Abstract
PURPOSE
To help resolve the clinical ambiguity between Duane syndrome with severe abduction deficit and abducens palsy, we performed orbital magnetic resonance imaging (MRI) to qualify abnormalities of the lateral rectus (LR) muscle in these entities.
DESIGN
Prospective observational case series.
METHODS
Orbital MRI was performed in 13 subjects with Duane syndrome (19 eyes), 10 subjects with chronic abducens palsy (10 eyes), and 10 orthotropic control subjects (18 eyes). High-resolution, surface coil, T1-weighted MRI was used to obtain contiguous, 2-mm thick quasi-coronal images of the orbits in central gaze. Digital image analysis was used to quantify cross-sectional area of the ipsilesional and contralesional LR to provide comparison with control measurements.
RESULTS
Mean maximum LR cross-sectional area in Duane syndrome was statistically similar to control (P = .454) and contralesional LR cross-sectional area (P = .227). However, in chronic abducens palsy, mean maximum ipsilesional LR cross-sectional area was markedly smaller than contralesional (P = .003) and control cross-sectional areas (P < .0001), as well as smaller than the LR in Duane syndrome (P= .0017).
CONCLUSIONS
The LR muscle in abducens palsy exhibits profound atrophy. The sparing of the LR in Duane syndrome from denervation atrophy despite absence of normal abducens innervation suggests existence of alternative LR innervation. High-resolution MRI can noninvasively demonstrate LR muscle size and distinguish Duane syndrome from chronic abducens palsy in uncertain cases.
Duane retraction syndrome is characterized by congenital abduction deficit, narrowing of the palpebral fissure on adduction, and globe retraction with occasional upshoot or downshoot in adduction.1,2 Diagnosis of Duane syndrome has traditionally been based on clinical examination and may not be difficult when patients who are orthotropic or nearly so in central gaze present with characteristic signs. However, the abduction deficits of Duane syndrome may mimic clinical findings of chronic abducens palsy.2– 4
Abducens innervation is deficient in both Duane syndrome and abducens palsy, although the pathophysiologic mechanisms differ. The pathophysiology of Duane syndrome is believed to be absence of the abducens nucleus, with innervation of the lateral rectus (LR) by branches of the oculomotor nerve.2,3,5,6 Differentiation of Duane syndrome from chronic abducens palsy is critically important because the surgical management of each condition is very different, and misdiagnosis can result in serious complications.3,5–7 The similarity of features of Duane syndrome and chronic abducens palsy is evidenced by electrooculography, which demonstrates similar slowing of abducting saccades in affected eyes in these two entities.8 Electromyographic differentiation of Duane syndrome from abducens palsy should be decisive,2 but is invasive and not widely available.
Modern imaging techniques can help resolve diagnostic dilemmas in ocular motility by enabling direct evaluation of the functional anatomy of extraocular muscles. Horton and associates9 first reported qualitative evidence of superior oblique muscle atrophy in a patient with trochlear palsy. Subsequent magnetic resonance imaging (MRI) studies have quantitatively demonstrated extraocular muscle atrophy in several forms of paralytic strabismus.10 –18 The purpose of this study was to use MRI to quantify LR changes in Duane syndrome and chronic abducens palsy, and to determine whether orbital MRI can help resolve the ambiguity between these disorders.
METHODS
All subjects gave written informed consent according to a protocol conforming to Declaration of Helsinki and approved by the Institutional Review Board at the University of California, Los Angeles, in conformity with Health Insurance Portability and Accountability Act (HIPAA) regulations. From an ongoing prospective study of strabismus, we selected seven patients with unilateral and six with bilateral Duane syndrome, 10 patients with chronic unilateral abducens palsy, and 10 orthotropic volunteers who had undergone high-resolution orbital MRI between 1997 and 2004 at the Jules Stein Eye Institute. Technical aspects of the methods and equipment conformed to the same prospective protocol during the study interval, and all MRI scans were performed by the same investigator with the same model of scanner. Characteristics of patients with Duane syndrome are listed in Table 1. All cases of Duane syndrome were congenital, and exhibited limited abduction with narrowing of the palpebral fissure on adduction. Most patients had type 1 Duane syndrome, with limitation only of abduction. One eye of patient 8 exhibited Duane syndrome type 2, with limited adduction only. Two patients had Duane syndrome type 3, with limitation of both abduction and adduction.
TABLE 1.
Characteristics of Subjects With Duane Retraction Syndrome
| Version
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Gender/Age (years) | Side | Central Gaze Alignment (Δ) | Abduction (R/L) | Adduction (R/L) | Fissure Narrowing | Globe Retraction | Up/downshoot (R/L) | Type DS (R/L) |
| 1 | M/16 | L | ET 30 | −3 | 0 | + | + | −/+ | Type 1 |
| 2 | M/33 | L | ET 20 | −4 | −1 | + | + | −/− | Type 1 |
| 3 | F/43 | L | ET 35, | −4 | 0 | + | + | −/− | Type 1 |
| RHT 6 | |||||||||
| 4 | F/5* | B | XT 20† | −1/−3 | 0/−0.5 | −/− | +/+ | −/−, −/− | Type 1/1 |
| 5 | M/42* | B | XT 10 | −2/−2 | −2/−1 | +/+ | +/+ | −/−, −/− | Type 1/1 |
| 6 | M/45* | B | XT† | −4/−1 | −1/−4 | +/+ | +/+ | −/+, −/− | Type 1/1 |
| 7 | M/17 | L | ET 30 | −4 | −0.5 | + | + | +/+ | Type 1 |
| 8 | M/2* | B | XT 20† | −2/−4 | −4/−4 | +/+ | +/+ | −/−, −/− | Type 2/3 |
| 9 | F/37 | L | LET 10† | −3 | 0 | + | + | +/+ | Type 1 |
| 10 | F/12 | R | ET 20, | −4 | −1 | + | + | −/+ | Type 1 |
| RHoT 20† | |||||||||
| 11 | M/13 | L | XT | −3 | 0 | + | + | −/− | Type 1 |
| 12 | F/4* | B | Ortho | −4/−3 | 0/−2 | +/+ | −/− | −/−, −/− | Type 1/1 |
| 13 | F/14* | B | Ortho | −4/−4 | 2/−2 | +/+ | +/+ | −/−, −/− | Type 3/3 |
+ = overaction; − = underaction; ET = esotropia; XT = exotropia; HT = hypertropia; HoT = hypotropia; R = right; L = left; DS = Duane retraction syndrome.
