Abstract
Hepatic resection in cirrhotic patients is associated with impaired liver regeneration and poor clinical outcome. Because experimental cirrhosis is associated with hepatic cell hypoxia, we herein investigated whether hypoxia might alter the mechanisms of liver regeneration in the cirrhotic liver. Cirrhosis was induced by diethylnitrosamine in rats. Immunohistochemistry was performed to assess hepatocellular hypoxia and proliferation 24 hours after a two-thirds partial hepatectomy (PH) in cirrhotic and control rats. Cultured hepatocytes and myofibroblastic hepatic stellate cells were submitted to hypoxia using anaerobic jars. Hepatocyte growth factor (HGF) and c-Met expressions were determined by reverse transcriptase-polymerase chain reaction, Northern blot, and Western blot. In control rats, hypoxia was restricted to perivenular hepatocytes, and PH induced a marked increase in hepatocyte proliferation and in liver HGF expression, whereas c-Met expression remained unchanged. In cirrhotic rats, hypoxia was detected virtually in all of the hepatocytes, and PH induced no significant change in hepatocyte proliferation and in liver HGF expression, whereas c-Met expression was decreased as compared to normal livers. In vitro, the expression of HGF in myofibroblastic hepatic stellate cells and of c-Met in hepatocytes underwent a dramatic decrease under hypoxia. Our results suggest that hepatocellular hypoxia causes inhibition of HGF (and of c-Met)-mediated proliferation and thereby might contribute to liver regeneration failure in cirrhotic liver.
Cirrhotic liver regenerates less actively than normal liver after partial hepatectomy (PH) 1-3 and liver resection in cirrhotic patients is often followed by higher morbidity and mortality than in noncirrhotic patients. 4 It was recently shown that liver regeneration in rats with CCl4-induced cirrhosis is deficient and associated with a lower level of signals that normally promote liver growth. 5 Yet, the molecular mechanisms responsible for impaired regeneration in the cirrhotic liver are still poorly known.
Hepatocyte growth factor (HGF), a ligand for the c-Met proto-oncogene product, is the most potent stimulator of hepatocyte proliferation. 6 HGF exerts multiple biological properties in the liver, including mitogenic, 7 anti-fibrotic, 8 and cytoprotective 9 activities. In the liver, HGF is produced by nonparenchymal cells, 10 such as hepatic stellate cells (HSCs), sinusoidal endothelial cells, and Kupffer cells, and targets parenchymal hepatocytes, 11 endothelial cells, 12 and bile duct epithelial cells. 13 HGF is one of the major mitogens early engaged in liver regeneration after PH and liver injury. 14-17
In a previous study, we have shown that experimental biliary cirrhosis is invariably associated with hepatocellular hypoxia. 18 To determine whether hypoxia might directly influence the mechanisms of liver regeneration in cirrhotic liver, we investigated the relationship between local hypoxia and posthepatectomy expression of HGF and of c-Met in experimental cirrhosis. We also tested whether in vitro hypoxia influenced the expression of HGF in myofibroblastic HSCs and that of c-Met in hepatocytes.
Materials and Methods
Animal Model
Male Wistar rats, weighing 200 to 225 g, received diethylnitrosamine (Sigma, St Quentin en Yvelines, France) at a dosage of 100 mg/kg of body weight (n = 5) or 0.9% sodium chloride (controls, n = 5) intraperitoneally once a week for 6 weeks. Two weeks after the last injection, a standard two-thirds PH was performed, as previously described. 19 Rats were killed 24 hours after hepatectomy. One hour before killing, they received a single intravenous injection of pimonidazole (Natural Pharmacia Int., Belmont, MA) at a dosage of 120 mg/kg of body weight. In this study involving animal experimentation, we provide assurance that all animals received humane care according to criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
Histology and Immunohistochemistry
Liver samples were fixed in 10% buffered formalin, paraffin-embedded, and sectioned at 4 μm. For standard histology, sections were stained with hematoxylin-eosin-safran. Immunolabeling was performed using monoclonal antibodies directed against pimonidazole (1:200; Natural Pharmacia Int.) and Ki-67 (1:200; Novocastra, France). An avidin-biotin-peroxidase technique was used for detection of immunostained cells. Pimonidazole immunoreactivity served as a marker of cell hypoxia, as previously described. 20 Hepatocyte proliferation was assessed by determining the percentage of Ki-67-positive hepatocyte nuclei, as previously described. 21
Cell Isolation and Culture
Hepatocytes were isolated from normal Wistar rats by a method derived from Seglen. 22 In brief, after in situ perfusion of the liver with 0.025% collagenase (Boehringer Mannheim, Meglan, France), dispersed hepatocytes were filtered through a 100-μm gauze, then centrifuged twice at 600 rpm. Hepatocytes were 85 to 90% pure, and cell viability exceeded 90% as tested by erythrosin exclusion. Cells were plated at an initial density of 1.0 × 10 5 cells/cm 2 in 100-mm collagen I-coated culture dishes. After 4 hours, the medium was replaced by a serum-free medium with 1 μmol/L of hydrocortisone hemisuccinate and insulin (0.25 U/ml medium). Medium was changed at 24 hours, then hepatocytes were subjected to hypoxia. HSCs were isolated from normal Wistar rats as previously described. 23 In brief, after in situ perfusion of the liver with 0.18% pronase and 0.025% collagenase (both from Boehringer Mannheim), dispersed cells were fractionated by centrifugation through a 8.2% Nycodenz density gradient (Sigma) and HSCs were removed from the upper fraction. HSCs were >99% pure, and cell viability exceeded 90% as tested by erythrosin exclusion. Culture-activated myofibroblastic HSCs were used within passages 3 and 5. Myofibroblastic HSCs were serum-starved 1 hour before hypoxic treatment.
