Abstract
Production of matrix-degrading proteases, particularly matrix metalloproteinases (MMPs), by endothelial cells is a critical event during angiogenesis, the process of vessel neoformation that occurs in normal and pathological conditions. MMPs are known to be highly regulated at the level of synthesis and activation, however, little is known about the regulation of MMP secretion by endothelial cells. We found that cultured human umbilical vein endothelial cells shed vesicles (300 to 600 nm) originating from localized areas of the cell plasma membrane, as revealed by ultrastructural analysis. Normal and reverse zymography, Western blot, and immunogold analyses of the vesicles showed two gelatinases, MMP-2 and MMP-9, in both the active and proenzyme forms, the MT1-MMP proenzyme located on the external side of the vesicle membrane and the two inhibitors TIMP-1 and TIMP-2. Serum and the angiogenic factors, fibroblast growth factor-2 and vascular endothelial growth factor, stimulated the shedding of MMPs as vesicle components. Shedding the vesicle was rapid, as it was already completed after 4 hours. Addition of shed vesicles to human umbilical vein endothelial cells resulted in autocrine stimulation of invasion through a layer of reconstituted basement membrane (Matrigel) and cord formation on Matrigel. We conclude that endothelial cells shed MMP-containing vesicles and this may be a mechanism for regulating focalized proteolytic activity vital to invasive and morphogenic events during angiogenesis.
Shedding macromolecules from the cell surface is an important mechanism of communication between eukaryotic cells and their environment. By definition, shedding is the release of soluble or vesicle-associated cell surface constituents, without affecting cell viability. Shedding membrane vesicles from the cell surface is generally a selective process and widespread in normal and tumor cells in vivo and in vitro. 1 Shedding is particularly active in proliferating and invasive cells, such as cancer cells, where cell surface components are continually released. 2 The consequences of vesicle shedding from cancer cells include effects on their ability to infiltrate, metastasize, and generally affect their microenvironment. Our previous studies demonstrated that vesicles shed by 8701-BC human breast carcinoma cells, 3 HT-1080 cells, 4 and CABA I ovarian carcinoma cells 5 contain matrix metalloproteinase (MMP)-9, MMP-2, and uPA.
Angiogenesis, the formation of new vessels from pre-existing ones, is a strictly regulated process necessary during reproduction, development, and wound repair. Deregulated angiogenesis is at the basis of several pathologies, including arthritis, diabetic retinopathy, and tumor growth and metastatic dissemination. 6 Remodeling of the extracellular matrix is a crucial event during angiogenesis. 7 Endothelial cells stimulated by angiogenic factors produce different matrix-degrading enzymes, including MMPs. MMPs are a family of secreted and membrane-associated zinc-dependent extracellular endopeptidases, which degrade extracellular matrix at physiological pH. 8 They are produced by a variety of cells. Endothelial cells have been described to produce MMP-1, MMP-2, MMP-9, MT1-MMP, all implicated in the regulation of angiogenesis. 9-12 The importance of these enzymes in angiogenesis is proved by evidence that endogenous and synthetic inhibitors of MMPs block the process 13,14 and by the impaired angiogenic response in mice deficient for MMPs. 7 However, the precise functions of MMPs in angiogenesis are not yet completely understood. Proteases enable endothelial cells to migrate through the basement membrane and into the interstitial stroma and participate in the final organization of the cells in tubular structures. However, besides this role in the breakdown of connective tissue barriers, proteases and their inhibitors are essential to endothelial cell attachment, proliferation, survival, and migration, acting either directly on cells or releasing matrix-associated angiogenic factors and active fragments of matrix components. 7,15
Production of MMPs by endothelial cells is strictly regulated and transcription and synthesis are crucial steps of this regulation. In contrast, little is known about the regulation of MMP secretion by endothelial cells. Although MMPs were once believed to be synthesized and rapidly secreted, recent evidence shows that active MMPs are stored in cytoplasmic secretory granules in endothelial cells, ready for rapid release by angiogenic stimuli. 16 We have previously reported that MMP production by endothelial cells was increased without de novo synthesis and was prevented by inhibitors of secretion, indicating that protease production may indeed be regulated at the level of secretion in these cells. 17
This study was designed to investigate whether shedding MMPs as membrane vesicle components might be a mechanism of rapid secretion of proteases during angiogenesis. We investigated whether endothelial cells shed MMP-containing vesicles, whether this release was modulated by angiogenic factors, and whether MMP-containing vesicles played a role in endothelial cell functions related to angiogenesis.
Materials and Methods
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cord veins and grown on 1% gelatin-coated flasks in M199 supplemented with 10% fetal calf serum, 10% newborn calf serum, 20 mmol/L HEPES, 6 U/ml heparin, 2 mmol/L glutamine, 50 μg/ml endothelial cell growth factor (crude extract from bovine brain), penicillin, and streptomycin. Cells were used between the third and fifth passage.
For the preparation of the supernatants, subconfluent cultures of HUVECs were rinsed once with serum-free medium, then exposed for 4 or 18 hours to medium containing the indicated amounts of serum and stimuli. To confirm that the MMPs did indeed come from endothelial cells and not serum, in some experiments serum was depleted of MMPs by chromatography on gelatin Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden). For the experiments with angiogenic factors, HUVECs were exposed to medium with 2.5% fetal calf serum, and either 10 ng/ml basic fibroblast growth factor (FGF-2; R&D Systems, Minneapolis, MN) or 10 ng/ml vascular endothelial growth factor (VEGF, R&D Systems). The supernatant was then collected and processed as described below. The remaining cells were counted. Vesicles isolated from at least two independent preparations of conditioned medium were analyzed for each experimental condition.
Isolation of Membrane Vesicles from Cell-Conditioned Medium
Vesicles were prepared as already described. 18 Conditioned medium obtained as above was centrifuged at 600 × g for 15 minutes and then at 1500 × g for 15 minutes to remove cells and large debris. The supernatants were centrifuged at 100,000 × g for 1 hour at 4°C. Pelleted vesicles were resuspended in phosphate-buffered saline (PBS), pH 7.5. The vesicles were quantified by measuring vesicle-associated proteins, using the method of Bradford (Bio-Rad, Milan, Italy) with bovine serum albumin (Sigma, St. Louis, MO) as the standard.