Bilateral Duane retraction syndrome.
Patients who received strabismus surgery before orbital magnetic resonance imaging.
Diagnosis of abducens palsy was made on the basis of limitation of abduction, slowing of the abducting saccade, and incomitant esotropia increasing in the field of action of the involved LR muscle without evidence of a restrictive myopathy of the medial rectus muscle. All cases of abducens palsy were unilateral and had been acquired more than 6 months previously. No patient had previously been treated with botulinum toxin. Characteristics of patients with abducens palsy and causative lesions are listed in Table 2.
TABLE 2.
Characteristics of Subjects With Abducens Palsy
| Subjects | Gender/Age (years) | Side | Cause | Duration (months) | Abduction Deficit | Esotropia in Central Gaze (Prism Diopters) |
|---|---|---|---|---|---|---|
| 1 | M/20 | R | Skull fracture | 9 | −4 | 70 |
| 2 | M/76 | L | Chordoma, clivus | 24 | −2 | 20 |
| 3 | F/43 | L | Complicated migraine | 13 | −4 | 55 |
| 4 | F/50 | L | Meningioma | 60 | −4 | 80 |
| 5 | F/75 | L | Cerebral Infarction | 7 | −2 | 25 |
| 6 | F/32 | L | Brainstem Infarction | 36 | −3.5 | 40 |
| 7 | M/33 | L | Chordoma, skull base | 6 | −4 | 65 |
| 8 | M/55 | R | Meningioma, skull base | 6 | 4 | 60 |
| 9 | M/46 | L | Chordoma, skull base | 24 | −4 | 60 |
| 10 | M/21 | L | Skull Fracture | 6 | −4 | 40 |
R = right; L = left.
All subjects underwent complete ophthalmologic examination, including measurement of binocular alignment by prism cover test and photography of ocular versions. Versions were quantified with a clinical scale of 0 ± 4, with 0 being normal, −4 representing maximal underaction, and +4 representing maximal overaction.
High-resolution, T1-weighted orbital MRI was performed by a 1.5-T general Electric Signa (Milwaukee, Wisconsin, USA) scanner. Crucial aspects of this technique, described in detail elsewhere,11,19 –21 include use of a dual-phased surface coil array (Medical Advances, Milwaukee, Wisconsin, USA) to improve signal-to-noise ratio and fixation target in central gaze to avoid motion artifacts during the approximately 3.5-minute scan time. To image the LR muscle, sets of contiguous 2-mm–thick quasi-coronal image planes were obtained perpendicular to the long axis of each orbit separately with a 256 × 256 matrix over an 8-cm square field of view, giving a pixel resolution of 312 μm. Some patients unable to voluntarily fixate were examined under deep sedation, as clinically indicated, with anesthesia monitoring. Deep sedation eliminated eye movement during scanning and resulted in a roughly central gaze position as determined from analysis of globe features in the images. Digital MRI images were transferred to a personal computer, converted to 8-bit tagged image file format (TIFF) by locally developed software, and quantified by the program NIH Image (Rasband W, National Institutes of Health).
In each MRI image, the border of the LR muscle was outlined manually with a digital cursor, and its cross-sectional area was computed by the “area” function of the NIH Image program. A single person performed all measurements. Anteroposterior LR portion in the orbit was normalized by selecting the image plane in each orbit designated zero, which included the globe– optic nerve junction in central gaze (Figure 1). Values for LR cross section were averaged across subjects in 2 mm thickness referenced to image plane zero. Ipsilesional and contralesional LR cross-sectional area was compared with normal controls. Ipsilesional LR cross-sectional area in Duane syndrome, abducens palsy, and normal control were also compared.
FIGURE 1.