Hypoxic Treatment
Hypoxic condition was achieved using AnaeroGen system (Oxoid, Dardilly, France), which catalytically reduces oxygen concentration to <1% within 30 minutes. 24 In brief, culture dishes were placed into a 2.5-L air-tight jar with an AnaeroGen sachet, and the lid was closed immediately. Then, the jar was incubated at 37°C until opening. Controls included parallel cell cultures in normoxia.
Assay of Cell Toxicity
Toxicity of hypoxia on hepatocytes and culture-activated HSCs was assessed by determining the release of lactate dehydrogenase activity in the medium as previously described. 25
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNAs were extracted from frozen tissue samples and cells by a guanidium thiocyanate-based method (Trizol; GIBCO BRL, Bethesda, MD). First strand cDNA was generated with MMLV reverse transcriptase using 5 μg of total RNAs and pd(N)6 primers (Pharmacia Biotech, Europe Orsay, France). The primers were designed according to the published rat cDNA sequences in EMBL: HGF, accession number D90102; c-Met, X96786; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), AF106860; vascular endothelial growth factor (VEGF), M32167; albumin, V01222; transforming growth factor (TGF)-β1, X52498; 28S rRNA, M11120 (primers sequences are available on request). Target genes were amplified using 75 ng of reverse-transcribed total RNAs. Each sense primer was labeled with a 5′-fluorescent dye (6-FAM). The PCR conditions were defined to assess the relative rate of each target during the ascendent amplification phase. PCR products were quantified by fluorodensitometry using the Genescan Software of an ABI model 373A automated DNA sequencer (Applied Biosystems, Applera France, Courtaboeuf, France).
Northern Blot Analysis
Five μg of total RNA were subjected to electrophoresis through a 1.2% agarose-formaldehyde gel, and blotted onto a nylon membrane filter. After prehybridization, the filter was incubated overnight at 42°C in a 50% formamide buffer with a 32P-labeled probe. cDNA probes for c-Met, VEGF, and albumin were generated by RT-PCR as described above. After washing, the filter was exposed on X-ray film and results were quantitated by scanning densitometry. A 28S rRNA oligonucleotide probe was used for normalization. 26
Western Blot Analysis
Proteins were extracted from cells as previously described. 18 Ten μg of protein were subjected to electrophoresis through a 9- to-12% sodium dodecyl sulfate-polyacrylamide gel, and transferred to a nitrocellulose membrane. After blocking of nonspecific binding sites, filters were incubated at 4°C with a polyclonal antibody directed against human HGF-α (1:300), murine c-Met (1:200), and human TGF-β1 (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA) overnight. Revelation was performed by a chemiluminescence-based method (ECL; Pharmacia Biotech Europe, Orsay, France).
Measurement of cAMP Level
Intracellular concentration of cAMP in culture-activated HSCs was measured using a radioimmunoassay kit according to the manufacturer’s instructions (NEN, Life Science Products, Paris, France).
Statistical Analyses
Independent means were compared by the Mann-Whitney U test. Paired means were compared by the Wilcoxon signed rank test. Data are expressed as means ± SEM. All reported P values are two-sided, and a P value <0.05 was considered statistically significant.
Results
Hepatocellular Hypoxia Is Associated with Impairment of Liver Regeneration
Liver regeneration was induced in normal and diethylnitrosamine-treated rats by a standard two-thirds PH. At the time of surgery, all diethylnitrosamine-treated rats had histologically demonstrated cirrhosis. Twenty-four hours after PH, hepatocellular hypoxia was evidenced by immunodetection of pimonidazole adducts on liver tissue sections (Figure 1, A and C) ▶ . Whereas pimonidazole immunolabeling was of low intensity and mainly detected in perivenular hepatocytes in control livers (Figure 1A) ▶ , almost all of the hepatocytes in cirrhotic livers were labeled and in ∼30% of them the labeling was highly intense (Figure 1C) ▶ .
Figure 1.
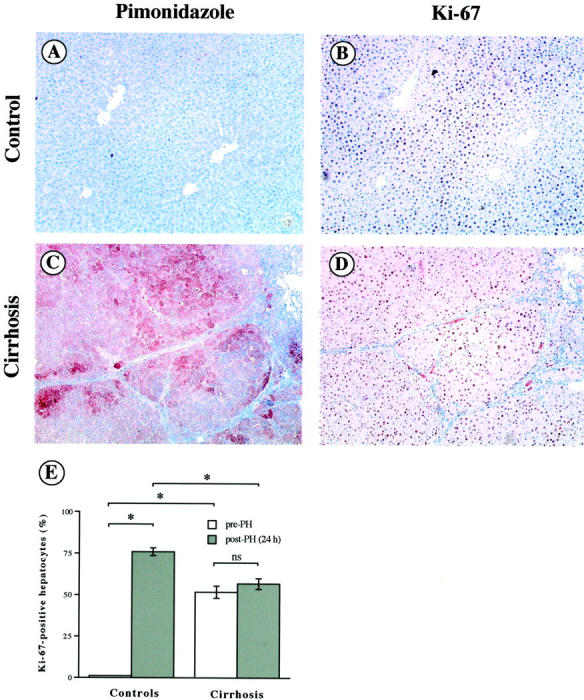
Hepatocellular hypoxia and posthepatectomy proliferation in control and cirrhotic livers on adjacent liver section samples. Hepatocellular hypoxia was assessed 24 hours after PH in control (A) and cirrhotic (C) liver samples by pimonidazole adducts immunolabeling (original magnifications, ×200). Hepatocellular proliferation was assessed 24 hours after PH in control (B) and cirrhotic (D) liver samples by Ki-67 immunolabeling (original magnifications, ×200). E: Percentage of Ki-67-positive hepatocytes (means ± SEM) was assessed on liver tissue sections from control and cirrhotic livers before (open bars) and 24 hours after PH (filled bars). *, P < 0.05.
