Abstract
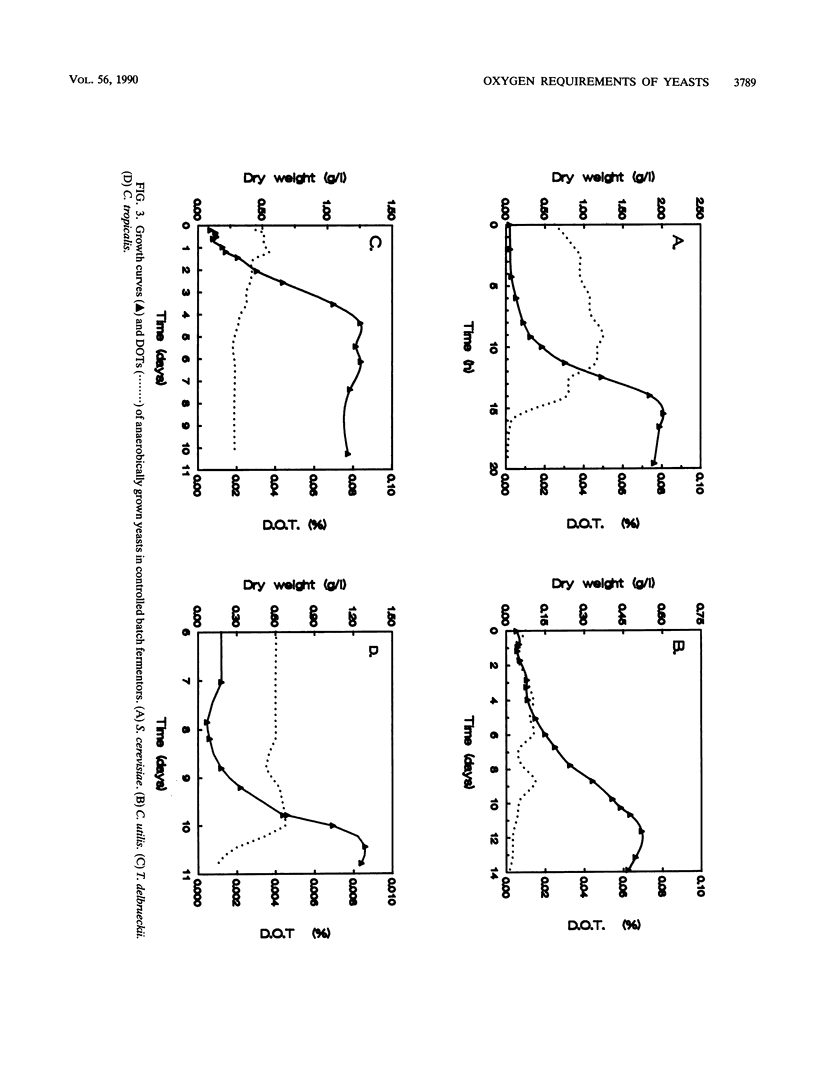
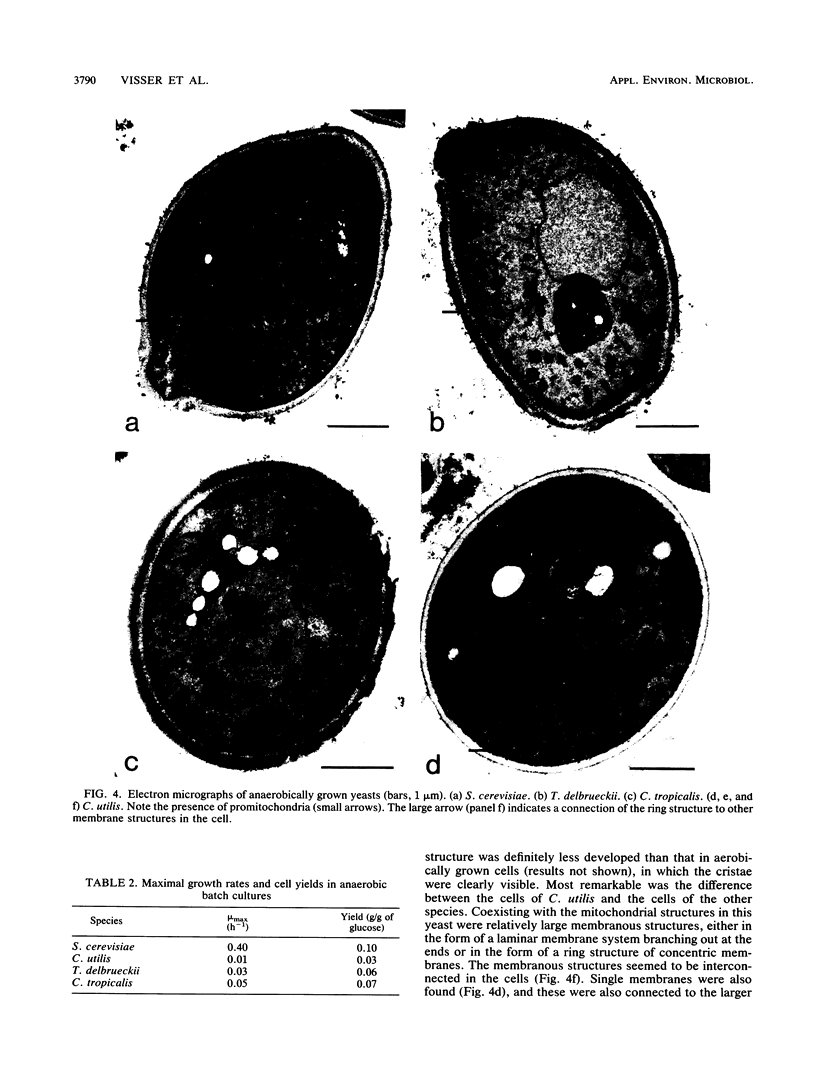
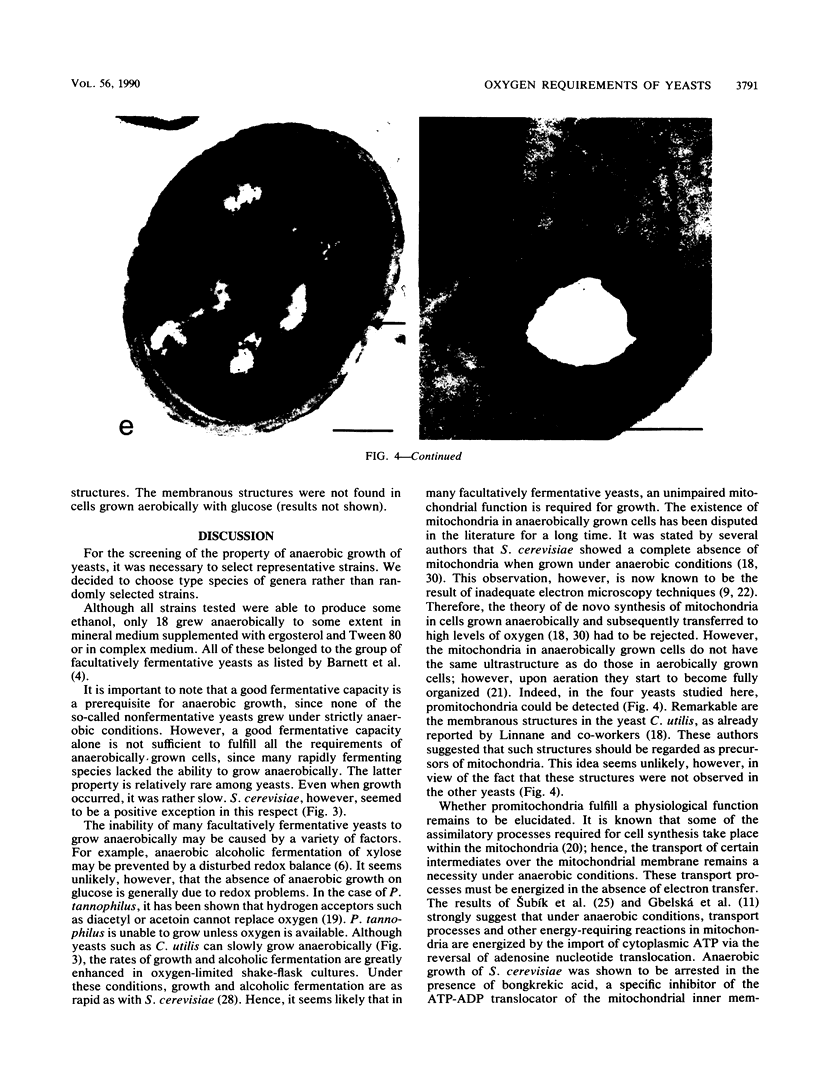
Type species of 75 yeast genera were examined for their ability to grow anaerobically in complex and mineral media. To define anaerobic conditions, we added a redox indicator, resazurin, to the media to determine low redox potentials. All strains tested were capable of fermenting glucose to ethanol in oxygen-limited shake-flask cultures, even those of species generally regarded as nonfermentative. However, only 23% of the yeast species tested grew under anaerobic conditions. A comparative study with a number of selected strains revealed that Saccharomyces cerevisiae stands out as a yeast capable of rapid growth at low redox potentials. Other yeasts, such as Torulaspora delbrueckii and Candida tropicalis, grew poorly mu max, 0.03 and 0.05 h-1, respectively) under anaerobic conditions in mineral medium supplemented with Tween 80 and ergosterol. The latter organisms grew rapidly under oxygen limitation and then displayed a high rate of alcoholic fermentation. It can be concluded that these yeasts have hitherto-unidentified oxygen requirements for growth.
