Abstract
An in vitro system of interleukin (IL)-4-induced human monocyte-derived macrophage fusion was used to investigate the cell/substrate adhesive mechanisms that support multinucleated foreign body giant cell (FBGC) formation. Monocytes were cultured for 3 days and IL-4 was added to induce macrophage fusion and FBGC formation by day 7. Functionally defined anti-integrin antibodies demonstrated that initial monocyte adhesion is mediated by β2 integrins, whereas during the induction of macrophage fusion by IL-4, an additional dependence on β1 integrins is acquired. The combination of anti-β1 plus anti-β2 was most effective, reducing macrophage/FBGC adhesion to 10% of controls. Consistent with integrin-mediated signaling, the tyrosine kinase inhibitor genistein and the phosphatidylinositol-3-kinase inhibitors wortmannin and LY294002 also attenuated macrophage/FBGC adhesion. Confocal microscopic analysis revealed that β2 integrins are present on monocytes after initial adhesion and are strongly expressed on fusing macrophages, particularly in peripheral cell areas, and on FBGCs. In contrast, β1 integrins are not detected on monocytes but begin to appear during macrophage development and are strongly expressed on fusing macrophages and FBGCs. For the first time, these results demonstrate the IL-4-induced acquisition of cooperation between β1 and β2 integrins in the cell/substrate adhesive interactions that are required for multinucleated FBGC formation.
Multinucleated giant cells are formed from macrophage fusion 1 and are recognized as hallmark histiological features of chronic inflammatory responses to persistent microbial infections or nonphagocytosable foreign objects. 2 As such, the foreign body giant cell (FBGC) is a prominent cell type on retrieved biomedical materials and has been observed on a variety of prosthetic materials after extended periods of implantation, eg, 15 years. 3 A role for these giant cells in biomaterial degradation involving oxidative mechanisms has been indicated by the discovery of material surface cracking on retrieved polyurethane directly, and only underneath adherent FBGCs 4 and on regions of failed pacemaker leads with high concentrations of inflammatory cells. 5
Biomaterial-associated FBGC formation requires the extravasation of blood monocytes and their subsequent adhesion to and macrophage development and fusion on implanted material surfaces. 6 Our previous research implicated β2 integrins (also known as the CD18 family of leukocyte-specific integrins) in initial monocyte adhesion to four different chemically modified polystyrene culture surfaces. 7 However, the cell/substrate adhesive interactions that promote macrophage development and support optimal FBGC formation have not been elucidated. Toward this end, and ultimately toward a fundamental mechanistic understanding of the macrophage fusion process, we developed an in vitro system of human monocyte-derived macrophage fusion and FBGC formation using interleukin (IL)-4 or IL-13 as the fusion-inducing cytokine. 8,9 This culture system requires the presence of autologous serum and a sufficient density of adherent macrophages; it does not generate significant macrophage fusion or FBGC formation in the absence of IL-4 or IL-13. The importance of IL-4 in FBGC formation on biomaterials in vivo was confirmed by antibody inhibition, 10 and a role for IL-4-induced mannose receptor activity 11 in the molecular mechanism of macrophage fusion was proposed. 12 Subsequently, we discovered that IL-4-induced macrophage fusion occurs considerably more readily and efficiently on culture surfaces to which the arginine-glycine-aspartate (RGD) integrin recognition sequence has been covalently attached than it does on standard cell culture polystyrene. 13 Immobilized RGD does not increase degrees of initial monocyte adhesion, and this more optimal macrophage fusion also requires the presence of as yet unidentified adsorbed serum components. Therefore, the immobilized RGD peptide is not, by itself, a sufficient substrate for FBGC formation. Nevertheless, this finding indicates an important role for integrin-mediated adhesion in IL-4-induced macrophage development and/or fusion.
Integrins comprise a large group of heterodimeric transmembrane molecules that mediate both cell-extracellular matrix and cell-cell interactions. 14 These adhesion molecules are now well known as important mediators of signal transduction between the extracellular and intracellular environments. 15,16 Several of the known integrins recognize the RGD sequence in their ligands, 17 with an interaction between fibronectin and α5/β1 as a classic example. 18 Monocytes/macrophages are believed to express three integrins in the β1 family, namely, the fibronectin receptors α4/β1 and α5/β1 and the laminin receptor α6/β1. In addition, there are four members of the leukocyte-specific β2 integrin family with αL, αM, αX, or αD in association with β2. αL/β2 and αD/β2 primarily appear to mediate intercellular adhesion. αX/β2 and, particularly, αM/β2 are capable of interactions with a diversity of ligands and mediate cell-particle or cell-substrate interactions. 19 These ligands include fragments of complement C3, fibrinogen, Factor X, and high-molecular weight kininogen, which might be predicted to adsorb from blood onto material surfaces during surgical implantation of a biomedical device. 20 αV/β3, which is known primarily as a vitronectin receptor, can also interact with certain other RGD-containing extracellular matrix proteins. 19
Both the β1 and β2 integrin families have been implicated in monocyte/macrophage adhesion to endothelium and extravasation to sites of inflammation. 21 αM/β2 is an important phagocytic receptor that may also mediate frustrated phagocytosis of a nonphagocytosable substance such as a biomaterial. 22 In early work, IL-4 was shown to increase expression of β2 integrins on monocytes 23 and, more recently, to increase β1 expression on and tyrosine phosphorylation in fibroblasts. 24 In neutrophils, the crosslinking of β2 integrins results in tyrosine kinase-dependent increases in β1 integrin expression. 25 Likewise, it has been proposed that integrin ligation and clustering in phagocytic cells activates cytoplasmic tyrosine kinases, that in turn leads to the activation of downstream signaling pathways involving phosphatidylinositol-3-kinase, phospholipases C and D, PKC, and others. These collective and highly regulated events control cytoskeletal rearrangements, focal contact formation, cell mobility, cell survival, and the synthesis of inflammatory mediators by phagocytic cells. 19
The present study was designed to investigate the nature of cell/substrate interactions that support optimal IL-4-induced macrophage fusion and FBGC formation. Specifically, we questioned integrin involvement in these adhesive interactions with an experimental approach that included anti-integrin antibody and pharmacological inhibition of adhesion, comparison of initial monocyte adhesion to macrophage/FBGC adhesion, and evaluation of integrin expression in monocytes, fusing macrophages, and FBGCs by fluorescence confocal scanning laser microscopy.
Materials and Methods
Materials
RGD-modified (Pronectin F-coated; Smartplastic) 96-well culture plates were from ICN, Irvine, CA. Lab Tek II 8-well chamber slides for confocal microscopy were from Nalge Nunc. Macrophage-serum-free medium (SFM) was from Life Technologies, Inc., Grand Island, NY. Human recombinant IL-4 (R & D Systems, Minneapolis, MN) was reconstituted in 0.5% bovine serum albumin (low endotoxin; Sigma Chemical Co., St. Louis, MO) according to the manufacturer’s instructions and stored in aliquots at −80°C. Anti-integrin antibodies (Table 1) ▶ were acquired as preservative-free or were dialyzed against phosphate-buffered saline (PBS) to remove preservative before functional studies. Additional reagents were obtained as follows: purified plasma fibronectin and its proteolytic fragments, Chemicon, Temecula, CA; nonspecific purified control IgGs, Chemicon; RNase A, Life Technologies, Inc.; Cy-5-conjugated goat anti-mouse IgG, Jackson ImmunoResearch, West Grove, PA; rhodamine-phalloidin and YO-YO-1, Molecular Probes, Eugene, OR; cell signaling reagents, Biomol (Plymouth Meeting, PA); Gel/Mount, Biomeda (Foster City, CA); all other reagents, Sigma.
