PIN, 4 cases (40%) of low-grade PIN, and 33 cases (45%) of benign glands (Table 1) ▶ . The number of cells staining in benign epithelium (mean, 3.8%) and low-grade PIN (mean, 3.5%) was much lower than in high-grade PIN (mean, 39.4%; P < 0.0001) or adenocarcinoma (mean, 37.5%; P < 0.0001) (Table 1) ▶ . There was no significant difference in the percentage of cells staining positive for sPLA2 between high-grade PIN and adenocarcinoma or between benign epithelium and low-grade PIN. The percentage of malignant cell in adenocarcinoma staining for sPLA-IIA was divided into two categories according to the mean percentage of cell staining (<37% and >37% of cancer cells staining), and analyzed against the degree of tumor differentiation. The adenocarcinomas were grouped into low grade (Gleason grade 4 to 6), intermediate grade (Gleason grade 7), and high grade (Gleason grade 8 to 10). No statistically significant difference was identified between percentage of sPLA-IIA-positive cancer cells and Gleason grade (P = 0.59).
Figure 1.
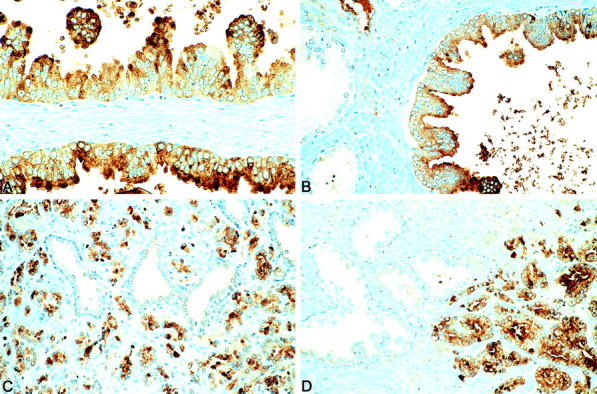
sPLA2 immunohistochemical staining of high-grade PIN (A and B) and prostatic adenocarcinoma (C and D). Immunoreactive sPLA2 was evident in malignant epithelial cells with a cytoplasmic staining pattern. In contrast, no or minimal immunoreactivity was seen in adjacent benign glands. For details, see the Materials and Methods section.
Table 1.
sPLA2 Immunoreactivity of Benign and Neoplastic Prostatic Tissues in Radical Prostatectomy Specimens
| No. cases | % of Cases staining | Mean % of cells staining ±SE | Range, % | |
|---|---|---|---|---|
| Benign epithelium | 74 | 45.2 | 3.8 ± 0.6 | 0 to 30 |
| Low-grade PIN | 10 | 40.0 | 3.5 ± 1.5 | 0 to 20 |
| High-grade PIN | 69 | 91.3 | 39.6 ± 2.9* | 0 to 95 |
| Adenocarcinoma | 78 | 89.7 | 37.5 ± 3.8* | 0 to 95 |
*, Indicates percentage of staining statistically higher compared to that of the benign epithelium and low-grade PIN with a P value < 0.0001 using analysis of variance. There was no difference in percentage of cell staining between high-grade PIN and adenocarcinoma or between benign epithelium and low-grade PIN.
The intensity of staining also differed among benign epithelium, low-grade PIN, high-grade PIN, and adenocarcinoma (Table 2) ▶ . The majority of benign glands (55%) and low-grade PIN (60%) did not show any reactivity. Moderate staining (intensity grade 2) was evident in 11% of the benign epithelium cases and no low-grade PIN cases. No benign epithelium or low-grade PIN showed strong reactivity (intensity grade 3). In contrast, strong staining was evident in 29% (20 cases) of high-grade PIN and 33% (26 cases) of adenocarcinoma. Only a minority of the cases was negative (6% for high-grade PIN and 9% for cancer) or demonstrated weak staining (10% for high-grade PIN and 22% for cancer). The percentage of staining was statistically higher compared to that of the benign epithelium and low-grade PIN (P < 0.0001). There was no difference in intensity of cell staining between high-grade PIN and adenocarcinoma or between benign epithelium and low-grade PIN. The staining intensity of adenocarcinomas was also further analyzed according to the Gleason grade. No statistically significant difference was identified between staining intensity and Gleason grade (P = 0.71).
Table 2.
