Abstract
INTRODUCTION
Chronic progressive external ophthalmoplegia (CPEO) is characterized by slowly progressive bilateral ophthalmoplegia and blepharoptosis. Molecular diagnosis is problematic because sporadic mitochondrial DNA deletions can be causative. We sought findings using magnetic resonance imaging (MRI) that might support the diagnosis of CPEO.
METHODS
Two men (ages 31 and 47 years) and 3 women (ages 40–49 years) with CPEO and symptom durations of 8 months to 28 years underwent high-resolution (2-mm slice thickness, 312 micron pixels), surface coil, T1-weighted orbital MRI in coronal planes. Images were analyzed quantitatively to determine extraocular muscle (EOM) sizes and were compared with 10 age- and gender-matched normal volunteers, one subject with myasthenia gravis, and with 30 subjects having EOM paralysis caused by oculomotor, trochlear,0 and abducens neuropathies.
RESULTS
EOM function was clinically diminished in CPEO, most markedly for the superior rectus (SR) and levator muscles. All EOMs in CPEO exhibited unusual qualitative T1 MRI signal abnormalities. Unlike the profound EOM atrophy typical of neurogenic paralysis, anterior volumes of medial rectus, lateral rectus, and inferior rectus muscles in CPEO were not smaller than normal ( p > 0.003). Anterior volumes of the SR muscle-levator complex and superior oblique were significantly reduced ( p < 0.003). Denervated EOMs exhibited statistically significant volume reduction when compared with normal and CPEO groups. Volume of the SR muscle-levator complex was the same in subjects with CPEO and oculomotor palsies.
CONCLUSIONS
CPEO is associated with minimal EOM volume reduction despite clinically severe weakness. This combination of findings may be specific for CPEO and could resolve the diagnostic dilemma in difficult cases.
Chronic progressive external ophthalmoplegia (CPEO) was first described by Von Graefe in 1868.1 It features insiduous onset of slowly progressive, typically symmetric, external ophthalmoplegia. The manifestations of CPEO range from involvement limited to the eyelids and extraocular muscles (EOMs) to systemic and encephalopathic features. Tissues with high oxidative metabolism, such as muscle, brain, heart, are the most affected.2–5
Molecular diagnosis of CPEO is problematic because most cases are caused by sporadic mitochondrial DNA deletions. Ragged red fibers, as demonstrated on modified trichrome stain, can be seen in limb and extraocular muscles in nearly all cases of Kearns Sayre syndrome and in some patients with isolated CPEO.6 However, this finding requires invasive biopsy justified only by a high index of suspicion for CPEO. We sought noninvasive findings that might support the diagnosis of CPEO.
Magnetic resonance imaging (MRI) now enables non-invasive visualization of living human EOMs.7 Until recently, activity of EOMs could only be indirectly inferred from examination of the orientation and movement of the eyes, supplemented in a limited way by electromyography and force generation testing. Within the last decade, improvements in MRI technology have enabled the direct examination of the locations, pulling directions, size, and contractility of each of the EOMs in living people.8 High-resolution MRI of the EOMs can now provide insight into specific causes of strabismus, challenging traditional concepts of etiology and suggesting alternative treatments.9–11
In the current study, we used orbital MRI to seek evidence of specific changes in the EOMs of patients with CPEO, comparing them with those of normal subjects, patients with ophthalmoplegia caused by well-defined cranial neuropathies, and one patient with myasthenia gravis.
Subjects and Methods
From an ongoing prospective protocol with cumulative enrollment of 303 strabismic patients undergoing high-resolution orbital MRI, the following cases were selected for analysis: (1) all 5 available subjects with CPEO (3 women, 2 men, mean ± SD age 41.8 ± 7 years); (2) the 10 most recently imaged subjects with oculomotor palsy (4 women, 6 men, mean age 33.9 ± 22.1 years); (3) the 10 most recently imaged subjects with trochlear palsy (3 women, 7 men, mean age 35.9 ± 17.7 years); (4) the 10 most recently imaged subjects with abducens palsy (4 women, 6 men, mean age 40.2 ± 15.5 years); (5) 10 age-matched normal volunteers (5 women, 5 men, mean age 47.9 ± 14.7 years); and (6) 1 subject with myasthenia gravis (a 47-year-old woman).
