Abstract
Purpose
Magnetic resonance imaging (MRI) was used to determine the effect of recessions and resections on horizontal extraocular muscle (EOM) paths and globe position.
Methods
Four adults with horizontal strabismus underwent contrast-enhanced, surface-coil MRI in central, secondary, and tertiary gazes, before and after horizontal EOM recessions and/or resections. EOM paths were determined from 2-mm thickness, quasicoronal MRI by analysis of cross-sectional area centroids in a normalized, oculocentric coordinate system. Globe displacement was determined by measuring the apparent shift of the bony orbit in eccentric gaze.
Results
In all subjects, the anteroposterior positions of the horizontal rectus pulleys shifted by less than 2 mm after surgery, indistinguishable from zero within measurement precision. In three subjects who underwent medial rectus (MR) recession or resection, postoperative globe position was similar in central gaze, but globe translation during vertical gaze shift changed markedly. There was no effect on globe translation in the subject who underwent only lateral rectus (LR) resection.
Conclusions
Recessions and resections of horizontal EOMs have minimal effect on anteroposterior EOM pulley positions. Because the pulley does not shift appreciably despite large alterations in the EOM insertion, the proximity of a recessed EOM to its pulley would be expected to introduce torsional and vertical actions in tertiary gazes. Connective tissue dissection during MR surgery may destabilize the globe’s vertical translational stability within the orbit, potentially changing the effective pulling directions of the rectus EOMs in vertical gazes. These changes may mimic oblique muscle dysfunction. LR surgery may avoid globe destabilization.
The relative stability of posterior extraocular muscle (EOM) paths despite large, surgically induced changes in the location of the EOM’s insertion provided one of the pivotal insights that led to the modern concept of rectus EOM pulleys.1 The transition in EOM path from stable posterior EOM belly to mobile tendon insertion defined the functional position of the EOM pulley after surgical transpositions.2,3 Further enhancements in magnetic resonance imaging (MRI) have allowed the functional position of the EOM pulleys to be detected, even during routine gaze changes in normal subjects.4,5 Histologic studies of the orbit in the region of EOM path inflections have revealed a complex, connective tissue matrix of smooth muscle, collagen, and elastin,6–10 with insertion by some fibers of the EOM’s orbital layer into the encircling connective tissue that has been said to act as each EOM’s functional origin,11,12—that is, its pulley. Abnormalities of the EOM pulleys, either in location13–17 or in stability,18,19 have been associated with several forms of incomitant strabismus.
Despite the wealth of published information on pulley location and stability in both normal subjects and subjects undergoing complex EOM surgery for incomitant strabismus, little is known about the effects on the EOM pulley caused by standard EOM surgery for horizontal strabismus. Most EOM surgery involves resecting (shortening the tendon while maintaining the same insertion) or recessing (moving the insertion posteriorly) the EOM without changing the direction of its path in central gaze. Although moving the EOM insertion anteriorly and posteriorly along its original path should not tangentially displace the posterior EOM belly, extensive surgical dissection of the orbital connective tissue may destabilize the EOM pulley during gaze shifts.5 Notwithstanding that the degree of mechanical coupling between the orbital and global layers of the rectus EOMs is quantitatively unknown, there is probably at least some transfer of force between them. The orbital and global layers of each rectus EOM form parallel laminae that, at least in midorbit, contain a similar maximum number of fibers. The orbital layer terminates well posterior to the sclera, with at least some orbital layer fibers terminating in connective tissue ensheathing the EOM at a location probably corresponding to the functional pulley.20 There is also some attachment of global layer fibers to the pulley tissues, although the global layer is generally thought to become contiguous with the tendon that ultimately inserts on the sclera.11 Because at least some orbital layer force would thus be directly coupled to the pulley and because pulleys are suspended from the anterior orbit by elastic bands of connective tissue,10 the posterior shift of the EOM insertion might be expected to shift its pulley posteriorly. Similarly, because the orbital and global layers of a rectus EOM are in close apposition throughout the entire extent of the former, mechanical coupling between the layers would shift attached structures such as pulleys in the same direction as the surgically shifted tendon insertion. If such interlaminar coupling were strong, resecting the EOM tendon anterior to its pulley would be expected to shift the pulley anteriorly. This study was designed to detect what effect, if any, standard recess or resect horizontal EOM surgery has on pulley position and stability.