Table 1.
Anti-Integrin Antibodies
| Clone | Species | Isotype | Source | |
|---|---|---|---|---|
| Anti-function-blocking | ||||
| β1 | JB1A | Mouse | IgG1 | Chemicon |
| 6S6 | Mouse | Unidentified | Chemicon | |
| β2 | YFC118.3 | Rat | IgG2b | Chemicon |
| MHM23 | Mouse | IgG1 | DAKO | |
| β3 | B3A | Mouse | IgG1 | Chemicon |
| 25E11 | Mouse | IgG2a | Chemicon | |
| αM | 60.1 | Mouse | IgG1 | P. Beatty, University of Utah |
| Anti-detecting | ||||
| β1 | 4B7R | Mouse | IgG1 | Santa Cruz Biotechnology |
| β2 | P4H9-A11 | Mouse | IgG3 | Chemicon |
| β3 | BB10 | Mouse | IgG1 | Chemicon |
Initial Monocyte Adhesion
Human monocytes were isolated by a nonadherent method as previously described 8 and added to polypropylene culture tubes in an ice bath at a concentration of 2 × 10 5 cells per tube in 0.1 ml of macrophage-SFM supplemented with 20% autologous serum. Various anti-integrin antibodies or nonspecific isotype-matched control IgGs, each at a concentration of 50 μg/ml, were added and the incubation was continued on ice with gentle shaking for 1 hour to allow antibodies to interact with monocytes. The cells were then transferred to 96-well culture plates and incubated at 37°C for 1 hour. Nonadherent cells were removed with two washes of 0.2 ml each of prewarmed (37°C) PBS containing calcium and magnesium ions (PBS++). Adherent cells were fixed in methanol for 1 minute, air-dried, and stained with May-Grunwald/Giemsa. For each sample, adhesion was evaluated by averaging the number of nuclei in two low-power fields (∼200 cells) and expressing the result as a percentage of untreated control monocytes. Results represent the mean ± SEM of data from three different monocyte donors.
Adhesion during the Induction of Macrophage Fusion and FBGC Formation
Macrophage fusion was induced with IL-4 using a modification of our previously described culture system 8 as described below. This protocol generates a mixture of fusing macrophages and FBGCs within 7 days and promotes relatively high fusion rates, eg, 67 ± 3% of nuclei within multinucleated cells of >2 nuclei per cell, n = 5 monocyte donors. Monocytes were added to 96-well RGD-modified culture plates at 2 × 10 5 cells per well in 0.1 ml of macrophage-SFM supplemented with 20% autologous serum. After 1.5 hours at 37°C, nonadherent cells and unadsorbed serum proteins were removed by aspirating the medium and washing with 0.2 ml of prewarmed PBS++. The remaining adherent monocytes were recovered with 0.2 ml of prewarmed, unsupplemented macrophage-SFM and incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C. On day 3, the medium was replaced with 0.2 ml of prewarmed macrophage-SFM. At this time, increasing concentrations of inhibitors, 50 μg/ml of functionally defined anti-integrin monoclonal antibodies (mAbs) (Table 1) ▶ , or the same concentrations and combinations of control IgGs as detailed in the figure legends were added. These were immediately followed by the addition of 15 ng/ml of IL-4 to induce macrophage fusion. The cultures were then incubated as above until day 7, when they were washed twice with prewarmed PBS++ to remove nonadherent cells and fixed with methanol. Macrophage/FBGC adhesion was determined as above on May-Grunwald/Giemsa-stained cells.
Integrin Detection by Immunofluorescence
To generate samples for confocal microscopy, monocytes were plated onto eight-well glass chamber slides at 5 × 10 5 cells per well in 0.25 ml of macrophage-SFM supplemented with 20% autologous serum. After 1.5 hours, nonadherent cells were removed by washing as above with 0.5 ml of prewarmed PBS++. Adherent cells were recovered with 0.5 ml per well of macrophage-SFM and incubated as above. On day 3, macrophage fusion was induced with IL-4 as above except that 10% heat-treated (1 hour × 56°C) autologous serum was used to supplement macrophage-SFM. On day 7, the cultures were washed with prewarmed PBS++ and fixed and permeabilized for 2 minutes with acetone at −20°C. Some cultures were also fixed after 1.5 hours (day 0) and on day 3 for comparison with day 7 cultures. Samples were stored at 4°C before immunofluorescent staining for integrins (day 0 and day 3 samples) or integrins, F-actin, and nuclei (day 7 samples) as outlined below.
The diluent and washing buffer for all procedures was PBS++. Day 7 samples were first treated with RNase A (1 μg/ml) for 1.5 hours at 37°C and washed three times for 5 minutes. Nonspecific sites were blocked with 10% goat serum for 1 hour at 37°C. Primary detecting antibodies (Table 1) ▶ or isotype-matched control IgGs were diluted to 20 μg/ml in 5% goat serum and applied for 1 hour at 37°C. After four 5-minute washes at room temperature, Cy-5-conjugated goat anti-mouse IgG diluted 1:100 (7.5 μg/ml) (day 0 and day 3 samples) or a mixture of Cy-5-conjugated goat anti-mouse IgG, rhodamine-phalloidin diluted 1:100 (2 U/ml), and YO-YO-1 diluted 1:10,000 (0.1 μmol/L) was added for 1 hour at room temperature. The samples were then washed three times for 10 minutes each and mounted under glass coverslips using Gel/Mount. Samples were viewed by confocal scanning laser microscopy (MRC-600; Bio-Rad, Richmond, CA) with settings adjusted to blacken any residual background fluorescence from the corresponding nonspecific control antibodies.
Results
Inhibition of Macrophage/FBGC Adhesion by Chelation of Divalent Cations or an RGD-Containing Proteolytic Fragment of Fibronectin
A classic feature of integrin-mediated adhesion is a dependence on divalent cations. 26 Therefore, we first asked whether the divalent cation chelator ethylenediaminetetraacetic acid (EDTA) or the more calcium-specific chelator ethyleneglycoltetraacetic acid (EGTA) would inhibit adhesion in our culture system. As is depicted in Figure 1 ▶ , both reagents completely or nearly completely inhibited both initial monocyte and macrophage/FBGC adhesion. If EDTA or EGTA was removed, adhesion was restored to control levels (not shown).
Figure 1.

The effects of EDTA or EGTA and various inhibitors of nonintegrin receptors on initial monocyte adhesion (A) and macrophage/FBGC adhesion (B). Freshly isolated monocytes or macrophages/FBGCs (day 3 to day 7) were treated with the indicated inhibitors as described in Materials and Methods. Results are expressed as the percent adhesion of untreated control cells, n = 3 monocyte donors. From left to right: EDTA, 10 mmol/L; EGTA, 10 mmol/L; heparin, 2 μmol/L; dextran sulfate, 25 μg/ml; lipopolysaccharide (LPS), 50 μg/ml; chondroitin sulfate A (CS-A), 1 mg/ml; chondroitin sulfate B (CS-B), 1 mg/ml.
