Gene expression profiling is now being used routinely to define complex biological events. The profiling of a large array of genes expressed in the progression of a biological response opens the door to our understanding the unique relationships between genes and their functions. Angiogenesis, the process of new blood vessel development, is a necessary component of both normal and pathological physiology. In this issue of The American Journal of Pathology, Shih and colleagues 1 have used quantitative molecular profiling of angiogenic-related factors to define some of the elements required for angiogenic profiling. Although presented as a technical advance, the basic concept of this work is that the use of quantitative molecular profiling of gene expression gives additional insight into functional interrelationships between the genes expressed during the angiogenic process. This approach can be applied in large array format as a diagnostic tool for experimental systems and pathological samples.
During the last two decades, an explosion in our understanding of angiogenesis at the molecular level has occurred. These advances have been facilitated by effective angiogenic models consisting of endothelial capillary tube-like formation in vitro, 2-7 in vivo angiogenesis in avian chorioallantoic membrane, 2,8,9 and angiogenesis associated with angiogenic or inflammatory cytokine-impregnated implants in mammals. 10-16 A significant addition to these models has been the use of genetic knockouts in mice to test the relevance of angiogenic genes in vivo. 17-21 Defects that occur, if any, in the developing vascular tree would indicate that the null gene is required for vasculogenesis or angiogenic events that occur during pre- and early postnatal development. From these and other functional models, the molecules involved in select events required for the angiogenic process to occur, including endothelial cell-specific proliferation, migration, cell-cell association, and vessel morphogenesis, have been partially defined.
Angiogenic cytokines and their receptors have been identified as key regulators of the angiogenic process. Central to the activation and maintenance of the neovasculature are members of the vascular endothelial growth factor family, VEGF-A, -B, -C, -D, and placental growth factor (PLGF), and the VEGF receptors, VEGF-R1, -R2, -R3, and R4. 7,22-26 In addition, the secreted angiopoietin, Ang-1 acts on the Tie-2 receptor to stabilize the vascular structure. Antagonism of the Tie-2 activity by the cytokine-induced Ang-2 is indicative of vascular destabilization and may be a early priming step in the angiogenic pathway. 5,27-29
Several important molecular profiling analyses have been performed on endothelium undergoing cytokine activation and during the angiogenic process in vitro and in vivo. 6,30-36 These studies have used a number of techniques including differential and subtractive hybridization, differential display, GeneCalling, 37 serial analysis of gene expression (SAGE), and cDNA arrays and microarrays. These studies have defined differentially expressed genes that are most likely to play a role in the angiogenic process. The genes identified fall into a number of protein subclasses such as secreted proteins, extracellular matrix, metalloproteinase, receptors, junctional molecules, protease inhibitors, transporters/channels, and miscellaneous cell surface proteins as was comprehensively presented by Peale et al. 34 These differential expression studies have provided an essential first step in defining the boundaries for the molecular characterization of the angiogenic process beginning from the initial activation events to vascular maturation and stabilization.
There are important distinctions, however, between genes induced in the process and how these genes might interact with each other to modulate function. For example, in the molecular profiling of angiogenic markers by Shih et al, 1 an important step toward defining the relationship between genes involved in the angiogenic process has been achieved. Taqman analysis was used for quantification, using standards that quantify the actual copy number of the target gene, compared to cyclophilin, an internal control. Data from this method allows for the direct comparison of the mRNA levels for several genes in the same sample. This can have important implications in the interpretation of the role for these genes in the angiogenic process. For example, a direct comparison of the expression of competing ligands for the same receptor, as is the case for Ang-1 and Ang-2, can be determined. One interesting observation from this study of two different prostatic tumors from the transgenic adenocarcinoma of mouse prostate (TRAMP) model has revealed an inverse relationship between Ang-2 and Tie-2 expression. The larger tumor had higher Ang-2 and less Tie-2 than the tumor of roughly half its size. Overexpression of both VEGF and Ang-2 also correlated with higher levels of the endothelial markers, kinase-domain receptor (KDR) (VEGF-R2), fems-like tyrosine kinase-1 (Flt-1) (VEGF-R1), VE-cadherin, platelet endothelial cell adhesion molecule-1 (PECAM-1), and Tie-1. Future studies such as these will likely provide the quantitative data for which mathematical algorithms may be designed to form a molecular profile for different stages in the tumor angiogenic process.