Methods
The study was designed as an observational case series. Four subjects were identified preoperatively in whom horizontal EOM recessions and/or resections were necessary to treat esotropia (ET) or exotropia (XT). All subjects underwent complete ophthalmic examinations. Then, after we obtained written informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the local Human Subject Protection Committee, each subject underwent high-resolution, T1-weighted MRI scanning before and after strabismus surgery via the limbal approach, with imaging techniques described in detail elsewhere.2–4 Dissection of connective tissues around the EOM tendons was intentionally conservative, using blunt technique minimally sufficient for tendon access.
Multiple contiguous quasicoronal MRI images 2 mm in thickness were obtained with a 256 × 256 matrix over an 8-cm2 field of view, giving a pixel resolution of 313 μm. Imaging was obtained in central gaze and also in secondary and tertiary gaze positions, with data collection in some subjects limited by fatigue in maintaining fixation in eccentric gaze positions for the 3.5 minutes required. Central gaze was determined by subjects based on self-report, and in all cases appeared reasonable to the experimenters.3 However, the central gaze position does not generally correspond to the kinematic primary position, defined as an eye orientation normal to Listing’s plane.
Digital MRI images were transferred to computer (Macintosh; Apple Computer, Cupertino, CA), converted into 8-bit tagged image file format (TIFF), and quantified on computer with NIH Image (Wayne Rasband, National Institutes of Health, Bethesda, MD; available by ftp from zippy.nimh.nih.gov). Left orbits were reflected to the configuration of right orbits to facilitate analysis.
Only images free from degradation by motion or other artifacts were analyzed quantitatively. A single point in each image plane, the “area centroid” (as defined by the NIH Image program, equivalent to the center of gravity of a shape of uniform density and thickness) was used to describe the location of each rectus EOM and the center of the bony orbit. The area centroid data were collected within the Cartesian coordinates of the MRI scanner and then were transformed to an oculocentric metric coordinate system to allow comparison of data before and after surgery.3
Central gaze EOM paths were compared before and after surgery, to measure tangential (vertical and horizontal) displacements in the posterior EOM path. The anteroposterior EOM path inflections in secondary and tertiary gaze were used to measure the functional anteroposterior pulley position.2,3 Finally, globe translation relative to the bony orbit was estimated by measuring the shift during eccentric gaze of the area centroid of the bony orbit at the globe–optic nerve junction. Because the bony orbit does not actually shift during gaze changes, movement of the orbital area centroid actually reflects translation of the globe center, defined as the origin of the oculocentric coordinate system.
Results
All four subjects were adults (average age, 49 years; range, 19–75 years) with horizontal strabismus.
Subject 1
This 59-year-old subject had intermittent, concomitant XT that decompensated after neurosurgical resection of a right cerebel-lopontine angle tumor to a constant angle of 95 to 100 prism diopters (approximately 45°). During surgery, both medial rectus (MR) muscles were resected 6 mm and both lateral rectus muscles (LR) were recessed 7 mm. One year after surgery, the subject was orthophoric at distance and had a residual 6-prism-diopter esotropia (ET) at near.
Preoperative MRI in central gaze demonstrated normal rectus EOM paths in both orbits, from which normal pulley positions can be inferred. Postoperative horizontal EOM paths were within 1 mm of preoperative locations in central gaze in both eyes, demonstrating minimal vertical displacement of the horizontal EOM pulleys after surgery (Fig. 1).
Figure 1.
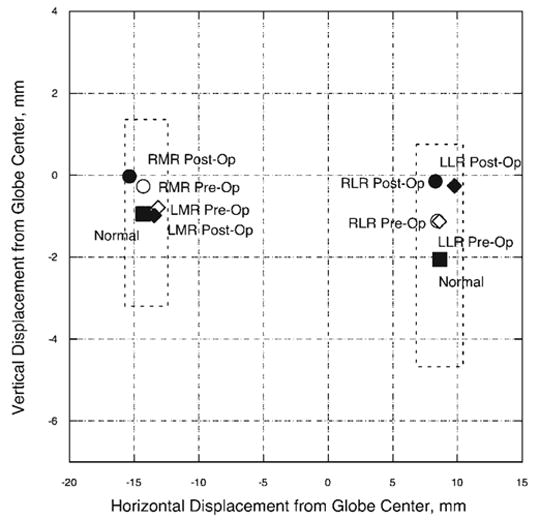
Horizontal and vertical horizontal rectus pulley positions in central gaze for the MR and LR extraocular muscles in subject 1. Pulley positions changed by less than 1 mm horizontally and vertically after 6.0 mm MR resections and 7.0 mm LR recessions. Dashed lines: two standard deviations around normal pulley positions for older adults.21 RMR, right medial rectus; LMR, left medial rectus; RLR, right lateral rectus; LLR, left lateral rectus.