Full text
PDF

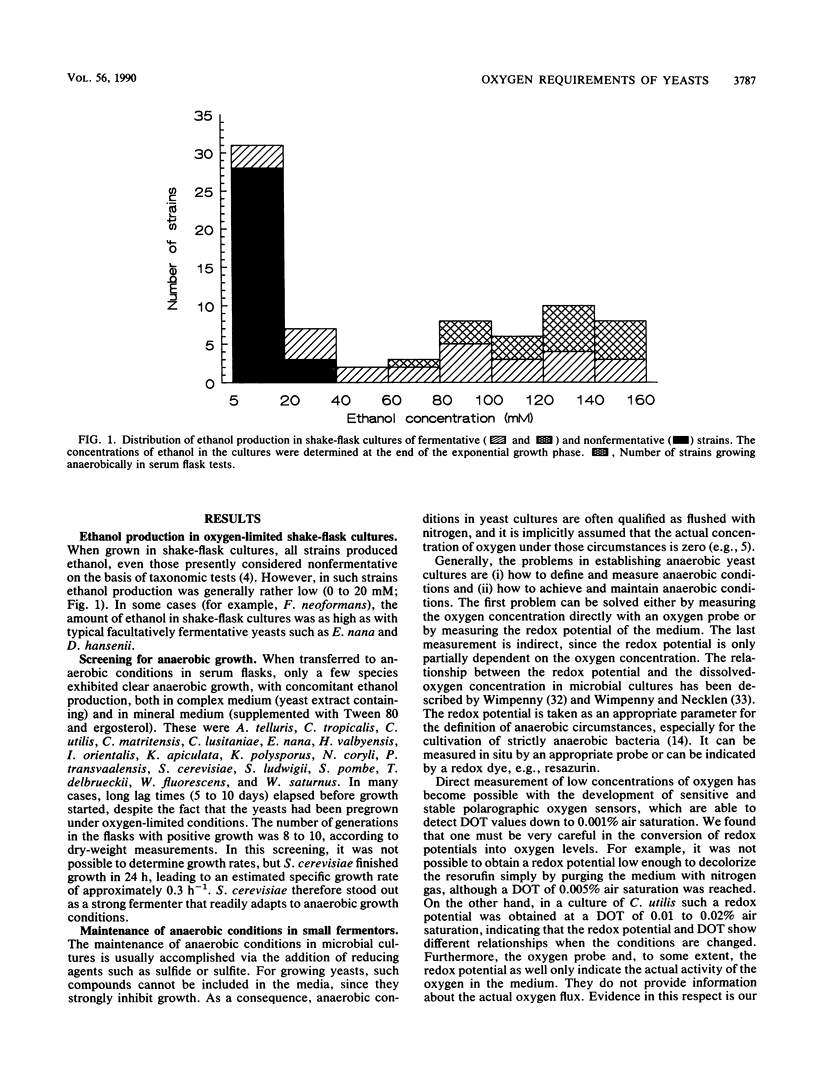
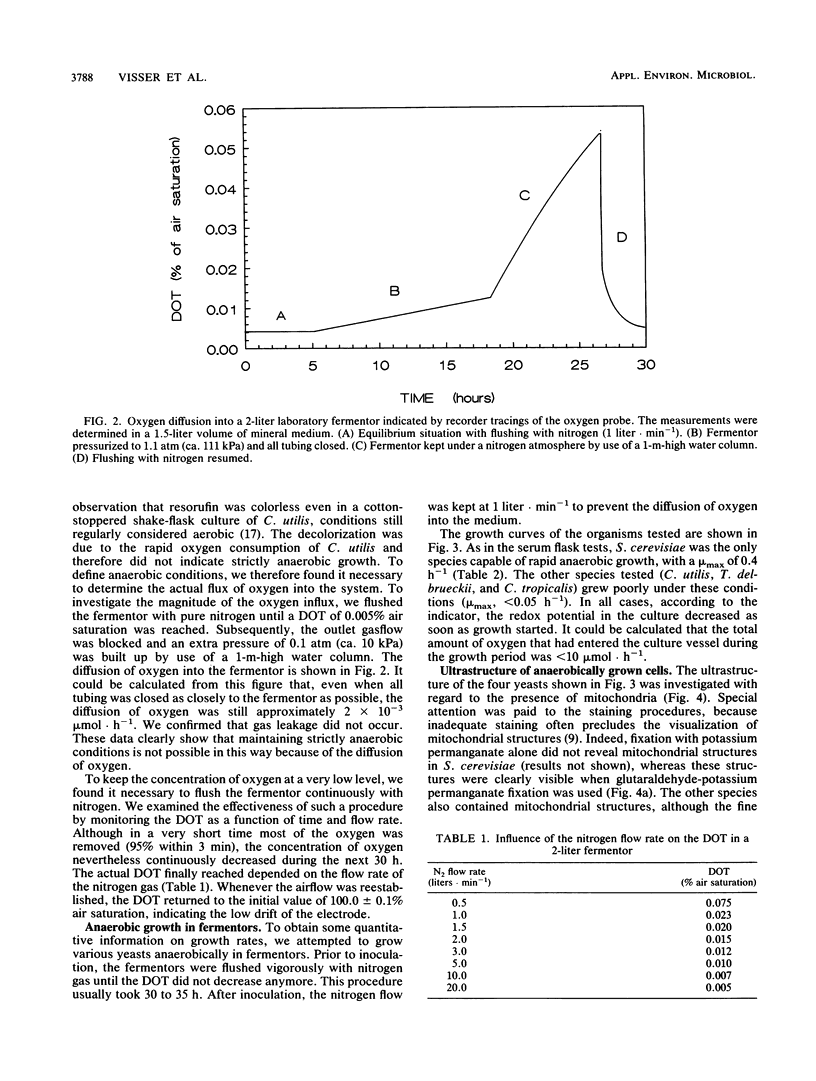

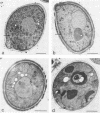
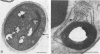
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J Cell Physiol. 1953 Feb;41(1):23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Babij T., Moss F. J., Ralph B. J. Effects of oxygen and glucose levels on lipid composition of yeast Candida utilis grown in continuous culture. Biotechnol Bioeng. 1969 Jul;11(4):593–603. doi: 10.1002/bit.260110407. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Johnson B. Influence of oxygen tension on the physiology of Saccharomyces cerevisiae in continuous culture. Antonie Van Leeuwenhoek. 1971;37(4):477–487. doi: 10.1007/BF02218518. [DOI] [PubMed] [Google Scholar]
- Bruinenberg P. M., van Dijken J. P., Scheffers W. A. An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol. 1983 Apr;129(4):965–971. doi: 10.1099/00221287-129-4-965. [DOI] [PubMed] [Google Scholar]