To date, most molecular profiling approaches have taken a differential expression approach, ie, comparing the relative expression patterns of genes induced or repressed genes under different physiological circumstances. Several groups have utilized computerized database extraction and functional assignment of genes found to be differentially expressed in the angiogenic process. 34,38,39 However, without quantitative analysis of each of these genes, it is difficult to interpret the stages in the angiogenic process. To capture the true state of a complex biological process with quantitative gene expression data, the interrelationship of genes expressed at any one time will have to be critically modeled and tested in experimental systems. Thus, differential expression combined with how the differentially expressed genes might interact together to influence a complex process such as angiogenesis will be required for effective molecular profiling. An example of the complexity presented by this approach is given in Table 1 ▶ , where the expression of genes that might be involved in the various stages of angiogenesis is tabulated as functional protein classes compared to the stages or targets in the angiogenic process. For example, during initial endothelial cell activation, the levels of angiogenic cytokines, such as VEGF, fibroblast growth factor (FGF)-2, and Ang-2, requires the action of VEGF-R, FGF-2, and Tie-2 on susceptible local microvasculature. The cytokine activation of local vasculature would then induce endothelial cell adhesion molecules such as E- and P-selectin and vascular cell adhesion molecule-1 (VCAM-1), thus promoting inflammatory cell infiltration (ie, monocytes and macrophage). Thus, the ratio of activators to inhibitors for functional events will help to identify the total angiogenic potential in the sample. It is anticipated that further definition of the interrelationship of a number of gene classes might define the angiogenic process, with respect to its physiological or pathophysiological mechanism, can be achieved.
Table 1.
Involvement of Functional Genes in Various Stages of the Angiogenic Process
| Protein type | Angiogenic processes | |||
|---|---|---|---|---|
| Endothelial cell activation | Stromal cell/matrix modulation | Proliferative expansion | Vascular morphogenesis/maturation | |
| Secreted proteins/lipids | VEGF, FGF-2, Ang-2, LPS, TNFa, HGF | IL-8, Gro-Ia, IL-2, MCP-1, TGFb, HGF, CTGF, MIP-1a | VEGF, FGF-2, HGF,PLGF, jagged | Ang-1, S-1-P, BMP-6,VEGF, ephrins |
| Receptors | VEGF-R2, FGF-R, Tie-2, E-selectin, VCAM-1 | FGF-R (flg) FGF-R (bek) CXCR4 | VEGF-R2 VEGF-R1, Tie-1, notch | VEGF-R1Tie-1, Edg-1, Eph B4, PDGF-R |
| Cell migration | Integrin aVb3, integrin a2, integrin a5, Fn, Vn, osteopontin | Collagen I, III, VI | Integrin aVb3, Fn, Vn, denatured collagen | SPARC, hevin, TSP-1,-2 |
| Extracellular matrix remodeling activators/inhibitors | t-PA, u-PA, MMP-2, MMP-9 | MMP-3, MMP-2, MMP-9, MMP-11, ADAMTS-1,-4 | u-PA, UPAR, Pai-1 | Laminin, collagen IV, TSP-1,-2, Pai-1, TFPI-1,-2 |
| Cell-cell junctions | PECAM-1 (CD31) | VE-cadherin, connexin 37, connexin 45 | ||
Matrix description of how genes involved in endothelial cell regulation would contribute to various functions over the course of angiogenic events ranging from early endothelial cell activation, proliferative expansion, and maturation. The stromal/inflammatory mediators will likely promote angiogenesis at any stage and thus are placed in the middle.