All subjects underwent complete ophthalmologic examination with particular attention to ocular motility. The study complied with the policies of the local institutional review board. All subjects gave written informed consent before participation.
Each subject underwent high-resolution orbital MRI using T1-weighted imaging with a 1.5-T General Electric Sigma scanner (Milwaukee, WI). Crucial aspects of this technique, described in detail elsewhere, include use of surface coils to improve signal to noise ratio.9 During imaging, subjects fixated at individual targets in central gaze. Images were obtained in sets of 17 contiguous, 2-mm thick, quasicoronal planes perpendicular to the long axis of each orbit separately, beginning just posterior to the posterior surface of the lens. Each image was obtained using a 256 × 256 matrix over an 8 cm2 field, giving pixel resolutions of 312 μm. Digital MRIs were transferred to Macintosh computers (Apple Computer, Cupertino, CA), converted to 8-bit tagged image file format and were quantitatively analyzed using the program NIH Image ( W. Rasband, National Institute of Health; available by FTP from zippy.nimh.nih.gov or on floppy disk from NTIS, Springfield, VA, part number PB 95-500195 GEI). The position of each rectus EOM was determined by its area centroid. Images of the left orbit were digitally reflected to the orientation of a right orbit for uniform analysis of EOM positions.
The SO muscle, medial rectus (MR) muscles, SR-levator complex, lateral rectus (LR) muscle, and IR muscles were imaged bilaterally over a total distance of 34 mm extending posteriorly from about the posterior lens surface. All measurements were performed by a single author, who was aware of subject diagnoses. Each EOM was outlined in each image plane to determine the cross-sectional area in square millimeters, and the 17 resulting cross-sectional areas of each orbit were summed and multiplied by the image plane thickness of 2 mm to compute EOM volume within the scanning range. It should be noted that the rectus EOMs are longer than 34 mm; therefore, EOM volumes in the orbital apex were not included in the scanning range and did not contribute to reported volumes. Analysis was performed in the 10 control subjects, 1 subject with myasthenia gravis, and 30 patients with neurogenic palsies.
Statistical results by Student t-test were considered significant at the 0.003 level (Bonferroni correction for multiple comparisons). Data for both eyes were pooled when normal or binocularly affected.
Cases of CPEO
Case 1
This 47-year-old man had progressive onset of nonfluctuating oblique binocular diplopia and bilateral ptosis since the age of 25 years. The administration of edrophonium did not alter ocular motility. Medical and family history was negative for systemic and ophthalmologic disease, indicating sporadic CPEO. In his central gaze, there was a committant 20Δ right hypertropia at distance and near and marked bilateral ophthalmoplegia. Electron microscopy of biopsied EOMs revealed abnormal mitochondria with a vacuolated appearance suggesting lipid.
Case 2
This 49-year-old woman reported a 2-year history of gradual difficulty in moving her eyes, particularly to read. There was no generalized weakness or ptosis. Her brain MRI was normal, as were her blood tests for dysthyroidism and myasthenia gravis. She presented marked ophthalmoplegia and 20Δ intermittent exotropia ( XT ) at near with fair control.
Case 3
This 40-year-old woman had a 12-year history of nonfluctuating, gradually progressive right XT and bilateral blepharoptosis. She underwent unsuccessful ptosis surgery and presented with horizontal, binocular diplopia. Her brain MRI and edrophonium testing were normal. There was no family history of CPEO. Her past medical history was positive for vertigo, arm and leg weakness, and fibromyalgia. There was marked deficiency of adduction in both eyes.
Case 4
This 40-year-old woman presented with blepharoptosis and progressive, nonfluctuating intermittent XT since the age of 14 years. There was no history of consanguinity, but a nephew in the maternal line had blepharoptosis. The patient had undergone 3 previous unsuccessful strabismus surgeries elsewhere. She complained of torsional binocular diplopia. There was marked ophthalmoplegia that was not improved by the administration of edrophonium.