- Crabtree H. G. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23(3):536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Nelson W. M., Claude A. Mitochondria in anaerobically-grown, lipid-limited brewer's yeast. J Cell Biol. 1969 Oct;43(1):174–179. doi: 10.1083/jcb.43.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiechter A., Fuhrmann G. F., Käppeli O. Regulation of glucose metabolism in growing yeast cells. Adv Microb Physiol. 1981;22:123–183. doi: 10.1016/s0065-2911(08)60327-6. [DOI] [PubMed] [Google Scholar]
- Gbelská Y., Subík J., Svoboda A., Goffeau A., Kovác L. Intramitochondrial ATP and cell functions: yeast cells depleted of intramitochondrial ATP lose the ability to grow and multiply. Eur J Biochem. 1983 Feb 1;130(2):281–286. doi: 10.1111/j.1432-1033.1983.tb07148.x. [DOI] [PubMed] [Google Scholar]
- Harder W., Visser K., Kuenen J. G. Laboratory fermenter with an improved magnetic drive. Lab Pract. 1974 Nov;23(11):644–645. [PubMed] [Google Scholar]
- LINNANE A. W., VITOLS E., NOWLAND P. G. Studies on the origin of yeast mitochondria. J Cell Biol. 1962 May;13:345–350. doi: 10.1083/jcb.13.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neirinck L. G., Maleszka R., Schneider H. The requirement of oxygen for incorporation of carbon from D-xylose and D-glucose by Pachysolen tannophilus. Arch Biochem Biophys. 