Hepatocellular proliferation was assessed 24 hours after PH in control (Figure 1B) ▶ and cirrhotic (Figure 1D) ▶ livers by immunolabeling of Ki-67 nuclear antigen. Hepatocyte proliferation was quantified by counting the Ki-67-positive hepatocyte nuclei on liver tissue sections (Figure 1E) ▶ . Before PH, the hepatocyte proliferation was higher in cirrhotic than in control livers (52 ± 4% versus 1 ± 0%, P < 0.05). After PH, there was a marked increase in hepatocyte proliferation in control livers (76 ± 2% versus 1 ± 0%, P < 0.05), whereas the percentage of proliferating hepatocytes remained unchanged in cirrhotic livers (57 ± 3% versus 52 ± 4%, ns) (Figure 1E) ▶ .
The percentage of Ki-67-positive hepatocytes was compared in five nodules showing no or a very faint pimonidazole cytoplasmic staining and in five nodules showing a strong pimonidazole staining (≥50% of the cells) in a representative cirrhotic liver sample before PH. An average of 100 to 250 hepatocytes in two independent fields by nodules was evaluated. The percentage of Ki-67-positive hepatocytes was significantly lower in pimonidazole-positive nodules (931 of 1698 hepatocytes; 54.8%) than in pimonidazole-negative nodules (1153 of 1376 hepatocytes; 83.3%; P < 0.001).
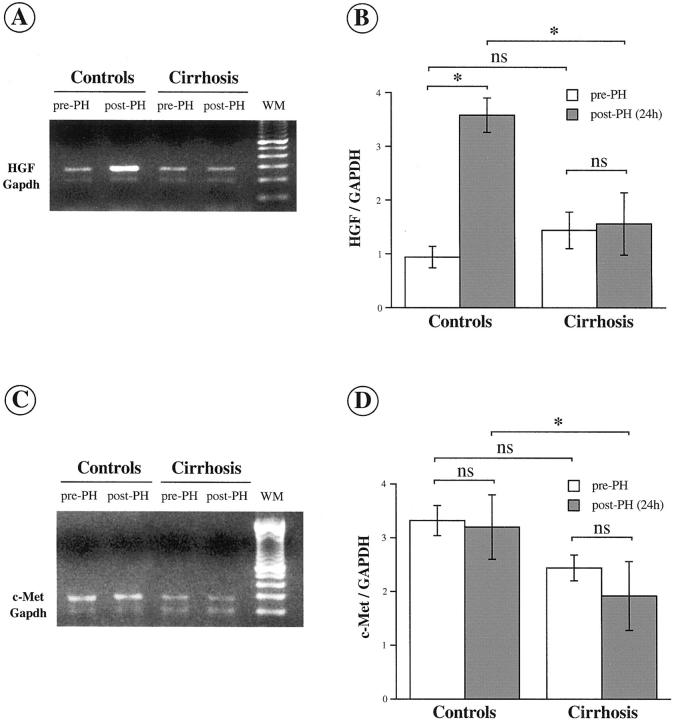
The expression of HGF (Figure 2A) ▶ and of c-Met (Figure 2C) ▶ transcripts was assessed by semiquantitative RT-PCR in control and cirrhotic livers at 0 hours and at 24 hours after PH. Results were normalized based on GAPDH expression (Figure 2, B and D) ▶ . PH was associated with a marked and significant increase in HGF mRNA level in control but not in cirrhotic livers (Figure 2B) ▶ . Whereas PH induced no significant change in c-Met mRNA level in control as well as in cirrhotic livers, the abundance of c-Met mRNA was significantly lower in cirrhotic than in control livers after PH (Figure 2D) ▶ .
Figure 2.
Changes in HGF and c-Met mRNA expression after PH in control and cirrhotic livers. HGF (A) and c-Met (C) transcripts were co-amplified with GAPDH mRNA by using RT-PCR in control and cirrhotic liver samples before and 24 hours after PH. RT-PCR products were quantified within the ascending phase of amplification by fluorodensitometry and the results were normalized using GAPDH amplification. Semiquantification of HGF (B) and c-Met (D) is shown as means ± SEM (n = 5 animals in each group). *, P < 0.05.
Hypoxia Induces Inhibition of HGF and c-Met Expressions in Hepatic Cells
Rat hepatocytes and culture-activated myofibroblastic HSCs were subjected to hypoxia (<1% oxygen tension), whereas control cells were maintained under normoxia. Toxicity of hypoxia was assessed in both cell types by determining the release of lactate dehydrogenase activity in the medium (data not shown). Noncytotoxic durations of hypoxia, ie, ≤2 hours in hepatocytes and ≤24 hours in myofibroblastic HSCs, were used in subsequent experiments.