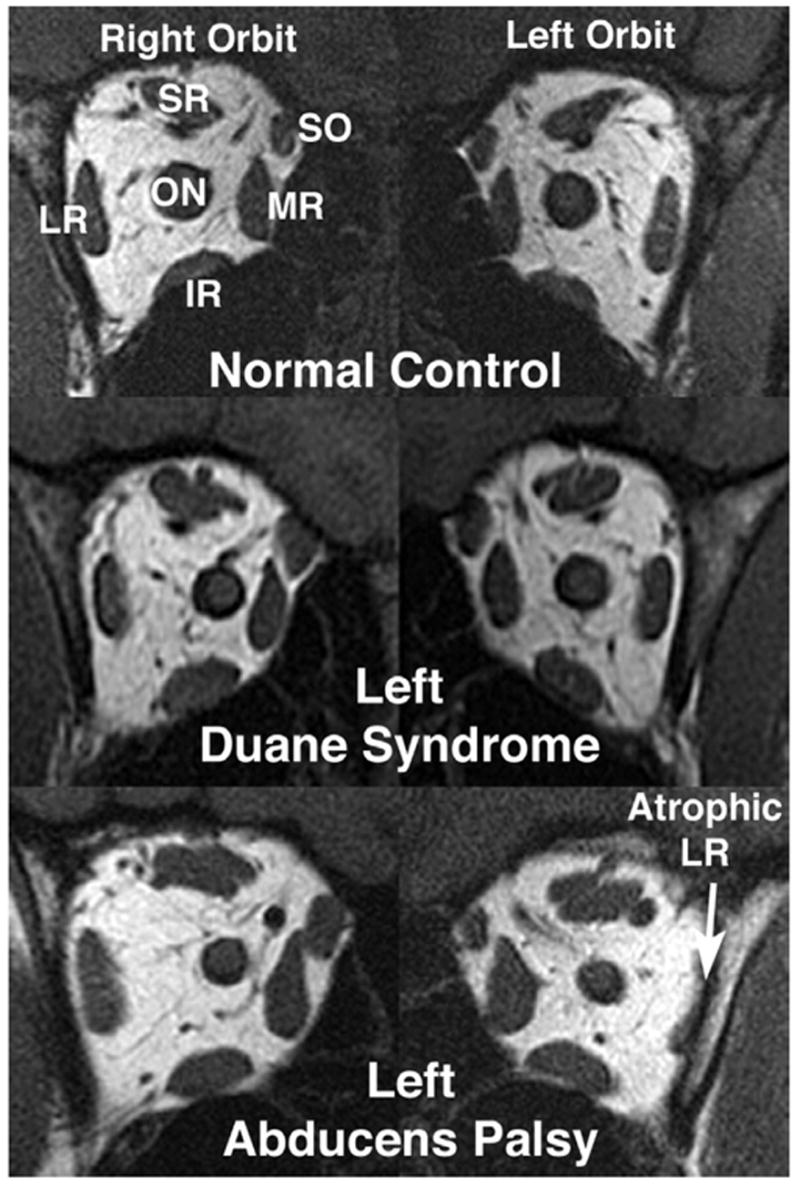
Quasi-coronal MRI scans of both orbits at the mid-orbit level in central gaze demonstrate preservation of lateral rectus (LR) muscle size in Duane syndrome (subject 9), but marked LR atrophy in chronic abducens paralysis (subject 7). Note similar cross-section of lateral rectus (LR) in both normal control orbits. IR = inferior rectus muscle; LR = lateral rectus muscle; MR = medial rectus muscle; ON = optic nerve; SO = superior oblique muscle; SR = rectus muscle.
Statistical analysis was performed by the two-tailed Student t test (Excel XP; Microsoft, Redmond, Washington, USA), and results were considered as significant at the level of .01. Data are expressed as mean ± SD.
RESULTS
A total of 18 orbits were imaged in 10 normal subjects of mean age 48 ± 15 years (range 31 to 69 years). In Duane syndrome, 26 orbits were imaged in 13 subjects having mean age 22 ± 16 years (range 2 to 55 years). Bilateral Duane syndrome was present in six subjects (12 orbits) who had mean age of 18 ± 19 years (range 2 to 45 years). The mean age of seven patients with unilateral Duane syndrome was 24 ± 12 years (range 12 to 43 years). Clinical characteristics of subjects with Duane syndrome are summarized in Table 1. All subjects with Duane syndrome exhibited showed a marked abduction deficit in one or both eyes, and most exhibited some globe retraction and palpebral fissure narrowing in adduction (Figure 2).
FIGURE 2.
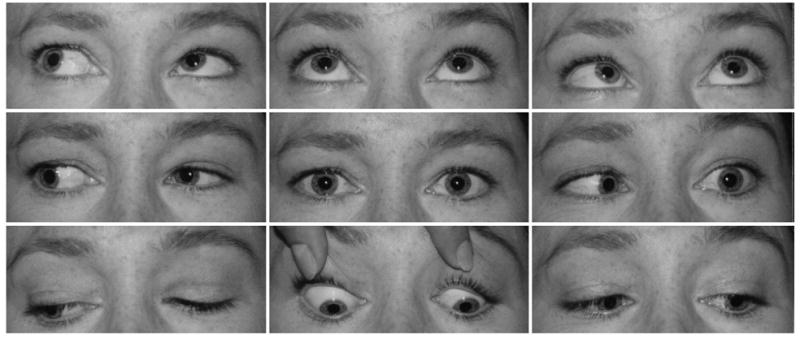
Abduction deficit in left Duane syndrome (subject 9; see Figure 1, middle row) associated with narrowing of palpebral fissure on adduction. Note the minimal esotropia in central gaze and presence of lid changes are the only clinical features distinguishing this presentation from subject 9 with left Duane syndrome illustrated in Figure 3.
A total of 20 orbits were imaged in 10 subjects who had unilateral abducens palsy and were of mean age 45 ± 20 years (range 20 to 76 years). Subject characteristics and causes of abducens palsy are detailed in Table 2. Mean duration of abducens palsy was 19.1 ± 17.6 months (range six to 60 months). All subjects with abducens palsy had severely limited abduction, but did not typically exhibit globe retraction or palpebral fissure narrowing on adduction (Figure 3). As may be seen by comparison of Figures 2 and 3, however, the clinical distinction between Duane syndrome and abducens palsy was not always obvious when based on motility examination alone.
FIGURE 3.