Case 5
This 31-year-old man had a 9-month history of nonfluctuating horizontal and vertical binocular diplopia and progressive blepharoptosis. He related a history of extreme fatigue, imbalance, ataxia, occasional paresthesias, and dystonia muscularis deformans. No family members were similarly affected. He had undergone bilateral thalamotomy at 9 years of age with marked improvement of his dystonia. Structural brain MRI and magnetic resonance angiography were normal. Pupils were 8 mm in diameter, round, and only microscopically reactive to light in each eye. There was bilateral blepharoptosis without fatigue or lid twitch, and marked ophthalmoplegia, with bilateral slowing of adducting saccades. Forced duction and forced generation testing showed no restriction to the full rotation of the right eye.
Results
The 5 patients with CPEO had symptom duration ranging from 9 months to 22 years. A striking and uniform finding on orbital imaging of these patients was an abnormal bright signal seen within EOMs on T1-weighted imaging, deep in the orbit, posterior to the optic nerve junction with the globe. In Figure 1, the signal is seen in the MR, IR, and LR of both eyes.
FIG 1.
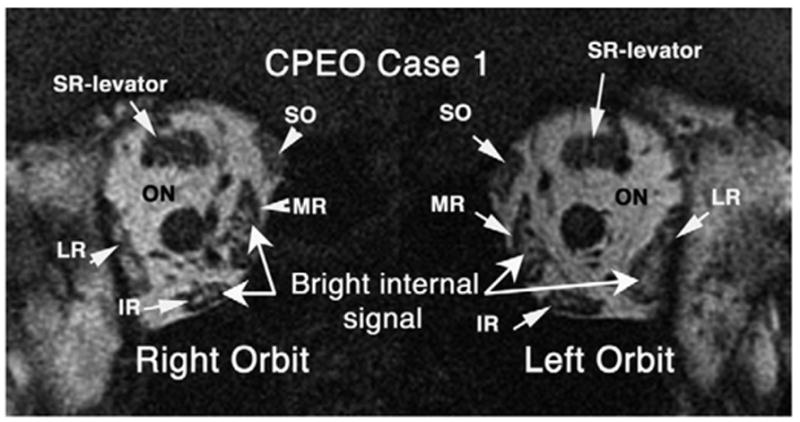
Coronal T1-weighted MRI of case 1 showing abnormal bright internal signal in multiple EOMs (right MR, right IR, left MR, left LR muscle) in the deep orbit of patient with CPEO. ON: optic nerve.
High-resolution MRI demonstrated profound atrophy of the SR-levator complex bilaterally in 3 cases. Reliability of this finding in the SR-levator complex was assured by confirmation in multiple contiguous image planes; consistent atrophy in multiple image planes was not observed in other EOMs. Figure 2 illustrates normal SR-levator size in the right orbit of a control subject, and the atrophic SR-levator of a patient with CPEO. Figure 2 also illustrates the bright internal signal in the MR and IR muscles of the patient with CPEO.
FIG 2.
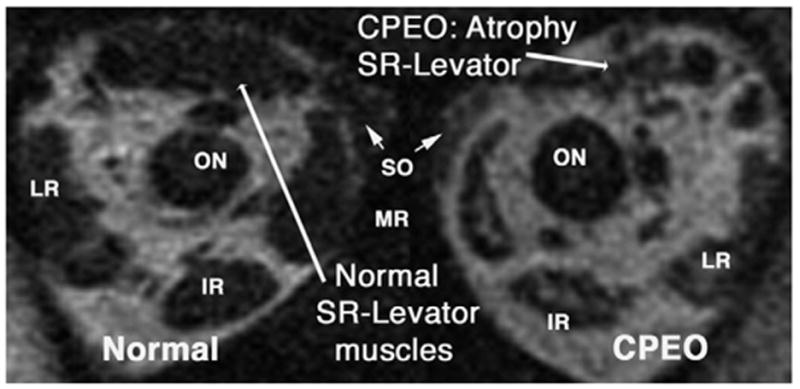
Coronal T1-weighted MRI in a normal subject and a subject with CPEO. The normal SR-levator complex size in the right orbit contrasts with atrophy in CPEO. Note bright internal signal in the left medial and IR muscles.