Further, to address the participation of selected nonintegrin receptors, we tested reagents that have been demonstrated to inhibit various other types of monocyte/macrophage interactions for their abilities to inhibit initial monocyte adhesion or macrophage/FBGC adhesion (Figure 1) ▶ . At least three different concentrations of each reagent at or higher than their reported effective concentrations were evaluated; data that are shown represent the highest concentration of each reagent tested. Heparin, which interferes with interactions mediated by the low-density lipoprotein receptor-related protein/α2-macroglobulin receptor, 27 did not reduce either initial monocyte or macrophage/FBGC adhesion. Dextran sulfate or bacterial lipopolysaccharide, which are polyanionic anions that bind to macrophage scavenger receptors, 28 also did not decrease adhesion. Chondroitin sulfates are ligands for CD44, an integral membrane protein that mediates cell-cell and cell-extracellular matrix interactions 29 and that also has been reported to participate in macrophage fusion. 30 However, neither chondroitin sulfate A or B reduced monocyte or macrophage/FBGC adhesion relative to control cells.
We then asked whether soluble whole fibronectin, a 120-kd fibronectin fragment containing the RGD integrin recognition sequence, or a 40-kd fibronectin fragment without this sequence would compete with the receptors mediating initial monocyte or macrophage/FBGC adhesion. Results from these experiments are shown in Figure 2 ▶ , in which it can be seen that neither of these proteins or fragments affected initial monocyte adhesion at three different concentrations. However, the 120-kd RGD-containing fibronectin fragment reduced adhesion in cultures of fusing macrophages/FBGCs in a concentration-dependent manner by ∼60% at 0.25 mg/ml. The 40-kd fragment was slightly inhibitory at this concentration, whereas whole plasma fibronectin or albumin again had no apparent effect. These results suggest that initial monocyte adhesion is mediated by interactions other than with substrate-adsorbed fibronectin or the RGD sequence. However, during macrophage development and the IL-4-induced onset of macrophage fusion, it seems that cell/substrate interactions are altered and may include adhesion to relevant regions of adsorbed fibronectin, other RGD-containing proteins, and/or immobilized RGD.
Figure 2.
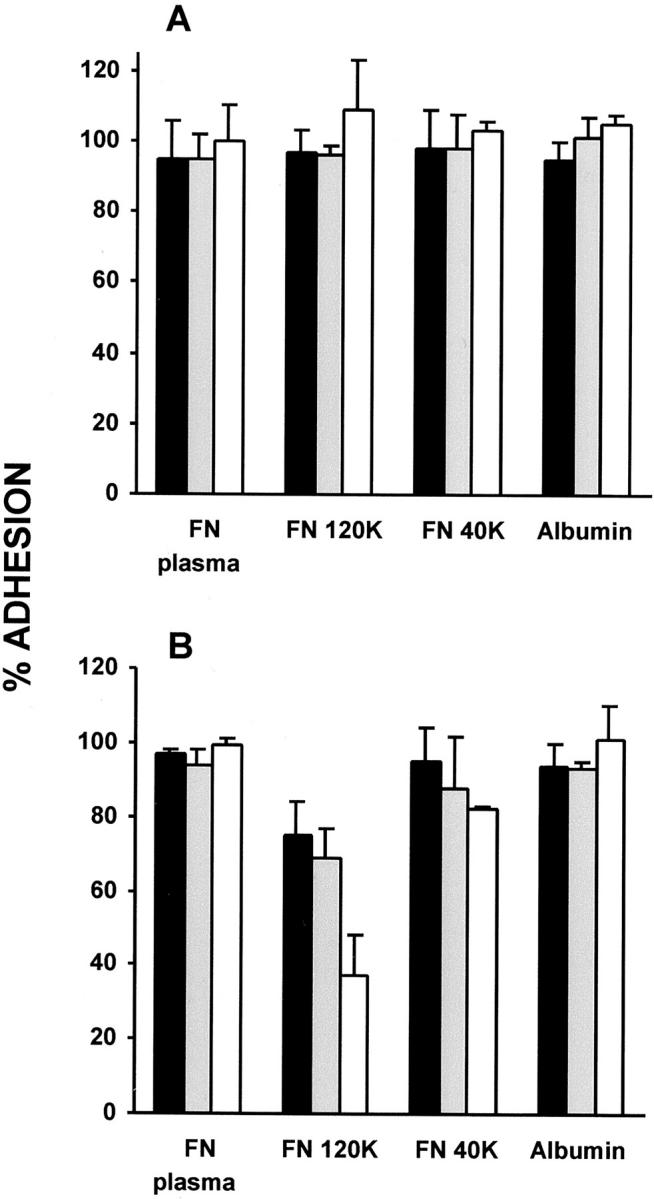
Effects of soluble fibronectin or its proteolytic fragments on initial monocyte adhesion (A) and macrophage/FBGC adhesion (B). Freshly isolated monocytes or macrophages/FBGCs (day 3 to day 7) were treated with 0.05 mg/ml (solid bars), 0.1 mg/ml (shaded bars), or 0.25 mg/ml (open bars) of purified plasma fibronectin (FN), the 120-kd FN fragment, the 40-kd FN fragment, or human serum albumin as described in Materials and Methods. Results are expressed as the percent adhesion of untreated control cells, n = 3 monocyte donors.
Inhibition of Monocyte or Macrophage/FBGC Adhesion by Functionally Defined Antibodies to β1 and β2 Integrins
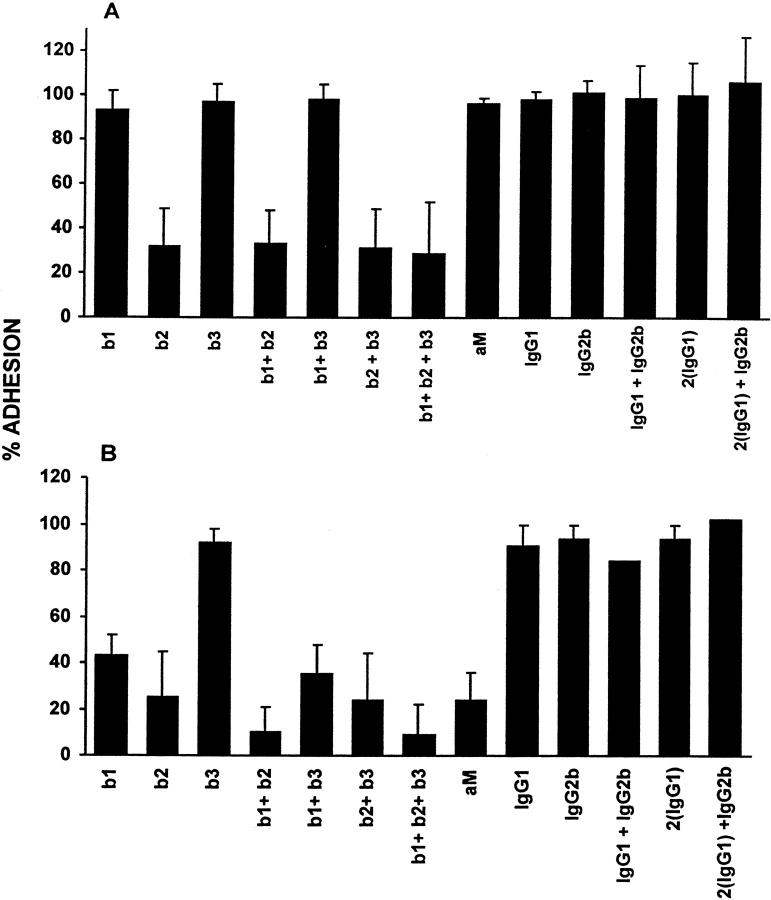
To address the identities of integrins that support monocyte or macrophage/FBGC adhesion, we used functionally defined anti-integrin antibodies (Table 1) ▶ . As is depicted in Figure 3A ▶ , neither anti-β1 (clone JB1a), anti-β3 (clone B3A), or these two antibodies in combination inhibited initial monocyte adhesion to culture surfaces. However, anti-β2 (clone YFC118.3) consistently reduced monocyte adhesion alone or in combination with anti-β1 and/or anti-β3 to ∼30% of control adhesion. In addition, the anti-β2 antibody MHM23 completely inhibited monocyte adhesion, whereas another anti-β1, clone 6S6, had no apparent effect (not shown). Isotype-matched control IgGs were not inhibitory.