Validation of the interrelationship models that might be developed from quantitative molecular profiling is not as easy as might be assumed. The molecular profiling of mRNA levels by arrays or quantitative analyses still does not define variations in protein synthesis and post-translational modifications that might affect their function. This is especially relevant for signal transduction kinases that are in a state of constant flux due to cellular signaling from the microenvironment and cytokine activation. Also, many proteins such as metalloproteinases are expressed in pro-forms, and thus the mRNA levels do not necessarily relate to their active levels. It is clear that validation of functional gene expression by direct in situ methods such as immunohistochemistry and activity assays will define cell-type specificity for key proteins involved in one or more elements of the angiogenic process and which of these may be appropriate markers for molecular profiling.
An additional complication with the analysis of total gene profiles in complicated biological processes such as angiogenesis is the diversity of cellular populations that are present within the tissue. For example, during exponential or late stage tumor growth, the types of endothelial markers would include those for infiltrating precursors from the circulation, 40,41 proliferating endothelial cells (ECs), newly formed tube structures, mature tube structures, and even vessels undergoing apoptosis. Thus, the total EC-specific gene expression profile would be a mixture of all of the endothelial pools that are likely to have very different gene expression ratios at any one time. The data presented by Shih et al, 1 give some insight into this possibility. Analysis of the two different sized prostatic tumors showed some similarities, but demonstrated hypoxic-induced VEGF in the larger tumor and the coordinate expression of the higher levels of Ang-2 and somewhat repressed Tie-2, compared to the smaller tumor.
A model of the stages in tumor angiogenesis and the implications for a tumor undergoing therapies directed at the angiogenic process alone or combined therapeutic targets is given in Figure 1 ▶ . The result of a partially effective anti-angiogenic therapy is likely to induce some tumor hypoxia and may potentiate tumor re-entry into an active angiogenic response by the up-regulation of VEGF and/or FGF-2. Alternatively, effective combination therapies directed toward angiogenic and tumor growth processes could lead to tumor regression which has remained an elusive goal with the anti-angiogenic strategies. The molecular profiles that are obtained from these different stages of tumor growth or treatment will be important for establishing the usefulness of this approach for evaluating the therapeutic effectiveness of anti-angiogenic therapies, either alone or in combination with other tumor-targeted approaches.
Figure 1.
Model of tumor angiogenic stages in relation to therapeutic intervention. Representation of the angiogenic stages in the initiation and expansion of tumor as well as the possible result for therapeutic intervention. Text indicates angiogenic events, cell types, and changes in microenvironment for each stage. Transitions between stages are indicated as 1: active and proliferative angiogenic response, 2: maturation into functional tumor vasculature, 3: effective anti-angiogenic therapy to derive a balance between tumor support and apoptosis, 4: re-entry of treated tumor into active angiogenesis due to increased expression of angiogenic stimuli from affected tumor or therapeutic relapse, 5: anti-angiogenic therapy alone that will benefit from self-reactivity to stable disease, 6: effective multi-modal therapeutics effective for blocking both angiogenic and tumor growth events, 7: long-term relapse from a small number of therapeutically resistant or static cells selected for survival.
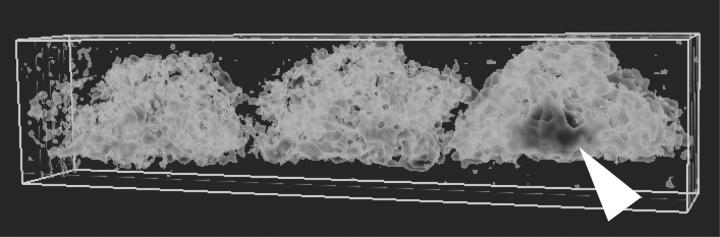
Combined interrelational algorithms applied to static molecular quantitative gene expression data will still have significant voids with regard to the variability in time and space within each sample. The future for resolving these kinds of temporal and spatial distinctions will likely be the combination of molecular profiling with a direct analysis of biological function. New methods for non-invasive imaging technologies that define functional vasculature and its relation to the level of tumor viability and growth are currently being developed. 42-46 The application of functional magnetic resonance imaging (MRI) has been a promising avenue to examine the angiogenic process. An example of one such application is shown in Figure 2 ▶ , where the use of a functional MRI screen based on the magnetic inhomogeneity related to the oxy/deoxy-hemoglobin conversion which can delineate differences between an animal with a prostatic tumor from a normal one. This non-invasive method does not require contrast agents and easily thresholds the angiogenic tumor-derived signals from that of surrounding normal tissue. These types of imaging technologies define areas where there is significant blood flow and utilization by the expanding tumor (delineated by dark gray in the tumor), and tumor areas that are not metabolically active or necrotic. Functional imaging combined with molecular and proteomic profiling analyses might provide a better overall interpretation of the angiogenic status of complex tumor physiology.