1984 Jan;228(1):13–21. doi: 10.1016/0003-9861(84)90041-9. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Intracellular localization of enzymes in yeast. Arch Biochem Biophys. 1970 Jan;136(1):245–259. doi: 10.1016/0003-9861(70)90348-6. [DOI] [PubMed] [Google Scholar]
- Plattner H., Schatz G. Promitochondria of anaerobically grown yeast. 3. Morphology. Biochemistry. 1969 Jan;8(1):339–343. doi: 10.1021/bi00829a047. [DOI] [PubMed] [Google Scholar]
- Scheffers W. A. Stimulation of fermentation in yeasts by acetoin and oxygen. Nature. 1966 Apr 30;210(5035):533–534. doi: 10.1038/210533a0. [DOI] [PubMed] [Google Scholar]
- Subík J., Kolarov J., Kovác L. Obligatory requirement of intramitochondrial ATP for normal functioning of the eucaryotic cell. Biochem Biophys Res Commun. 1972 Oct 6;49(1):192–198. doi: 10.1016/0006-291x(72)90028-9. [DOI] [PubMed] [Google Scholar]
- Van Urk H., Mak P. R., Scheffers W. A., van Dijken J. P. Metabolic responses of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 upon transition from glucose limitation to glucose excess. Yeast. 1988 Dec;4(4):283–291. doi: 10.1002/yea.320040406. [DOI] [PubMed] [Google Scholar]
