Abstract
Cellular mechanisms contributing to renewal of terminal bronchioles remain poorly defined. Our previous studies identified pollutant-resistant Clara cell secretory protein (CCSP)-expressing stem cells that localize to the neuroepithelial body (NEB) and contribute to renewal of the proximal bronchiolar epithelium. However, activation of NEB-associated stem cells is unlikely to contribute to renewal of terminal bronchiolar epithelium because of the paucity of NEBs at this location. Goals of this study were to determine the location and properties of cells contributing to renewal of terminal bronchioles after Clara cell depletion. Pollutant-resistant CCSP-expressing cells were identified that localized to the bronchoalveolar duct junction (BADJ) and contribute to restoration of a phenotypically diverse epithelium. CCSP-expressing cells comprise the predominant proliferative population in initial terminal bronchiolar repair and include a population of label-retaining cells suggesting that they maintain characteristics of a stem cell population. Furthermore, immunohistochemical co-localization studies involving CCSP and the NEB-specific marker calcitonin gene-related peptide indicate that BADJ-associated CCSP-expressing stem cells function independently of NEB microenvironments. These studies identify a BADJ-associated, NEB-independent, CCSP-expressing stem cell population in terminal bronchioles and support the notion that regiospecific stem cell niches function to maintain epithelial diversity after injury.
Dramatic differences in the cellular composition of conducting airway epithelia exist in a continuum between the trachea and terminal bronchioles; a feature that contributes to functional heterogeneity essential for normal airway homeostasis. 1-8 Although many studies have focused on regeneration of this tissue after injury, only recently have the intricacies of renewing such a complex epithelium begun to be elucidated. Injury models that target terminally differentiated cell populations identify the importance of the transit-amplifying (TA) subset of progenitor cells in the regeneration process. Proliferation and differentiation of TA cells results in rapid restoration of a normal epithelium. In contrast, selective depletion of an abundant TA cell pool results in activation of rare stem cell populations. 9-15 To date, stem cells have been identified that are restricted to the trachea, submucosal gland ducts, and neuroepithelial bodies (NEBs) of the bronchiolar epithelium. 16-20 Even though cells that contribute to the renewal of terminal bronchioles have also been shown to exist using a model of lung progenitor cell depletion, the precise location and characteristics of putative stem cells have not been described.
The terminal bronchiole is a particularly resilient region of the airway that exhibits rapid epithelial renewal in response to depletion of either terminally differentiated (ciliated) or TA cell populations. Clara cells function as the sole TA cell that participates in maintenance of both secretory and ciliated cell types after oxidant-mediated damage. 21 Importantly, depletion of this TA cell population through administration of the Clara cell-specific cytotoxicant naphthalene fails to diminish the regenerative potential of terminal bronchioles. 20,22,23 After Clara cell depletion, residual cells of terminal bronchioles are fully capable of epithelial renewal and restoration of differentiated cell phenotypes appropriate for this region. 22,23 These data support the notion that a population of pollutant-resistant stem cells participate in regeneration of the surrounding terminal bronchiolar epithelium after TA cell depletion.
Even though the molecular and phenotypic characteristics of stem cell populations vary according to the tissue in which they reside, a number of unifying properties are used to distinguish this category of regenerative cells from their TA counterparts. 13 Characteristics of stem cells include a relatively undifferentiated phenotype, pluripotent differentiation potential, a low rate of steady-state proliferation, an extended life span, and localization to a specific microenvironment commonly referred to as a stem cell niche. 10,13,24,25 Using these criteria, we have previously demonstrated the existence of a Clara cell secretory protein (CCSP)-expressing (CE) cell population within NEB microenvironments of the bronchiolar epithelium that functions as an airway stem cell. 20 CE stem cells and TA cell populations within the airway epithelium have been distinguished primarily by differences in pollutant susceptibility, proliferative and differentiation potential, and spatial localization. Depletion of both of these populations via transgene-mediated ablation of all CCSP-positive cells results in an overall failure of bronchiolar epithelial renewal. 19,26 These results indicate the importance of CE cells in bronchiolar regeneration and led us to hypothesize that phenotypically similar populations of stem cells may regulate epithelial renewal in both the proximal and terminal bronchioles. 19,20 Goals of the present study were to investigate this hypothesis through defining the molecular phenotype of cells contributing to repair of terminal bronchioles, their localization, and their rate of proliferation in the steady-state and after injury. Results indicate that regeneration of the terminal bronchiole after injury is mediated by CE stem cells with characteristics similar to those located within NEB microenvironment, but that this stem cell functions within a unique NEB-independent niche located at the bronchoalveolar duct junction (BADJ).
Materials and Methods
Animal Housing
Adult male FVB/n wild-type mice (2 to 4 months of age) were housed under specific pathogen-free conditions and tested quarterly using a 16-agent serological sentinel-screening program. Animals were maintained under a 12-hour light/dark cycle, and allowed access to both food and water ad libitum.
Naphthalene Exposures
Naphthalene (Sigma, St. Louis, MO) was dissolved in Mazola corn oil and administered to mice via intraperitoneal injection before 10:00 a.m. Animals were briefly anesthetized with aerosolized halothane before exposure to facilitate weighing and injections. Mice received 275 mg of naphthalene/kg body weight; control animals received a comparable volume (10 ml/kg body weight) of corn oil alone.
Pulse and Continuous Labeling
[3H]-Thymidine (2.5 mCi/kg) was injected intraperitoneally to mice 1 hour before sacrifice for pulse labeling. For continuous labeling studies, [3H]-thymidine (1.0 mCi/ml) was administered throughout a 7-day period via a subcutaneous miniosmotic pump (Alzet model 2002, 0.5 μl/hour). Alzet pumps were implanted 6 hours after naphthalene administration and removed after the labeling period.
Immunohistochemistry and Immunofluorescence
Paraffin-embedded lung tissue was sectioned at 5 μm to reveal the major axial pathway and adhered to glass slides. Slides were blocked with 0.5% bovine serum albumin (Sigma)/5% normal goat serum (Vector Laboratories, Burlingame, CA) in phosphate-buffered saline (PBS) (blocking solution) for 30 minutes. Adjacent serial sections were incubated with polyclonal rabbit anti-CCSP (1:30,000) 26 or rabbit anti-calcitonin gene-related peptide (CGRP) (l:16,000, Sigma) 26 overnight at 4°C in blocking solution. Slides were washed three times in PBS, and incubated with biotinylated goat anti-rabbit Ig (1:4000 in blocking solution, Sigma). Slides were washed and streptavidin-horseradish peroxidase (Sigma) was added at a 1:4000 dilution in PBS. Antigen-antibody complexes were detected using a diaminobenzidine substrate detection kit (Vector Laboratories). For detection of incorporated [3H]-thymidine, immunostained slides were washed overnight in PBS, dehydrated, and dried. Slides were coated with NTB-2 emulsion (Kodak, Rochester, NY), dried, stored at 4°C for 2 weeks, and developed according to the manufacturer’s instructions. All slides are counterstained with Mayer’s hematoxylin to identify nuclei.