Figure 3.
The effects of anti-integrin antibodies on initial monocyte adhesion (A) or macrophage/FBGC adhesion (B). Freshly isolated monocytes or macrophages/FBGCs (day 3 to day 7) were treated with 50 μg/ml each of the indicated anti-integrin antibodies (b1, β1; b2, β2; b3, β3; aM, αM) individually or in various combinations or with nonspecific control IgGs as described in Materials and Methods. Results are expressed as the percent adhesion of untreated control cells, n = 3 monocyte donors.
In Figure 3B ▶ , it can be seen that upon the IL-4 induction of macrophage fusion and FBGC formation, additional adhesive interactions operate to mediate the attachment of these cells to culture surfaces. At the day 7 time point, anti-β1 alone reduced macrophage/FBGC adhesion to 40% of untreated controls. Again, anti-β2 was a strong inhibitor and reduced adhesion to ∼30% of untreated controls. The combination of anti-β1 and anti-β2 was most effective and essentially abrogated adhesion by its reduction to 10% of untreated controls. Interestingly, a mAb to αM, which did not affect the initial adherence of monocytes, also strongly inhibited macrophage/FBGC adhesion. Neither anti-β3, clone B3A, another anti-β3, clone 25E11 (not shown), or isotype-matched control IgGs were inhibitory.
To ask whether anti-β1 or anti-β2 affected macrophage fusion as well as macrophage/FBGC adhesion, we examined the remaining adherent cells after antibody treatments. After inhibition of adhesion by anti-β1, the 40% remaining cells were clearly observed with multiple pseudopodial extensions and to be fusion competent. These cells had undergone degrees of fusion (67 ± 4%, n = 3 experiments) that were comparable to untreated control cultures (67 ± 3%, n = 5 experiments). In the same experiments after anti-β2 treatment, the majority of the 30% remaining adherent cells were mononuclear macrophages with few pseudopods (22 ± 10% fusion, n = 3 experiments).
Integrin-Mediated Signaling during the Induction of Macrophage Fusion/FBGC Formation
Integrin-mediated phagocytic cell signaling pathways have been demonstrated to involve tyrosine and phosphatidylinositol-3-kinases and phosphatidylinositide turnover via PLC activation. 19 Therefore, we asked whether macrophage/FBGC adhesion would be disrupted by the inclusion of various inhibitors of these pathways. Genistein, a widely used inhibitor of tyrosine phosphorylation, reduced adhesion in a concentration-dependent manner (Figure 4A) ▶ . In addition, increasing doses of the phosphatidylinositol-3-kinase inhibitors LY294002 (Figure 4B) ▶ or wortmannin (Figure 4C) ▶ completely abrogated macrophage/FBGC adhesion, whereas the PKG and PKA inhibitor H-8, at concentrations of up to 10 μmol/L, did not (not shown). These collective results extend our antibody inhibition experiments and are consistent with integrin-mediated signaling during the induction of macrophage fusion by IL-4.
Figure 4.
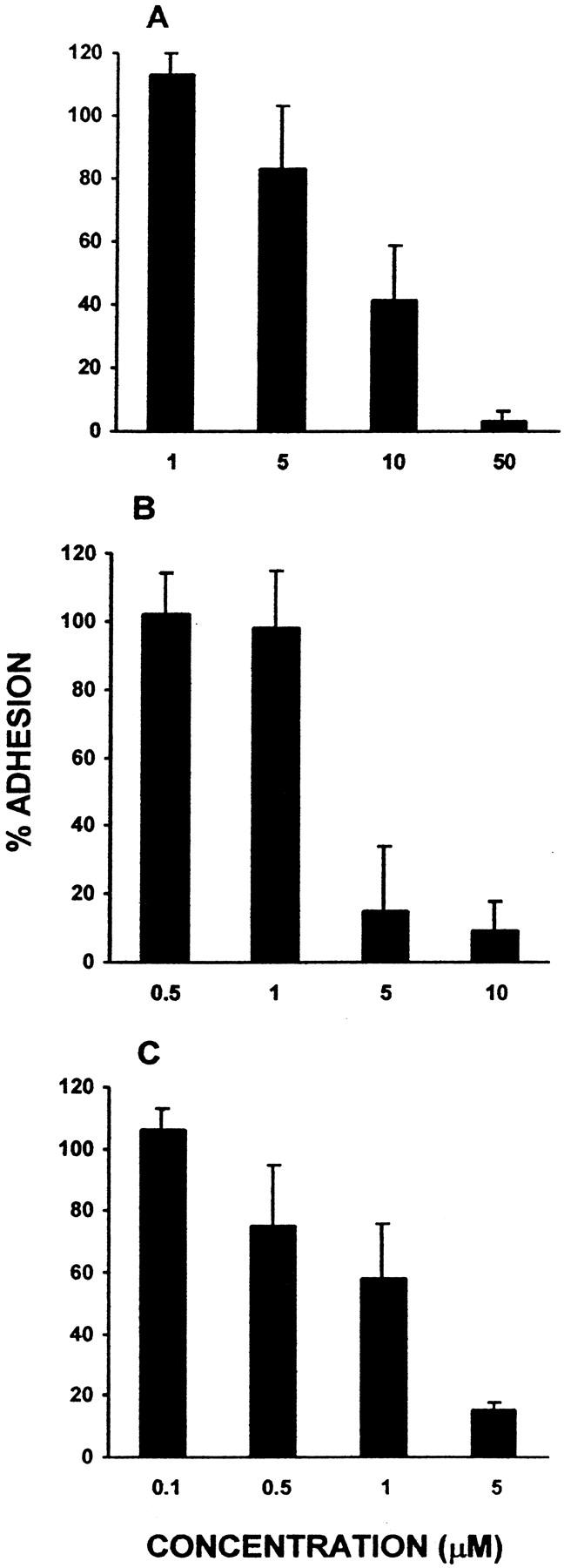
The effects of inhibitors of integrin-mediated cell signaling. Increasing concentrations of the tyrosine kinase inhibitor genistein (A) or the phosphatidylinositol-3-kinase inhibitors LY294002 (B) and wortmannin (C) were added during the induction of macrophage fusion/FBGC formation (day 3 to day 7) as described in Materials and Methods. Results are expressed as the percent adhesion of untreated control cells, n = 3 monocyte donors.