The architecture of the EOMs on the normal orbit does not show the typical “spongiform” bright signal pattern observed in the EOMs with CPEO. Cranial nerve palsy was associated with marked atrophy of the involved EOMs, as seen in the left abducens palsy illustrated in Figure 3. Figure 4 shows a patient with partial right oculomotor palsy with atrophy of the right MR muscle and right IR EOMs. None of these EOMs exhibited the bright internal signal observed in CPEO. Despite the severe ophthalmoplegia exhibited by subjects with CPEO, their rectus EOM cross sections did not appear greatly diminished in coronal plane MRI images. This impression was substantiated by quantitative analysis of rectus and SO EOM volumes.
FIG 3.
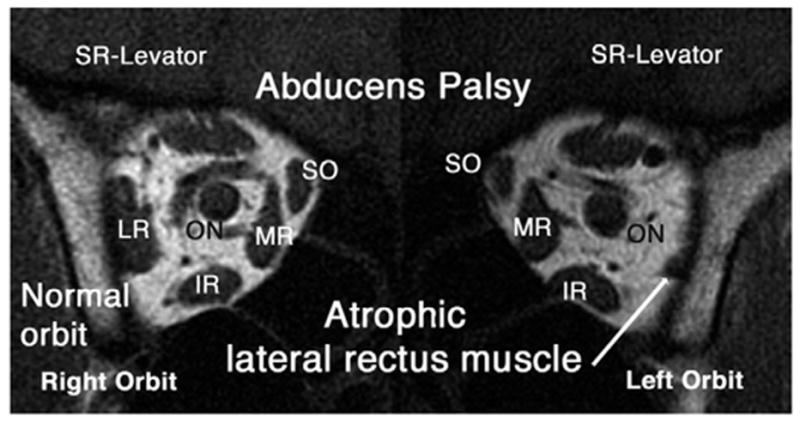
Coronal T1-weighted MRI in subject with left abducens palsy showing atrophic left lateral rectus (LLR) compared with normal right lateral rectus (RLR) muscle. No bright internal signal is present in these EOMs.
FIG 4.
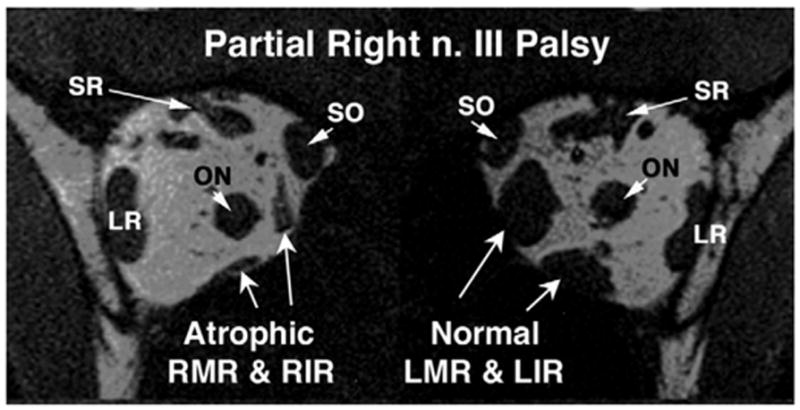
Coronal T1-weighted MRI in subject with partial right oculomotor nerve palsy. The right medial (MR) and IR muscles are atrophic, as compared with the normal left MR and IR muscles.
Table 1 summarizes mean EOM volume data for the SO, MR, SR-levator complex, LR and IR EOMs. Except for the SR and SO, the EOMs in CPEO generally were comparable in size with those of control subjects. Only the SR and SO muscles had significantly subnormal mean volume (p = 0.0001 and 0.001, respectively) when compared with the control group. Although the MR and IR muscles in patients with CPEO had volumes smaller than control, the differences were not statistically significant ( p = 0.02 and 0.03, respectively). The difference in LR volume between the CPEO and control groups was not statistically significant ( p = 0.36).
Table 1.