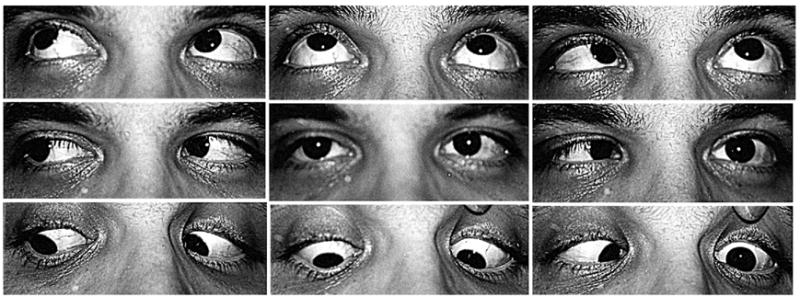
Abduction deficit in left abducens palsy (subject 7, see Figure 1, lower row) showing esotropia in primary gaze and inability to abduct the left eye past the midline. Note the greater degree of esotropia in central gaze and absence of lid changes are the only clinical features distinguishing this presentation from subject 9 with left Duane syndrome palsy illustrated in Figure 2.
Cross-sectional area of the LR was measured over a wide anteroposterior extent from orbital apex to a region anterior to the globe equator. In all LR muscles, cross-sectional area was maximal in midorbit, which is approximately 8 mm posterior to the globe– optic nerve junction, and was smaller anteriorly and posteriorly (Figure 4). Mean maximum LR cross-sectional area in normal subjects was 38.8 ± 4.8 mm2, with a 95% confidence interval of 29.4 to 48.2 mm2. The mean maximum ipsilesional LR cross-sectional area in Duane syndrome occurred at midorbit and was similar to normal at 36.3 ± 13.3 mm2; cross-sectional area closely approximated normal in image planes anterior to this and was slightly, but not significantly, subnormal more posteriorly (P > .05). The cross-sectional area of the presumably unaffected contralesional LR showed a trend toward being even less that the cross-sectional area of the ipsilesional LR in the deep orbit (Figure 4), although it was still not markedly subnormal. Mean maximum ipsilesional LR cross-sectional area was significantly subnormal at 22.5 ± 4.7 mm2 in chronic abducens palsy (P < .0001, Table 3).
FIGURE 4.
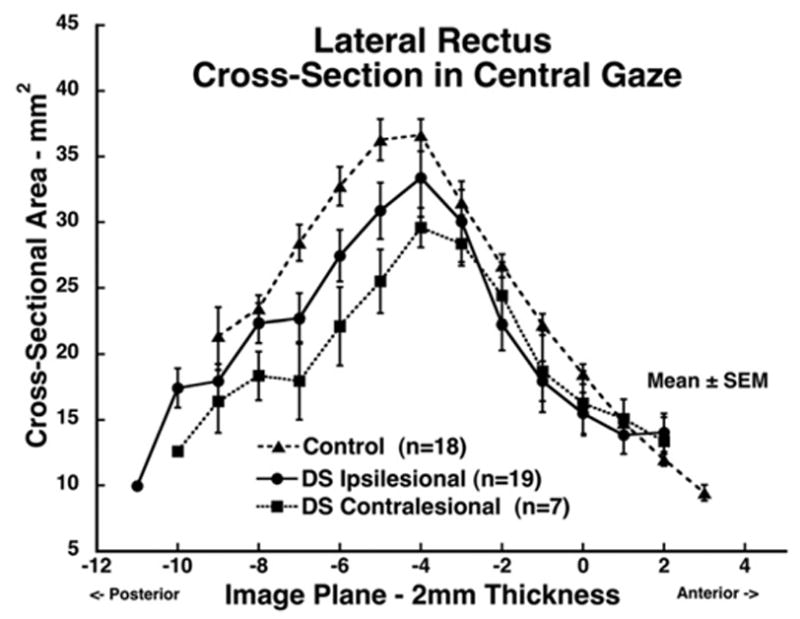
Mean lateral rectus (LR) muscle cross-sectional area in central gaze for 19 muscles ipsilesional to Duane syndrome, seven muscles contralesional to Duane syndrome, and 18 normal control muscles. Cross-sections are plotted as function of 2-mm thickness image plane number, referenced to image plane zero at the globe–optic nerve junction in central gaze. Ipsilesional LR cross-sectional area did not markedly differ from normal control and contralesional LR values.
TABLE 3.
Mean Maximum Lateral Rectus Cross-Sectional Area
| Characteristic | Mean ± SD Maximum Area (mm2) | No. eyes | P Value vs Normal |
|---|---|---|---|
| Normal control | 38.8 ± 4.8 | 18 | — |
| Ipsilesional Duane syndrome | 36.3 ± 13.3 | 19 | .4537 |
| Ipsilesional abducens palsy | 22.7 ± 4.7 | 10 | .0001 (.0004*) |
Significantly different from ipsilesional Duane syndrome by Student t test.
When we averaged over all image planes in which the LR was identified, we found that the mean overall cross-sectional area of 18 normal control LR muscles was 25.9 ± 8.9 mm2, with a 95% confidence interval of 8.5 to 43.4 mm2. In Duane syndrome, the mean overall LR cross-sectional area was 23.5 ± 10.7 mm2 on the ipsilesional side (P = .16) and 21.5 ± 7.5 mm2 on the contralesional side (P = .10), both similar to normal (P = .1). In abducens palsy, mean overall LR cross-sectional area was significantly subnormal at 15.5 ± 6.0 mm2 on the ipsilesional side (P < .0001), but normal at 23.5 ± 9.8 mm2 on the contralesional side. In abducens palsy, the LR atrophy was located mainly posterior to the globe– optic nerve junction; LR muscles in the anterior orbit did not exhibit notable atrophy.