β1 and β2 Integrins Are Expressed on Fusing Macrophages and FBGCs
To evaluate the expression of integrins during monocyte-to-macrophage development and in fusing macrophages/FBGCs, we used fluorescence confocal scanning laser microscopy and anti-integrin detecting antibodies (Table 1) ▶ applied to permeabilized cells at various time points in the 7-day culture period. Rhodamine phalloidin and the nucleic acid stain YO-YO-1 were also used to visualize F-actin and nuclei, respectively, in fusing macrophages/FBGCs. Representative results are presented in Figure 5 ▶ for monocytes after initial adhesion (1.5 hours) and after a period of monocyte-to-macrophage development (day 3). β1 integrins were not detected on adherent monocytes, but are apparently induced during macrophage development and were observable on day 3 of the culture period. In contrast, β2 integrins were readily detectable on freshly adherent monocytes and were even more strongly expressed with macrophage development on day 3. β3 integrins were undetectable or very weakly expressed at either time point.
Figure 5.
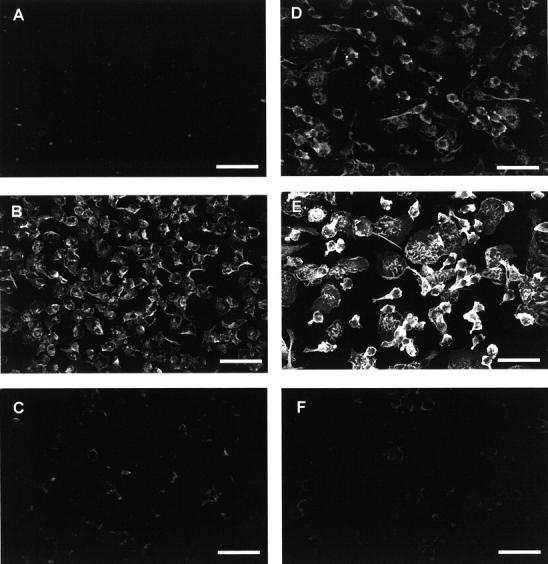
Expression of integrins on monocytes or monocyte-derived macrophages. Integrin expression was analyzed on monocytes after initial adhesion after 1.5 hours (A, B, and C) or on day 3 of culture (D, E, and F). Permeabilized cells were stained with mouse monoclonal anti-integrin detecting antibodies (Table 1) ▶ and anti-mouse IgG conjugated to Cy-5. A and D, β1 integrin; B and E, β2 integrin; and C and F, β3 integrin. Representative images are of single 2-μm slices at the cell/substrate interface. Scale bars, 50 μm.
After the IL-4 induction of macrophage fusion and FBGC formation, the expression of β1 integrins was much more striking (Figure 6A) ▶ . In fusing macrophages, β1 integrins were observed throughout the cytoplasm. In multinucleated FBGCs, β1 integrins were observed to be most concentrated in central cytoplasmic areas. They were also detectable throughout the cell and in the extreme periphery just outside of the peripheral rings of punctate actin that are characteristic of these giant cells. 31 β2 integrins were also strongly expressed on fusing macrophages, particularly in peripheral cell areas, and were frequently co-localized with F-actin (yellow fluorescence, Figure 6B ▶ ). Similarly to β1, upon FBGC formation, β2 integrins were observed to be most concentrated in central cytoplasmic regions together with the nuclei. Further, β2 was also expressed directly outside of FBGC punctate actin rings, appearing to distinctly outline these multinucleated giant cells at the cell/substrate interface. Finally, as was the case for monocytes and day 3 macrophages, β3 integrins were essentially undetectable in cultures of macrophages/FBGCs, which were readily visualized with F-actin and nuclear staining (Figure 6C) ▶ .
Figure 6.
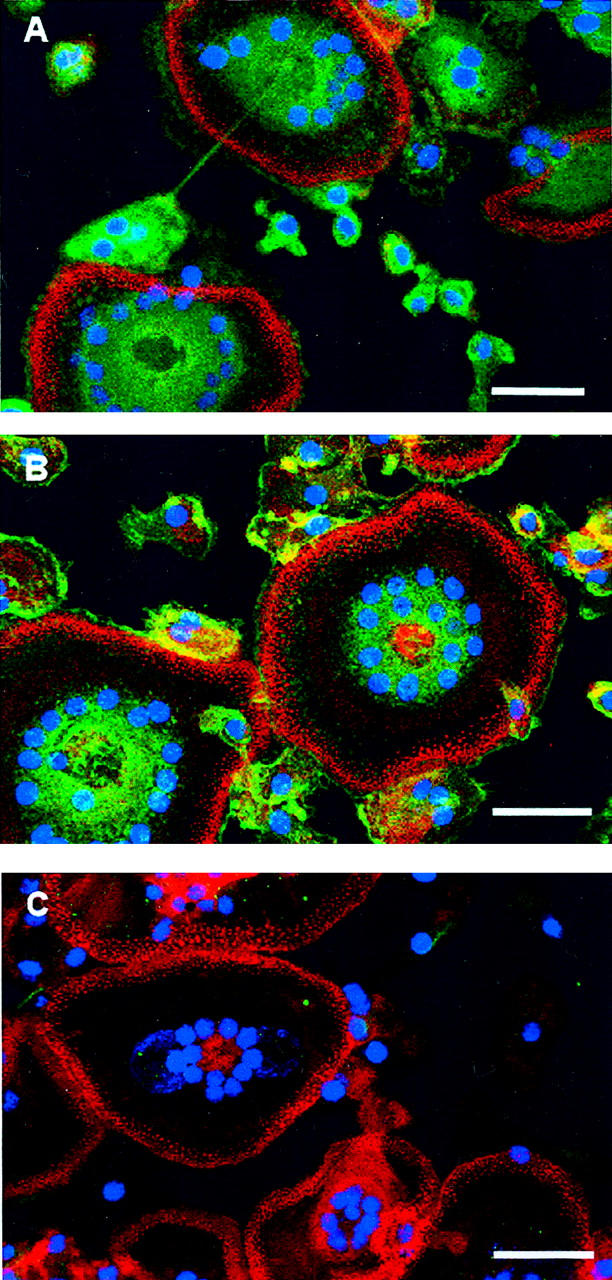
Expression of integrins on fusing macrophages/FBGCs. A, β1 integrin; B, β2 integrin; and C, β3 integrin. Cells were fixed and permeabilized on day 7 after the induction of macrophage fusion by IL-4. Fusing macrophages/FBGCs were then stained with mouse monoclonal anti-integrin detecting antibodies (Table 1) ▶ followed by a mixture of anti-mouse IgG conjugated to Cy-5 (green, integrins), rhodamine phalloidin (red, F-actin), and YO-YO-1 (blue, nuclei). Representative micrographs are shown of merged Z-series images. Scale bars, 50 μm.