Electron Microscopy
To visualize the shedding we used scanning and transmission electron microscopic analyses.
Transmission electron microscopic analysis was performed using a standard technique. Briefly, cells were fixed with 2% glutaraldehyde in flasks, scraped, postfixed with 1% OsO4, dehydrated with ethanol, and embedded in Epon 812. Samples were then sectioned, poststained with uranyl acetate and lead citrate, and examined under an electron microscope (Philips CM10, Eindhoven, The Netherlands). For analysis of the ultrastructural morphology of the shed vesicles, the ultracentrifugation pellet, which contained the membrane vesicles, was resuspended in PBS and then applied to collodion-coated grids. After washing, the vesicles were negatively stained with 1% phosphotungstic acid, brought to pH 7.0 with NaOH, and examined by transmission electron microscopy. 19 The immunogold labeling was performed after vesicles were applied to collodion-coated grids: rabbit anti-MT1-MMP antibody (20 μg/ml) (Chemicon International Inc., Temecula, CA) in PBS was added, and samples were incubated for 1 hour at room temperature. After the washings, samples were further incubated with gold-conjugated anti-rabbit antibody (10 nm, Sigma Chemical Co.) for 1 hour. After the washings, samples were negatively stained with 1% phosphotungstic acid, brought to pH 7.0 with NaOH and examined by transmission electron microscopy with a Philips CM100 instrument.
For scanning electron microscopy cells were grown on coverslips and fixed with 2% glutaraldehyde in PBS for 30 minutes. Samples were, after critical point dried, glued onto stubs, coated with gold in a SCD040 Balzer Sputterer and observed using a Philips 505 scanning electron microscope at 10 to 30 kV.
Zymographic Analysis
Zymography was performed using sodium dodecyl sulfate-polyacrylamide (7.5%) gels co-polymerized with 1 mg/ml gelatin type B (Sigma Chemical Co.). Vesicle samples were diluted in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer in nonreducing conditions without heating. After electrophoresis, gels were washed twice for 30 minutes in 2.5% Triton X-100 at room temperature and incubated overnight in collagenase buffer (50 mmol/L Tris-HCl, pH 7.5, 10 mmol/L CaCl2, 150 mmol/L NaCl) at 37°C. Gels were stained in Coomassie Blue R 250 (Bio-Rad, Milan, Italy) in a mixture of methanol:acetic acid:water (4:1:5) for 1 hour and destained in the same solution without dye. Gelatinase activities were visualized as distinct bands, indicating proteolysis of the substrate. Supernatant of WM983A cells was used as a reference standard for proMMP-2 and proMMP-9.
Reverse Zymography
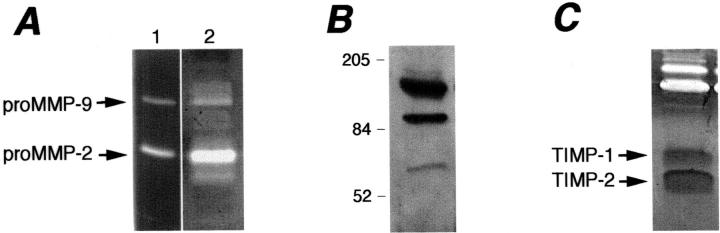
Vesicles were applied to a 14% polyacrylamide gel polymerized with 1 mg/ml gelatin and 30% (v/v) 6× concentrated conditioned medium of HT-1080 cells, used as a source of gelatinases, as described. 20 After electrophoresis, gels were washed, incubated in collagenase buffer, and stained as described above. TIMP-1 and TIMP-2 appeared as dark bands at, respectively, 28 and 21 kd, corresponding to the areas where gelatin degradation by the gelatinases added to the gel is prevented by the inhibitors. Supernatant of WM983A cells was used as a reference standard for TIMPs.
Western Blot Analysis
MMP-9, MT1-MMP, and β1 integrin presence were analyzed by Western blot using specific antibodies. Briefly, samples were electrophoresed and transferred to Hybond nitrocellulose paper (Schleicher & Schuell, Dassel, Germany). Nonspecific binding sites were blocked for 1 hour in 5% nonfat dry milk in a Tris buffer containing 20 mmol/L Tris, 137 mmol/L NaCl, and 0.05% Tween 20 (pH 7.6). Blots were incubated with 5 μg/ml of polyclonal antibodies against MMP-9 (Oncogene Research Products, Calbiochem, Darmstadt, Germany), with anti-β1-integrin monoclonal antibody MAR4 21 (a kind gift of S. Canevari, Milan, Italy) or with rabbit anti-MT1-MMP (Chemicon International, Inc.) for 1 hour, then with secondary antibody anti-mouse and anti-rabbit horseradish peroxidase-conjugated IgG diluted 1:5000 in blocking solution for 1 hour. After washes, reactive bands were visualized by a chemiluminescence detection kit (Pierce, Rockford, IL).
Invasion Assay
HUVEC invasion was assayed using the modified Boyden chamber. 22 The lower compartment contained serum-free medium or conditioned medium of NIH-3T3 cells used as a reference attractant. The filters (8-μm pore size polycarbonate polyvinylpyrolidone-free Nucleopore filters) were coated with a layer of Matrigel (0.5 mg/ml). HUVECs were detached, washed once with Dulbecco’s modified Eagle’s medium-0.1% bovine serum albumin, resuspended in this medium at the concentration of 5 × 105/ml and added to the upper compartment of the chamber. Vesicles, at the concentration indicated, were added to the endothelial cells. To verify the role of MMPs in this system, the MMP inhibitors TIMP-1 and TIMP-2 (both from R&D Systems), BB-94 (batimastat; kindly provided by British Biotech, Oxford, UK) and GM3001 (Chemicon), at the indicated concentrations, were added to the endothelial cells together with vesicles (9 μg/well) and incubated throughout the assay. After 6 hours of incubation at 37°C, filters were stained with Diff-Quik (Marz-Dade, Dudingen, Switzerland), and the migrated cells in 10 high-power fields were counted.