Standard immunofluorescence methods were used to simultaneously detect CGRP and CCSP on 5-μm tissue sections. 19 Rabbit anti-CGRP (1:4000) and goat-anti-CCSP (1:10,000) were diluted in 3% bovine serum albumin/PBS. After a 24-hour incubation at 4°C, slides were incubated simultaneously with Alexa 488-conjugated donkey anti-goat Ig and Alexa 594-conjugated donkey anti-rabbit Ig (1:125 dilution; Molecular Probes, Eugene, OR) overnight at 4°C in the dark. All slides were mounted with 2 μg/ml of 4,6-diamidino-2-phenylindole (Sigma) in Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL) and visualized with an AX70 microscope equipped with a 4,6-diamidino-2-phenylindole/Texas Red dual optical excitation filter cube and a fluorescein isothiocyanate optical excitation filter cube (Olympus, Melville, NY).
In Situ Hybridization
CCSP mRNA was detected on 5-μm paraffin sections of lung tissue using [35S] anti-sense riboprobes. Conditions for hybridization were essentially as described. 27 Slides were washed under high stringency conditions, coated with Kodak NTB2 emulsion, exposed for 16 hours, and developed according to the manufacturer’s instructions.
Morphometric Analysis and Labeling Index
Terminal bronchioles were defined by the presence of an intact BADJ, at least 200 μm of conducting airway epithelium, and a visible alveolar duct. All cells within 200 μm of the BADJ exhibiting a nuclear profile and clear attachment to the basement membrane were included in cell density analyses. Previous studies have used a similar methodology for analysis of neuroepithelial body (NEB) microenvironments. 20 For proliferation analysis, cells were defined as “labeled” if they contained at least five grains localized over a visible nucleus. “Label retaining cells” were defined as those containing >15 grains/nucleus. Mean values for cellular density, percent Clara cells, and proliferative index were compared to control values using the Student’s t-test. Statistical significance for all comparisons was accepted at P < 0.05.
Results
Pollutant-Resistant CCSP-Expressing Cells Occupy Multiple Distinct Microenvironments within the Conducting Airway Epithelium
The terminal bronchiolar epithelium of mice is a particularly sensitive site for naphthalene toxicity, exposure to which results in rapid and extensive depletion of Clara cells. 22,28 Although Clara cells are the principal progenitor for this region of the conducting airway, 21 repair of terminal bronchioles is rapidly induced and virtually complete 2 weeks after administration of 200 mg/kg of naphthalene. 22,28-31 Even though NEB-associated CCSP-expressing (CE) cells have been identified as a naphthalene-resistant stem cell pool that contributes to renewal of proximal bronchiolar epithelium, there is still debate over the identity and properties of cells contributing to renewal of terminal bronchiolar (TB) epithelium. To identify regions of the TB epithelium that maintained naphthalene-resistant cells, mice were exposed to 275 mg/kg of naphthalene, recovered 24 hours, and the phenotype of pollutant-resistant cells assessed by dual-color fluorescence immunostaining for the Clara cell-specific marker (CCSP, green immunofluorescence) or the pulmonary neuroendocrine cell (PNEC)-specific marker calcitonin gene-related peptide (CGRP, red immunofluorescence). In the distal airway of untreated animals, CE cells accounted for 60 to 90% of epithelial cells whereas PNECs comprise only a small percentage of this population (Figure 1A) ▶ . Naphthalene exposure resulted in extensive exfoliation of CE cells within 24 hours (Figure 1B) ▶ . Clusters of pollutant-resistant CE cells that have been previously shown to serve as regenerative foci are maintained adjacent to NEBs of bronchi (Figure 1B ▶ , asterisk). 19,20 Interestingly, a second population of pollutant-resistant CE cells that did not appear to co-localize with CGRP-immunoreactive PNECs was located within TBs adjacent to the BADJ. (Figure 1B ▶ , arrow). This observation suggested that the BADJ may represent a second distinct regenerative microenvironment within bronchioles in addition to the previously characterized NEB microenvironment.
Figure 1.
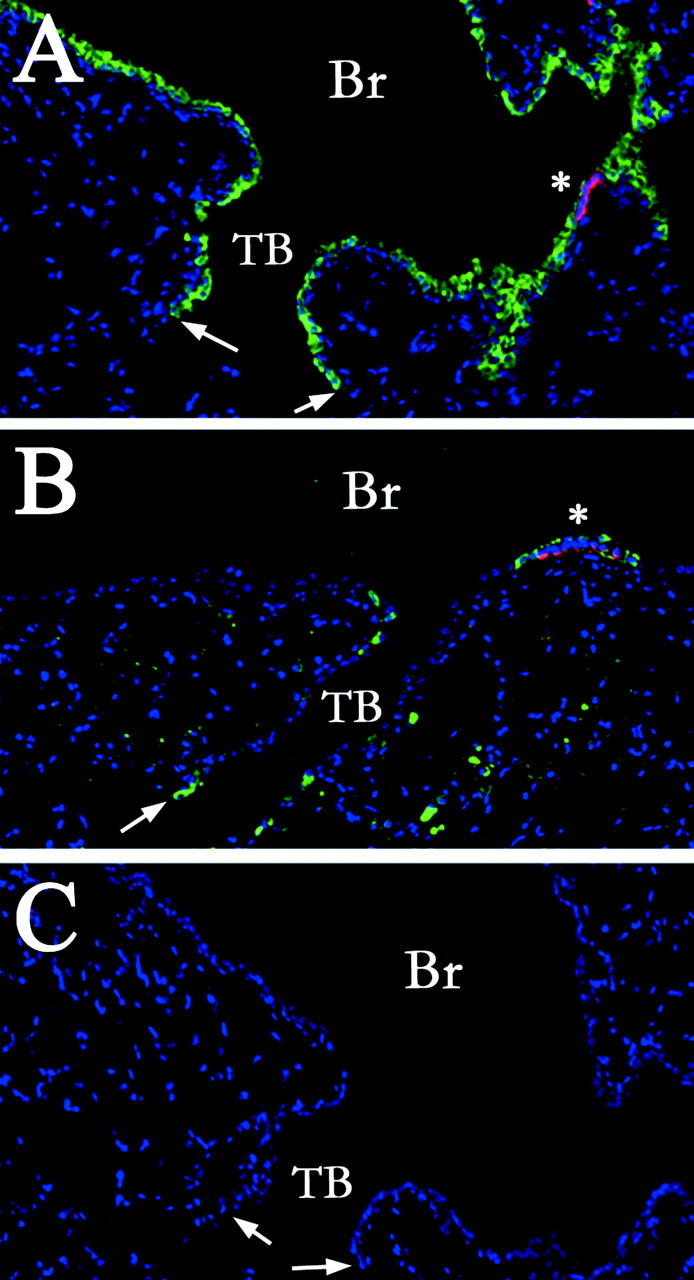
Pollutant-resistant CE cells occupy multiple distinct microenvironments within the conducting airway epithelium. CCSP (green)- and CGRP (red)-immunopositive cells were detected by dual-color immunofluorescent staining of lung tissue sections from either untreated control mice (A) or mice treated with 275 mg/kg of naphthalene and recovered for 24 hours (B). Primary antibodies against CCSP and CGRP were omitted from untreated lung samples as a control for antibody specificity (C). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (blue fluorescence, A–C). Immunofluorescent cellular debris (lacking nuclei) in the alveolar space (B) is an artifact of inflation fixation. Analysis revealed a significant decrease in the number of CCSP-positive cells in both the bronchiole (Br) and terminal bronchiolar (TB) airways of naphthalene-treated mice (B). Rare populations of naphthalene-resistant CCSP-immunoreactive cells were located directly adjacent to CGRP-positive NEBs (A and B, asterisks), or at the BADJ (A and B, arrows). Original magnifications, ×100.