Intensity of sPLA2 Staining of Benign and Neoplastic Prostate in Radical Prostatectomy Specimens
| Staining intensity | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Benign epithelium | 40 (54.8%) | 25 (34.2%) | 8 (11.0%) | 0 (0%) |
| Low-grade PIN | 6 (60.0%) | 4 (40.0%) | 0 (0%) | 0 (0%) |
| High-grade PIN* | 6 (8.7%) | 7 (10.1%) | 36 (52.2%) | 20 (28.9%) |
| Adenocarcinoma* | 7 (9.0%) | 17 (21.8%) | 27 (34.6%) | 27 (34.6%) |
*, Indicates percentage of staining statistically higher compared to that of the benign epithelium and low-grade PIN using Cochran-Mantel-Haenszel tests (P < 0.0001 for benign epithelium and P < 0.02 for low-grade PIN). There was no difference in percentage of cell staining between high-grade PIN and adenocarcinoma or between benign epithelium and low-grade PIN.
There was no significant correlation between the percentage of cells staining and patient age (r = 0), tumor stage (r = 0.21), lymph node metastasis (r = 0.17), Gleason grade (r = 0.05), or extent of tumor involvement in the prostatectomy specimens (r = 0.16).
Discussion
To our knowledge, this is the first report to characterize the immunohistochemical staining of group IIA sPLA2 in the high-grade PIN and prostatic adenocarcinoma. We found more intense cytoplasmic immunoreactivity for sPLA2 in neoplastic prostate tissues (high-grade PIN and adenocarcinoma) compared to benign glands and low-grade PIN. The percentage of positively stained high-grade PIN and adenocarcinoma cells was significantly higher than observed in the benign prostatic glands and low-grade PIN. There were no significant differences in the extent or intensity of sPLA2 immunoreactivity between high-grade PIN and adenocarcinoma or between benign epithelium and low-grade PIN. Neither the extent nor the intensity of sPLA2 immunoreactivity in prostatic adenocarcinomas was related to Gleason grade.
Increased immunoreactivity for group IIA sPLA2 in prostatic neoplasia seen in the present study agrees with previously published observations by Faas and colleagues. 15 Using enzymatic analysis, these investigators demonstrated a two-fold enhancement of sPLA2 activity in human prostatic adenocarcinoma compared to benign prostatic tissue. Evidence indicates that malignant prostatic tissue contains significantly lower levels of arachidonic acid in phospholipids. 17,18 Altered phospholipid metabolism in malignant prostatic tissues may result from increased utilization of arachidonic acid for the formation of prostaglandins and eicosanoids. These arachidonic acid metabolites may be crucial for the growth and progression of malignant lesions. Chaudry and colleagues 19 demonstrated a 10-fold increase in prostaglandin E2 synthesis from labeled arachidonic acid in malignant human prostatic tissues. Shaw and colleagues 20 also showed increased prostaglandin E2 levels in the effusions of a fast-growing, metastasizing subline of the Dunning R-3327 rat prostatic adenocarcinoma when compared to a slow-growing derivative. Taken together, these findings are consistent with our observation of enhanced expression of sPLA2 immunoreactivity in high-grade PIN and prostatic adenocarcinoma.
sPLA2 has previously been localized using immunohistochemistry using human Paneth cells, chondrocytes, amniotic epithelial cells, and lacrimal gland cells. 21-23 Expression of different sPLA2 mRNA isoforms have been evaluated in various tissues. 24 Comparative expression of sPLA2 protein expression in other organs and their malignant counterparts has been published. 25-29 The distinctive staining pattern of group IIA sPLA2 between the benign and malignant prostatic glands suggests a utility of this antigen as a potentially useful diagnostic maker. Given the significant differences evident in the staining patterns, sPLA2 immunostaining might have an advantage over prostate-specific antigen and prostate-specific membrane antigen to differentiate between benign epithelium, high-grade PIN, and malignant prostatic cells. If present at all, benign epithelia show mainly focal sPLA2 staining. Even when positive, the staining intensity in benign epithelium is usually much weaker (one or two grades) than in adjacent malignant tissues. Because a minority (8.7% for high-grade PIN and 9.0% for adenocarcinoma) of the neoplastic cases were not immunoreactive, sPLA2 staining is not able to detect all of the prostatic cancers or high-grade PIN lesions. Further, a number of cases showed weak or moderate sPLA2 immunoreactivity in benign epithelium. Therefore, the utility of sPLA2 as a diagnostic marker to distinguish benign from neoplastic glands is still limited.