Imaging was also performed before and after surgery in secondary gaze positions in the right eye and in tertiary gaze positions in the left eye. In many of these gaze positions, inflections in the MR and LR paths could be defined by the intersections of anterior and posterior regression lines, based on segmentation of area centroid points defining the anterior and posterior segments of the EOM paths to maximize their summed linear correlation coefficients. The inflections in EOM path determined by the anterior and posterior regression lines defined the anteroposterior locations of the horizontal rectus pulleys. The preoperative anteroposterior positions of the MR and LR pulleys were similar to published values for older adults.21 After surgery, the anteroposterior positions of the pulleys were within one image plane (2 mm) of preoperative positions (Fig. 2). Secondary gaze positions, however, suggested large apparent changes in posterior EOM path (Fig. 2) that actually reflected larger globe translation than occurred before surgery (Table 1), substantially exceeding the submillimeter translation typical of normal subjects.3 Although postoperative globe position in central gaze was similar to preoperative position, the globe translated significantly in all secondary and tertiary gaze positions. After surgery, the globe translated superiorly in both supraduction and infraduction. This movement is in contrast to normal globe translation, which is superior in infraduction and inferior in supraduction, opposite the direction of gaze change.3
Figure 2.
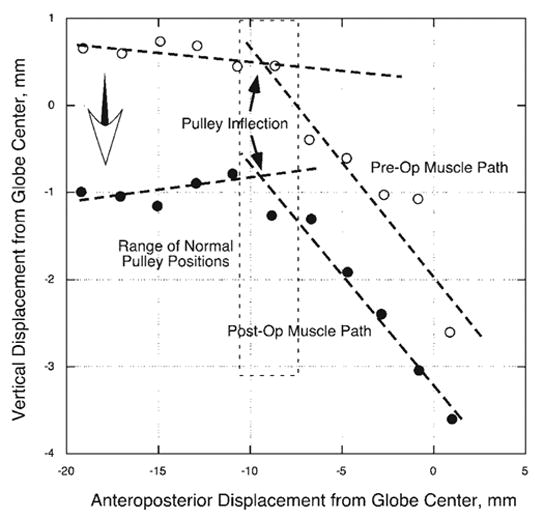
Right MR path of subject 1 in an oculocentric coordinate system before and after 6-mm MR resection and 7-mm LR recession. The path inflections between the stable posterior MR belly and down-wardly displaced MR insertion occurred at essentially the same antero-posterior position before and after surgery, 8 to 9 mm posterior to globe center (solid arrows). After surgery, however, the posterior MR belly shifted inferiorly during infraduction (large open arrow), reflecting superior displacement of the globe. A similar effect was observed during supraduction, and in the fellow orbit. Dashed lines: two standard deviations around normal MR pulley position for older adults.21
Table 1.
Postoperative Vertical Change in Globe Position
| Central Gaze
|
Infraduction
|
Supraduction
|
||||
|---|---|---|---|---|---|---|
| Subject | Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye |
| 1 | 0.9 | 0.2 | 2.2 | 1.3 | 1.5 | 1.4 |
| 2 | −0.4 | −0.6 | 0.5 | 1.0 | 1.4 | 1.5 |
| 3 | −0.3 | 0.0 | 1.5 | 1.0 | 0.6 | 0.1 |
| 4 | 0.1 | −0.1 | 0.6 | 0.0 | 0.8 | 0.3 |
Positive values represent superior globe displacement. Data are expressed in millimeters.
Subject 2
This 19-year-old subject had an incomitant XT of 20 prism diopters in central gaze that increased to 65 prism diopters on supraduction and decreased to 10 prism diopters on infraduction. There was marked bilateral overelevation in adduction. The subject had undergone one prior strabismus surgery, involving bilateral LR recessions of 9 mm. More than 10 months after a reoperation consisting of bilateral 4-mm MR resections and bilateral 10-mm inferior oblique recessions, the subject had a residual, concomitant ET measuring 6 to 8 prism diopters in all gaze positions.
Preoperative orbital MRI was performed in central and secondary gazes for the right eye and central and tertiary gazes for the left eye. The preoperative MRI in central gaze demonstrated a superiorly displaced right MR and inferiorly displaced right LR muscle more than two standard deviations from normal (Fig. 3). Previous work has shown that these abnormal horizontal EOM pulley locations can result in overelevation and overdepression of the globe during adduction without postulating any oblique EOM dysfunction.14
Figure 3.