Cord Formation Assay
Ice-cold Matrigel (10 mg/ml) was layered in a 96-well plate and incubated at 37°C for 30 minutes to allow polymerization. HUVECs (2 × 104/200 μl) in medium with 5% serum were added to the wells in the presence of the indicated amounts of vesicles. After 2 and 24 hours the formation of cords was scored by two blinded observers. Pictures were taken after 24 hours.
Results
Shedding Membrane Vesicles by Endothelial Cells
Scanning electron microscopic analysis of HUVECs reveals that these cells shed membrane vesicles originating from specific areas of the cell plasma membrane (Figure 1, A and B) ▶ . Vesicle shedding was also confirmed by transmission electron microscopic analysis of HUVECs. Figure 1C ▶ shows a cross-section of a cell in the process of shedding several small membrane vesicles from the cell surface.
Figure 1.
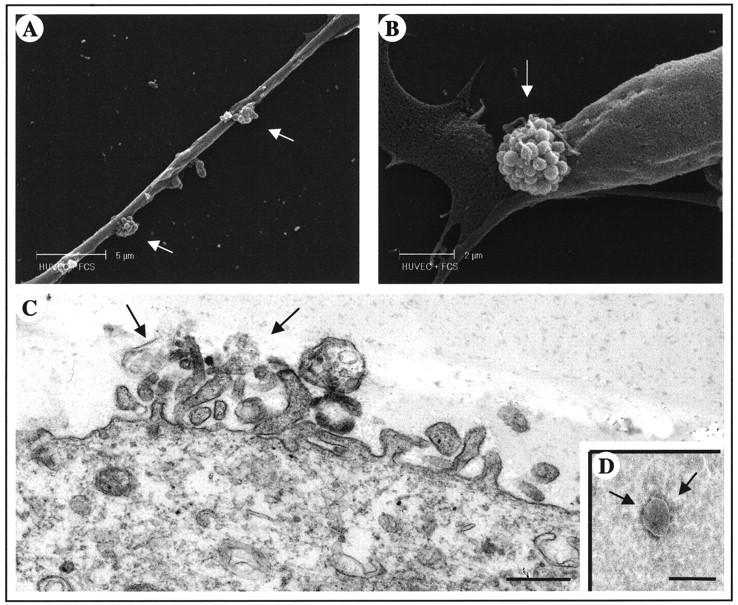
Ultrastructural analyses of the vesicle-shedding process. A and B: Scanning electron micrograph of HUVEC-releasing vesicles. The phenomenon occurs in two different areas of cell surface (arrows). C: Transmission electron microscopic picture of the surface of HUVEC, in cross-section, showing vesicle release from the plasma membrane. Scale bar, 0.5 μm. D: Morphological picture of isolated membrane vesicle (350 nm) with negative staining. Scale bar, 0.5 μm.
Shed vesicles were isolated by ultracentrifugation of HUVEC-conditioned medium. Negative staining under the electron microscope revealed membrane vesicles with a roughly spherical shape, varying in size ranging from 300 to 600 nm, with an integral membrane (Figure 1D) ▶ .
Vesicle-Associated Proteolytic Activity
The presence of matrix-degrading proteases in HUVEC-derived vesicles was analyzed by gelatin zymography (Figure 2A) ▶ . Lytic bands corresponding to proMMP-9 and proMMP-2 were observed. The presence of lower molecular weight species indicated activation of both enzymes. In addition, unidentified bands with a molecular weight more than 200 kd were detected. Specific inhibitors were used to confirm the nature of the bands. Incubation of the gelatin gels with the MMP inhibitor 1,10-phenanthroline completely prevented the appearance of the gelatinolytic bands (not shown). The serine protease inhibitor phenylmethyl sulfonyl fluoride and the serine and cysteine proteinase inhibitors leupeptin and aprotinin had no effect (not shown), indicating that all gelatinolytic bands are MMPs.
Figure 2.
Vesicle-associated proteolytic activity. A: Zymographic analysis of gelatinases. Bands corresponding to the proenzyme forms or the gelatinases (proMMP-2 and proMMP-9) were detected in the isolated vesicles (20 μg, lane 2). Lower molecular weight bands correspond to the activated enzymes. Supernatant of WM983A cells (lane 1) was used as a standard for proMMP-2 and proMMP-9. B: Western blot analysis of MMP-9. Vesicles (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with an anti-MMP-9 antibody as described in Materials and Methods. C: Reverse zymographic analysis of TIMPs in vesicles (40 μg). TIMP-1 and TIMP-2 appear as dark bands of, respectively, 28 and 21 kd.
The presence of MMP-9 in HUVEC-derived vesicles was confirmed by Western blot analysis (Figure 2B) ▶ . The antibody detected both the proenzyme (97 kd), a complex with high molecular weight (∼150 kd), and a truncated form (∼60 kd), as previously reported. 19 A similar zymographic pattern of gelatinases was observed when HUVECs were incubated in medium supplemented with gelatin Sepharose-treated MMP-free serum (not shown), confirming that the MMPs in the preparation do not come from serum, but are really produced by endothelial cells. The presence of TIMP-1 and TIMP-2 was checked by reverse zymography. Both inhibitors were clearly detectable in the vesicle preparation (Figure 2C) ▶ .
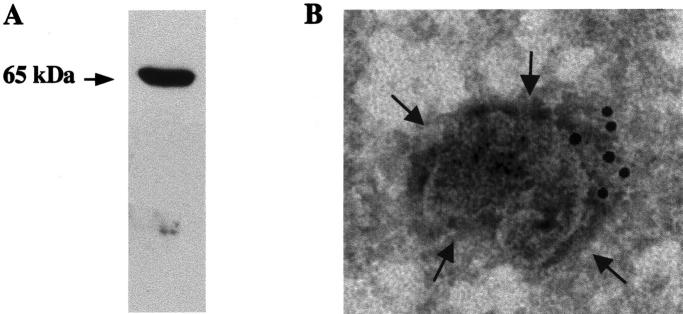
The proenzymatic form of MT1-MMP (65 kd) was also present in HUVEC-shed vesicles, as revealed by Western blot analysis (Figure 3A) ▶ . Negative immunogold staining (10-nm gold particles) of fresh shed vesicles with anti-MT1-MMP antibodies identified the molecule on the external side of vesicles.