DISCUSSION
Abduction is deficient in both duane syndrome and chronic abducens palsy. In this study, we used high-resolution orbital MRI to evaluate LR size in both disorders, comparing it with LR size in normal orthotropic subjects. High-resolution MRI enables direct visualization of human extraocular muscles, often demonstrating the pathophysiology. Simonsz and associates22 first used computed tomography for evaluation of rectus extraocular muscle paths. After the pioneering study of Miller,23 surface-coil MRI has been used to evaluate the size, contractility, and paths of extraocular muscles and has revealed previously unsuspected anatomic abnormalities such as atrophy, heterotopy, or absence of muscles.12,13,16,24 –32 There have been few MRI reports on Duane syndrome.33–39 These reports mainly described absence or hypoplasia of the abducens nerve, and have generally not evaluated LR muscular size. However, MRI has been used to demonstrate a skull-base meningioma in the setting of Duane syndrome, reporting marked LR atrophy in the orbit affected by Duane syndrome presumably due to tumor compression of anomalous branches of the oculomotor nerve.34
The current study used MRI with quantitative image analysis to measure LR size. The mean maximum LR cross-sectional area was similar to normal in Duane syndrome, but markedly subnormal in abducens palsy. Visual inspection of coronal MRI images of the orbits in this study demonstrated obvious atrophy of the posterior LR in abducens palsy, but not in Duane syndrome (Figure 1), a finding confirmed to be significant by quantitative analysis of cross-sectional area (Table 3).
In the current study, mean maximum LR cross-sectional area was found to be 38.8 mm2 in normal subjects, 36.3 mm2 ipsilesional to Duane syndrome, but only 22.7 mm2 ipsilesional to chronic abducens palsy. The normal values reported here are comparable to those published. In Nakagawa’s40 cadaver study, maximal LR cross-sectional area was 37.6 mm2, whereas in Miller’s23 MRI study of four normal subjects, the value was 32.3 mm2. By means of MRI, Tian and associates41 found the LR cross-sectional area in 42 orbits of 21 normal orthotropic subjects to be 41.2 mm2. The concordance of these findings supports the notion that MRI measurements are reliable for LR cross-sectional area.41
The current study also compared mean overall LR cross-sectional area, averaged over the entire length of the LR, and confirmed that the ipsilesional LR in abducens palsy was markedly smaller than the contralesional LR, smaller than normal control LR muscles, and smaller than the ipsilesional LR of Duane syndrome (Figures 5 and 6). In Duane syndrome, LR size was normal by all comparisons (Figure 4). It is notable that in abducens palsy, LR atrophy was mainly evident in the portion of the LR posterior to the globe– optic nerve junction, not in the anterior orbit. The decreased LR cross-sectional area in abducens palsy is suggestive of denervation atrophy. The posterior distribution of atrophy in abducens palsy could be another clinically useful clue distinguishing this entity from Duane syndrome in cases where the overall severity of LR atrophy is not obvious from clinical inspection.
FIGURE 5.
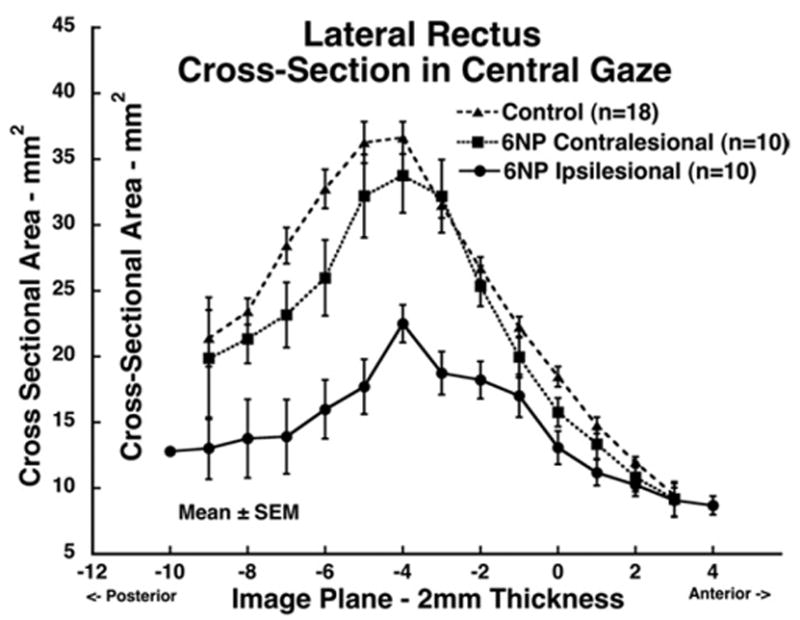
Mean lateral rectus (LR) muscle cross-sectional area in central gaze for 10 muscles ipsilesional to abducens palsy, 10 muscles contralesional to abducens palsy, and 18 normal control muscles. Cross-sections are plotted as function of 2-mm thickness image plane number, referenced to image plane zero at the globe–optic nerve junction in central gaze. Ipsilesional LR cross-sectional area was significantly smaller than normal control and contralesional LR values (P < .0001).
FIGURE 6.