Figure 2.
Diagnostic three-dimensional functional MRI identification of angiogenic prostate tumor in mice. Three mice were scanned without contrast agent in a 1.5 Tesla MR using a peripheral coil across the lower abdomen for development of orthotopically injected PC-3 prostate tumors. Functional MRI parameters were computed for the inverse transition rate (R2′) and the images aligned and reconstructed in 3-D. Animal on right indicates a tumor with prominent R2′ signal (gray, arrow) on the periphery of a defined tumor later confirmed by dissection and histological analysis. Note the differential levels of gray signal ranging from light to dark foci. The normal tissues appear as mosaic white/light gray.
The future of molecular profiling will likely use detailed information from advanced imaging, molecular and proteomic profiling, and in situ analyses to define interpretive models that are predictive of biological behavior. Multiplex analysis of the angiogenic process is presented in Figure 3 ▶ , and implies that analyses of a complex biological process will likely require a multi-modal approach. Integration of data from multiple methodologies by advanced computational models will likely be needed to validate a smaller number of key molecular and proteomic profile gene targets necessary to interpret the angiogenic status of any particular sample. Although many of these candidate genes might be known at the current time, the actual expression ratio between each of them or between different functional classes combined with their positive or negative effects on angiogenic events remain to be determined. In short, the ability to provide quantitative data with respect to both molecular and proteomic profiling of angiogenic markers is essential to the interpretation of this complex biological process.
Figure 3.
A multiplex approach to profiling the complex angiogenic process. Analytical and quantitative profiling methodologies are defined on the spokes around the central angiogenic processes. Given the variability in physiological and pathophysiological angiogenic activators, some of which are indicated around the central process, the analysis of the angiogenic responses in each condition may define specific molecular profiles for each.
Acknowledgments
I thank Ia Gomez-Garcia, Jason Kirk, and Lahmer Lynds for graphic and technical assistance.
Footnotes
Address reprint requests to Kevin P. Claffey, University of Connecticut Health Center, Center for Vascular Biology, E5018, Department of Physiology, 263 Farmington Avenue, MC3505, Farmington, CT 06030-3505. E-mail: claffey@sun.uchc.edu.
Supported by National Institutes of Health:National Cancer Institute grant CA64436.
References
- 1.Shih S-C, Robinson GS, Peruzzi C, Smith LE, Senger D: Molecular profiling of angiogenic markers. Am J Pathol 2002, 161:35-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J, Haudenschild C: Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K 1980, 100:346-353 [PubMed] [Google Scholar]
- 3.Kubota Y, Kleinman HK, Martin GR, Lawley TJ: Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 1988, 107:1589-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamble JR, Matthias LJ, Meyer G, Kaur P, Russ G, Faull R, Berndt MC, Vadas MA: Regulation of in vitro capillary tube formation by anti-integrin antibodies. J Cell Biol 1993, 121:931-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ: Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie-2. Cardiovasc Res 2001, 49:659-670 [DOI] [PubMed] [Google Scholar]
- 6.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE: Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation, and G-protein signaling. J Cell Sci 2001, 114:2755-2773 [DOI] [PubMed] [Google Scholar]