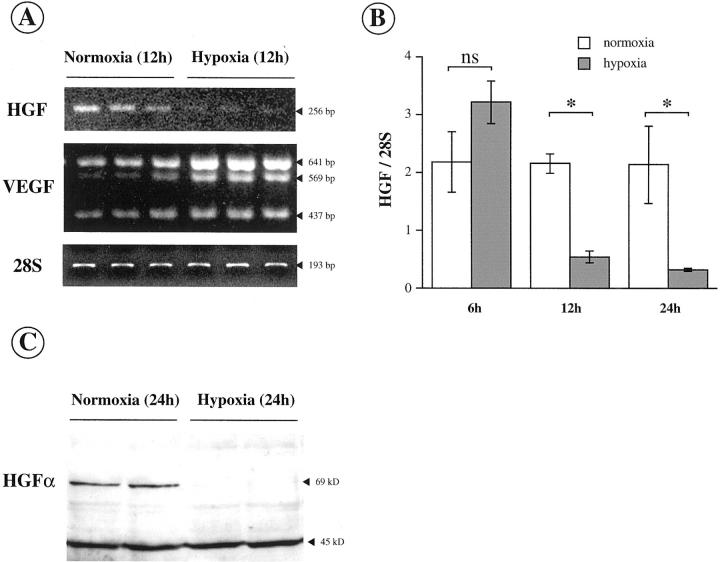
HGF expression was evaluated in activated HSCs under hypoxic and normoxic conditions by semiquantitative RT-PCR (Figure 3A) ▶ and by Western blot (Figure 3C) ▶ . VEGF mRNA expression, which is known to be hypoxia-induced, and the amount of 28S rRNA, used as an internal standard, were determined in parallel by RT-PCR (Figure 3A) ▶ . HGF mRNA level was significantly decreased at 12 hours and at 24 hours of hypoxia (Figure 3B) ▶ , whereas VEGF mRNA level was increased in the same conditions (Figure 3A) ▶ . Consistent with these RT-PCR results, HGF expression was at the limit of detection by Western blot at 24 hours of hypoxia (Figure 3C) ▶ .
Figure 3.
Hypoxia inhibits HGF expression in culture-activated myofibroblastic HSCs. A: HGF mRNA expression was assessed by semiquantitative RT-PCR in HSCs maintained under normoxia or exposed to hypoxia. VEGF was used as a control of hypoxia-induced gene. 28S rRNA served as an internal standard. B: Semiquantification of HGF mRNA level (means ± SEM, n = 3) in HSCs exposed to normoxia (open bars) or hypoxia (filled bars) for 6, 12, and 24 hours. C: HGF expression was estimated by Western blot in HSCs exposed to normoxia or hypoxia for 24 hours. Additional evidence for the specificity of the results is the detection of nonspecific bands sized at 45 kd that are not influenced by hypoxia.
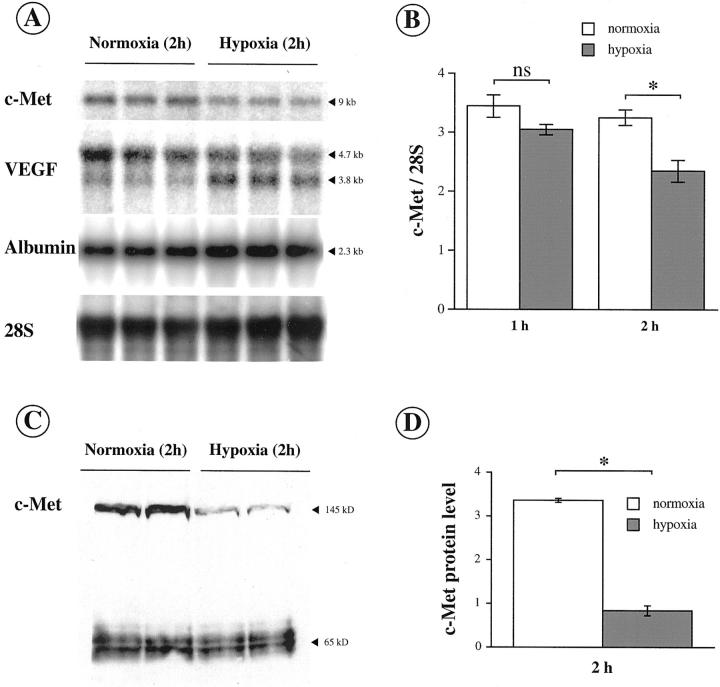
C-Met expression was assessed in hepatocytes under hypoxic and normoxic conditions by Northern blot (Figure 4, A and B) ▶ and by Western blot (Figure 4, C and D) ▶ . The amounts of VEGF, albumin and 28S RNA were quantified by Northern blot in the same time (Figure 4A) ▶ . C-Met mRNA level was significantly decreased after 2 hours of hypoxia (Figure 4B) ▶ , whereas in the same conditions VEGF level was induced and albumin mRNA level, an index of hepatocyte function, remained unchanged (Figure 4A) ▶ . Consistent with these results, Western blot data confirmed the specific and significant decrease in c-Met expression after 2 hours of hypoxia (Figure 4, B and D) ▶ .
Figure 4.