Cells within Terminal Bronchioles Support Regeneration of Airways of Appropriate Cellular Diversity
To determine whether NEB-independent pollutant-resistant cells of terminal bronchioles are capable of appropriate epithelial renewal, airways of mice were compared 45 days after administration of 275 mg/kg of naphthalene or vehicle (corn oil). Efficacy of epithelial renewal within terminal bronchioles was determined by comparison of epithelial cell density and the representation of CCSP-immunoreactive cells in untreated and treated animals. No qualitative differences in the abundance of CCSP within immunoreactive cells of corn oil and naphthalene-exposed groups were observed (Figure 2, A and B ▶ , respectively). A small yet significant reduction in terminal bronchiolar cell density was observed in treated animals (Figure 2C) ▶ . This decrease in cell density was not associated with a shift in the representation of CE cells at this location (Figure 2D) ▶ . These data demonstrate that terminal bronchioles contain a regenerative cell population capable of restoring an airway of appropriate cellular diversity after depletion of the TA (Clara) cell population.
Figure 2.
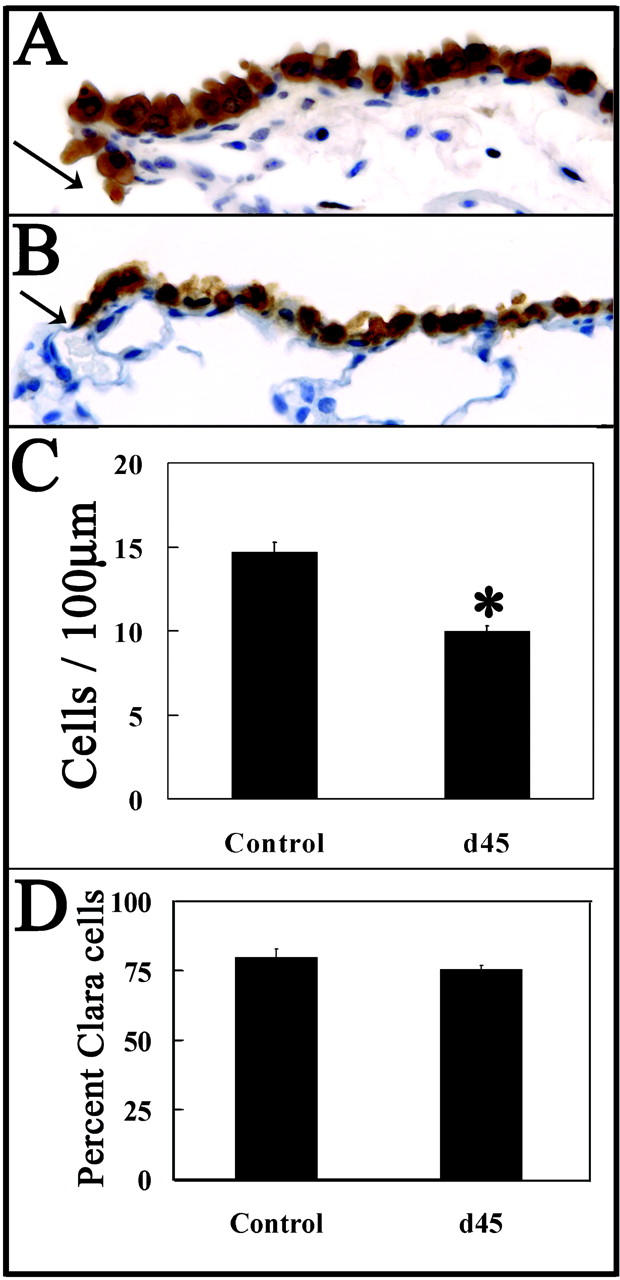
Cells within terminal bronchioles support regeneration of airways of appropriate cellular diversity. CCSP-immunopositive cells in terminal bronchioles were detected by immunohistochemistry 45 days after intraperitoneal injection of vehicle (A) or 275 mg/kg of naphthalene (B). Nuclei were counterstained with hematoxylin; arrows indicate the BADJ (A and B). Morphometric analyses of cell density (total cells/100 μm basement membrane, C), and representation of Clara cells (percent Clara cells/total epithelial cells, D) were performed as described in Materials and Methods. Eight to 16 terminal bronchioles were analyzed per lung (n = lungs from three mice). Significant changes in overall cell density (C, ✻, P < 0.005) but not percentage of Clara cells (D) indicates an appropriate differentiation potential of epithelial cells within terminal bronchioles after naphthalene-induced injury. Original magnifications, ×400.
Pollutant-Resistant CCSP-Expressing Cells Represent the Initial Proliferative Population in Terminal Bronchioles
To investigate the kinetics of Clara cell ablation within terminal bronchioles and to identify the location and cell type responsible for initial epithelial renewal, mice were exposed to either corn oil or 275 mg/kg of naphthalene, recovered for 6, 12, 24, or 36 hours, and injected with 2.5 mCi/kg [3H]-thymidine (TdR) 1 hour before sacrifice. Clara cells were identified by immunohistochemistry for CCSP and proliferating cells were identified by autoradiographic detection of incorporated [3H]-TdR (Figure 3) ▶ . Six hours after naphthalene exposure Clara cells exhibited a loss of apical projections without significant alterations in overall cell density as determined by CCSP immunoreactivity (Figure 3 ▶ , compare A and B). Exfoliating CCSP-immunoreactive cells were evident beginning at the 12-hour time point (Figure 3C) ▶ . This change was associated with a reduction in both the overall cell density and percentage of Clara cells within the epithelial population. Within 24 hours, maximal CE cell ablation was achieved as determined by the absence of exfoliating cell bodies (Figure 3D) ▶ . The previously identified population of pollutant-resistant CE cells (Figure 1B) ▶ was identifiable directly adjacent to the BADJ at this time point (Figure 3D) ▶ . Although no epithelial proliferation was observed in any terminal bronchiolar cell populations at this early recovery time point, proliferating fibroblasts that serve as a control for appropriate [3H]-TdR incorporation and detection were identified at this and all other time points analyzed (Figure 3, B, E ▶ arrowheads). Proliferative epithelial cells were first observed 36 hours after naphthalene administration and exhibited a rounded morphology (Figure 3E) ▶ . At this time point 0.6 ± 0.1 proliferative cells were detected per 100 μm (approximately one labeled cell per terminal bronchiole), of which CE pollutant-resistant cells represent 90 ± 15%. These data suggest a critical role for these cells in renewal of the distal conducting airway.
Figure 3.