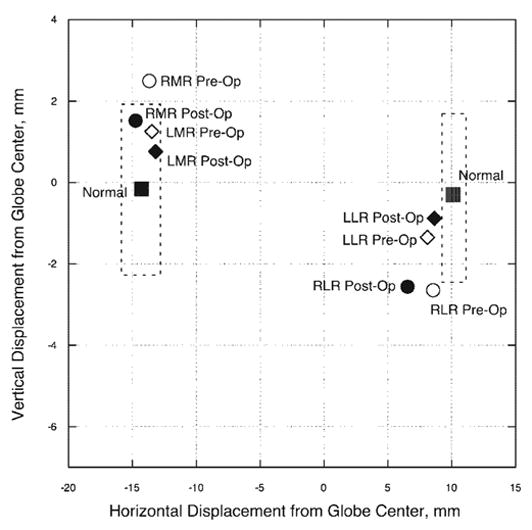
Subject 2: horizontal and vertical positions in central gaze for the MR and LR muscles in the region of their respective pulleys. Pulley positions changed by less than 1 mm horizontally and vertically after 4-mm resections of each MR tendon. Dashed lines: two standard deviations around normal pulley positions for younger adults.3 The preoperative right MR position and pre- and postoperative right LR position fall outside of the normal range of pulley positions. Abbreviations are as in Figure 1.
Postoperative MRI was performed 3 weeks after the second surgery and revealed that the horizontal EOM paths and globe position in central gaze were essentially unchanged in both orbits, although the slight inferior shift of the right MR now placed its pulley within the normal range (Fig. 3). Based on EOM path inflections in eccentric gaze, anteroposterior pulley positions were also unchanged. The globe, however, displaced superiorly on supraduction by more than 1 mm and superiorly on infraduction by 0.5 to 1.0 mm in both eyes (Table 1).
Subject 3
This 42-year-old subject had previously untreated, concomitant infantile ET measuring 60 prism diopters. There was no A- or V-pattern strabismus, and the left eye demonstrated only minimal overelevation and overdepression in adduction. Five months after bilateral 6.5-mm MR recessions, the subject had a residual concomitant ET of 12 to 15 prism diopters.
Preoperative MRI in central gaze demonstrated normal horizontal rectus EOM paths in both orbits, from which normal pulley positions can be inferred. After surgery, MRI demonstrated that the horizontal EOM paths and globe position in central gaze and the functional anteroposterior pulley positions in secondary gaze were essentially unchanged in both eyes (Fig. 4). The globe, however, translated superiorly by 1.0 to 1.5 mm on infraduction and superiorly by less than 1 mm on supraduction (Table 1).
Figure 4.
Subject 3: horizontal and vertical positions in central gaze for the MR and LR extraocular muscles in the region of their respective pulleys. Pulley positions changed by less than one mm horizontally and vertically after 6.5-mm recessions of each MR tendon. Dashed lines: two standard deviations around normal pulley positions for older adults.21 Abbreviations are as in Figure 1.
Subject 4
This 75-year-old subject had acquired, concomitant ET of the divergence-insufficiency type, measuring 20 prism diopters in the distance and 4 prism diopters at near. There was no A- or V-pattern strabismus, but both eyes demonstrated slightly diminished abduction. Seven months after bilateral 7-mm LR resections, the subject was orthophoric at near, with a residual concomitant distance ET of 12 prism diopters.
The preoperative MRI in central gaze demonstrated an inferiorly displaced right LR more than two standard deviations from normal and a left LR that was slightly inferiorly displaced as well (Fig. 5). After surgery, MRI demonstrated that the horizontal EOM paths and globe position in central gaze and the functional anteroposterior pulley positions in secondary gaze were essentially unchanged in both eyes (Fig. 5).
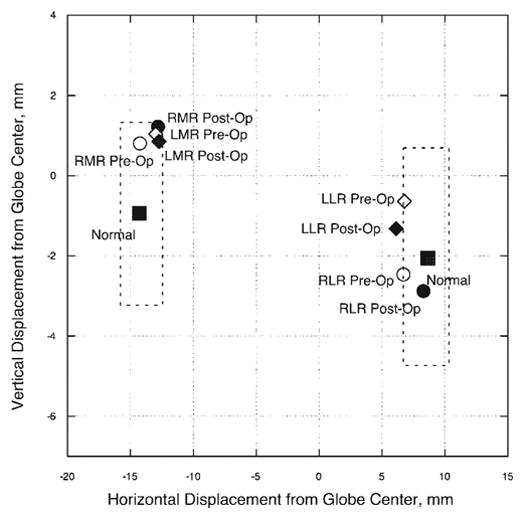
Figure 5.