Hypoxia inhibits c-Met expression in hepatocytes. A: c-Met mRNA expression was determined by Northern blot in hepatocytes in primary culture maintained under normoxia or exposed to hypoxia. VEGF was used as a control of hypoxia-induced gene. Albumin was used as a control gene of hepatocellular function. 28S rRNA served as an internal standard. B: Quantification of c-Met mRNA level (means ± SEM, n = 3) in hepatocytes under normoxia (open bars) or hypoxia (filled bars) for 1 and 2 hours. C: c-Met expression was estimated by Western blot in hepatocytes exposed to normoxia or to hypoxia for 2 hours. Additional evidence for the specificity of the results is the detection of nonspecific bands sized at 65 kd that are not influenced by hypoxia. D: Quantification of c-Met protein level (means ± SEM, n = 3) in hepatocytes under normoxia (open bars) or hypoxia (filled bars) for 2 hours. *, P < 0.05.
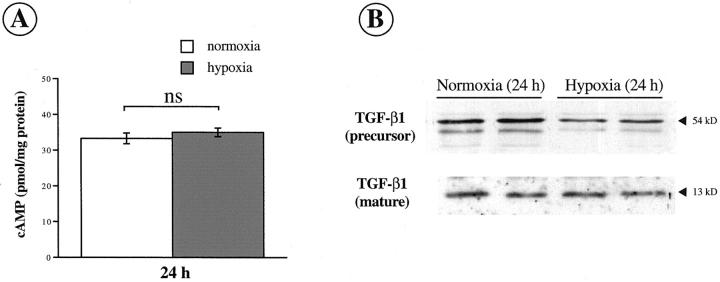
Inhibition of HGF by Hypoxia in Activated HSCs Is Not Mediated by cAMP or TGF-β1
It has been shown that cAMP stimulated HGF production 27,28 whereas TGF-β1 down-regulated HGF expression in different cell types. 29-31 To determine the influence of hypoxia on these two factors, we measured the concentration of cAMP by radioimmunoassay and assessed the expression of TGF-β1 by Western blot in myofibroblastic activated HSCs under normoxic and hypoxic conditions (Figure 5, A and B) ▶ . The concentration of intracellular cAMP remained unchanged after 24 hours of hypoxia (Figure 5A) ▶ , whereas HGF mRNA and protein were decreased in the same conditions (see Figure 3, B and C ▶ ). Similarly, the amount of TGF-β1 protein (precursor and mature forms) did not increase after 24 hours of hypoxia (Figure 5B) ▶ . Northern blot data confirmed the absence of increase in TGF-β1 expression in myofibroblastic HSCs after 24 hours of hypoxia (data not shown).
Figure 5.
Inhibition of HGF by hypoxia in activated HSCs is not mediated by cAMP or TGF-β1. A: Quantification of cAMP concentration by radioimmunoassay in HSCs under normoxia (open bars) and hypoxia (filled bars) for 24 hours (means ± SEM, n = 3). B: The expression of precursor (54 kd) and mature (13 kd) forms of TGF-β1 was studied by Western blot in HSCs maintained under normoxia and exposed to hypoxia for 24 hours.
Discussion
The present study, in keeping with our previous observations, 18 shows that local hypoxia is a constant event in cirrhotic livers. We show that hepatocyte proliferation is decreased in hypoxic nodules as compared to normoxic nodules in the same representative cirrhotic liver sample. We herein demonstrate that HGF expression and hepatocyte proliferation in the cirrhotic livers do not increase after PH as opposed to normal livers. In addition, we show that c-Met expression after PH is significantly lower in cirrhotic than in normal livers. Finally, we demonstrate that in vitro hypoxia directly inhibits the expression of HGF in myofibroblastic HSCs and that of c-Met in hepatocytes. Taken together, these results indicate that hepatocellular hypoxia, through the inhibition of HGF and of its receptor c-Met, might contribute to liver regeneration failure in cirrhosis.
We observe, first, that hepatocyte proliferation activity is increased in diethylnitrosamine-induced cirrhotic livers as compared to control livers, and secondly, that cirrhotic livers show a significantly depressed capacity for regeneration after PH. These findings are consistent with data obtained in other experimental models of cirrhosis. 1,3 In addition, our in vivo data show that local hypoxia is associated with an impaired capacity of the liver to regenerate. Hepatocellular hypoxia in cirrhotic livers might be explained by the impairment of sinusoidal permeability and perfusion, 32-34 which could result from several mechanisms, including intrahepatic shunts, 35,36 venoocclusive lesions, 37,38 and capillarization of sinusoids. 39,40 Indirect evidence of hypoxia influence on hepatic function has been provided by pharmacological studies showing that oxygen supply in patients with cirrhosis increased the hepatic clearance of drugs and restored hepatocyte energy status. 41,42
In keeping with our findings, a decrease in c-Met expression has been observed in CCl4-induced cirrhotic liver after PH. 5 Furthermore, a direct effect of hypoxia has been shown on c-Met expression in isolated rat pancreatic islets 43 and on HGF expression in vascular cells. 44 Consistent with these observations, our results support the hypothesis that the hypoxia that occurs in the cirrhotic liver exerts a direct inhibitory effect on both HGF and c-Met expressions in hepatic cells. Moreover, because HGF was found to up-regulate its own receptor, 45,46 one can suggest that decreased expression of c-Met in the cirrhotic liver might result from both a direct effect of local hypoxia and HGF decrease. Finally, as shown for VEGF, hypoxia might down-regulate c-Met expression not only at the transcriptional level but also at the translational and/or posttranslational levels resulting in the different decreases observed between c-Met protein and mRNAs. The same comment applies to HGF protein and mRNAs but further studies are required to confirm this hypothesis.