- 7.Koolwijk P, Peters E, van der Vecht B, Hornig C, Weich HA, Alitalo K, Hicklin DJ, Wu Y, Witte L, van Hinsbergh VW: Involvement of VEGFR-2 (kdr/flk-1) but not VEGFR-1 (flt-1) in VEGF-A and VEGF-C-induced tube formation by human microvascular endothelial cells in fibrin matrices in vitro. Angiogenesis 2001, 4:53-60 [DOI] [PubMed] [Google Scholar]
- 8.Vu MT, Smith CF, Burger PC, Klintworth GK: An evaluation of methods to quantitate the chick chorioallantoic membrane assay in angiogenesis. Lab Invest 1985, 53:499-508 [PubMed] [Google Scholar]
- 9.Wilting J, Christ B, Bokeloh M, Weich HA: In vivo effects of vascular endothelial growth factor on the chicken chorioallantoic membrane. Cell Tissue Res 1993, 274:163-172 [DOI] [PubMed] [Google Scholar]
- 10.Ryu S, Albert DM: Evaluation of tumor angiogenesis factor with the rabbit cornea model. Invest Ophthalmol Vis Sci 1979, 18:831-841 [PubMed] [Google Scholar]
- 11.Hayek A, Culler FL, Beattie GM, Lopez AD, Cuevas P, Baird A: An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem Biophys Res Commun 1987, 147:876-880 [DOI] [PubMed] [Google Scholar]
- 12.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ: A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 1996, 37:1625-1632 [PubMed] [Google Scholar]
- 13.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR: A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 1992, 67:519-528 [PubMed] [Google Scholar]
- 14.Rubin PA, Nicaeus TE, Warner MA, Remulla HD: Effect of sucralfate and basic fibroblast growth factor on fibrovascular ingrowth into hydroxyapatite and porous polyethylene alloplastic implants using a novel rabbit model. Ophthalmic Plast Reconstr Surg 1997, 13:8-17 [DOI] [PubMed] [Google Scholar]
- 15.Lichtenberg J, Hansen CA, Skak-Nielsen T, Bay C, Mortensen JT, Binderup L: The rat subcutaneous air sac model: a new and simple method for in vivo screening of anti-angiogenesis. Pharmacol Toxicol 1997, 81:280-284 [PubMed] [Google Scholar]
- 16.Claffey KP, Abrams K, Shih SC, Brown LF, Mullen A, Keough M: Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest 2001, 81:61-75 [DOI] [PubMed] [Google Scholar]
- 17.Fong GH, Rossant J, Gertsenstein M, Breitman ML: Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376:66-70 [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P: Gene targeting and gene transfer to unravel the molecular basis of the formation and disorders of blood vessels. Verh K Acad Geneeskd Belg 2000, 62:31-68 [PubMed] [Google Scholar]
- 19.Peng J, Zhang L, Drysdale L, Fong GH: The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. Proc Natl Acad Sci USA 2000, 97:8386-8391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M: Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA 2002, 99:3657-3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmeliet P, Collen D: Transgenic mouse models in angiogenesis and cardiovascular disease. J Pathol 2000, 190:387-405 [DOI] [PubMed] [Google Scholar]
- 22.Siemeister G, Martiny-Baron G, Marme D: The pivotal role of VEGF in tumor angiogenesis: molecular facts and therapeutic opportunities. Cancer Metastasis Rev 1998, 17:241-248 [DOI] [PubMed] [Google Scholar]
- 23.Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol 1999, 237:97-132 [DOI] [PubMed] [Google Scholar]
- 24.Olofsson B, Jeltsch M, Eriksson U, Alitalo K: Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol 1999, 10:528-535 [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N: Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol 2001, 280:C1358-C1366 [DOI] [PubMed] [Google Scholar]
- 26.Robinson CJ, Stringer SE: The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 2001, 114:853-865 [DOI] [PubMed] [Google Scholar]
- 27.Lauren J, Gunji Y, Alitalo K: Is angiopoietin-2 necessary for the initiation of tumor angiogenesis? Am J Pathol 1998, 153:1333-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis S, Yancopoulos GD: The angiopoietins: yin and yang in angiogenesis. Curr Top Microbiol Immunol 1999, 237:173-185 [DOI] [PubMed] [Google Scholar]