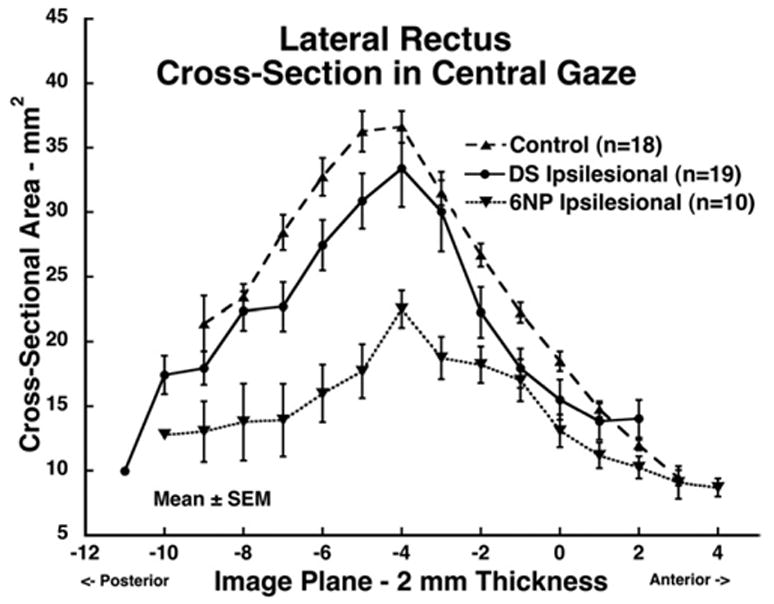
Mean lateral rectus (LR) muscle cross-sectional area in central gaze for 19 muscles ipsilesional to Duane syndrome, 10 muscles ipsilesional to abducens palsy, and 18 normal control muscles. Cross-sections are plotted as function of 2-mm thickness image plane number, referenced to image plane zero at the globe–optic nerve junction in central gaze. Maximum LR cross-sectional area was 8 mm posterior to the globe–optic nerve junction. The abducens palsy LR cross-sectional area was significantly smaller than normal control (P < .0001) and Duane syndrome (P < .004) values, but in Duane syndrome did not significantly differ from normal.
There have been some prior applications of MRI to demonstrate extraocular muscle atrophy in diagnosis of paralytic strabismus. Horton and associates9 reported MRI findings of superior oblique atrophy in acquired trochlear palsy. Bloom and associates18 assessed reduction in size of rectus muscle size by means of conventional coronal MRI in horizontal rectus muscle palsies. Some early studies used gross size, diameter, or muscle bulk for assessment by qualitative comparisons rather than quantitative measurements. A previous study measured superior oblique muscle size in acquired superior oblique palsy.15 Quantitative MRI has been used to measure extraocular muscle size and contractility in superior oblique palsy.10,14,17 These studies quantified superior oblique muscle cross-sectional area and found in chronic palsy that maximum cross-sectional area in primary gaze was markedly subnormal, consistent with chronic denervation atrophy. All of these studies concluded that the palsied extraocular muscles exhibit decreased size, diameter, and bulk compared with their normal counterparts, and these features were attributed to denervation atrophy. In the current study, LR cross-sectional area was precisely measured by digital image analysis over a wide anteroposterior extent encompassing almost the whole orbit by thin 2-mm image sections. This approach is sensitive to changes in LR muscle size along nearly its entire length.
It seems reasonable to attribute the reduction in size of LR in abducens palsy observed in this study to denervation atrophy. It is unclear when extraocular muscle atrophy would develop after denervation. Although denervation atrophy is commonly observed in skeletal muscles, the extraocular muscles show unique denervation responses. The inferior oblique muscle of rabbit exhibited maximal transient hypertrophy in its central layer 4 to 5 weeks after experimental denervation, with subsequent atrophy.42 In denervated canine extraocular muscles, there was reduction in mean diameter of coarse and granular fibers, but fine fibers were relatively spared.43 Porter and associates44 found hypertrophy 1 month after extraocular muscle denervation in months, with profound atrophy by 4 months. In studies of extraocular muscle changes in beagles, persistent atrophy of singly innervated fibers in global and orbital layers of denervated muscles were found, but the multienervated fibers were spared.45
Abducens innervation is deficient in both abducens palsy and in Duane syndrome type 1. The pathogenesis of Duane syndrome type 1 is proposed to be the absence or hypoplasia of the abducens nucleus with anomalous innervation of the LR by branches of the oculomotor nerve, perhaps complicated by secondary mechanical changes in extraocular muscles.2,5,46,47 Most cases of isolated unilateral abducens palsy resolve spontaneously within 6 months, but in nonresolving abducens palsy, chronic orbital mechanical changes have been hypothesized to occur, such as contracture of the medial rectus, lengthening of the LR, and changes in orbital connective tissues.2,38 These may increase the abduction limitation and increase the esotropia in central gaze, similar to Duane syndrome. The current finding of absence of detectable LR atrophy in Duane syndrome suggests some other innervation may exist to prevent rescue the LR from denervation atrophy, perhaps arising from the oculomotor nerve.
Relatively little is known of the effects of denervation on extraocular muscles. Injury to the motor nerve innervating a striated muscle results in loss of muscle bulk due to of shrinkage of sarcomeres, loss of myofibrils, and replacement by fatty tissue.48 The response of extraocular muscles to denervation may differ from that of striated muscle. Four months after denervation by oculomotor nerve transection at the cavernous sinus in monkey, cross-sectional area of orbital layer fibers in the medial rectus decreased to approximately half of control, but fibers in the global layer were unaffected.44 Histologic studies are needed of human extraocular muscles in paralytic strabismus.
The present study demonstrated profound LR atrophy in abducens palsy and generally normal LR size in Duane syndrome. Imperfect matching of the age distribution of the comparison groups does not undermine this conclusion because aging does not have an appreciable influence on extraocular muscle size.41 Patients with Duane syndrome were slightly younger than other two groups, but they nevertheless had mean LR cross-sectional area and the mean maximum LR cross-sectional area not markedly different from normal control values. Imaging of LR size thus appears to be a useful diagnostic tool for discriminating Duane syndrome from chronic abducens palsy. This might be of particular clinical use in children, in whom motility examination can be limited by poor cooperation.
Biographies
 Joseph L. Demer, MD, PhD, is the Leonard Apt Professor of Ophthalmology and Professor of Neurology at UCLA. He has a PhD in Biomedical Engineering from Johns Hopkins. An active ophthalmic surgeon, Dr Demer completed fellowship in Pediatric Ophthalmology at Texas Children’s Hospital. He received the Friedenwald Award from ARVO in 2003 for his work on extraocular muscles. Dr Demer laboratories are funded for study of amblyopia, strabismus, vestibulo-ocular reflex function, and genetics of cranial innervation.