Although we found a similar expression of HGF and c-Met transcripts in cirrhotic livers as compared to control livers before PH, the percentage of Ki-67-positive hepatocytes in cirrhotic nodules was significantly increased, illustrating a low but permanent increased regenerative activity in cirrhotic livers. This regenerative activity might be because of other growth factors such as epidermal growth factor and TGF-α, which are also involved in the liver regeneration 6 and are increased in cirrhotic livers unlike HGF. Furthermore, it has been observed that endogenous HGF expression in rat cirrhotic livers could be enhanced by human HGF gene therapy, suggesting that the very low basal expression of HGF in cirrhotic livers is probably not maximal and might be stimulated. 46 These data and our present results support the hypothesis that HGF is significantly decreased in cirrhotic livers both before and after PH.
It has been shown that cirrhosis is associated with a progressive increase in epidermal growth factor expression and a trend toward TGF-α up-regulated expression in rats. 47 In addition, increased TGF-α expression has been observed in vivo in the serum and in the liver of most cirrhotic patients, mainly in regenerating hepatocytes of cirrhotic nodules. 48-50 Moreover, it has been recently shown that TGF-α might be hypoxia-induced (as well as VEGF) through the von Hippel-Lindau tumor suppressor. 51 However, although epidermal growth factor and TGF-α are increased in cirrhotic livers (maybe in part as a result of hypoxia), liver regeneration remains impaired suggesting that hypoxia-induced HGF deficiency might play a significant role in this negative effect.
Our hypoxic conditions in vitro are probably more severe than those observed in cirrhotic livers as ascertained by pimonidazole assay. This may explain the difference in c-Met decreases observed in vivo and in vitro. However, the residual expression of c-Met in the cirrhotic liver is sufficient to allow cell proliferation. Indeed, HGF administration in rats treated with dimethylnitrosamine prevents the development of cirrhosis when administrated along with dimethylnitrosamine, accelerates the recovery from liver cirrhosis, and prevents death because of hepatic dysfunction when given later. 8 Similar results have been obtained after HGF gene therapy. 46 In addition, continuous intravenous infusion of HGF enhances the growth and function of the remnant liver in rats with cirrhosis after PH. 17 These findings support the hypothesis that hypoxia-mediated regeneration failure depends on reduction of HGF production in myofibroblastic-activated HSCs rather than on reduced c-Met expression in hepatocytes.
The molecular mechanism of HGF inhibition in HSCs in response to hypoxia is still unclear. It has been suggested that down-regulation of HGF by hypoxia in vascular cells might be because of an early decrease in cAMP concentration, 44 and potentially to a late increase in TGF-β, a potent growth inhibitor of hepatocytes that inhibits HGF expression. 29,52-54 In the present study, we did not observe a significant decrease in cAMP concentration or an increase in TGF-β1 expression in culture-activated HSCs after 24 hours of hypoxia, whereas HGF expression was down-regulated. These results suggest that cAMP and TGF-β1 do not play a major role in the inhibition of HGF by hypoxia in our experimental conditions.
In conclusion, our study provides evidence that hepatocellular hypoxia inhibits HGF and to a lesser extent c-Met expressions and might thereby interfere with the regeneration of the cirrhotic liver. These data suggest that treatments susceptible to reduce hepatic cell hypoxia might improve liver regeneration and prevent the onset of liver failure in cirrhotic patients.
Acknowledgments
We thank Colette Rey for expert assistance and Nils Kinnman for his contribution.
Footnotes
Address reprint requests to Olivier Rosmorduc, M.D., Ph.D., Service d’Hépatologie, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, 184 rue du Faubourg Saint-Antoine, 75571 Paris Cedex 12, France. E-mail: olivier.rosmorduc@sat.ap-hop-paris.fr.
Supported by a grant from Association pour la Recherche contre le Cancer (grant ARC 9553).