- WALLACE P. G., LINNANE A. W. OXYGEN-INDUCED SYNTHESIS OF YEAST MITOCHONDRIA. Nature. 1964 Mar 21;201:1191–1194. doi: 10.1038/2011191a0. [DOI] [PubMed] [Google Scholar]
- Wijsman M. R., van Dijken J. P., van Kleeff B. H., Scheffers W. A. Inhibition of fermentation and growth in batch cultures of the yeast Brettanomyces intermedius upon a shift from aerobic to anaerobic conditions (Custers effect). Antonie Van Leeuwenhoek. 1984;50(2):183–192. doi: 10.1007/BF00400180. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Necklen D. K. The redox environment and microbial physiology. I. The transition from anaerobiosis to aerobiosis in continuous cultures of facultative anaerobes. Biochim Biophys Acta. 1971 Dec 7;253(2):352–359. doi: 10.1016/0005-2728(71)90039-9. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W. The effect of Eh on regulatory processes in facultative anaerobes. Biotechnol Bioeng. 1969 Jul;11(4):623–629. doi: 10.1002/bit.260110409. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., van den Bosch E., Hermans J. J., de Miranda L. R., Scheffers W. A. Alcoholic fermentation by 'non-fermentative' yeasts. Yeast. 1986 Jun;2(2):123–127. doi: 10.1002/yea.320020208. [DOI] [PubMed] [Google Scholar]