Figure 3.
Analysis of MT1-MMP on shed vesicles. A: Western blot analysis of MT1-MMP. Vesicles (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with an anti-MT1-MMP antibody as described in Materials and Methods. B: Electron microscopy localization of MT1-MMP in the vesicles. The application of heavy metal salt solution on the sample reveals an electron-translucent membrane (arrow) on a dark background and the gold particles (10 nm) show the presence of MT1-MMP on the external site of the vesicle.
To assess the rapidity of the shedding, the supernatants of HUVECs collected after 4 and 18 hours of incubation were compared. The same amounts of shed vesicles and patterns of gelatinolysis were observed (not shown), indicating that vesicle shedding by HUVECs is rapid and that 4 hours are sufficient to achieve the maximal level.
Vesicle-Associated β1 Integrin
We previously reported that the tumor-derived vesicles contain the β1-integrin chain. 18 Because β1 integrins mediate endothelial cell interaction with the surrounding extracellular matrix and are important in angiogenesis 23,24 we tested whether HUVEC-derived vesicles contained this integrin chain. The β1 integrin was detected in Western blot analysis (Figure 4) ▶ , suggesting also that in this cellular model the vesicles might be associated with the extracellular matrix.
Figure 4.
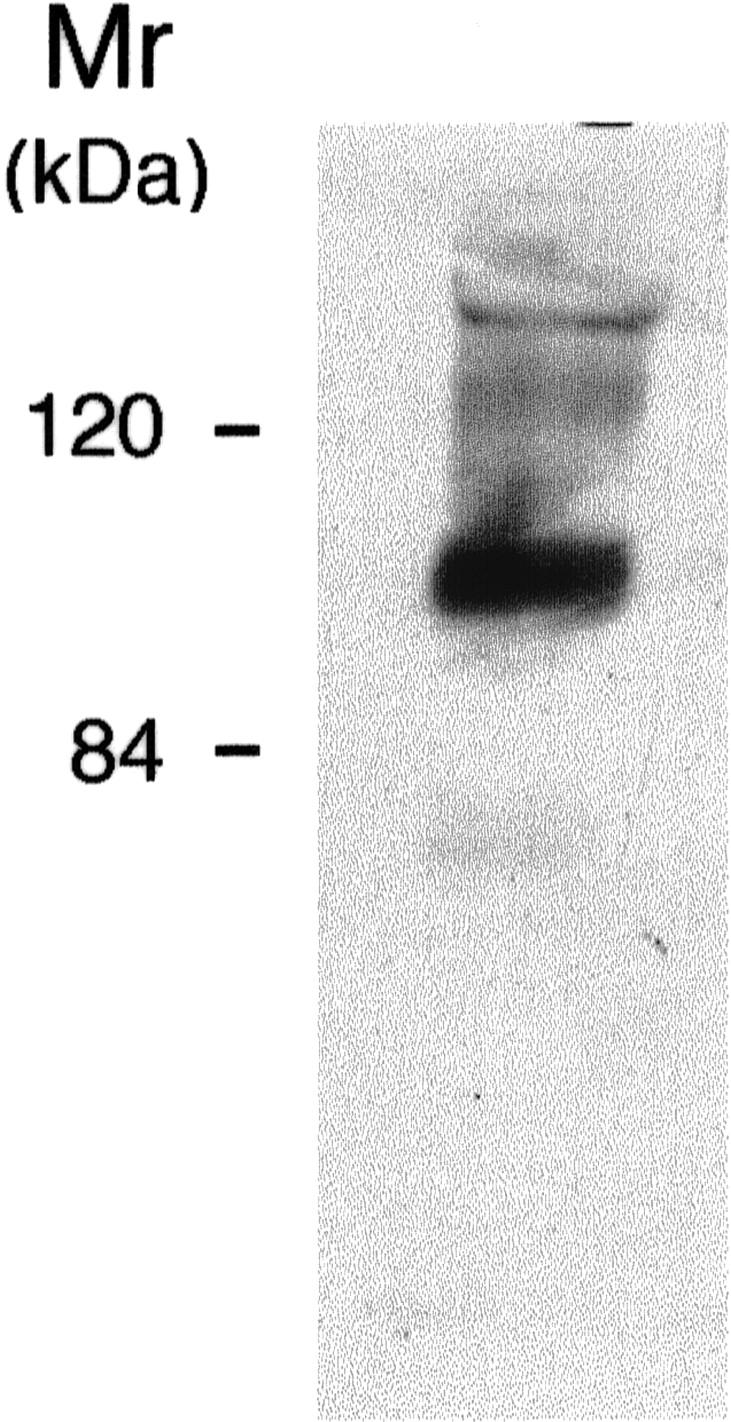
Expression of β1 integrin on HUVEC-shed vesicles. Isolated vesicles were subjected to Western blot analysis as described in Materials and Methods.
Modulation of Vesicle Shedding by Angiogenesis Regulatory Factors
Because the production of MMPs is critical in angiogenesis and is modulated by angiogenic factors, we investigated whether shedding of MMP-containing vesicles by HUVECs was modulated by growth and angiogenic factors.
Exposure of endothelial cells to escalating serum concentrations increased the content of vesicle-associated pro-MMPs and activated MMPs (Figure 5A) ▶ . Serum concentrations of 2.5% and 10% induced respectively 3- and 22-fold increments in proMMP-9 compared to the absence of serum, and threefold and sevenfold increases in proMMP-2. Similar increments in MMP content were observed when the volumes of vesicle samples loaded onto the zymogram were corrected either for cell number (not shown) or for vesicle protein content (Figure 5A) ▶ .
Figure 5.