References
- 1.Chijiiwa K, Nakano K, Kameoka N, Nagai E, Tanaka M: Proliferating cell nuclear antigen, plasma fibronectin, and liver regeneration rate after seventy percent hepatectomy in normal and cirrhotic rats. Surgery 1994, 116:544-549 [PubMed] [Google Scholar]
- 2.Hashimoto M, Watanabe G: Functional capacity of the cirrhotic liver after partial hepatectomy in the rat. Surgery 1999, 126:541-547 [PubMed] [Google Scholar]
- 3.Andiran F, Ayhan A, Tanyel FC, Abbasoglu O, Sayek I: Regenerative capacities of normal and cirrhotic livers following 70% hepatectomy in rats and the effect of alpha-tocopherol on cirrhotic regeneration. J Surg Res 2000, 89:184-188 [DOI] [PubMed] [Google Scholar]
- 4.Franco D, Capussotti L, Smadja C, Bouzari H, Meakins J, Kemeny F, Grange D, Dellepiane M: Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology 1990, 98:733-738 [PubMed] [Google Scholar]
- 5.Masson S, Scotte M, Francois A, Coeffier M, Provot F, Hiron M, Teniere P, Fallu J, Salier JP, Daveau M: Changes in growth factor and cytokine mRNA levels after hepatectomy in rat with CCl(4)-induced cirrhosis. Am J Physiol 1999, 277:G838-G846 [DOI] [PubMed] [Google Scholar]
- 6.Michalopoulos GK, DeFrances MC: Liver regeneration. Science 1997, 276:60-66 [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Nawa K, Ichihara A: Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun 1984, 122:1450-1459 [DOI] [PubMed] [Google Scholar]
- 8.Matsuda Y, Matsumoto K, Yamada A, Ichida T, Asakura H, Komoriya Y, Nishiyama E, Nakamura T: Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology 1997, 26:81-89 [DOI] [PubMed] [Google Scholar]
- 9.Okano J, Shiota G, Kawasaki H: Protective action of hepatocyte growth factor for acute liver injury caused by D-galactosamine in transgenic mice. Hepatology 1997, 26:1241-1249 [DOI] [PubMed] [Google Scholar]
- 10.Maher JJ: Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest 1993, 91:2244-2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T: Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology 1992, 16:1227-1235 [PubMed] [Google Scholar]
- 12.Seto S, Kaido T, Yamaoka S, Yoshikawa A, Arii S, Nakamura T, Niwano M, Imamura M: Hepatocyte growth factor prevents lipopolysaccharide-induced hepatic sinusoidal endothelial cell injury and intrasinusoidal fibrin deposition in rats. J Surg Res 1998, 80:194-199 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ: Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology 1994, 20:376-382 [PubMed] [Google Scholar]
- 14.Lindroos PM, Zarnegar R, Michalopoulos GK: Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology 1991, 13:743-750 [PubMed] [Google Scholar]
- 15.Shiota G, Wang TC, Nakamura T, Schmidt EV: Hepatocyte growth factor in transgenic mice: effects on hepatocyte growth, liver regeneration and gene expression. Hepatology 1994, 19:962-972 [PubMed] [Google Scholar]
- 16.Patijn GA, Lieber A, Schowalter DB, Schwall R, Kay MA: Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology 1998, 28:707-716 [DOI] [PubMed] [Google Scholar]
- 17.Kaibori M, Kwon AH, Nakagawa M, Wei T, Uetsuji S, Kamiyama Y, Okumura T, Kitamura N: Stimulation of liver regeneration and function after partial hepatectomy in cirrhotic rats by continuous infusion of recombinant human hepatocyte growth factor. J Hepatol 1997, 27:381-390 [DOI] [PubMed] [Google Scholar]
- 18.Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R: Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol 1999, 155:1065-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins G, Anderson R: Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 1931, 12:186-202 [Google Scholar]
- 20.Arteel GE, Thurman RG, Yates JM, Raleigh JA: Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 1995, 72:889-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach C, Sakkab D, Scholzen T, Dabler R, Alison M, Gerdes J: Ki-67 expression during rat liver regeneration after partial hepatectomy. Hepatology 1997, 26:573-578 [DOI] [PubMed] [Google Scholar]
- 22.Seglen PO: Preparation of isolated rat liver cells. Methods Cell Biol 1976, 13:29-83 [DOI] [PubMed] [Google Scholar]
- 23.Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C: PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest 2000, 80:697-707 [DOI] [PubMed] [Google Scholar]
- 24.Imhof A, Heinzer I: Continuous monitoring of oxygen concentrations in several systems for cultivation of anaerobic bacteria. J Clin Microbiol 1996, 34:1646-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamiya T, Kwon AH, Kanemaki T, Matsui Y, Uetsuji S, Okumura T, Kamiyama Y: A simplified model of hypoxic injury in primary cultured rat hepatocytes. In Vitro Cell Dev Biol Anim 1998, 34:131-137 [DOI] [PubMed] [Google Scholar]
- 26.Barbu V, Dautry F: Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res 1989, 17:7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto K, Okazaki H, Nakamura T: Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. J Biochem (Tokyo) 1995, 117:458-464 [DOI] [PubMed] [Google Scholar]
- 28.Morishita R, Higaki J, Hayashi SI, Yo Y, Aoki M, Nakamura S, Moriguchi A, Matsushita H, Matsumoto K, Nakamura T, Ogihara T: Role of hepatocyte growth factor in endothelial regulation: prevention of high D-glucose-induced endothelial cell death by prostaglandins and phosphodiesterase type 3 inhibitor. Diabetologia 1997, 40:1053-1061 [DOI] [PubMed] [Google Scholar]