- 29.Jones N, Iljin K, Dumont DJ, Alitalo K: Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2001, 2:257-267 [DOI] [PubMed] [Google Scholar]
- 30.Nelson PS, Hawkins V, Schummer M, Bumgarner R, Ng WL, Ideker T, Ferguson C, Hood L: Negative selection: a method for obtaining low-abundance cDNAs using high-density cDNA clone arrays. Genet Anal 1999, 15:209-215 [DOI] [PubMed] [Google Scholar]
- 31.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW: Genes expressed in human tumor endothelium. Science 2000, 289:1197-1202 [DOI] [PubMed] [Google Scholar]
- 32.Kahn J, Mehraban F, Ingle G, Xin X, Bryant JE, Vehar G, Schoenfeld J, Grimaldi CJ, Peale F, Draksharapu A, Lewin DA, Gerritsen ME: Gene expression profiling in an in vitro model of angiogenesis. Am J Pathol 2000, 156:1887-1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Cardena G, Comander JI, Blackman BR, Anderson KR, Gimbrone MA: Mechanosensitive endothelial gene expression profiles: scripts for the role of hemodynamics in atherogenesis? Ann NY Acad Sci 2001, 947:1-6 [PubMed] [Google Scholar]
- 34.Peale FV, Jr, Gerritsen ME: Gene profiling techniques and their application in angiogenesis and vascular development. J Pathol 2001, 195:7-19 [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Zhang L, Shao ZM, Beatty P, Sartippour M, Lane TF, Barsky SH, Livingston E, Nguyen M: Identification of a novel endothelial-derived gene EG-1. Biochem Biophys Res Commun 2002, 290:602-612 [DOI] [PubMed] [Google Scholar]
- 36.Glienke J, Schmitt AO, Pilarsky C, Hinzmann B, Weiss B, Rosenthal A, Thierauch KH: Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem 2000, 267:2820-2830 [DOI] [PubMed] [Google Scholar]
- 37.Shimkets RA, Lowe DG, Tai JT, Sehl P, Jin H, Yang R, Predki PF, Rothberg BE, Murtha MT, Roth ME, Shenoy SG, Windemuth A, Simpson JW, Simons JF, Daley MP, Gold SA, McKenna MP, Hillan K, Went GT, Rothberg JM: Gene expression analysis by transcript profiling coupled to a gene database query. Nature Biotechnol 1999, 17:798-803 [DOI] [PubMed] [Google Scholar]
- 38.Novatchkova M, Eisenhaber F: Can molecular mechanisms of biological processes be extracted from expression profiles? Case study: endothelial contribution to tumor-induced angiogenesis. Bioessays 2001, 23:1159-1175 [DOI] [PubMed] [Google Scholar]
- 39.Khatri P, Draghici S, Ostermeier GC, Krawetz SA: Profiling gene expression using onto-express. Genomics 2002, 79:266-270 [DOI] [PubMed] [Google Scholar]
- 40.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S: Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000, 95:952-958 [PubMed] [Google Scholar]
- 41.Isner JM, Kalka C, Kawamoto A, Asahara T: Bone marrow as a source of endothelial cells for natural and iatrogenic vascular repair. Ann NY Acad Sci 2001, 953:75-84 [DOI] [PubMed] [Google Scholar]
- 42.Abramovitch R, Frenkiel D, Neeman M: Analysis of subcutaneous angiogenesis by gradient echo magnetic resonance imaging. Magn Reson Med 1998, 39:813-824 [DOI] [PubMed] [Google Scholar]
- 43.Fanelli M, Locopo N, Gattuso D, Gasparini G: Assessment of tumor vascularization: immunohistochemical and non-invasive methods. Int J Biol Markers 1999, 14:218-231 [DOI] [PubMed] [Google Scholar]
- 44.Weber WA, Haubner R, Vabuliene E, Kuhnast B, Wester HJ, Schwaiger M: Tumor angiogenesis targeting using imaging agents. Q J Nucl Med 2001, 45:179-182 [PubMed] [Google Scholar]
- 45.Padhani AR, Neeman M: Challenges for imaging angiogenesis. Br J Radiol 2001, 74:886-890 [DOI] [PubMed] [Google Scholar]
- 46.Anderson H, Price P, Blomley M, Leach MO, Workman P: Measuring changes in human tumour vasculature in response to therapy using functional imaging techniques. Br J Cancer 2001, 85:1085-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]