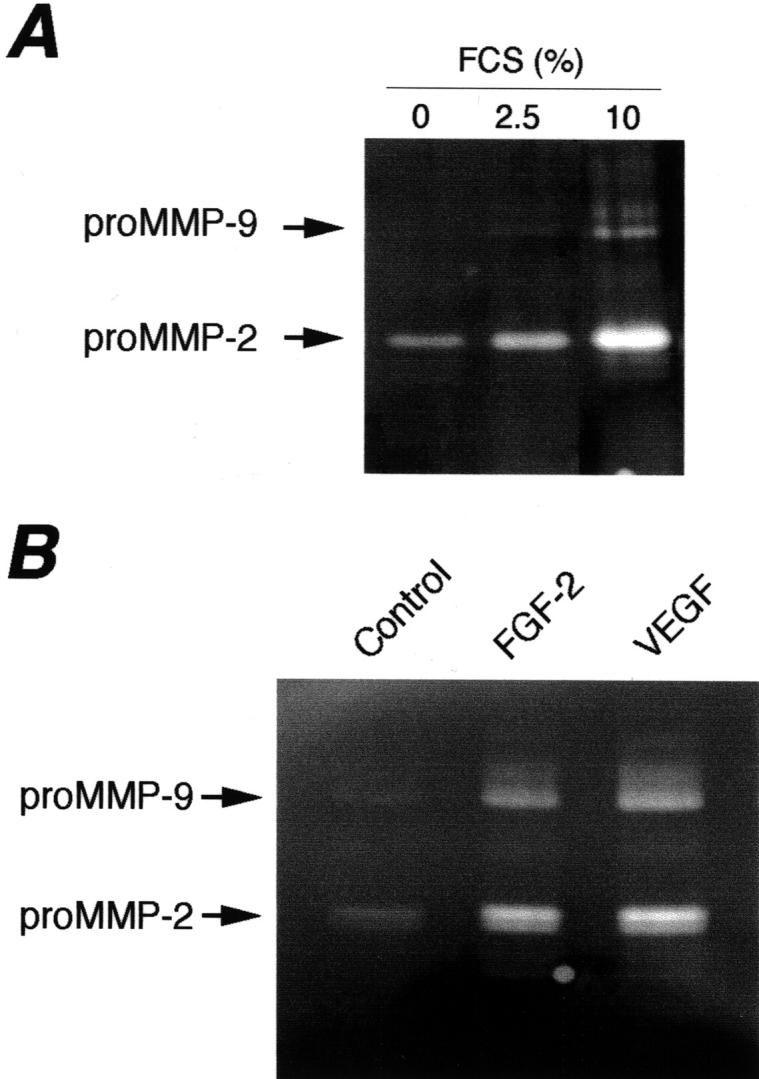
Modulation of vesicle-associated proteolytic activity by serum and angiogenesis regulatory factors. Zymographic analysis of vesicles isolated from supernatants of HUVECs exposed for 4 hours to medium containing the indicated concentrations of serum (A) or FGF-2 (10 ng/ml) or VEGF (10 ng/ml) (B).
We next evaluated the effect of FGF and VEGF, two angiogenic factors that stimulate MMP production and endothelial cell invasiveness. Both factors markedly increased the amount of vesicle-associated MMPs (Figure 5B) ▶ . FGF-2 and VEGF, at a concentration that stimulates endothelial cell functions, induced respectively, 7- and 12-fold increases in proMMP-9, and sixfold and eightfold increases in proMMP-2. The two factors also stimulated MMP activation, as shown by the increased intensity of the bands corresponding to activated MMP-9 and MMP-2 (Figure 5B) ▶ . Also in this case results were similar when supernatants were collected after 4 or 18 hours of incubation (not shown).
Effect of Vesicles on HUVEC Invasion and Cord Formation
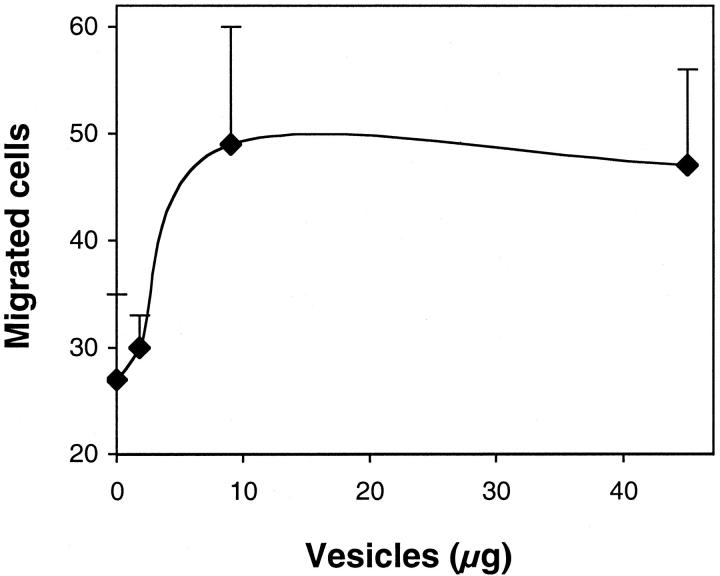
MMPs are involved in endothelial cell invasion and capillary structure formation. We therefore tested whether the released vesicles, containing MMPs, exerted an autocrine stimulatory effect on endothelial cells. In the absence of other stimuli, addition of vesicles to endothelial cells increased their ability to invade a layer of matrix (Matrigel) in the Boyden chamber assay (Figure 6) ▶ . The effect was dose-dependent, and stimulation was maximal at 9 μg/well. In three independent experiments the addition of vesicles increased endothelial cell invasion 2.1 ± 0.7-fold compared to control invasion, without stimuli. Supernatant of NIH-3T3 cells, used as a reference attractant, stimulated invasion 2.9 ± 1.8-fold (not shown). The requirement for MMPs in vesicle-induced endothelial cell invasion was confirmed by the activity of MMP inhibitors (Table 1) ▶ . Both the broad-spectrum inhibitors BB-94 and GM3001, as well as TIMP-1 and TIMP-2 significantly prevented cell invasion (Table 1) ▶ .
Figure 6.