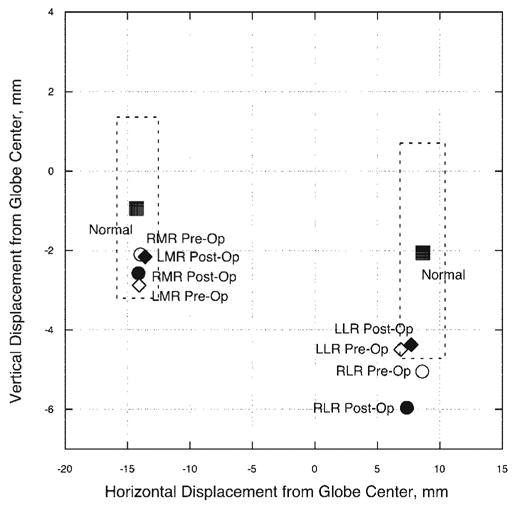
Subject 4: horizontal and vertical positions in central gaze for the MR and LR extraocular muscles in the region of their respective pulleys. Pulley positions changed by less than one mm horizontally and vertically after 7 mm LR resections. Dashed lines: represent two standard deviations around normal pulley positions for older adults.21 The pre- and postoperative right LR positions fall outside of the normal range of pulley positions. Abbreviations are as in Figure 1.
In addition, for this subject, the magnitude of the postoperative globe translation in both central and secondary gaze positions was within the normal range,3 measuring less than 1 mm superior displacement on supraduction and infraduction in both eyes. The direction of globe translation, however, was superior, regardless of the change in vertical gaze position, different from the finding in normal subjects,3 but similar to results observed in the other subjects in this study.
Discussion
The results demonstrate that, as predicted from the effects of insertional transposition,4,22 conventional recession and resection of horizontal rectus EOMs did not result in horizontal or vertical displacement of their pulleys. Like insertional transposition, dissections performed during conventional strabismus surgery do not sever the stiff connective tissue suspensions responsible for the resistance of pulleys to sideslip. However, normal rectus pulleys make large, physiologic anteroposterior shifts during EOM contraction and relaxation.12 In view of the close apposition between the orbital and the global layers and the attachment of the pulleys to the EOMs, we were surprised to find that shifting EOM insertions posteriorly or anteriorly (combined with tendon shortening) by 4 to 7 mm did not result in detectable anteroposterior shifts of the corresponding EOM pulleys, at least at the level of the 2-mm sensitivity of this experiment.
The orbital layer of each rectus EOM does not continue into direct continuity with the scleral insertion. The orbital layer is not found anterior to the pulley, and some orbital layer fibers insert directly onto the immediately adjacent orbital surface of the pulley.20 The orbital layer does not take a separate and distinct path from the global layer, but parallels the global layer in close apposition as far as the orbital layer extends anteriorly. It must be assumed that some fraction of global layer force is transmitted to the orbital layer, and vice versa, as emphasized by Dimitrova et al.23 The amount of this force coupling is currently unknown, but the present result perhaps gives some sense of the magnitude of interlayer coupling. Even under the anatomically unrealistic assumption of zero friction between the orbital and global layers, the existence in some gaze positions of an inflection of the global layer’s path at the pulley would couple transverse force from the global layer to the orbital layer. The current finding of no detectable effect of EOM tendon surgery on anteroposterior pulley location suggests that the global layer fibers do not transmit enough translational force to shift pulley position by even as much as 2 mm, the minimum shift measurable by the current technique.