Extraocular muscle volumes
| Group | Age (Years) Mean ± SD | Superior oblique Mean ± SD (mm3) | Medial rectus Mean ± SD (mm3) | Superior rectus Mean ± SD (mm3) | Lateral rectus Mean ± SD (mm3) | Inferior rectus Mean ± SD (mm3) |
|---|---|---|---|---|---|---|
| CPEO | 42 ± 7 | 209 ± 36 | 516 ± 75 | 400 ± 78 | 575 ± 64 | 400 ± 76 |
| Control | 48 ± 15 | 253 ± 32
p < 0.0018 |
582 ± 68
p < 0.02 |
541 ± 56
p < 0.0001 |
603 ± 83
p < 0.36 |
448 ± 42
p < 0.03 |
| Neurogenic Palsies | 37 ± 18 | 109 ± 48
p < 0.0001 |
310 ± 123
p < 0.0003 |
304 ± 103
p < 0.03 |
370 ± 148
p < 0.0008 |
245 ± 121
p < 0.003 |
| Myasthenia gravis | 47 yr. | 210 ± 24
p < 0.96 |
453 ± 25
p < 0.28 |
330 ± 53
p < 0.24 |
400 ± 48
p < 0.004 |
323 ± 51
p < 0.20 |
p-values for comparisons by Student t-test between EOMs in CPEO, and normal, neurogenic palsies and myasthenia gravis.
SD : Standard deviation; CPEO : chronic progressive external ophthalmoplegia; EOM : extraocular muscle.
Comparisons showed that EOM volumes were significantly larger in CPEO than in EOMs denervated by cranial neuropathies. Mean EOM volumes in CPEO were significantly larger than denervated EOMs in cranial palsies ( p < 0.003) except for the SR. The SR had a mean volume of 400 ± 78 mm3 in CPEO compared with 304 ± 103 mm3 in oculomotor palsies, larger but not significantly so at p = 0.03.
Volumes of EOMs in CPEO and the subject with myasthenia gravis did not differ statistically and in that subject did not show the bright internal signal observed in CPEO. Statistically comparing the subject with myasthenia gravis with subjects having CPEO by Student t-test, the p-values for the SR, SO, MR, LR, and IR muscles were 0.24, 0.96, 0.28, 0.004, and 0.2, respectively.
Discussion
The present study indicates that CPEO can be associated with normal or nearly normal volumes of most EOMs despite clinically severe ophthalmoplegia. This finding, and the presence of bright signal within EOMs on high-resolution T1 MRI in the absence of orbital or EOM inflammmation, may be clinically useful in distinguishing CPEO from other conditions causing severe ophthalmoplegia. Unlike CPEO, chronic denervation produces significant EOM atrophy. We have noted elsewhere that, after acute denervation, MRI shows that the reduction in EOM cross section is evident within 6 weeks and reaches a complete state within 1 year.12,13 We analyzed 1 subject with myasthenia gravis who did not show internal abnormalities in the EOMs.14
Chronic progressive external ophthalmoplegia can present with signs and symptoms similar to multiple cranial nerve palsies, disorders of the neuromuscular junction, orbital myositis, thyroid orbitopathy, and the Miller Fisher variant of Guillain Barré syndrome.14–17 Because duction limitations frequently are symmetric in CPEO, diplopia may not occur. In addition, asymmetric ptosis can occlude the visual axis of one eye and prevent diplopia. Nevertheless, in our study four patients presented with diplopia.
The high mitochondrial content of the EOMs is associated with elevated oxidative enzyme activity, high vascularity, and high blood flow. These characteristics may explain their high fatigue resistance and enhancement with gadolinium contrast during MRI.18–27
Ringel at al28 studied EOM biopsy specimens of 8 patients with CPEO. They noted an increase in the number of ragged red fibers, as well as a marked variation in the size and distribution of fine, granular, and coarse muscle fibers. The relationship between mitochondrial DNA (mtDNA) abnormalities and the functional and morphological changes that lead to the CPEO phenotye is still poorly understood. Carta et al29 studied the relationship between mtDNA abnormalities and ultrastructural aspects of EOMs in longstanding CPEO. Electron microscopy (EM) revealed focal areas of both disruption and abnormality of mitochondria in only some of the EOM fibers, producing selective vacuolization. Low-magnification EM of resected EOMs revealed a heterogeneous ultrastructural pattern, with normal EOM fibers flanked by other fibers having diffuse sarcoplasmic vacuolization. The number of mitochondria was not grossly increased. In fact, the sarcoplasm of damaged EOM fibers was filled with ultrastructurally normal myofibrils, whereas mitochondria displayed different degrees of disruption of their cristae, leading from partial to complete mitochondrial matrix emptying, with preservation of the external membrane.