Discussion
This study provides the first description of the cell/substrate adhesive interactions that support macrophage fusion and multinucleated FBGC formation in vitro. We report that initial monocyte adhesion is strongly dependent on β2 integrins, but that both β1 and β2 integrins are required to mediate adhesion during IL-4-induced macrophage fusion and FBGC formation. Consistent with integrin-mediated intracellular signaling, inhibitors of tyrosine kinase and phosphatidylinositol-3-kinase activities abrogate macrophage/FBGC adhesion. Integrin localization in cultures of fusing macrophages and FBGCs further demonstrates that β1 and β2 integrins play cooperative roles in cell adhesion during the formation of these multinucleated giant cells.
Initial monocyte adhesion results with anti-β2 integrin antibodies are similar to our earlier findings using several chemically modified culture surfaces and different mAbs to β2. 7 β2 integrins, particularly αM/β2, are able to interact with a variety of serum components that are expected to be present in our culture system. Therefore, β2-mediated adhesion may occur to adsorbed fragments of complement C3 (C3bi) 7 and/or residual coagulation factors such as fibrinogen, Factor X, or high molecular weight kininogen. It is also possible that β2 integrins may associate with undefined protein domains that have been denatured by adsorption to material surfaces 32 or with IgG in a Fc receptor-independent manner. 33
During IL-4-induced macrophage fusion and FBGC formation, we find that the requirement for β2 integrins is maintained, but that an additional dependence on β1 integrins is acquired. Anti-β1 alone has no apparent effect on initial monocyte adhesion, but it inhibits macrophage/FBGC adhesion by ∼60%. Likewise, the 120-kd RGD-containing proteolytic fragment of fibronectin does not reduce initial monocyte adhesion, but it inhibits macrophage/FBGC adhesion by ∼60%. The combination of function-blocking anti-β1 and anti-β2 antibodies severely reduces macrophage/FBGC to 10% of control adhesion. Although it is possible that residual degrees of adhesion are mediated by other receptors that were not addressed in this study, we interpret the present results to indicate that functional β1 and β2 integrins are necessary and likely sufficient for the adhesion of these multinucleated giant cells.
Our data are consistent with a report that engagement of β2 integrins induces cell surface expression of β1 integrin receptors in neutrophils. 25 In addition, macrophage development has been demonstrated to involve an interaction between secreted fibronectin and α5/β1 integrins 34 and to be associated with increases in β1 integrin expression. 35 Therefore, the engagement of β2 integrin on initial monocyte adhesion may subsequently induce the expression of β1 integrin and its binding to immobilized RGD, adsorbed fibronectin from serum, and/or other adsorbed serum components. The combined influences of IL-4 and adhesion-driven macrophage development likely promote the cellular synthesis and deposition of RGD-containing matrix proteins such as cell-derived fibronectin. 36-38 These or other secreted components may present an even more favorable adhesive substrate for β1 and β2 integrins to cooperatively strengthen and increase the cell receptor-substrate interactions required for the adhesion and considerable cytoplasmic spreading of these large multinucleated cells. 39 The ability of RGD-modified culture surfaces to promote and support FBGC formation more efficiently than standard cell culture polystyrene 13 supports this perspective.
Confocal microscopic analysis served to complement and confirm our antibody inhibition data. Function-blocking antibodies to β1 integrins, which are not detectable on monocytes after initial adhesion (1.5 hours), but appear with macrophage development and are strongly expressed in day 7 cultures of fusing macrophages, inhibited adhesion on day 7 only. Likewise, β2 integrins are readily observed on monocytes after initial adhesion as well as on fusing macrophages, and function-blocking anti-β2 integrin antibodies inhibited cell adhesion at both time points. Of interest, we note that β2 integrins are particularly evident in peripheral cell areas. This may be related to the observation that after anti-β2 inhibition of macrophage/FBGC adhesion, the remaining adherent cells were mostly mononuclear macrophages with few pseudopodial extensions, whereas the remaining adherent cells after anti-β1 treatment displayed pseudopods and were as competent to undergo fusion as their untreated counterparts. Therefore, the present results point to a role for β1 integrins in mediating cell/substrate, rather than cell/cell, interactions during FBGC formation. Similarly, they demonstrate the importance of β2 integrins in the cell/substrate interactions that support both initial monocyte adhesion and adhesion during IL-4-induced FBGC formation. A further role for β2 integrins, however, may encompass cell/cell interactions in the macrophage fusion process. This is consistent with other reports that macrophage fusion induced with a cytokine-rich supernatant from mitogen-activated mononuclear cells 40 or with T-lymphocyte-derived cytokines and mycobacteria 41 is also inhibited by anti-β2 antibodies.
Interestingly, we observed an acquired sensitivity of macrophage/FBGC adhesion to an anti-αM antibody (mAb 60.1) that was not apparent during initial monocyte adhesion. This may be related to a report that the cross-linking of α5/β1 causes the activation of αM/β2 on monocytes. 42 Accordingly, the engagement of β1 integrins during the induction of macrophage fusion may cause conformational changes in αM/β2, resulting in exposure of previously inaccessible ligand-binding sites that are important for macrophage/FBGC adhesion. Thus, during IL-4-induced macrophage fusion, it is possible that the β2-integrin interactions mediating initial monocyte adhesion are altered or enhanced. This may be another mechanism that operates to promote further macrophage phenotypic development, giant cell cytoplasmic spreading, and/or focal adhesion formation. 43
Evidence from cell signaling studies continues to provide information on how integrins transduce mechanical and biochemical environmental signals to regulate cell functions. The effects of signaling by IL-4 or IL-13 have been associated with phosphatidylinositol-3-kinase activity, 44 which also has been linked to cytoskeletal rearrangements 45 and rescue from apoptosis. 46 In macrophages, β1 integrin signaling involves phosphatidylinositol-3-kinase, and its pharmacological inhibition prevents spreading on fibronectin surfaces, 39 phagocytosis, 47 and pseudopod formation. 48 Similarly, the interaction of α5/β1 and cell-derived fibronectin 49 and the adhesion of macrophage U937 cells to plastic or to fibronectin are accompanied by tyrosine phosphorylation of a variety of proteins. 34 Likewise, the β1-mediated adhesion and mobility of epithelial cells are inhibited by tyrosine kinase inhibitors. 50 The present results with inhibitors of phosphatidylinositol-3-kinase or tyrosine kinase activities are consistent with integrin-mediated adhesion during IL-4-induced macrophage fusion and FBGC formation.
These integrin signaling pathways are believed to involve a p125 focal adhesion kinase and to facilitate the formation of focal adhesion contacts at the cell/substrate interface. 16 In FBGCs, the punctate actin adhesive structures that concentrate in peripheral cytoplasmic areas have been identified as podosomes, although the p125 focal adhesion kinase was not detected in these structures. 31 Interestingly, however, a proline-rich tyrosine kinase with considerable sequence homology to the p125 focal adhesion kinase has been found to be activated by β2 integrin ligation and to co-localize in macrophage podosomes with αM/β2. 43 Therefore, this proline-rich tyrosine kinase may participate in podosome assembly during FBGC formation, and, based on our observed inhibition of macrophage/FBGC adhesion with anti-αM, it is possible that αM/β2 plays a role in this assembly process.