If the rectus pulleys were completely coupled to the global layers and their contiguous tendons, a shift much larger than 2 mm would have been anticipated after, for example, the 7-mm recession of the scleral insertion performed in subject 1. Such a recession represents more than 40% of the normal distance between the insertion and the pulley.12 Despite obvious anatomic indications of mechanical intercouplings, the current finding thus suggests a degree of partial mechanical independence between the global layer and the pulley, and between the orbital and global layers. It should be emphasized that mechanical couplings are still likely to function to a partial extent, but their effects should be considered working against the putatively high stiffness of the pulley suspensions. Computational simulations assuming only passive elastic constraints on pulley positions suggest that the stiffness of these suspensions would have to be in the range of 40 g/mm to account for the high resistance of pulleys to sideslip.12 With such high stiffness, the hypothetical passive anteroposterior shift of pulleys attributable to frictionless passage of EOMs through pulleys has been calculated not to exceed 0.1 mm for even ± 35° ductions.12 The observation that actual rectus pulley shifts are in the range of 14 mm for such ductions has been taken as evidence that pulley shifts are actively rather than passively mediated.12 More complex biomechanical modeling of bilaminar EOMs and realistic connective tissue couplings would be required to interpret quantitatively the present finding of a less than 2-mm pulley shift after EOM recession and resection. It would be desirable to infer computationally, for example, the approximate value of a putative a subunity coupling coefficient between the orbital and global layers. Such a worthwhile computational investigation, however, is beyond the scope of the present study.
After resection, where the EOM tendon is disinserted, shortened, and reinserted at the original location on the sclera, anteroposterior pulley stability should support normal ocular kinematics. The three-dimensional relationship between the functional origin of the EOM, the pulley, and its insertion onto the globe is preserved after surgery. After recession, however, the EOM insertion is brought much closer to the pulley, potentially introducing torsional and vertical actions in tertiary gaze positions.
Figure 6, a schematic lateral view of a horizontal rectus EOM and its pulley, illustrates the kinematic consequences of EOM recession.11 Before surgery (Fig. 6, left), the distance D2 from globe center to insertion is maintained equal to distance D1 from pulley to globe center. For a trigonometrically small angle of supraduction α typical of the ocular motor system, this relationship causes the rotational velocity axis imposed on the globe by the EOM to tilt posteriorly by angle α/2. This “half angle” dependence of ocular rotational velocity axis on eye position is a sufficient condition for the eye to remain in compliance with Listing’s Law of ocular torsion.24 Listing’s Law is a quantitative description of ocular torsion whose corollary and original statement is that, when the head is upright and stationary, any eye orientation can be reached from a primary position by rotation about a single axis lying in Listing’s plane.24 Because three-dimensional eye velocity is imparted by the direction of EOM force, maintenance of the mechanical arrangement of the rectus EOM pulleys accounts for Listing’s Law without any explicit neural computation of eye torsion25,26 and also makes the ocular motor periphery appear to the brain to be mathematically commutative with respect to the sequence of ocular rotations.27 This kinematic analysis is supported by recent neurophysiological observations in monkeys. Motoneurons innervating vertical rectus and oblique EOMs do not encode the torsion corresponding to half-angle behavior during pursuit,28 whereas direct electrical stimulation of the abducens nerve without the possibility of downstream neural processing evoke horizontal saccades conforming to Listing’s Law.29 Known violations of Listing’s Law during convergence30 and ocular counterrolling31 have been demonstrated by MRI in humans to be associated with rotation of the rectus pulley array in the coronal plane in coordination with ocular torsion. Thus, even after ocular torsion has been driven out of Listing’s plane by a vestibular stimulus, subsequent visual saccades conform to Listing’s Law in a new Listing’s plane paralleling the original one,32 and modulation of the orientation of Listing’s plane by gravity33 can be explained by rotation of the rectus pulley array.31
Figure 6.
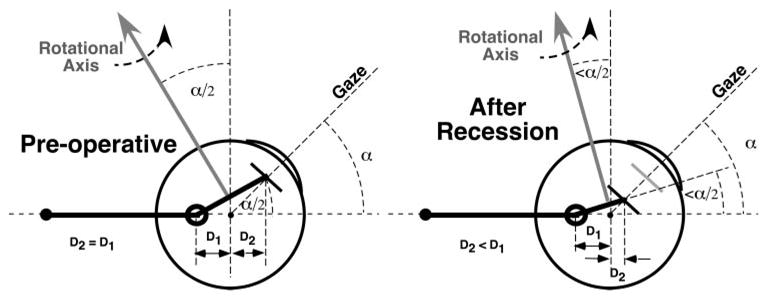
Lateral view of schematic horizontal rectus extraocular muscle (EOM) and pulley in supraduction. Before surgery (left), distance D2 from globe center to insertion is maintained equal to distance D1 from pulley to globe center. For a small angle of supraduction α typical of the ocular motor system, the rotational velocity axis imposed on the globe by the EOM tilts posteriorly by angle α/2. In the absence of globe translation, recession of the EOM’s scleral insertion (right) reduces its distance D2 from globe center without significantly altering distance D1 from pulley to globe center. After recession, supraduction to angle α causes the EOM’s rotational velocity axis to tilt posteriorly by less than angle α/2, with the actual amount depending on the recession distance. Globe translation would further alter the EOM’s rotational axis.