Early CPEO is readily confused with multiple other clinical entities. It is unclear why EOM volume is not substantially decreased in CPEO. High-quality surface coil T1 MRI is sensitive enough to resolve the architecture of the EOMs, and in CPEO typically shows preservation of EOM volume out of proportion to the severity of ophthalmoplegia, with abnormal internal bright signal not associated with inflammation. This finding has not been seen in other ophthalmologic subjects imaged in the prospective protocol who exhibited normal EOM sizes and were free of orbital inflammation.13 The bright internal signal also has been observed in congenital fibrosis of the extraocular muscles (CFEOM), but in that case was associated with smaller EOMs.30 Selective atrophy of the SR-levator complex is mysterious but typically is seen in patients with CFEOM. The bright internal signal also is observed in active thyroid ophthalmopathy, but in that case is associated with enlarged EOMs or enlargement of the orbital fat compartment.31 Okamato et al32 compared orbital CT scans of 4 patients with mitochondrial myopathy (2 cases each with CPEO and Kearns Sayre syndrome) and 3 patients with myasthenia gravis. Orbits were scanned using 5 mm thickness axial CT planes and reformatted 2.5 mm coronal images at midorbit in 4 cases. They concluded that orbital CT cannot help to clinically differentiate between mitochondrial myopathies and myasthenia gravis. Such CT technique has much lower spatial resolution than the current MRI study, and lacks the ability of MRI to resolve soft-tissue contrast. Our experience suggests that high-resolution orbital MRI may have specificity for CPEO and could help resolve the diagnostic dilemma in difficult cases.
Footnotes
Presented at the 30th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, Washington DC, March 27-31, 2004.
Supported by the USPHS NIH EY 08313 & Research to Prevent Blindness. J.L.D. received an unrestricted award from Research to Prevent Blindness and is the Leonard Apt Professor of Ophthalmology.
References
- 1.Von Graefe A. Verhandlungen arztlicher Gesselschaften. Berlin Klin Wochenschr. 1868;5:125. [Google Scholar]
- 2.Miller NR, editor. Walsh and Hoyt’s clinical neuro-ophthalmology. 4. Vol. 2. Baltimore (MD): Williams and Wilkins; 1985. pp. 811–23. [Google Scholar]
- 3.Rowland LP. Progressive external ophthalmoplegia and ocular myopathies. In: Rowland LP, Di Mauro S, editors. Handbook of clinical neurology, revised series. Vol. 18. Amsterdam: Elsevier; 1992. pp. 287–329. [Google Scholar]
- 4.Biousse V, Newman NJ. Neuro-ophthalmology of mitochondrial diseases. Semin Neurol. 2001;21:275–291. doi: 10.1055/s-2001-17945. [DOI] [PubMed] [Google Scholar]
- 5.Biousse V, Newman NJ. Neuro-ophthalmology of mitochondrial diseases. Curr Opin Neurol. 2003;16:35–43. doi: 10.1097/01.wco.0000053592.70044.57. [DOI] [PubMed] [Google Scholar]
- 6.Vilarinho L, Santorelli FM, Cardoso ML, Coelho T, Guimaraes A, Coutinho P. Mitochondrial DNA analysis in ocular myopathy. Observations in 29 Portuguese patients. Eur Neurol. 1998;39:148–53. doi: 10.1159/000007925. [DOI] [PubMed] [Google Scholar]
- 7.Demer JL. The orbital pulley system—a revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 8.Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–59. doi: 10.1016/s0002-9394(01)01099-6. [DOI] [PubMed] [Google Scholar]
- 9.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical strabismus management: principles and techniques. Philadelphia: Saunders; 1999. pp. 84–98. [Google Scholar]
- 10.Shin GS, Demer JL, Rosenbaum AL. High resolution, dynamic, magnetic resonance imaging in complicated strabismus. J Pediatr Ophthalmol Strabismus. 1996;33:282–90. doi: 10.3928/0191-3913-19961101-03. [DOI] [PubMed] [Google Scholar]