A functional role for β3 integrins in either initial monocyte or macrophage/FBGC adhesion is not supported by the results of this study. Although the present data cannot definitively exclude β3, they are consistent with other reports in which investigators describe very low levels of macrophage αV/β3 compared to α5/β1 34 and no detectable αV compared to α4, α5, β1, and β2. 43 It is interesting to note, however, that β3 integrins are apparently required for the adhesion of osteoclasts, which are multinucleated giant cells that play a critical role in normal bone physiology. 51 β3 integrins have been detected in osteoclastoma tissue 52 and in an in vitro system of osteoclast formation from human monocytes. 53 In addition, αV/β3 has been demonstrated to mediate the spreading of osteoclast precursors before fusion and thus has been suggested to participate in osteoclast differentiation. 54 Therefore, this study supports the perspective that although osteoclasts and IL-4-induced FBGCs are both multinucleated giant cells of monocyte-derived macrophage origin, their formation may occur under the influences of different cell/substrate adhesive mechanisms as well as cytokine mediators. Accordingly, these multinucleated cells may have undergone differential pathways of development leading toward functional specializations in bone and at sites of chronic inflammation, respectively.
Multinucleated giant cells have long been observed in chronically inflamed tissues, 55 and yet their pathophysiological significance remains unknown. There is considerable evidence for IL-4 and IL-13 as negative modulators of monocyte/macrophage proinflammatory functions. 56 However, the demonstrated ability of FBGCs to mediate biomaterial degradation in vivo 4 argues for the concept that FBGC formation represents a joining of phagocytic cell forces against a nonphagocytosable material. To resolve this apparent conflict, it is speculated that IL-4-induced macrophage fusion leading to a state of multinucleation facilitates the formation of a highly differentiated cell type with acquired polarized functional capabilities. This is also suggested by the localization of a lysosomal antigen at the cell/substrate interface and a Na+K+-ATPase in the nonadherent domain of multinucleated macrophages. 57 Thus, FBGCs may sequester phagocytic cell activities at the cell/substrate interface and express anti-inflammatory or wound healing functional capacities at the cell/host tissue interface. The adhesive interactions that are engaged during the differentiation and development of multinucleated macrophages must transduce critical environmental signals that support and facilitate these complex processes. Our in vitro system of FBGC formation provides a means for further mechanistic investigation that may lead to new design criteria for biomedical materials and, ultimately, to an understanding of the physiological roles of multinucleated giant cells at sites of chronic inflammation.
Footnotes
Address reprint requests to Dr. James M. Anderson, Institute of Pathology, Case Western Reserve University, Cleveland, Ohio 44106. E-mail: akm2@po.cwru.edu.
Supported by National Heart, Lung, and Blood Institute, Devices and Technology Branch, grants HL33849 and HL55714.
References
- 1.Murch AR, Grounds MD, Marshall CA: Direct evidence that inflammatory multinucleate giant cells form by fusion. J Pathol 1980, 137:177-180 [DOI] [PubMed] [Google Scholar]
- 2.Chambers TJ, Spector WG: Inflammatory giant cells. Immunobiology 1982, 161:2283-2289 [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM: Inflammatory response to implants. Am Soc Artif Intern Organs 1988, 11:101-107 [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q, Topham NS, Anderson JM, Hiltner A, Lodoen G, Payet R: Foreign body giant cells and polyurethane biostability: in vivo correlation of cell adhesion and surface cracking. J Biomed Mater Res 1991, 25:177-183 [DOI] [PubMed] [Google Scholar]
- 5.Wiggins MJ, Wilkoff B, Anderson JM, Hiltner A: Biodegradation of polyether polyurethane inner insulation in bipolar pacemaker leads. J Biomed Mater Res 2001, 58:302-307 [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM: Multinucleated giant cells. Curr Opin Hematol 2000, 7:40-47 [DOI] [PubMed] [Google Scholar]
- 7.McNally AK, Anderson JM: Complement C3 participation in monocyte adhesion to different surfaces. Proc Natl Acad Sci USA 1994, 91:10119-10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNally AK, Anderson JM: Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol 1995, 147:1487-1499 [PMC free article] [PubMed] [Google Scholar]
- 9.DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM: Interelukin-13 induces monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol 1997, 158:3385-3390 [PubMed] [Google Scholar]
- 10.Kao WJ, McNally AK, Hiltner A, Anderson JM: Role for interleukin-4 in foreign body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res 1995, 29:1267-1275 [DOI] [PubMed] [Google Scholar]
- 11.Stein M, Keshav S, Harris N, Gordon S: Interleukin-4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992, 176:287-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNally AK, Anderson JM: Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol 1996, 149:975-985 [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JM, DeFife K, McNally A, Collier T, Jenney C: Monocyte, macrophage and foreign body giant cell interactions with molecularly engineered surfaces. J Mater Sci Mater Med 1999, 10:579-588 [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Amaro R, Sanchez-Madrid F: Cell adhesion molecules: selectins and integrins. Crit Rev Immunol 1999, 19:389-429 [PubMed] [Google Scholar]
- 15.Schwartz MA, Shaller MD, Ginsberg MH: Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995, 11:549-559 [DOI] [PubMed] [Google Scholar]
- 16.Giancotti FG, Ruoslahti E: Integrin signaling. Science 1999, 285:1028-1032 [DOI] [PubMed] [Google Scholar]
- 17.Ruoslahti E: RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 1996, 12:697-715 [DOI] [PubMed] [Google Scholar]
- 18.Pytela R, Pierschbacher MD, Ruoslahti E: Identification and isolation of a 140 kilodalton cell surface glycoprotein with properties of a fibronectin receptor. Cell 1985, 40:191-198 [DOI] [PubMed] [Google Scholar]
- 19.Berton G, Lowell CA: Integrin signaling in neutrophils and macrophages. Cell Signal 1999, 11:621-635 [DOI] [PubMed] [Google Scholar]
- 20.Anderson JM, Ziats NP, Bonfield TL, McNally AK, Topham NS: Human blood protein and cell interactions with cardiovascular materials. Akutsu T Koyanagi H eds. Artificial Heart, 1991, vol 3.:pp 45-55 Springer-Verlag, New York [Google Scholar]
- 21.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA: Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol 1994, 125:1417-1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henson PM, Henson JE, Fittschen C, Kimani G, Bratton DL, Riches DWH: Phagocytic cells: degranulation and secretion. Gallin JI Goldstein IM Snyderman R eds. Inflammation: Basic Principles and Clinical Correlates. 1988, :pp 363-390 Raven New York [Google Scholar]