Joseph L. Demer, MD, PhD, is the Leonard Apt Professor of Ophthalmology and Professor of Neurology at UCLA. He has a PhD in Biomedical Engineering from Johns Hopkins. An active ophthalmic surgeon, Dr Demer completed fellowship in Pediatric Ophthalmology at Texas Children’s Hospital. He received the Friedenwald Award from ARVO in 2003 for his work on extraocular muscles. Dr Demer laboratories are funded for study of amblyopia, strabismus, vestibulo-ocular reflex function, and genetics of cranial innervation.
 Nam-Yeo Kang, MD, PhD, is an Assistant Professor of Ophthalmology at the Holy Family Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. Dr Kang has completed a fellowship in Pediatric Ophthalmology and Strabismus at the Jules Stein Eye Institute, UCLA, 2004-2005. Dr Kang is interested in restrictive and complicated strabismus, adjustable suture under propofol anesthesia, and the pathophysiology and treatment of intermittent exotropia.
Nam-Yeo Kang, MD, PhD, is an Assistant Professor of Ophthalmology at the Holy Family Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. Dr Kang has completed a fellowship in Pediatric Ophthalmology and Strabismus at the Jules Stein Eye Institute, UCLA, 2004-2005. Dr Kang is interested in restrictive and complicated strabismus, adjustable suture under propofol anesthesia, and the pathophysiology and treatment of intermittent exotropia.
Footnotes
Supported by Grant EY08313 from the United States Public Health Service, National Institutes of Health, National Eye Institute. J.L.D. received an unrestricted award from Research to Prevent Blindness and is the Leonard Apt Professor of Ophthalmology.
References
- 1.Duane A. Congenital deficiency of abduction associated with impairment of adduction, retraction movements, contraction of the palpebral fissure and oblique movements of the eye. Arch Ophthalmol. 1905;34:133–159. doi: 10.1001/archopht.1996.01100140455017. [DOI] [PubMed] [Google Scholar]
- 2.Huber A. Electrophysiology of the retraction syndromes. Br J Ophthalmol. 1974;58:293–300. doi: 10.1136/bjo.58.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caputo AR, Wagner RS, Guo S, Santiago AP. Infantile abduction deficit: Duane’s retraction syndrome or abducens palsy? A study of 24 cases. Binocul Vis Strabismus Q. 1996;11:213–218. [Google Scholar]
- 4.Galeta SL, Smith JL. Chronic isolated sixth nerve palsies. Arch Neurol. 1989;46:79 –82. doi: 10.1001/archneur.1989.00520370081024. [DOI] [PubMed] [Google Scholar]
- 5.Jampolsky A. In: Duane syndrome. Clinical strabismus management: principles and surgical techniques. Rosenbaum AL, Santiago AP, editors. Philadelphia, Pennsylvania: WB Saunders; 1999. pp. 325–346. [Google Scholar]
- 6.DeRespinis PA, Caputo AR, Wagner RS, Guo S. Duane’s retraction syndrome. Surv Ophthalmol. 1993;38:257–288. doi: 10.1016/0039-6257(93)90077-k. [DOI] [PubMed] [Google Scholar]
- 7.Souza-Dias C. Correspondence: congenital VI nerve palsy is Duane’s syndrome until disproven. Binocul Vis Q. 1992;7:70. [Google Scholar]
- 8.Yang MC, Bateman JB, Yee RD, Apt L. Electrooculography and discriminant analysis in Duane’s syndrome and sixth-cranial-nerve palsy. Graefes Arch Clin Exp Ophthalmol. 1991;229:52–56. doi: 10.1007/BF00172261. [DOI] [PubMed] [Google Scholar]
- 9.Horton JC, Tsai RK, Truwit CL, Hoyt WF. Magnetic resonance imaging of superior oblique muscle atrophy in acquired trochlear nerve palsy [letter] Am J Ophthalmol. 1990;110:315–316. doi: 10.1016/s0002-9394(14)76358-5. [DOI] [PubMed] [Google Scholar]
- 10.Demer JL, Miller MJ, Koo EY, et al. True versus masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G, editor. Update on strabismus and pediatric ophthalmology. Boca Raton, Florida: CRC Press; 1995. pp. 303–306. [Google Scholar]
- 11.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical strabismus management: principles and techniques. Philadelphia, Pennsylvania: WB Saunders; 1999. pp. 84–98. [Google Scholar]
- 12.Demer JL. Anatomy of strabismus. In: Taylor D, Hoyt C, editors. Pediatric ophthalmology and strabismus. London, United Kingdom: Elsevier; 2005. pp. 849–861. [Google Scholar]
- 13.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–347. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 14.Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology. 2003;110:1219 –1229. doi: 10.1016/S0161-6420(03)00331-2. [DOI] [PubMed] [Google Scholar]
- 15.Ozkan S, Aribal ME, Sener EC, et al. Magnetic resonance imaging in evaluation of congenital and acquired superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1997;34:29 –34. doi: 10.3928/0191-3913-19970101-07. [DOI] [PubMed] [Google Scholar]
- 16.Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: a new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–188. doi: 10.3928/0191-3913-19940501-10. [DOI] [PubMed] [Google Scholar]
- 17.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906 –913. [PubMed] [Google Scholar]
- 18.Bloom JN, Cadera W, Heiberg E, Karlik S. A magnetic resonance imaging study of horizontal rectus muscle palsies. J Pediatr Ophthalmol Strabismus. 1993;30:296 –300. doi: 10.3928/0191-3913-19930901-07. [DOI] [PubMed] [Google Scholar]