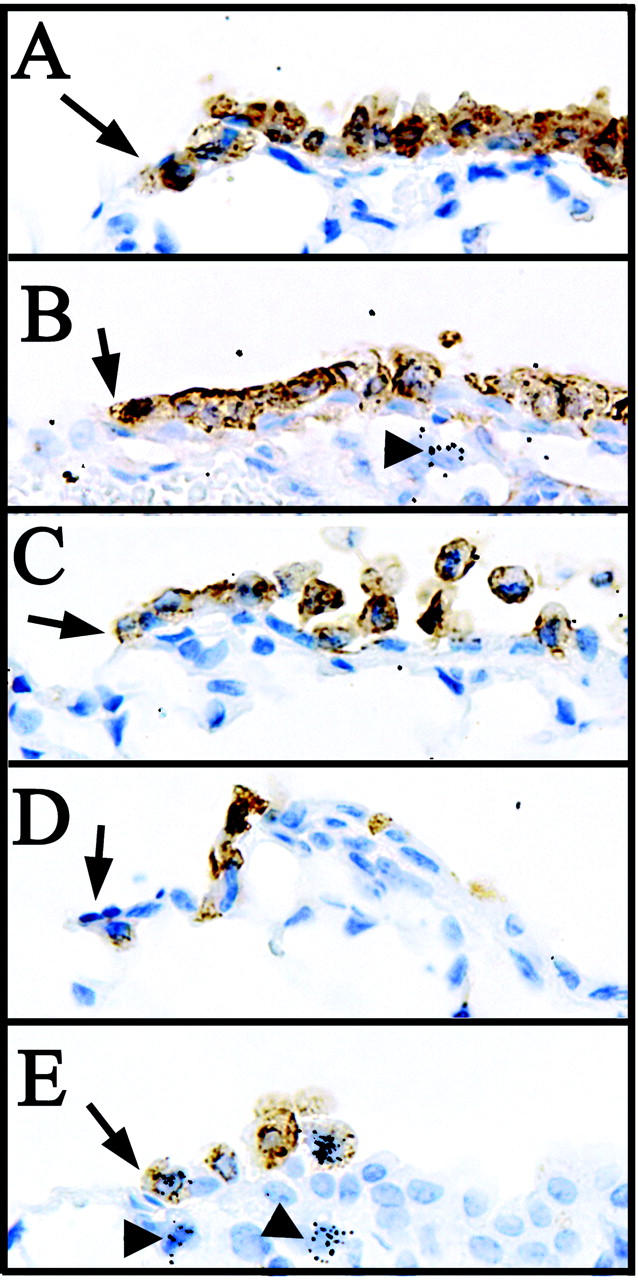
CE cells adjacent to the BADJ are pollutant-resistant and represent the initial proliferative epithelial population. Mice were exposed to 275 mg/kg of naphthalene and recovered for 0, 6, 12, 24, and 36 hours (A to E, respectively). Proliferating mitotic cells were labeled with 2.5 mCi of [3H]-TdR/kg body weight 1 hour before sacrifice. CE cells were detected by immunohistochemistry using diaminobenzidine detection (brown) and sites of [3H]-TdR incorporation identified by autoradiography (black grains). Nuclei were counterstained with hematoxylin. Naphthalene-resistant CE cells are detectable adjacent to the BADJ at all time points (A–E, arrows), and are the initial proliferative epithelial cell type 36 hours after naphthalene administration (E, black grains). Arrowheads in B and E indicate underlying proliferative fibroblasts. Original magnifications, ×400.
Pollutant-Resistant BADJ-Associated Cells Maintain CCSP mRNA Expression
A caveat to identification of BADJ-associated CCSP-positive cells as CE cells is the relatively long half-life of CCSP that may result in immunoreactivity in cells that have undergone a de- or trans-differentiative process. 32,33 To ascertain whether pollutant-resistant, BADJ-associated, CCSP-immunopositive cells actively transcribe CCSP, as well as to more fully characterize their molecular phenotype, the distribution of CCSP mRNA in TBs of control and naphthalene-exposed mice was determined by in situ hybridization (Figure 4, A and B) ▶ . CE cells present 24 hours after exposure were analyzed as these cells represent a residual, resistant population rather than nascent cells that may be the product of epithelial regeneration. Nonspecific hybridization signal was minimal as determined by anti-sense [35S]-labeled CCSP probe hybridization to tissue from CCSP−/− mice (Figure 4C) ▶ . CCSP mRNA was detected throughout the terminal bronchiole of untreated wild-type mice (Figure 4A) ▶ . In contrast, terminal bronchioles from naphthalene-treated mice maintained a subset of CCSP mRNA-positive cells directly adjacent to the BADJ (Figure 4B) ▶ . The levels of CCSP mRNA in this region were reduced compared with those of control, yet they are significantly greater than background levels. Although the cellular specificity of this transcript cannot be determined using [35S]-labeled probes, the proximity of CCSP mRNA-positive cells to the BADJ correlates with immunohistochemical data (Figures 1 and 3) ▶ ▶ . These data support the notion that pollutant-resistant cells of the BADJ are indeed a CE population.
Figure 4.
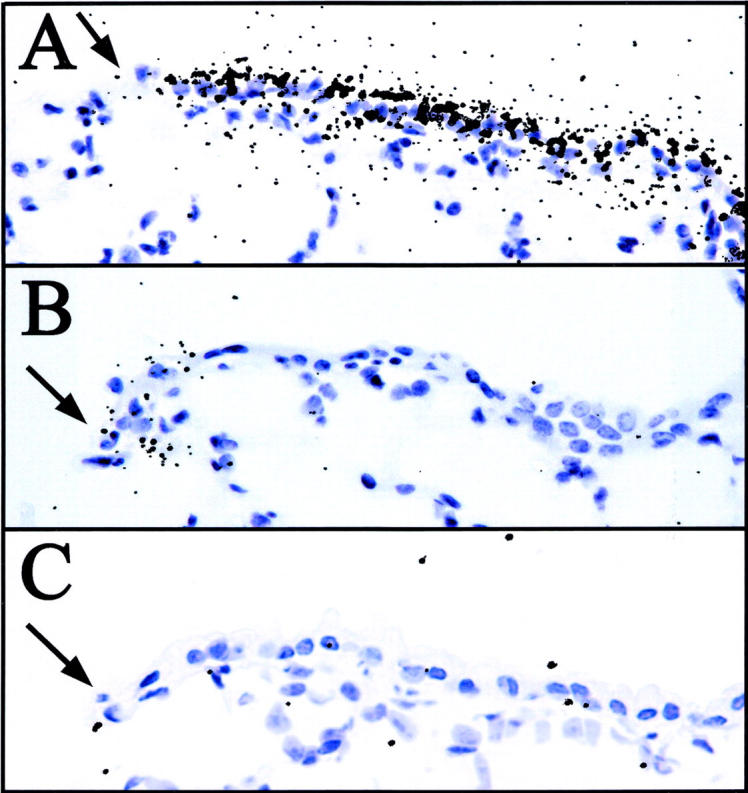
BADJ-associated pollutant-resistant cells maintain CCSP gene expression. CCSP gene expression in untreated control (A) and during the period of maximum injury 24 hours after 275 mg/kg of naphthalene treatment (B) was determined by in situ hybridization using an anti-sense [35S]-labeled CCSP riboprobe. Untreated, CCSP−/− tissue was used as a negative control (C). Hybridization was detected by autoradiography (black grains), and indicates a population of pollutant-resistant CCSP mRNA-positive cells immediately adjacent to the BADJ (A–C, arrows). Panels are representative of terminal bronchioles of three animals per exposure group. Original magnifications, ×400.