Effect of vesicles on endothelial cell invasion, assessed in the Boyden chamber, using a Matrigel-coated filter. HUVECs were added to the upper compartment of the chamber with or without the indicated amount of vesicles. Results are expressed as the number of migrated endothelial cells in 10 high-power fields (mean and SD of triplicates). The results are from one experiment representative of three.
Table 1.
Effect of MMP Inhibitors on Vesicle-Induced Endothelial Cell Invasion
| Vesicles | Inhibitor | Concentration | Migrated cell (mean ± SD) |
|---|---|---|---|
| − | − | − | 12.1 ± 7.4 |
| + | − | − | 47.3 ± 14.3 |
| + | TIMP-1 | 1 μg/ml | 15.3 ± 6.0* |
| + | “ | 0.1 μg/ml | 22.7 ± 7.5* |
| + | TIMP-2 | 1 μg/ml | 19.0 ± 4.2† |
| + | “ | 0.1 μg/ml | 23.5 ± 10.6† |
| + | BB-94 | 0.3 μmol/L | 12.3 ± 8.0* |
| + | “ | 0.03 μmol/L | 23.7 ± 15.6† |
| + | GM6001 | 2.5 μmol/L | 13.5 ± 6.4† |
Endothelial cell invasion was assessed in the Boyden chamber using a Matrigel-coated filter. HUVECs were added to the upper compartment of the chamber, with or without vesicles (9 μg/well) and the indicated concentration of inhibitor. Results are expressed as the number of migrated endothelial cells in 10 high-power fields (mean and SD of triplicates).
*P ≤ 0.01.
†P ≤ 0.05 (Mann-Whitney U test).
Vesicles also stimulated the formation of capillary-like structures by endothelial cells seeded on a three-dimensional matrix (Matrigel). The addition of vesicles to HUVECs in medium containing a low concentration of serum (Figure 7A) ▶ stimulated the formation of cords to much the same extent as in complete medium (Figure 7B) ▶ , supplemented with a high concentration of serum and growth factors. The addition of as little as 0.01 μg of vesicle protein to the cells stimulated cord formation (Figure 7C) ▶ . However, the addition of larger amounts of vesicles had an inhibitory effect on cord formation (Figure 7D) ▶ , suggesting that the process requires an optimal concentration of proteases.
Figure 7.
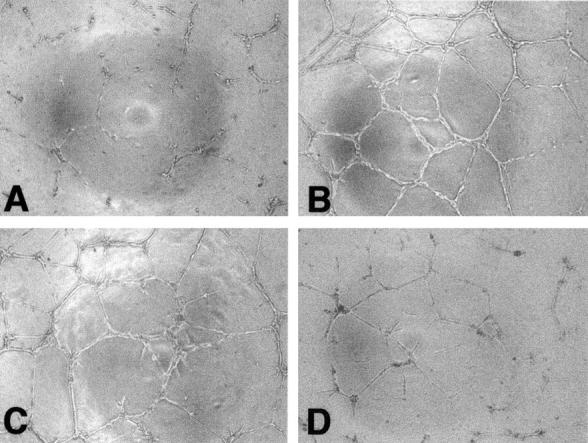
Effect of vesicles on the formation of capillary-like structures by endothelial cells. HUVECs were plated on Matrigel, in medium with 5% serum (A), complete medium (B), medium with 5% serum containing vesicles 0.01 μg/well (C), or 1 μg/ml (D). Pictures were taken after 24 hours. Original magnifications, ×100.
Discussion
Although enormous progress has been made in the last few years in elucidating the mechanisms of how blood vessels are formed in normal and pathological processes, further studies are needed to determine the molecular mechanisms that regulate angiogenesis. In particular, MMPs have been recognized as vital mediators of angiogenesis and hence as a potential target for therapies, 13,14 but little is known about the mechanisms that regulate the proteolytic activity of endothelial cells during angiogenesis. This study provides the first evidence that endothelial cells shed protease-containing vesicles that might play a role in endothelial proteolytic activity during angiogenesis.
The release of vesicles from the cell plasma membrane is considered an additional mechanism of membrane component turnover. It occurs in specific membrane portions in which selected molecules are concentrated. 19 Ultrastructural analysis shows shedding in endothelial cells; vesicles originate from the cell plasma membrane and are morphologically similar to those produced by tumor cells. The process is rapid, and in previous studies the maximum amount of shed vesicles was recovered from conditioned medium within 3 hours of addition of fresh medium. After longer incubation the amount of recovered vesicles decreased, indicating that these structures are quickly degraded. 25 Accordingly, in endothelial cells we found that 4 hours were sufficient for the maximal release of vesicles. This speed is crucial during the early phases of angiogenesis, when endothelial cells, stimulated by angiogenic factors, promptly release proteases that allow the focal matrix degradation necessary for the early process of migration of the sprouting vessel.
Endothelial cell-derived vesicles contain MMP-2 and MMP-9 in both the active and proenzyme forms. This finding is in agreement with our previous reports that shed vesicles act as proteases carriers. 4,5,26 The rapidity of the vesicle-shedding suggests that these enzymes come from existing intracellular pools, and are not synthesized ex novo. This is borne out by the finding that both inactive and activated MMP-2 and MMP-9 accumulate in the cytosol of human endothelial cells, 16 confirming the existence of intracellular storage compartments and activation mechanisms. In addition, our previous finding that angiogenesis-stimulating factors rapidly induced secretion of MMP-2 by endothelial cells, independently of protein synthesis, 17 further suggests that endothelial cell proteolytic activity can be stimulated by quick mobilization of intracellular MMP pools.
TIMP-1 and TIMP-2, endogenous inhibitors of MMPs, present in the same shed vesicles, could exert a regulatory effect on the activity of the proteases. Their function however, extends beyond protease inhibition because TIMP-2 participates in the MT1-MMP-dependent activation of MMP-2, and both inhibitors directly affect cell functions such as proliferation and survival. 7 Therefore their presence in the vesicles could have different, unforeseeable functional consequences.