- 23.Te Velde AA, Klomp JPG, Yard BA, de Vries JE, Figdor CG: Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol 1988, 140:1548-1554 [PubMed] [Google Scholar]
- 24.Doucet C, Brouty-Boye D, Pottin-Clemenceau C, Jasmin C, Canonica GW, Azzarone B: IL-4 and IL-13 specifically increase adhesion molecule and inflammatory cytokine expression in human lung fibroblasts. Int Immunol 1988, 10:1421-1433 [DOI] [PubMed] [Google Scholar]
- 25.Werr J, Eriksson AA, Hedqvist P, Lindbom L: Engagement of beta2 integrins induces surface expression of beta1 integrin receptors in human neutrophils. J Leukoc Biol 2000, 68:553-560 [PubMed] [Google Scholar]
- 26.Ruoslahti E, Pierschbacher MD: New perspectives in cell adhesion: RGD and integrins. Science 1987, 238:491-497 [DOI] [PubMed] [Google Scholar]
- 27.Chappell DA, Fry GL, Waknitz MA, Muhonen LE, Pladet MW, Iverius PH, Strickland DK: Lipoprotein lipase induces catabolism of normal triglyceride-rich lipoproteins via the low density lipoprotein receptor-related protein/a2-macroglobulin receptor in vitro. A process facilitated by cell-surface proteoglycans. J Biol Chem 1993, 268:14168-14175 [PubMed] [Google Scholar]
- 28.Krieger M, Acton S, Ashkenas J, Pearson A, Penman M, Resnick D: Molecular flypaper, host defense. J Biol Chem 1993, 268:4569-4572 [PubMed] [Google Scholar]
- 29.Naor D, Sionov VR, Ish-Shalom D: CD44: structure, function, and association with the malignant process. Adv Cancer Res 1997, 71:241-319 [DOI] [PubMed] [Google Scholar]
- 30.Sterling H, Saginario C, Vignery A: CD44 occupancy prevents macrophage multinucleation. J Cell Biol 1998, 143:837-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeFife KM, Jenney CR, Colton E, Anderson JM: Cytoskeletal and adhesive structural polarizations accompany IL-13-induced human macrophage fusion. J Histochem Cytochem 1999, 47:65-74 [DOI] [PubMed] [Google Scholar]
- 32.Davis GE: The Mac-1 and p150,95 beta2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res 1992, 200:242-252 [DOI] [PubMed] [Google Scholar]
- 33.Jenney CR, Anderson JM: Adsorbed IgG: a potent adhesive substrate for human macrophages. J Biomed Mater Res 2000, 50:281-290 [DOI] [PubMed] [Google Scholar]
- 34.Laouar A, Collart FR, Chubb CBH, Xie B, Huberman E: Interaction between a5b1 integrin and secreted fibronectin is involved in macrophage differentiation of human HL-60 myeloid leukemia cells. J Immunol 1999, 162:407-414 [PubMed] [Google Scholar]
- 35.Ammon C, Meyer SP, Scharzfischer L, Krause SW, Andreesen R, Kreutz M: Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology 2000, 100:364-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Postlewaite AE, Holness MA, Katai H, Raghow R: Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin-4. J Clin Invest 1992, 90:1479-1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller R, Krahl T, Sarvetnick N: Tissue-specific expression of interleukin-4 induces extracellular matrix accumulation and extravasation of B cells. Lab Invest 1997, 76:117-128 [PubMed] [Google Scholar]
- 38.Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S: Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix proteins betaIG-H3. Scand J Immunol 2001, 53:386-392 [DOI] [PubMed] [Google Scholar]
- 39.Meng F, Lowell CA: A beta 1 integrin signaling pathway involving Src-family kinases, Cb1 and PI-3 kinase is required for macrophage spreading and migration. EMBO J 1998, 17:4391-4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Most J, Neumayer HP, Deierich MP: Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-gamma and expression of LFA-1. Eur J Immunol 1990, 20:1661-1667 [DOI] [PubMed] [Google Scholar]
- 41.Gasser A, Most J: Generation of multinucleated giant cells in vitro by culture of human monocytes with Mycobacterium bovis BGC in combination with cytokine-containing supernatants. Infect Immun 1999, 67:395-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Berg BM, van Furth R, Hazenbos WL: Activation of complement receptor 3 on human monocytes by cross-linking of very late antigen-5 is mediated via protein tyrosine kinases. Immunology 1999, 98:197-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duong LT, Rodan GA: PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by beta2 integrin ligation. Cell Motil Cytoskel 2000, 47:174-188 [DOI] [PubMed] [Google Scholar]
- 44.Ceponis PJM, Botelho F, Richards CD, McKay DM: Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. J Biol Chem 2000, 275:29132-29137 [DOI] [PubMed] [Google Scholar]
- 45.Corvera S, Czech MP: Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol 1998, 8:442-446 [DOI] [PubMed] [Google Scholar]
- 46.Chen RH, Chang MC, Su YH, Fsai YT, Kuo ML: Interleukin-6 inhibits transforming growth factor-beta-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem 1999, 274:23013-23019 [DOI] [PubMed] [Google Scholar]
- 47.Araki N, Johnson MT, Swanson JA: A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol 1996, 135:1249-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox D, Tseng CC, Bjeke G, Greenberg S: A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem 1999, 274:1240-1247 [DOI] [PubMed] [Google Scholar]
- 49.Koyama Y, Norose-Toyoda K, Hirano S, Kobayashi M, Ebihara T, Someki I, Fujisaki H, Irie S: Type I collagen is a non-adhesive extracellular matrix for macrophages. Arch Histol Cytol 2000, 63:71-79 [DOI] [PubMed] [Google Scholar]
- 50.Petit V, Boyer B, Thiery JP, Vallas AM: Characterization of the signaling pathways regulating alpha2beta1 integrin-mediated events by a pharmacological approach. Cell Adhesion Commun 1999, 7:151-165 [DOI] [PubMed] [Google Scholar]
- 51.Teitelbaum SL: Bone resorption by osteoclasts. Science 2000, 289:1504-1508 [DOI] [PubMed] [Google Scholar]
- 52.James IE, Dodds RA, Lee-Rykaczewski E, Eichman CF, Connor JR, Hart TK, Maleeff BE, Lackman RD, Gowen M: Purification and characterization of fully functional human osteoclast precursors. J Bone Miner Res 1996, 11:1608-1618 [DOI] [PubMed] [Google Scholar]
- 53.Fujikawa Y, Quinn JM, Sabokbar A, McGee JO, Athanasou NA: The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 1996, 137:4058-4060 [DOI] [PubMed] [Google Scholar]
- 54.Boissy P, Machuca I, Pfaff M, Ficheux D, Jurdic P: Aggregation of mononucleated precursors triggers cell surface expression of alphavbeta3 integrin, essential to formation of osteoclast-like multinucleated cells. J Cell Sci 1998, 111:2563-2574 [DOI] [PubMed] [Google Scholar]
- 55.Langhans T: Uber riezenzellen mit wandstandigen kernen in tuberkeln und die fibrose form des tuberkels. Arch Pathol Anat 1868, 42:382-404 [Google Scholar]
- 56.Malefyt RW: Role of interleukin-10, interleukin-4, and interleukin-13 in resolving inflammatory responses. Gallin JI Snyderman R eds. Inflammation: Basic Principles and Clinical Correlates. 1999, :pp 837-849 Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 57.Vignery A, Niven-Fairchild T, Ingbar DH, Caplan M: Polarized distribution of Na+K+-ATPase in giant cells elicited in vivo and in vitro. J Histochem Cytochem 1989, 37:1265-1271 [DOI] [PubMed] [Google Scholar]