In summary, group IIA sPLA2 expression is elevated in the neoplastic human prostatic tissue raising the possibility that dysregulation of this enzyme may play a role in prostatic carcinogenesis. Our findings may have implications for target validation and development of therapeutic strategies modulating phospholipid metabolic pathways in prostatic neoplasia.
Footnotes
Address reprint requests to Liang Cheng, M.D., Department of Pathology, University Hospital 3465, Indiana University School of Medicine, 550 North University Blvd., Indianapolis, IN 46202. E-mail: lcheng@iupui.edu.
Supported in part by Clarion Health Value Fund grant and Indiana Biomedical Research Fund grant (to L.C.).
References
- 1.Anderson KM, Roshak A, Winkler JD, McCord M, Marshall LA: Cytosolic 85-kDa phospholipase A2-mediated release of arachidonic acid is critical for proliferation of vascular smooth muscle cells. J Biol Chem 1997, 272:30504-30511 [DOI] [PubMed] [Google Scholar]
- 2.Dethlefsen SM, Shepro D, Amore PA: Arachidonic acid metabolites in BFGF-, PDGF-, and serum-stimulated vascular cell growth. Exp Cell Res 1994, 212:262-273 [DOI] [PubMed] [Google Scholar]
- 3.Fafeur V, Jiang ZP, Bohlen P: Signal transduction by bFGF, but not TGF beta 1, involves arachidonic acid metabolism in endothelial cells. J Cell Physiol 1991, 149:277-283 [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Jiang XR, Newland AC, Kelsey SM: Failure to activate cytosolic phospholipase A2 causes TNF resistance in human leukemic cells. J Immunol 1998, 160:5929-5935 [PubMed] [Google Scholar]
- 5.De Valck D, Vercammen D, Fiers W, Beyaert R: Differential activation of phospholipases during necrosis or apoptosis: a comparative study using tumor necrosis factor and anti-Fas antibodies. J Cell Biochem 1998, 71:392-399 [PubMed] [Google Scholar]
- 6.Enari M, Hug H, Hayakawa M, Ito F, Nishimura Y, Nagata S: Different apoptotic pathways mediated by Fas and the tumor-necrosis-factor receptor. Cytosolic phospholipase A2 is not involved in Fas-mediated apoptosis. Eur J Biochem 1996, 236:533-538 [DOI] [PubMed] [Google Scholar]
- 7.Mayer RJ, Marshal LA: New insights on mammalian phospholipase A2(s): comparison of arachidonyl-selective and-nonselective enzymes. FASEB J 1993, 7:339-348 [DOI] [PubMed] [Google Scholar]
- 8.Kramer RM: Structure, function and regulation of mammalian phospholipases A2. Adv Sec Messenger Phosphoprotein Res 1993, 28:81-89 [PubMed] [Google Scholar]
- 9.Clark JD, Schievella AR, Nalefski EA, Lin LL: Cytosolic phospholipase A2. J Lipid Mediators Cell Signal 1995, 12:83-117 [DOI] [PubMed] [Google Scholar]
- 10.Anderson KM, Seed TM, Wilson DE, Harris JE: 5,8,11,14-eicosatetraenoic acid-induced destruction of mitochondria destruction of mitochondria in human prostate cells (PC-3). In Vitro Cell Dev Biol 1992, 28A:410-414 [DOI] [PubMed] [Google Scholar]
- 11.Rose DP, Connolly JM: Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate 1991, 18:243-254 [DOI] [PubMed] [Google Scholar]
- 12.Rose DP, Cohen LA: Effects of dietary menhaden oil and retinyl acetate on the growth of DU 145 human prostatic adenocarcinoma cells transplanted into athymic nude mice. Carcinogenesis 1988, 9:603-605 [DOI] [PubMed] [Google Scholar]
- 13.Schirner MP, Schneider MR: The prostacyclin analogue cicaprost inhibits metastasis of tumours of R 3327 MAT Lu prostate carcinoma and SMT 2A mammary carcinoma. J Cancer Res Clin Oncol 1992, 118:497-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann NJ, Warrick GE, O’Dea K, Knapp HR, Sinclair AJ: The effect of linoleic, arachidonic and eicosapentaenoic acid supplementation on prostacyclin production in rats. Lipids 1994, 29:157-162 [DOI] [PubMed] [Google Scholar]