In view of the preceding anatomic and physiologic evidence indicating Listing’s Law to be an emergent property of EOM path geometry, surgical alteration of this geometry as illustrated in Figure 6 (right) would be anticipated to alter ocular kinematics. The current results demonstrate that recession of an EOM’s scleral insertion (Fig; 6, right) reduces its distance D2 from globe center, without significantly altering distance D1 from pulley to globe center. After recession, supraduction to angle α causes the EOM’s rotational velocity axis to tilt posteriorly by less than angle α/2, with the actual amount depending on the recession distance. If, as evidence suggests, Listing’s Law is an emergent property of EOM paths as dependent on the orbital pulleys, then Listing’s Law would be expected to be violated after rectus EOM recessions. Specifically, this violation would manifest as abnormal ocular torsion in tertiary gaze positions where the recessed EOM contributes significant force. Binocular alignment in tertiary gaze positions should also be affected by abnormal vertical and torsional actions of recessed horizontal rectus EOMs. The extent of such putative abnormalities depends on both the amount of EOM recession and the tertiary gaze angle. Because the horizontal rectus pulley positions did not change after resections of their tendons, such surgery would not be predicted to alter ocular kinematics and would not be predicted to result in violations of Listing’s Law.
This study also demonstrated translational destabilization of the globe in eccentric gaze after both MR resection and recession. Translational globe position in central gaze was similar before and after surgery, within 1 mm of the preoperative position for all subjects (Table 1), but the globe displaced superiorly on both supraduction and infraduction by as much as 2 mm. This amount of superior globe displacement (and resultant relative inferior displacement of the horizontal EOMs) has been shown to mimic oblique EOM dysfunction.14 Translational globe destabilization was not observed in subject 4, the only subject who only underwent LR surgery.
Of note, subject 4 also appeared to have inferiorly displaced LR pulleys both before and after surgery. Clinically, the subject was diagnosed with divergence palsy and apparent mild LR underaction. These clinical findings can be at least partially explained by the abnormal inferior placement of the LR pulleys. Much of the contractile force of the LR would be directed toward infraduction instead of abduction. This conclusion is supported by the relatively poor clinical response to LR resection surgery, which left the subject with substantial ET in the distance only.
All the participants chosen were adults, because the level of concentration and cooperation required during orbital MRI precluded the use of young children. Horizontal EOM surgery, however, is frequently performed in young children, who typically have denser connective tissue within the region of the EOM pulleys. Because this connective tissue thins with age,10 older subjects may be more susceptible to globe destabilization during strabismus surgery than are children.
This study illustrates the importance of both controlling gaze during orbital imaging and obtaining images in multiple positions of gaze. The connective tissue components of the orbit shift position during changes of gaze, maintaining the relationship between the globe and EOM pulleys. In this group of subjects, our conclusions would have been very different if we had only imaged in central gaze, because we would have erroneously concluded that standard horizontal EOM recessions and resection had very little effect on orbital connective tissue and EOM pulleys. Likewise, if these subjects had simply closed their eyes and relaxed during imaging, allowing Bell’s phenomenon to rotate both eyes into supraduction, we may have erroneously concluded that recessions and resections somehow introduced superior displacements in globe position without appreciating the relationship of globe displacement to change of gaze position.
In conclusion, this study demonstrates that recessions and resections of horizontal EOMs have minimal effect on antero-posterior, vertical, and horizontal EOM pulley positions within the bony orbit. Since recession places the horizontal rectus EOM insertion near its pulley, vertical and torsional actions are predicted to occur in tertiary gaze positions. In addition, connective tissue dissection or other factors associated with MR recession or resection may destabilize the globe’s vertical position within the bony orbit. Superior displacement of the globe changes the effective pulling directions of the horizontal rectus EOMs on supraduction and infraduction, possibly mimicking oblique EOM dysfunction. LR surgery alone may avoid globe destabilization.
Footnotes
Presented in part at the American Association for Pediatric Ophthalmology and Strabismus meeting, Orlando, Florida, March 2005.