- 11.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic rectus muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 12.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: a new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–88. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 13.Demer JL, Clark RA, Kono R, Wright W, Velez F, Rosenbaum AL. A 12-year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2002;6:337–47. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 14.Brodsky MC, Baker RS, Hamed LM. Neuro-ophthalmologic manifestations of systemic and intracranial disease. In: Brodsky MC, Baker RS, Hamed LM, editors. Pediatric neuro-ophthalmology. New York: Springer; 1996. pp. 390–92. [Google Scholar]
- 15.Holt IJ, Harding AE, Cooper JM, et al. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989;26:699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- 16.Suomalainen A, Kaukonen J, Amati P, et al. An autosomal locus predisposing to deletions of mitochondrial DNA. Nat Genet. 1995;9:146–51. doi: 10.1038/ng0295-146. [DOI] [PubMed] [Google Scholar]
- 17.Porter JD, Baker RS. Muscles of a different “color” The unusual properties of the extraocular muscles may predispose or protect them in neurogenic or myogenic disease . Neurology. 1996;46:30–7. doi: 10.1212/wnl.46.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Oh SY, Poukens V, Cohen MS, Demer JL. Structure-function correlation of laminar vascularity in human rectus extraocular muscles. Invest Ophthalmol Vis Sci. 2001;42:17–22. [PubMed] [Google Scholar]
- 19.Durston JH. Histochemistry of primate extraocular muscles and the changes of denervation. Br J Ophthalmol. 1974;58:193–216. doi: 10.1136/bjo.58.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer RF, Porter JD. Structural organization of the extraocular muscles. Rev Oculomot Res. 1988;2:33–70. [PubMed] [Google Scholar]
- 21.Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39:451–84. doi: 10.1016/s0039-6257(05)80055-4. [DOI] [PubMed] [Google Scholar]
- 22.Koornneef L. The architecture of the musculo-fibrous apparatus in the human orbit. Acta Morphol Neerl Scand. 1977;15:35–64. [PubMed] [Google Scholar]
- 23.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–36. [PubMed] [Google Scholar]
- 24.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–85. [PubMed] [Google Scholar]
- 25.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–90. [PubMed] [Google Scholar]
- 26.Oh SY, Poukens V, Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–6. [PubMed] [Google Scholar]
- 27.Kaissar G, Kim JH, Bravo S, Sze G. Histologic basis for increased extraocular muscle enhancement in gadolinium-enhanced MR imaging. Radiology. 1991;179:541–2. doi: 10.1148/radiology.179.2.2014307. [DOI] [PubMed] [Google Scholar]
- 28.Ringel SP, Wilson WB, Barden MT. Extraocular muscle biopsy in chronic progressive external ophthalmoplegia. Ann Neurol. 1979;6:326–39. doi: 10.1002/ana.410060406. [DOI] [PubMed] [Google Scholar]
- 29.Carta A, D’Adda T, Carrara F, Zeviani M. Ultrastructural analysis of extraocular muscle in chronic progressive external ophthalmoplegia. Arch Ophthalmol. 2000;118:1441–5. doi: 10.1001/archopht.118.10.1441. [DOI] [PubMed] [Google Scholar]
- 30.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–9. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 31.Thacker NM, Velez FG, Demer JL, Rosenbaum AL. Superior oblique muscle involvement in thyroid ophthalmopathy. J AAPOS. 2005;9:174–8. doi: 10.1016/j.jaapos.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto K, Ito J, Tokiguchi S, Furusawa T. Atrophy of bilateral extraocular muscles. Journal of Neuro-Ophthalmology. 1996;16:286–8. [PubMed] [Google Scholar]