Regenerative CCSP-Expressing Cells Are Preferentially Associated with the BADJ of Terminal Bronchioles
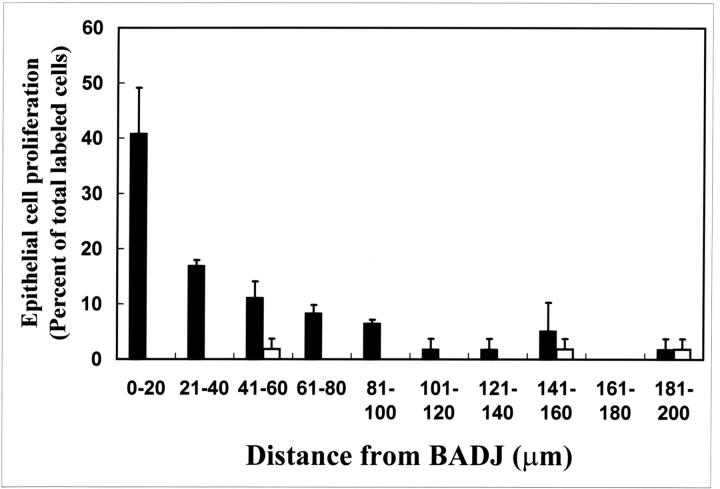
The phenotype, frequency, and spatial distribution of the initial cohort of proliferating cells within the TBs were determined 36 hours after naphthalene exposure using the BADJ as a reference point. CE cells were identified by immunohistochemical detection of CCSP and proliferating cells were identified by autoradiography. At this time point, 57% of labeled CE cells were located within 40 μm (approximately six cell diameters) of the BADJ and >75% of proliferating CE cells were located within 80 μm of the BADJ ( ∼11 cells; Figure 5 ▶ , black bars). As noted previously, CE cells accounted for 90 ± 15% proliferative cells within the TBs. A small number of labeled CE cells were randomly distributed throughout the TBs and were most likely rare CGRP-immunoreactive PNECs similar to those previously identified within regenerative domains of more proximal airways (Figure 5 ▶ , white bars). Based on these results, we conclude that the predominant proliferative population during the initial phase of epithelial regeneration within TBs are naphthalene-resistant CE cells and that these cells are spatially restricted to the BADJ microenvironment.
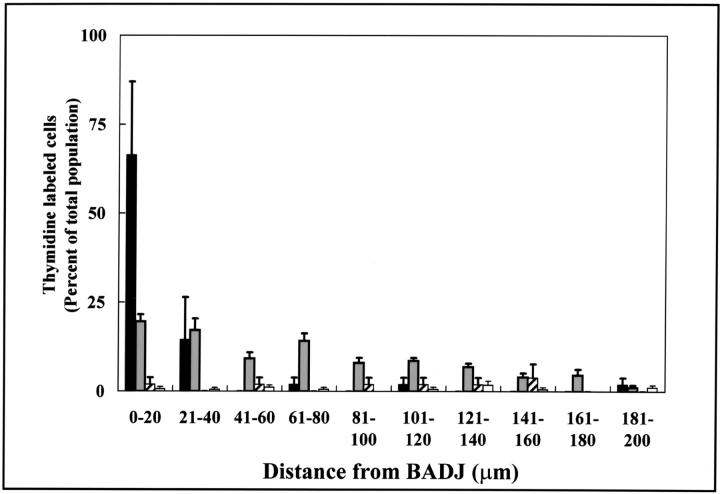
Figure 5.
Proliferative CE cells are preferentially associated with the BADJ. Distribution of [3H]-TdR-labeled cells was determined within the terminal bronchiole 36 hours after 275 mg/kg of naphthalene exposure. Terminal bronchioles were divided into 20-μm segments originating from the BADJ and the number of labeled CE) (black bars) or CCSP-negative (white bars) cells in each segment enumerated. Fifty-seven percent of labeled CE cells were found within 40 μm (6 cell diameters) of the BADJ. In contrast, CCSP-negative cells contribute only slightly to this initial proliferative population and were not spatially restricted within the terminal bronchiole. Data represent the summation of 8 to 10 terminal bronchioles/section (n = 3 animals) ± SEM.
Two Distinct Microenvironments Support Regeneration of the Terminal Bronchiolar Epithelium
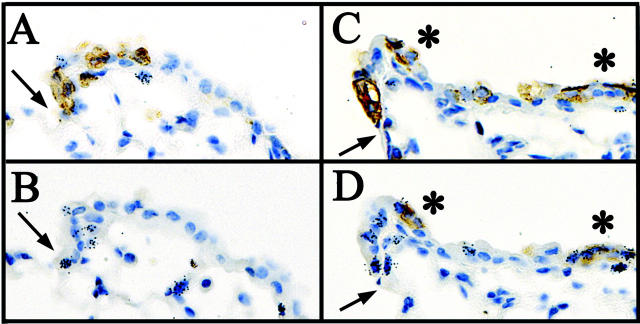
Previous studies have demonstrated that NEBs serve as microenvironments for proliferation and the maintenance of bronchial stem cells. 19,20,34,35 Studies by Peake and co-workers 36 determined that the abundance of CGRP-immunoreactive cells per unit airway surface area does not change substantially along the proximal/distal axis of the airway. As such, the number of CGRP-immunopositive cells within an airway segment decreases in direct proportion to airway surface area, raising the possibility that not all terminal bronchioles contain a NEB microenvironment. To determine whether NEBs have a role in either maintenance or activation of BADJ-associated CE cells, mice were exposed to 275 mg/kg of naphthalene, recovered for 36 hours, and treated with [3H]-TdR 1 hour before sacrifice to identify proliferating cells. Adjacent serial sections were stained for either CCSP or CGRP and proliferating cells were visualized by autoradiography (Figure 6) ▶ . Analysis of 23 terminal bronchioles containing at least one labeled cell (n = 5 animals) demonstrated that 75% of regenerating terminal bronchioles lack CGRP-immunopositive cells in an adjacent serial section, suggesting that the BADJ-microenvironment is distinct from that defined by NEBs (Figure 6, A and B) ▶ . The remaining 25% of regenerating terminal bronchioles analyzed by this method contained CGRP-immunoreactive cells (Figure 6, C and D) ▶ . These data indicate that TBs contain two microenvironments that harbor regenerative pollutant-resistant CE cells, the BADJ and the NEB.
Figure 6.
Proliferative CE cell populations function independently of PNECs/NEBs in the terminal bronchiole. Adjacent serial section immunostaining for CCSP (A and C) and CGRP (B and D) was coupled with autoradiography (black grains) to determine whether terminal bronchiolar proliferation was preferentially associated with CGRP-immunopositive PNECs/NEBs. The majority (75%) of terminal bronchioles contain numerous mitotic CE cells adjacent to the BADJ (A) yet lack any CGRP-positive PNECs/NEBs in the adjacent serial section (B) 36 hours after 275 mg/kg of naphthalene. A minority of terminal bronchioles (25%) appear to contain both CCSP (C)- and CGRP (D)-positive mitotic cells by adjacent serial section analysis. Interestingly, the occurrence of NEBs (C and D, asterisks) appeared to relax the requirement for BADJ association among proliferating CE cells of the terminal bronchiole (black grains; A and B versus C and D).