MT1-MMP, another MMP reportedly involved in angiogenesis, was also present in the vesicles on the external side of the membrane. The importance of this membrane-associated enzyme in morphogenic processes is confirmed by a recent study showing that MT1-MMP is able to directly confer cells with the ability to invade the matrix and to generate tubular structures. 27 However, we could not demonstrate a preferential contribution of MT1-MMP to Matrigel degradation in our system because, among other MMP inhibitors vesicle-stimulated cell invasion was also prevented by TIMP-1, which does not effectively inhibit MT1-MMP. MT1-MMP mediates activation of pro-MMP-2 through the formation of a ternary complex with TIMP-2. 7 It is interesting to note that the proenzyme rather than the active form of MT1-MMP was found associated with the vesicles. We cannot however exclude that some activated enzyme is present in the vesicles, either transiently or in low amounts, hence undetectable in our analysis.
All together these findings suggest that HUVEC-shed vesicles are equipped with 1) active proteases (MMP-2 and MMP-9), and are therefore ready to promote matrix degradation; and 2) the machinery (pro-MT1-MMP, pro-MMP-2, and TIMP-2) to generate active MMP-2, presumably initiated by stimuli from the environment.
Vesicles also contained the β1-integrin chain. These integrins are the main receptors for matrix components, including collagens and fibronectin, and have been implicated in the regulation of angiogenesis. 23,24 In tumor cell-derived vesicles it was suggested that this integrin promoted the interaction of the vesicles with the extracellular matrix, helping localize the degradation by proteases. 18 In this case also the presence of this integrin might indicate an association of the protease-containing vesicles with matrix components resulting in focal degradation of the matrix.
Vesicle shedding has been described in transformed and normal cells. We found that in normal (endothelial) cells this process differs in many aspects from that observed in tumor cells. Although in the latter shedding of vesicles involves the whole plasma membrane, 19 in endothelial cells it is confined to discrete regions of the plasma membrane, suggesting a more focalized and controlled process. Secondly, although in tumor cells vesicle shedding is constitutive, in normal cells the process is activated only by specific stimuli. 2 Accordingly, we found that shedding of vesicles by endothelial cells was modulated by serum and angiogenic factors. Serum, known to increase vesicle production by tumor cells, 3,4,19,25 is required for shedding by endothelial cells, because in the absence of serum the production of vesicles is hardly detectable. In addition, angiogenic factors such as VEGF and FGF-2 increase vesicle shedding. These two factors are considered the main angiogenic modulators stimulating several endothelial cell functions relevant to angiogenesis, including invasiveness and production of matrix-degrading enzymes. 10,12,28,29 While confirming the stimulatory effect of these factors on the proteolytic activity of endothelial cells, our study provides the first evidence that angiogenic factors stimulate the secretion of MMPs as membrane vesicle components.
Vesicles shed by endothelial cells are involved in the autocrine regulation of cell interactions with the extracellular matrix because they increase endothelial cell invasion and cord formation. Little is known about the mechanisms of shed vesicle-enhanced cell invasiveness. 5,30 A pioneer study in this field by Poste and Nicolson 31 indicated that fusion of vesicles with the plasma membrane of tumor cells (obtained with agents causing membrane fusion) was required to increase their metastatic potential. It is therefore plausible that also in our case a direct interaction of the vesicles with the endothelial cells occur. In agreement, we found that after a relatively short incubation with the vesicles (30 minutes at 37°C) endothelial cells can be washed and still retain the increased invasive potential at levels comparable to nonwashed cells (not shown). This finding strongly indicates that a rapid association of vesicles with the cell is the basis for the functional activation. Further studies are needed to clarify the nature of cell-vesicle interaction, to verify whether the vesicles are adsorbed to the cell surface or become actually incorporated in the target cell. Although other factors in the shed vesicles may contribute, MMPs are certainly involved in the stimulation of endothelial cell invasion and cord formation.
Interestingly, although large amounts of vesicles were required to increase HUVEC invasiveness, very low concentrations stimulated cord formation, higher ones being actually inhibitory. This is in agreement with studies on MMP-2 and MT1-MMP that reported that small amounts of these MMPs are essential for the onset of the morphogenetic program, whereas an excess of proteolytic activity prevents tubulogenesis and cause the reabsorption of the formed vasculature—through loss of the matrix-supporting scaffold necessary for maintaining the structure—and promotes cell invasion. 27,32-34 In this respect, the need for a delicate balance between proteases and their inhibitors in each phase of the angiogenic process could provide an addition functional explanation for the presence of TIMPs in vesicles shed by endothelial cells.
In conclusion our findings suggest that the shedding of protease-containing membrane vesicles by stimulated endothelial cells is a means of achieving rapid, directional proteolysis during cell migration and three-dimensional morphological organization during angiogenesis.
Acknowledgments
We thank W. G. Stetler-Stevenson for helpful comments, Sadie Aznavoorian-Cheshire for reagents and suggestions concerning the analysis of MT1-MMP, and M. Giammatteo for technical support in scanning electron microscopic analysis.
Footnotes
Address reprint requests to Dr. Vincenza Dolo, Ph.D., Dipartimento di Medicina Sperimentale, Università di L’Aquila Via Vetoio-Coppito 2, 67100 L’Aquila, Italy. E-mail: dolo@univaq.it.
Supported by grants from the Italian Ministry of University and Scientific and Technological Research (MURST), the Ministero del Lavoro e Previdenza Sociale n. 792, the Italian Association for Cancer Research (AIRC), and the Italian Foundation for Cancer Research (FIRC).