- 29.Ramadori G, Neubauer K, Odenthal M, Nakamura T, Knittel T, Schwogler S, Meyer zum Buschenfelde KH: The gene of hepatocyte growth factor is expressed in fat-storing cells of rat liver and is downregulated during cell growth and by transforming growth factor-beta. Biochem Biophys Res Commun 1992, 183:739-742 [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto K, Tajima H, Okazaki H, Nakamura T: Negative regulation of hepatocyte growth factor gene expression in human lung fibroblasts and leukemic cells by transforming growth factor-beta 1 and glucocorticoids. J Biol Chem 1992, 267:24917-24920 [PubMed] [Google Scholar]
- 31.Yo Y, Morishita R, Yamamoto K, Tomita N, Kida I, Hayashi S, Moriguchi A, Kato S, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Actions of hepatocyte growth factor as a local modulator in the kidney: potential role in pathogenesis of renal disease. Kidney Int 1998, 53:50-58 [DOI] [PubMed] [Google Scholar]
- 32.Huet P-M, Goresky C, Villeneuve J-P, Marleau D: Assessment of liver microcirculation in human cirrhosis. J Clin Invest 1982, 70:1234-1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varin F, Huet P-M: Hepatic microcirculation in the perfused cirrhotic rat liver. J Clin Invest 1985, 76:1904-1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villeneuve J-P, Dagenais M, Huet P-M, Roy A, Lapointe R, Marleau D: The hepatic microcirculation in the isolated perfused human liver. Hepatology 1996, 23:24-31 [DOI] [PubMed] [Google Scholar]
- 35.Rappaport AM, MacPhee PJ, Fisher MM, Phillips MJ: The scarring of the liver acini (cirrhosis). Tridimentional and microcirculatory consideration. Virchows Arch (Pathol Anat) 1983, 402:107-137 [DOI] [PubMed] [Google Scholar]
- 36.Gaudio E, Pannarale L, Onori P, Riggio O: A scanning electron microscopic study of liver microcirculation disarrangement in experimental rat cirrhosis. Hepatology 1993, 17:477-485 [PubMed] [Google Scholar]
- 37.Goodman ZD, Ishak KG: Occlusive venous lesions in alcoholic liver disease. A study of 200 cases. Gastroenterology 1982, 83:786-796 [PubMed] [Google Scholar]
- 38.Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G: Hepatic and portal vein thrombosis in cirrhosis: possible role in the development of parenchymal extinction and portal hypertension. Hepatology 1995, 21:1238-1247 [PubMed] [Google Scholar]
- 39.Reichen J, Egger B, Ohara N, Zeltner TB, Zysset T, Zimmermann A: Determinants of hepatic function in liver cirrhosis in the rat. Multivariate analysis. J Clin Invest 1988, 82:2069-2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onori P, Morini S, Franchitto A, Sferra R, Alvaro D, Gaudio E: Hepatic microvascular features in experimental cirrhosis: a structural and morphometrical study in CC14-treated rats. J Hepatol 2000, 33:555-563 [DOI] [PubMed] [Google Scholar]
- 41.Froomes PR, Morgan DJ, Smallwood RA, Angus PW: Comparative effects of oxygen supplementation on theophylline and acetaminophen clearance in human cirrhosis. Gastroenterology 1999, 116:915-920 [DOI] [PubMed] [Google Scholar]
- 42.Harvey PJ, Gready JE, Yin Z, Le Couteur DG, McLean AJ: Acute oxygen supplementation restores markers of hepatocyte energy status and hypoxia in cirrhotic rats. J Pharmacol Exp Ther 2000, 293:641-645 [PubMed] [Google Scholar]
- 43.Vasir B, Reitz P, Xu G, Sharma A, Bonner-Weir S, Weir GC: Effects of diabetes and hypoxia on gene markers of angiogenesis (HGF, cMET, uPA and uPAR, TGF-alpha, TGF-beta, bFGF and vimentin) in cultured and transplanted rat islets. Diabetologia 2000, 43:763-772 [DOI] [PubMed] [Google Scholar]
- 44.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, Taiji M, Noguchi H, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation 1999, 100:II301-II308 [DOI] [PubMed] [Google Scholar]
- 45.de Juan C, Sanchez A, Nakamura T, Fabregat I, Benito M: Hepatocyte growth factor up-regulates met expression in rat fetal hepatocytes in primary culture. Biochem Biophys Res Commun 1994, 204:1364-1370 [DOI] [PubMed] [Google Scholar]
- 46.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J: Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 1999, 5:226-230 [DOI] [PubMed] [Google Scholar]
- 47.Napoli J, Prentice D, Niinami C, Bishop GA, Desmond P, McCaughan GW: Sequential increases in the intrahepatic expression of epidermal growth factor, basic fibroblast growth factor, and transforming growth factor beta in a bile duct ligated rat model of cirrhosis. Hepatology 1997, 26:624-633 [DOI] [PubMed] [Google Scholar]
- 48.Komuves LG, Feren A, Jones AL, Fodor E: Expression of epidermal growth factor and its receptor in cirrhotic liver disease. J Histochem Cytochem 2000, 48:821-830 [DOI] [PubMed] [Google Scholar]
- 49.Harada K, Shiota G, Kawasaki H: Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver 1999, 19:318-325 [DOI] [PubMed] [Google Scholar]
- 50.Nalesnik MA, Lee RG, Carr BI: Transforming growth factor alpha (TGFalpha) in hepatocellular carcinomas and adjacent hepatic parenchyma. Hum Pathol 1998, 29:228-234 [DOI] [PubMed] [Google Scholar]
- 51.de Paulsen N, Brychzy A, Fournier MC, Klausner RD, Gnarra JR, Pause A, Lee S: Role of transforming growth factor-alpha in von Hippel-Lindau (VHL)(−/−) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci USA 2001, 98:1387-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A: Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun 1985, 133:1042-1050 [DOI] [PubMed] [Google Scholar]
- 53.Gohda E, Matsunaga T, Kataoka H, Yamamoto I: TGF-beta is a potent inhibitor of hepatocyte growth factor secretion by human fibroblasts. Cell Biol Int Rep 1992, 16:917-926 [DOI] [PubMed] [Google Scholar]
- 54.Okajima A, Miyazawa K, Kitamura N: Characterization of the promoter region of the rat hepatocyte-growth-factor/scatter-factor gene. Eur J Biochem 1993, 213:113-119 [DOI] [PubMed] [Google Scholar]