- 15.Faas FH, Dang AQ, Polland M, Hong XM, Fan K, Luckert PH, Schutz M: Increased phospholipid fatty acid remodeling in human and rat prostatic adenocarcinoma tissues. J Urol 1996, 156:243-248 [PubMed] [Google Scholar]
- 16.Bostwick DG: Neoplasms of the prostate. ed 1 Bostwick DG Eble JN eds. Urologic Surgical Pathology, 1997, :pp 343-422 Mosby, St. Louis [Google Scholar]
- 17.Narayan P, Dahiya R: Alterations in sphingomyelin and fatty acids in human benign prostatic hyperplasia and prostatic cancer. Biophys Biochim Acta 1991, 50:1099-1108 [PubMed] [Google Scholar]
- 18.Chaudry A, McClinton S, Moffat LE, Wahle KW: Essential fatty acid distribution in the plasma and tissue phospholipids of patients with benign and malignant prostatic disease. Br J Cancer 1991, 64:1157-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudry AA, Wahle KW, McClinton S, Moffat LE: Arachidonic acid metabolism in benign and malignant tissue in vitro: effects of fatty acids and cyclooxygenase inhibitors. Int J Cancer 1994, 57:176-180 [DOI] [PubMed] [Google Scholar]
- 20.Shaw MW, Ablin RJ, Ray P, Rubenstein M, Guinan PD, Mckiel CF: Immunobiology of the Dunning R-3327 rat prostate adenocarcinoma sublines: plasma and tumor effusion prostaglandins. Am J Reprod Microbiol 1985, 8:77-79 [DOI] [PubMed] [Google Scholar]
- 21.Nevalainen TJ, Aho HJ, Peuravuori H: Secretion of group 2 phospholipase A2 by lacrimal glands. Invest Opthalmol Vis Sci 1994, 35:417-421 [PubMed] [Google Scholar]
- 22.Nevalainen TJ, Meri KM, Niemi M: Synovial-type (group II) phospholipase A2 human seminal plasma. Andrologia 1993, 25:355-358 [DOI] [PubMed] [Google Scholar]
- 23.Nevalainen TJ, Marki F, Kortesuo PT, Grutter MG, Di Marco S, Schmitz A: Synovial type (group II) phospholipase A2 in cartilage. Rheumatology 1993, 20:325-330 [PubMed] [Google Scholar]
- 24.Cupillard L, Koumanove K, Mattei MG, Lazdunski M, Lambeau G: Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J Biol Chem 1997, 272:15745-15752 [DOI] [PubMed] [Google Scholar]
- 25.Ogawa M, Yamashita S, Sakamoto K, Ikei S: Elevation of serum group IIa phospholipase A2 in patients with cancers of digestive organs. Res Commun Chem Pathol Pharmacol 1991, 74:241-244 [PubMed] [Google Scholar]
- 26.Murata K, Egami H, Kiyohara H, Oshima S, Kurizaki T, Ogawa M: Expression of group-II phospholipase A2 in malignant and non-malignant human gastric mucosa. Br J Cancer 1993, 68:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita S, Yamashita J, Sakamoto K, Inada K, Nakashima Y, Murata K, Saishoji T, Normura K, Ogawa M: Increased expression of membrane-associated phospholipase A2 shows malignant potential of human breast cancer cells. Cancer 1993, 71:3058-3064 [DOI] [PubMed] [Google Scholar]
- 28.Kiyohara H, Egami H, Kako H, Shibata Y, Murata K, Ohshima S, Sei K, Suko S, Kurano R, Ogawa M: Immunohistochemical localization of group II phospholipase A2 in human pancreatic carcinomas. Int J Pancreatol 1993, 13:49-57 [DOI] [PubMed] [Google Scholar]
- 29.Abe T, Sakamoto K, Kamohara H, Hirano Y, Kuwahara N, Ogawa M: Group II phospholipase A2 is increased in peritoneal and pleural effusions in patients with various types of cancer. Int J Cancer 1997, 74:245-250 [DOI] [PubMed] [Google Scholar]