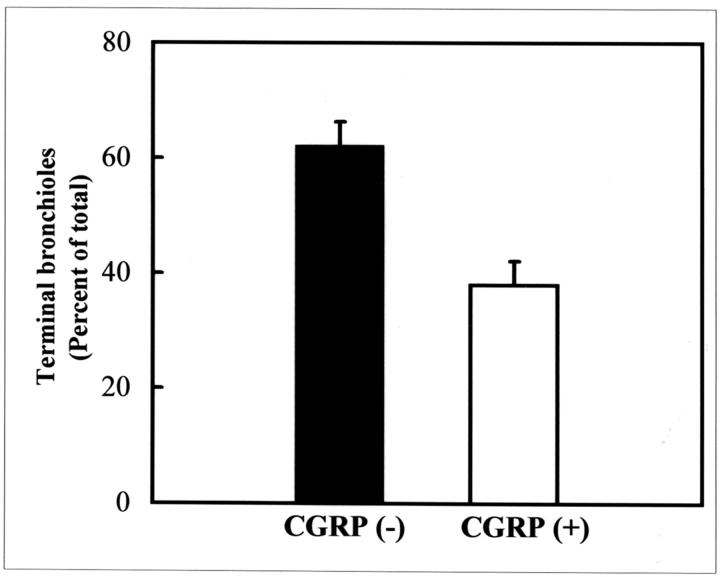
The Majority of Terminal Bronchioles Lack CGRP-Immunoreactive Pulmonary Neuroendocrine Cells after Pollutant Injury
To determine whether subpopulations of TBs can be distinguished by the presence or absence of a NEB microenvironment, the incidence of CGRP- and CCSP-immunoreactive cells was assessed by dual-color immunofluorescent analysis of 20 adjacent 5-μm serial sections through 32 TBs (n = 5 animals) from mice exposed to 275 mg/kg of naphthalene and recovered 24 hours. Terminal bronchioles containing a visible BADJ were scored for the presence of CCSP and CGRP in at least 1 of the 20 adjacent serial sections. Results of this analysis indicate that all terminal bronchioles contain several CCSP-immunoreactive cells closely associated with the BADJ. Thirty-eight percent of terminal bronchioles contain at least one detectable CGRP-positive cell. These PNEC were located some distance from the BADJ and did not appear to co-localize with BADJ-associated CE cell populations. In contrast, 62 percent of TBs lacked detectable NEBs or individual CGRP-immunoreactive cells (Figure 7) ▶ . These data indicate that the majority of TBs lack a NEB and support the notion that the BADJ functions as a distinct microenvironment for maintenance of pollutant-resistant regenerative cells.
Figure 7.
The majority of terminal bronchioles lack PNECs/NEBs after naphthalene injury. Dual-color immunofluorescent staining for CCSP and CGRP proteins was used to co-localize CE and PNEC populations within terminal bronchioles 24 hours after exposure to 275 mg/kg of naphthalene. Twenty consecutive 5-μm-adjacent serial lung sections were stained for the presence of CCSP and CGRP and all terminal bronchioles containing a visible BADJ were analyzed (minimum of five terminal bronchioles/animal; n = 5 animals). Although all terminal bronchioles analyzed contained detectable CCSP-immunoreactive cells at or near the BADJ, 62 ± 5% lacked any CGRP-positive PNECs/NEBs at any location within the terminal bronchiole. Error bars represent SEM.
BADJ-Associated CCSP-Expressing Cells Are an Infrequently Cycling (Label Retaining) Population
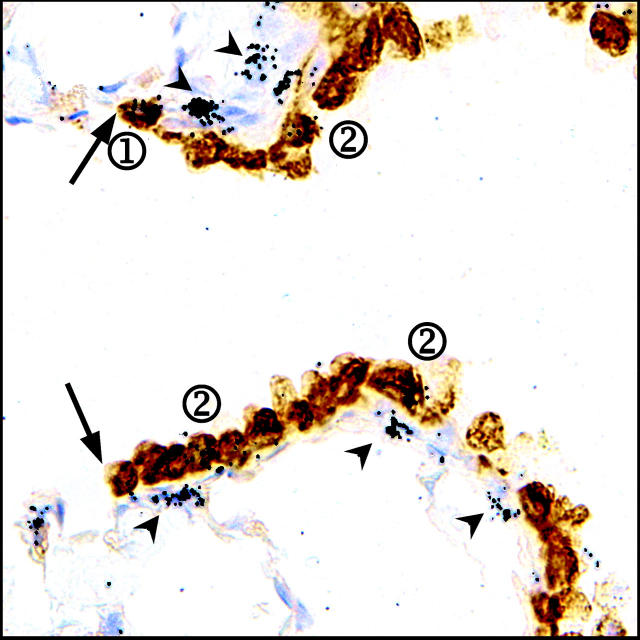
In addition to a relatively undifferentiated phenotype, a low steady-state rate of proliferation is a commonly cited property of stem cells. To assess the mitotic rate of cells in the terminal bronchiole after exposure to 275 mg/kg of naphthalene, cells proliferating during the first 7 days of recovery were continuously labeled with [3H]-TdR via a subcutaneous miniosmotic pump and the animals allowed to recover a total of 45 days. As the period of [3H]-thymidine incorporation was followed by a significant recovery time, a negative correlation between number of silver grains and the number of rounds of proliferation after initial recovery is observed. 10,19 Numerous fibroblast-like cells bore a large number of silver grains 45 days after naphthalene exposure indicating the efficacy of the labeling and chase protocol used in this experiment (Figure 8 ▶ , arrowheads). Two distinct populations of [3H]-thymidine-labeled cells were distinguished by their number of grains/nucleus: label-retaining cells that contained >15 grains/nucleus (Figure 8 ▶ , circled 1) and labeled cells that contained 5 to 10 grains/nucleus (Figure 8 ▶ , circled 2). Infrequently cycling cells (label-retaining) comprise 12% of all labeled cells observed (21 cells total versus 198 total cells, n = 4 animals). This finding suggests that two mitotic cell populations that differ in their proliferation rate are found within terminal bronchioles after acute naphthalene exposure.
Figure 8.
BADJ-associated CE cells represent an infrequently cycling (label-retaining) cell population. Mice were exposed to 275 mg/kg of naphthalene and [3H]-TdR was administered continuously for the subsequent 7 day period via a miniosmotic pump. Recovering mice were sacrificed 45 days after naphthalene exposure, at which time CCSP protein expression and label retention was visualized by immunohistochemistry and autoradiography of lung tissue sections. The majority of labeled cells contained an average of 5 to 10 grains/nuclei, and were composed of both CCSP-immunopositive (circled 2) and -immunonegative cells (not evident in field). A rare population of CE cells located near the BADJ (arrow) were more heavily labeled with an average of >15 grains/nuclei (circled 1). Fibroblasts, identified by location and nuclear morphology, also contained large numbers of grains/nuclei (arrowheads). Original magnification, ×1000.
To determine the molecular phenotype of the labeled or label-retaining cells and whether these populations were restricted to a particular region of the terminal bronchiole, sections were stained for CCSP and the distribution of each cell type evaluated using the BADJ as a reference point (Figures 8 ▶ , arrow, and 9 ▶ ). CE label-retaining cells exhibited a nonrandom distribution within TBs (Figure 9 ▶ , solid black bars). Greater than 60% of label-retaining CCSP-immunopositive cells were specifically localized in the 20-μm region (three cell diameters) adjacent to the BADJ. CCSP-negative label-retaining, as well as CCSP-positive-labeled, and CCSP-negative-labeled cells were randomly distributed throughout the terminal bronchiole (Figure 9 ▶ , hatched, gray and white bars, respectively). Spatial restriction of label-retaining CE cells to the BADJ microenvironment supports the notion that these cells represent a stem cell pool important for terminal bronchiolar renewal.
Figure 9.
CE label-retaining cells are preferentially located at the BADJ. The distribution of [3H]-TdR-labeled (5 to 10 grains/nuclei) and label-retaining (>15 grains/nuclei) cells in terminal bronchioles of animals exposed to 275 mg/kg of naphthalene and recovered for 45 days were assessed by morphometric analysis. The percentage of each thymidine-labeled cell population was plotted independently as a function of distance in 20-μm increments originating from the BADJ. A correlation was observed between proximity to the BADJ and the abundance of CE, label-retaining cell populations (black bars). Very few CCSP-negative cells label-retaining cells (hatched white bars) were identified within terminal bronchioles, and these cells were not associated with the BADJ. Both CCSP-immunopositive (gray bars) and -negative (white bars) labeled (5 to 10 grains/nuclei) cell populations were distributed randomly throughout the terminal bronchiole. Data are the summation of 23 terminal bronchioles (n = 4 mice).