References
- 1.Taylor DD, Black PH: Shedding of plasma membrane fragments. Steinberg M eds. Developmental Biology. 1986, :pp 33-57 Plenum Press, New York [DOI] [PubMed] [Google Scholar]
- 2.Black PH: Shedding from the cell surface of normal and cancer cells. Advances in Cancer Research. 1980, :pp 75-197 New York, Academic Press [DOI] [PubMed] [Google Scholar]
- 3.Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML: Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. J Submicrosc Cytol Pathol 1994, 26:173-180 [PubMed] [Google Scholar]
- 4.Ginestra A, Monea S, Seghezzi G, Dolo V, Nagase H, Mignatti P, Vittorelli ML: Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT-1080 fibrosarcoma cells. J Biol Chem 1997, 272:17216-17221 [DOI] [PubMed] [Google Scholar]
- 5.Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A: Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis 1999, 7:131-140 [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 7.Stetler-Stevenson WG: Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 1999, 103:1237-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagase H, Woessner JF: Matrix metalloproteinases. J Biol Chem 1999, 274:21491-21494 [DOI] [PubMed] [Google Scholar]
- 9.Mignatti P, Tsuboi R, Robbins E, Rifkin DB: In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol 1989, 108:671-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelius LA, Nehring LC, Roby JD, Parks WC, Welgus HG: Human dermal microvascular endothelial cells produce matrix metalloproteinases in response to angiogenic factors and migration. J Invest Dermatol 1995, 105:170-176 [DOI] [PubMed] [Google Scholar]
- 11.Hanemaaijer R, Koolwijk P, Le Clercq L, de Vree WJA, van Hinsbergh VWM: Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells. Biochem J 1993, 296:803-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unemori EN, Bouhana KS, Werb Z: Vectorial secretion of extracellular matrix proteins, matrix-degrading proteinases, and tissue inhibitor of metalloproteinases by endothelial cells. J Biol Chem 1990, 265:445-451 [PubMed] [Google Scholar]
- 13.Giavazzi R, Taraboletti G: Preclinical development of metalloproteasis inhibitors in cancer therapy. Crit Rev Oncol Hematol 2000, 37:53-60 [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo M, Eckhardt SG: Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 2001, 93:178-193 [DOI] [PubMed] [Google Scholar]
- 15.Werb Z: ECM and cell surface proteolysis: regulating cellular ecology. Cell 1997, 91:439-442 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen M, Arkell J, Jackson CJ: Active and tissue inhibitor of matrix metalloproteinase-free gelatinase B accumulates within human microvascular endothelial vesicles. J Biol Chem 1998, 273:5400-5404 [DOI] [PubMed] [Google Scholar]
- 17.Taraboletti G, Sonzogni L, Vergani V, Hosseini G, Ceruti R, Ghilardi C, Bastone A, Toschi E, Borsotti P, Scanziani E, Giavazzi R, Pepper MS, Stetler-Stevenson WG, Bani MR: Posttranscriptional stimulation of endothelial cell matrix metalloproteinases 2 and 1 by endothelioma cells. Exp Cell Res 2000, 258:384-394 [DOI] [PubMed] [Google Scholar]
- 18.Dolo V, Adobati E, Canevari S, Picone MA, Vittorelli ML: Membrane vesicles shed into the extracellular medium by human breast carcinoma cells carry tumor-associated surface antigens. Clin Exp Metastasis 1995, 13:277-286 [DOI] [PubMed] [Google Scholar]
- 19.Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, Nagase H, Canevari S, Pavan A, Vittorelli ML: Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res 1998, 58:4468-4474 [PubMed] [Google Scholar]
- 20.Vergani V, Garofalo A, Bani MR, Borsotti P, Parker MP, Drudis T, Mazzarol G, Viale G, Giavazzi R, Stetler-Stevenson WG, Taraboletti G: Inhibition of matrix metalloproteinases by over-expression of tissue inhibitor of metalloproteinase-2 inhibits the growth of experimental hemangiomas. Int J Cancer 2001, 91:241-247 [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini R, Bazzini P, Tosi E, Tagliabue E, Conforti G, Dejana E, Ménard S, Colnaghi MI: Production and characterization of two monoclonal antibodies directed against the integrin β1 chain. Tumori 1992, 78:1-4 [DOI] [PubMed] [Google Scholar]
- 22.Taraboletti G, Morbidelli L, Donnini S, Parenti A, Granger HJ, Giavazzi R, Ziche M: The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB J 2000, 14:1674-1676 [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Bell K, Mousa SA, Barner JA: Regulation of angiogenesis in vivo by ligation of integrin 5b1 with the central cell-binding domain of fibronectin. Am J Pathol 2000, 156:1345-1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo CR, Pihlajaniemi T, Alitalo K, Vuori K: Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci USA 2001, 98:1024-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassara D, Ginestra A, Dolo V, Miele M, Caruso G, Lucania G, Vittorelli ML: Modulation of vesicle shedding in 8701 BC human breast carcinoma cells. J Submicrosc Cytol Pathol 1998, 30:45-53 [PubMed] [Google Scholar]
- 26.Angelucci A, D’Ascenzo S, Festuccia C, Gravina GL, Bologna M, Dolo V, Pavan A: Vesicle-associated uPA system promotes invasion in prostate cancer cells. Clin Exp Metastasis 2000, 18:157-164 [DOI] [PubMed] [Google Scholar]
- 27.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ: Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol 2000, 149:1309-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamoreaux WJ, Fitzgerald ME, Reiner A, Hasty KA, Charles ST: Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res 1998, 55:29-42 [DOI] [PubMed] [Google Scholar]
- 29.Mignatti P, Tsuboi R, Robbins E, Rifkin DB: In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induce proteinases. J Cell Biol 1989, 108:671-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zucker S, Wieman JM, Lysik RM, Wilkie DP, Ramamurthy N, Lane B: Metastatic mouse melanoma cells release collagen-gelatin degrading metalloproteinases as components of shed membrane vesicles. Biochim Biophys Acta 1987, 924:225-237 [DOI] [PubMed] [Google Scholar]
- 31.Poste G, Nicolson GL: Arrest and metastasis of blood-born tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci USA 1980, 77:399-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D’Orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, Quigley JP, Kleinman HK: Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol 1993, 156:235-246 [DOI] [PubMed] [Google Scholar]
- 33.Zhu WH, Guo X, Villaschi S, Nicosia RF: Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest 2000, 80:545-555 [DOI] [PubMed] [Google Scholar]
- 34.Quaranta V: Cell migration through extracellular matrix: membrane-type metalloproteinases make the way. J Cell Biol 2000, 149:1167-1170 [DOI] [PMC free article] [PubMed] [Google Scholar]