Disclosure: R.A. Clark, None; J.L. Demer, None
Supported by National Eye Institute Grant EY-08313 and Core Grant EY-00331 and an unrestricted award from Research to Prevent Blindness (JLD). JLD is the Leonard Apt Professor of Ophthalmology.
References
- 1.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 2.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys: muscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 3.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 4.Clark RA, Rosenbaum AL, Demer JL. Magnetic resonance imaging after surgical transposition defines the anteroposterior location of the rectus muscle pulleys. J AAPOS. 1999;3:9–14. doi: 10.1016/s1091-8531(99)70088-1. [DOI] [PubMed] [Google Scholar]
- 5.Clark RA, Demer JL. Rectus extraocular muscle pulley displacement after surgical transposition and posterior fixation for paralytic strabismus. Am J Ophthalmol. 2002;133:119–128. doi: 10.1016/s0002-9394(01)01264-8. [DOI] [PubMed] [Google Scholar]
- 6.Koornneef L. New insights in the human orbital connective tissue: result of a new anatomical approach. Arch Ophthalmol. 1977;95:1269–1273. doi: 10.1001/archopht.1977.04450070167018. [DOI] [PubMed] [Google Scholar]
- 7.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 8.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. [PubMed] [Google Scholar]
- 9.Demer JL. The orbit pulley system: a revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 10.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 11.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 12.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gaze. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 13.Demer JL, Miller MJ, Koo EY, Rosenbaum AL, Bateman JB. True versus masquerading superior oblique palsies: muscle mechanisms revealed by magnetic resonance imaging. In: Lennerstrand G, editor. Update on Strabismus and Pediatric Ophthalmology. Boca Raton, FL: CRC Press; 1995. pp. 303–306. [Google Scholar]
- 14.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 15.Demer JL, Clark RA, Miller JM. Role of orbital connective tissue in the pathogenesis of strabismus. Am Orthoptic J. 1998;48:56 –64. [Google Scholar]
- 16.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Amsterdam: Swets; 1999. pp. 91–94. [Google Scholar]
- 17.Demer JL, Clark RA, Kono R, Wright W, Velez F, Rosenbaum AL. A 12-year prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2002;6:337–347. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 18.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–2178. [PubMed] [Google Scholar]
- 19.Demer JL, Kono R, Wright W, Oh SY, Clark RA. Gaze-related orbital pulley shift: a novel cause of incomitant strabismus. In: de Faber JT, editor. Progress in Strabismology. Lisse, The Netherlands: Swets and Zeitlinger; 2003. pp. 207–210. [Google Scholar]
- 20.Oh SY, Poukens V, Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–16. [PubMed] [Google Scholar]
- 21.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Amer J Ophthalmol. 2002;134:872–878. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 22.Miller JM, Demer JL, Rosenbaum AL. Effect of transposition surgery on rectus muscle paths by magnetic resonance imaging. Ophthalmology. 1993;100:475–487. doi: 10.1016/s0161-6420(93)31618-0. [DOI] [PubMed] [Google Scholar]
- 23.Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol. 2003;90:3809–3815. doi: 10.1152/jn.00622.2003. [DOI] [PubMed] [Google Scholar]
- 24.Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–127. doi: 10.1016/0042-6989(90)90131-4. [DOI] [PubMed] [Google Scholar]
- 25.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 26.Raphan T. Modeling control of eye orientation in three dimensions. I. Role of muscle pulleys in determining saccadic trajectory. J Neurophysiol. 1998;79:2653–2667. doi: 10.1152/jn.1998.79.5.2653. [DOI] [PubMed] [Google Scholar]
- 27.Quaia C, Optican LM. Commutative saccadic generator is sufficient to control a 3-D ocular plant with pulleys. J Neurophysiol. 1998;79:3197–3215. doi: 10.1152/jn.1998.79.6.3197. [DOI] [PubMed] [Google Scholar]
- 28.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:1–13. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Klier EM, Meng H, Angelaki DE. Abducens nerve/nucleus stimulation produces kinematically correct three-dimensional eye movements. Soc Neurosci Abstr. 2005:475.4. [Google Scholar]
- 30.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;90:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 31.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. [DOI] [PubMed] [Google Scholar]
- 32.Crane BT, Tian JR, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809. doi: 10.1167/iovs.05-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hess BJ, Angelaki DE. Gravity modulates Listing’s plane orientation during both pursuit and saccades. J Neurophysiol. 2003;90:1340–1345. doi: 10.1152/jn.00167.2003. [DOI] [PubMed] [Google Scholar]