Discussion
Results of this study identify a novel stem cell compartment that contributes to renewal of the terminal bronchiolar epithelium after acute progenitor (Clara) cell depletion. Rare, pollutant-resistant, CE cells were identified in numerous terminal bronchioles both by immunohistochemistry and in situ hybridization, and were present at all time points after naphthalene administration. Interestingly, these pollutant-resistant CE cells were spatially restricted to the most distal conducting airway epithelium immediately adjacent to the BADJ. After depletion of naphthalene-sensitive Clara cells, BADJ-associated CE cells account for all proliferative cells within terminal bronchioles. At later time points, progeny of BADJ-associated cells appear to migrate proximally and contribute to the restoration of a terminal bronchiolar epithelium of appropriate cellular composition. In addition to rapidly proliferating cells, a subset of BADJ-associated CE cells rapidly enters a quiescent state and remains spatially restricted to this region of the airway. These BADJ-associated cells function independently of NEBs, and thus define a novel regenerative microenvironment. We conclude that epithelial CE cells that are enriched within the BADJ microenvironment of terminal bronchioles represent a pollutant-resistant, infrequently cycling population with multipotent differentiation potential. Based on these findings we suggest that BADJ-associated CE cells represent a stem cell pool.
Although this study is the first to define a specific stem cell of terminal bronchioles, the kinetics of injury and repair within the terminal bronchiolar epithelium have been extensively characterized. The time course and magnitude of Clara cell ablation noted in the present study are similar to previously published findings. 28,37,38 Despite differences in the dose of naphthalene, the temporal relationship between initial injury and the subsequent onset of repair were quite similar for each model. However, the present findings are not fully consistent with previous suggestions regarding the identity of cells that actively contribute to the proliferative fraction of the renewing epithelium. Studies by Van Winkle and co-workers 22 demonstrate the existence of squamated cells, some of which possess cilia, within the residual terminal bronchiolar epithelium of naphthalene-exposed mice. The rapid pace of epithelial renewal, coupled with a lack of cells exhibiting morphological characteristics of Clara cells, led this group to suggest that residual ciliated cells represented the most likely progenitor pool for renewal of naphthalene-injured terminal bronchioles. 22,39 In the present study we demonstrate that residual squamated epithelial cells that resist naphthalene-induced injury include a CE subset and that this population of pollutant-resistant CE cells proliferate to effect renewal of terminal bronchioles. This finding is consistent with our previous studies in which systemic delivery of gancyclovir was used to achieve conditional ablation of CE cells in transgenic mice (CCSP-HSVtk) expressing the protoxin gene Herpes simplex virus thymidine kinase under the regulatory control of the mouse CCSP promoter. 19,26 Ablation of all CE cell populations in those studies was accompanied by proliferation of PNECs of the bronchial and bronchiolar epithelium, but was not associated with renewal of the injured epithelium. Importantly, neither proliferative epithelial cells nor airway regeneration was observed within CE cell-depleted terminal bronchioles of CCSP-HSVtk transgenic mice during recovery from acute gancyclovir exposure, 19 indicating the loss of a critical stem cell population. Therefore, CE cell populations within NEB and terminal bronchioles represent an essential component of epithelial renewal after injury.
Our current and previous findings identify pollutant resistance and CCSP expression as criteria that are unique properties of stem cell pools within both terminal and proximal bronchiolar epithelium. Mechanisms that might contribute to resistance of the stem cell pool from pneumotoxic compounds such as naphthalene may either be intrinsic to the cell, such as differences in pollutant metabolizing enzymes, or as a result of its physical sequestration. A precedent exists for each model. A growing consensus is that stem cells exhibit a relatively undifferentiated phenotype that may confer protection from the potentially genotoxic consequences of metabolizing xenobiotic compounds. 13,14,40 CE cells localizing to the NEB microenvironment of proximal bronchioles included a subset showing no detectable immunoreactive CYP2F2 protein, the cytochrome P450 isoenzyme primarily responsible for metabolism of naphthalene to a cytotoxic epoxide. 20 Alternatively, the fact that stem cells of regenerative epithelia are generally sequestered within secluded microenvironments, as is the case for stem cells found in the limbus of the cornea and crypts of the small intestine, 41,42 also raises the possibility that stem cells may be physically isolated from toxic pollutants. The relative contribution of intrinsic pollutant resistance versus anatomical sequestration cannot be easily discerned for stem cells of the NEB microenvironment and both mechanisms may in fact contribute to their resistance from naphthalene-induced injury. However, BADJ-associated stem cells described herein are localized to a region of the conducting airway that is most sensitive to the effects of parenterally administered naphthalene. Even though the expression of CYP2F2 and other pollutant-metabolizing enzymes were not measured within this population of stem cells, their exposed location at the BADJ suggests that intrinsic resistance to xenobiotic agents is an important determinant contributing to their maintenance after naphthalene exposure.
Other insights into the molecular phenotype of regenerative cells contributing to renewal of naphthalene-injured airways have come from studies investigating expression of the growth factors epidermal growth factor and transforming growth factor-α, and the epidermal growth factor receptor, during airway repair. 23 Elevated expression of epidermal growth factor receptor was observed among a population of squamated epithelial cells located within terminal bronchioles during repair from naphthalene exposure. Moreover, cells with abundant epidermal growth factor receptor also incorporated the nucleoside analogue bromodeoxyuridine, suggesting a role for epidermal growth factor in regulation of progenitor cell proliferation. 23 Findings of Van Winkle and co-workers investigating the kinetics, distribution and morphology of cells expressing epidermal growth factor receptor early in the response to naphthalene exposure show a close correlation with the distribution and proliferative kinetics of CE cells described in the present study that exhibit properties of stem cells. Clearly, microenvironmental cues are involved in both regulation of stem cell phenotype, stem cell maintenance, and their activation after progenitor cell depletion.
Microenvironments that support the maintenance of adult stem cells have recently been identified within both the surface and glandular epithelium of pulmonary airways. The best characterized stem cell microenvironments include those of the submucosal gland in proximal airways, 16 the NEB microenvironment, 20 and the BADJ microenvironment identified in the present study. However, very little is known at present of the molecular and cellular mechanisms that account for stem cell maintenance within these lung microenvironments. The composition of airway epithelia at each location differs quite dramatically, a feature that raises the question of whether intrinsic or extrinsic factors influence stem cell maintenance and the fate of daughter cells. In this regard, it is interesting to note that basic features of stem cells located within BADJ and NEB microenvironments are similar despite clear differences in the cellular milieu at these locations. More thorough analysis of the molecular phenotype of stem cells located within these disparate microenvironments is needed and may, in turn, shed light on molecular mechanisms contributing to their maintenance.
Acknowledgments
We thank Kyung U. Hong for insightful discussions during preparation of this manuscript and Jennifer LaRuffa for animal husbandry.
Footnotes
Address reprint requests to Barry R. Stripp, Department of Environmental and Occupational Health, Room 314, 3343 Forbes Ave., University of Pittsburgh, Pittsburgh, PA 15260. E-mail: brs2@pitt.edu.
Suported by National Institute of Health research grants HL64888, ES08964, and HL70575, and toxicology training grant ES07026.