- 19.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys inferred from muscle paths. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 20.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212. [PubMed] [Google Scholar]
- 21.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 22.Simonsz HJ, Harting F, de Waal BJ, Verbeeten BWJ. Sideways displacement and curved path of recti eye muscles. Arch Ophthalmol. 1985;103:124 –128. doi: 10.1001/archopht.1985.01050010130036. [DOI] [PubMed] [Google Scholar]
- 23.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 24.Demer JL, Miller JM, Poukens V. Surgical implications of the rectus extraocular muscle pulleys. J Pediatr Ophthalmol Strabismus. 1996;33:208 –218. doi: 10.3928/0191-3913-19960701-03. [DOI] [PubMed] [Google Scholar]
- 25.Demer JL, Miller JM, Poukens V, et al. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 26.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 27.Demer JL, Kono R, Wright W, et al. Gaze-related orbital pulley shift: a novel cause of incomitant strabismus. In: de Faber JT, editor. Progress in strabismology. Lisse: Swets and Zeitlinger; 2002. pp. 207–210. [Google Scholar]
- 28.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in strabismology. Buren, The Netherlands: Aeolus Press; 1999. pp. 91–94. [Google Scholar]
- 29.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Cur Opin Neurol. 2006;19:4 –13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald Lecture. Invest Ophthalmol Vis Sci. 2004;45:729 –738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 31.Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann N Y Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 32.Demer JL. Extraocular muscles. In: Jaeger EA, Tasman PR, editors. Duane’s clinical ophthalmology. Philadelphia, Pennsylvania: Lippincott; 2000. pp. 1–23. [Google Scholar]
- 33.Ozkurt H, Basak M, Oral Y, Ozkurt Y. Magnetic resonance imaging in Duane’s retraction syndrome. J Pediatr Ophthalmol Strabismus. 2003;40:19 –22. doi: 10.3928/0191-3913-20030101-07. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg M, Demer JL. Duane’s syndrome with compressive denervation of the lateral rectus muscle. Am J Ophthalmol. 2001;131:146 –148. doi: 10.1016/s0002-9394(00)00714-5. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Hwang JM. Presence of abducens nerve according to the type of Duane’s retraction syndrome. Ophthalmology. 2005;112:109 –113. doi: 10.1016/j.ophtha.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Parsa CF, Grant E, Dillon WP, et al. Absence of the abducens nerve in Duane syndrome verified by magnetic resonance imaging. Am J Ophthalmol. 1998;125:399 – 401. doi: 10.1016/s0002-9394(99)80158-5. [DOI] [PubMed] [Google Scholar]
- 37.Carteret M, Massin M, Cabanis EA. Stilling Duane syndrome and MRI: 2 preliminary results. Fr J Ophthalmol. 1992;15:537–542. [PubMed] [Google Scholar]
- 38.Miller JM, Demer JL, Rosenbaum AL. Two mechanisms of up-shoots and downshoots in Duane’s syndrome revealed by a new magnetic resonance imaging (MRI) technique. In: Campos EC, editor. Strabismus and ocular motility disorders. London, United Kingdom: MacMillan; 1990. pp. 229–234. [Google Scholar]
- 39.Bloom JN, Graviss ER, Mardelli PG. A magnetic resonance imaging study of the upshoot-downshoot phenomenon of Duane’s retraction syndrome. Am J Ophthalmol. 1991;111:548 –554. doi: 10.1016/s0002-9394(14)73696-7. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa T. Topographic anatomical studies on the orbit and its contents. Acta Soc Ophthalmol Jpn. 1965;69:2155. [Google Scholar]
- 41.Tian S, Nishida Y, Isberg B, Lennerstrand G. MRI measurements of normal extraocular muscles and other orbital structures. Graefe’s Arch Clin Exp Ophthalmol. 2000;238:393– 404. doi: 10.1007/s004170050370. [DOI] [PubMed] [Google Scholar]
- 42.Asmussen G, Kiessling A. Diameter changes of muscle fiber types of the inferior oblique muscle of the rabbit following denervation. Acta Anat (Basel) 1976;96:386 –403. [PubMed] [Google Scholar]
- 43.Baker RS, Millett AJ, Young AB, Markesbery WR. Effects of chronic denervation on the histology of canine extraocular muscle. Invest Ophthalmol Vis Sci. 1982;22:701–705. [PubMed] [Google Scholar]
- 44.Porter JD, Burns LA, McMahon EJ. Denervation of primate extraocular muscle: a unique pattern of structural alterations. Invest Ophthalmol Vis Sci. 1989;30:1894 –1908. [PubMed] [Google Scholar]
- 45.Christiansen SP, Baker S, Madhat M, Terrell B. Type-specific changes in fiber morphometry following denervation of canine extraocular muscle. Exp Mol Pathol. 1992;56:87–95. doi: 10.1016/0014-4800(92)90026-8. [DOI] [PubMed] [Google Scholar]
- 46.Miller NR, Kiel SM, Green WR, Clark AW. Unilateral Duane’s retraction syndrome (type I) Arch Ophthalmol. 1982;100:1468 –1472. doi: 10.1001/archopht.1982.01030040446016. [DOI] [PubMed] [Google Scholar]
- 47.Hotchkiss MG, Miller NR, Clark AW, Green WM. Bilateral Duane’s retraction syndrome: a clinical-pathologic case report. Arch Ophthalmol. 1980;98:870 – 874. doi: 10.1001/archopht.1980.01020030864013. [DOI] [PubMed] [Google Scholar]
- 48.Kakulas BA, Adams RD. Disease of muscle. 4. Philadelphia, Pennsylvania: Harper and Row; 1985. pp. 129–158. [Google Scholar]