References
- 1.Barth PJ, Wolf M, Ramaswamy A: Distribution and number of Clara cells in the normal and disturbed development of the human fetal lung. Pediatr Pathol 1994, 4:637-651 [DOI] [PubMed] [Google Scholar]
- 2.Plopper CG, Mariassy AT, Hill LH: Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: I. A comparison of rabbit, guinea pig, rat, hamster, and mouse. Exp Lung Res 1980, 1:139-154 [DOI] [PubMed] [Google Scholar]
- 3.Plopper CG, Mariassy AT, Hill LH: Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: II. A comparison of horse, steer, sheep, dog, and cat. Exp Lung Res 1980, 1:155-169 [DOI] [PubMed] [Google Scholar]
- 4.Plopper CG, Hill LH, Mariassy AT: Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res 1980, 1:171-180 [DOI] [PubMed] [Google Scholar]
- 5.Plopper CG, Mariassy AT, Wilson DW, Alley JL, Nishio SJ, Nettesheim P: Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res 1983, 5:281-294 [DOI] [PubMed] [Google Scholar]
- 6.Plopper CG, Halsebo JE, Berger WJ, Sonstegard KS, Nettesheim P: Distribution of nonciliated bronchiolar epithelial (Clara) cells in intra- and extrapulmonary airways of the rabbit. Exp Lung Res 1983, 5:79-98 [DOI] [PubMed] [Google Scholar]
- 7.Massaro GD, Singh G, Mason R, Plopper CG, Malkinson AM, Gail DB: Biology of the Clara cell. Am J Physiol 1994, 266:L101-L106 [DOI] [PubMed] [Google Scholar]
- 8.Plopper CG, Buckpitt A, Evans M, Van Winkle L, Fanucchi M, Smiley-Jewell S, Lakritz J, West J, Lawson G, Paige R, Miller L, Hyde D: Factors modulating the epithelial response to toxicants in tracheobronchial airways. Toxicology 2001, 160:173-180 [DOI] [PubMed] [Google Scholar]
- 9.Enver T, Greaves M: Loops, lineage, and leukemia. Cell 1998, 94:9-12 [DOI] [PubMed] [Google Scholar]
- 10.Lehrer MS, Sun TT, Lavker RM: Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci 1998, 111:2867-2875 [DOI] [PubMed] [Google Scholar]
- 11.Petkov PM, Kim K, Sandhu J, Shafritz DA, Dabeva MD: Identification of differentially expressed genes in epithelial stem/progenitor cells of fetal rat liver. Genomics 2000, 68:197-209 [DOI] [PubMed] [Google Scholar]
- 12.Saam JR, Gordon JI: Inducible gene knockouts in the small intestinal and colonic epithelium. J Biol Chem 1999, 274:38071-38082 [DOI] [PubMed] [Google Scholar]
- 13.Watt FM, Hogan BL: Out of Eden: stem cells and their niches. Science 2000, 287:1427-1430 [DOI] [PubMed] [Google Scholar]
- 14.Watt FM: Stem cell fate and patterning in mammalian epidermis. Curr Opin Gene Dev 2001, 11:410-417 [DOI] [PubMed] [Google Scholar]
- 15.Weissman IL, Anderson DJ, Gage F: Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001, 17:387-403 [DOI] [PubMed] [Google Scholar]
- 16.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH: Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001, 24:662-670 [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L: Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995, 121:2031-2046 [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt JF: Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol 2001, 24:649-652 [DOI] [PubMed] [Google Scholar]
- 19.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR: Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001, 24:671-681 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds SD, Giangreco A, Power JH, Stripp BR: Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000, 156:269-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans MJ, Cabral-Anderson LJ, Freeman G: Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest 1978, 38:648-653 [PubMed] [Google Scholar]
- 22.Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG: Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol 1995, 269:L800-L818 [DOI] [PubMed] [Google Scholar]
- 23.Van Winkle LS, Isaac JM, Plopper CG: Distribution of epidermal growth factor receptor and ligands during bronchiolar epithelial repair from naphthalene-induced Clara cell injury in the mouse. Am J Pathol 1997, 151:443-459 [PMC free article] [PubMed] [Google Scholar]
- 24.Spradling A, Drummond-Barbosa D, Kai T: Stem cells find their niche. Nature 2001, 414:98-104 [DOI] [PubMed] [Google Scholar]
- 25.Quesenberry PJ, Becker PS: Stem cell homing: rolling, crawling, and nesting. Proc Natl Acad Sci USA 1998, 95:15155-15157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR: Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol 2000, 278:L1256-L1263 [DOI] [PubMed] [Google Scholar]
- 27.Wert SE, Glasser SW, Korfhagen TR, Whitsett JA: Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol 1993, 156:426-443 [DOI] [PubMed] [Google Scholar]
- 28.Stripp BR, Maxson K, Mera R, Singh G: Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995, 269:L791-L799 [DOI] [PubMed] [Google Scholar]
- 29.Born SL, Fix AS, Caudill D, Lehman-McKeeman LD: Selective Clara cell injury in mouse lung following acute administration of coumarin. Toxicol Appl Pharmacol 1998, 151:45-56 [DOI] [PubMed] [Google Scholar]
- 30.Van Winkle LS, Isaac JM, Plopper CG: Repair of naphthalene-injured microdissected airways in vitro. Am J Respir Cell Mol Biol 1996, 15:1-8 [DOI] [PubMed] [Google Scholar]
- 31.Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG: Early events in naphthalene-induced acute Clara cell toxicity: comparison of membrane permeability and ultrastructure. Am J Respir Cell Mol Biol 1999, 21:44-53 [DOI] [PubMed] [Google Scholar]
- 32.Miele L, Cordella-Miele E, Mantile G, Peri A, Mukherjee AB: Uteroglobin and uteroglobin-like proteins: the uteroglobin family of proteins. J Endocrinol Invest 1994, 17:679-692 [DOI] [PubMed] [Google Scholar]
- 33.Warburton D, Wuenschell C, Flores-Delgado G, Anderson K: Commitment and differentiation of lung cell lineages. Biochem Cell Biol 1998, 76:971-995 [PubMed] [Google Scholar]
- 34.Adriaensen D, Scheuermann DW: Neuroendocrine cells and nerves of the lung. Anat Rec 1993, 236:70-85 [DOI] [PubMed] [Google Scholar]
- 35.Stevens TP, McBride JT, Peake JL, Pinkerton KE, Stripp BR: Cell proliferation contributes to PNEC hyperplasia after acute airway injury. Am J Physiol 1997, 272:L486-L493 [DOI] [PubMed] [Google Scholar]
- 36.Peake JL, Reynolds SD, Stripp BR, Stephens KE, Pinkerton KE: Alteration of pulmonary neuroendocrine cells during epithelial repair of naphthalene-induced airway injury. Am J Pathol 2000, 156:279-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A: Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J Pharmacol Exp Ther 1992, 261:353-363 [PubMed] [Google Scholar]
- 38.Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR: Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest 1992, 67:553-565 [PubMed] [Google Scholar]
- 39.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG: Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury-distal airways. Am J Pathol 2002, 160:315-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slack JM: Stem cells in epithelial tissues. Science 2000, 287:1431-1433 [DOI] [PubMed] [Google Scholar]
- 41.Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D: Retinal stem cells in the adult mammalian eye. Science 2000, 287:2032-2036 [DOI] [PubMed] [Google Scholar]
- 42.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H: Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 1998, 19:379-383 [DOI] [PubMed] [Google Scholar]