Abstract
Geminin interacts with a DNA replication initiation factor, Cdt1p, to suppress initiation of DNA replication in a Xenopus egg extract based cell-free system, leading to the expectation that the protein acts as an inhibitor of cell proliferation. Immunohistochemistry and immunoblotting for geminin, however, reveals that the protein is expressed specifically in proliferating lymphocytes and epithelial cells. This pattern is in contrast to the expression of a bona fide cell cycle inhibitor like p21/WAF1 that is specifically expressed in quiescent cells. Geminin is widely expressed in several malignancies and the number of geminin-expressing cells is directly proportional to the cell proliferation index as measured by Ki-67 expression. Therefore, instead of being a suppressor of cell proliferation, geminin expression is positively correlated with cell proliferation. Consistent with this observation, transient overexpression of wild-type geminin in cancer cells in culture did not produce a cell cycle block. A point mutation in the destruction box of geminin, however, results in a protein that is stabilized in G1 and capable of arresting cells at the G1-S transition.
The proliferation of any cell, normal or malignant, involves the passage of the cell through a discrete set of ordered phases that together constitute the cell cycle. Cells prepare for DNA replication during G1, replicate their DNA in S phase, prepare for mitosis in G2, and finally segregate the daughter chromosomes in M phase. Due to the central role of the cell cycle in maintaining genomic integrity, it is not surprising that several key cell cycle regulatory proteins have been implicated in the pathology of cancers. 1-4 In particular, molecules that inhibit passage through specific stages of the cell cycle are frequently found to be tumor suppressors that are mutated or repressed in a large fraction of human malignancies. Classic examples of such molecules are the retinoblastoma (Rb) protein, the p53 protein, or the p16INK4a cdk inhibitor, all of which, when overexpressed, suppress the passage of a cell from G1 into S. Therefore, the identification of a new inhibitor of the G1-S transition immediately raises the question as to whether it functions as a tumor suppressor.
DNA replication is initiated at discrete sites on chromosomes through the coordinate action of a number of replication initiation factors. 5,6 Based on analogy with yeast, where this process is best understood, it is believed that a complex of proteins called the origin recognition complex (ORC) associates with specific DNA sequences near origins of replication to recruit other replication initiation factors during the G1 phase of the cell cycle. The other replication initiation factors, Cdc6p, Cdt1p, and the Mcm2–7p complex, associate with the origin sequence in an ORC-dependent reaction to form a pre-replicative complex (pre-RC). At the G1-S transition, the activation of cyclin-dependent kinases leads to the recruitment of elongation factors, CDC45, DNA polymerases, and RPA to the pre-RCs at origins. The action of these replication elongation proteins leads to the initiation of DNA synthesis, the hallmark of S phase. Although the proteins involved in pre-RC assembly (ORC, Cdc6p, Cdt1p, and Mcm2–7p) persist in the human cell even after the G1-S transition, new pre-RCs are not assembled on already fired origins during the late S, G2 and early M phases of the cell cycle. It is not until the subsequent G1 phase of the cell cycle that new pre-RCs are assembled onto origins. This inhibition of pre-RC assembly during the latter half of the cell cycle ensures that the same origin is not activated twice in the same cell cycle and thus acts to maintain genomic integrity by preventing abnormal re-replication of DNA. 7
Geminin is a central regulator of the process that inhibits re-replication. It interacts with Cdt1p and prevents the recruitment of the Mcm2–7p complex to origins during S, G2, and early M phases of the cell cycle and thereby inhibits replication initiation. 8,9 In the normal cell cycle, however, geminin is not present in G1 and thus does not impede the normal establishment of pre-RCs. Geminin expression begins as the origins are triggered at the G1-S transition and the protein level rises through the rest of the cell cycle to reach a maximum in mitosis. An ubiquitin ligase called the anaphase promoting complex (APC) is activated specifically in mitosis and leads to the proteolysis of geminin. 8 This careful orchestration of geminin expression and destruction ensures that, although the protein does not prevent normal pre-RC assembly in G1, it is present in significant amounts in late S and G2 when it can inhibit the Cdt1p protein and prevent the abnormal establishment of pre-RC on already fired origins.
Addition of geminin to cell-free DNA replication reactions derived from Xenopus egg extracts suppresses replication initiation and prevents cell cycle progression. 8-10 Overexpression of geminin might therefore suppress cancer cell proliferation. In addition, since inactivation of geminin would lead to re-replication of DNA in the same cell cycle and since polyploidy and gene amplification are often seen in cancers, one might expect mutations that inactivate geminin or suppress its expression to be selected for during cancer progression. Based on these arguments, it has been hypothesized that geminin might be a classic tumor suppressor protein. 11 If geminin is a tumor suppressor, its expression in tumors or normal cells might correlate with decreased cell proliferation and one might see subsets of tumors that have suppressed expression of the cell cycle inhibitor. In addition, overexpression of geminin should suppress cell proliferation in culture. In this paper, we report that neither of these expectations are supported by experiment. In contrast, geminin is expressed specifically in proliferating cells, leading to the conclusion that geminin does not behave as a classical tumor suppressor and inhibitor of cell proliferation.
Materials and Methods
Immunoblotting for Geminin
Multi-tissue Western blots were purchased from Geno Technology, Inc (St. Louis, MO). These blots were probed with anti-geminin antibodies that had been affinity-purified using recombinant human geminin using the protocol described in Lane and Harlow. 12 Immunoblotting experiments shown in Figure 6A ▶ were done using unpurified geminin antibodies.
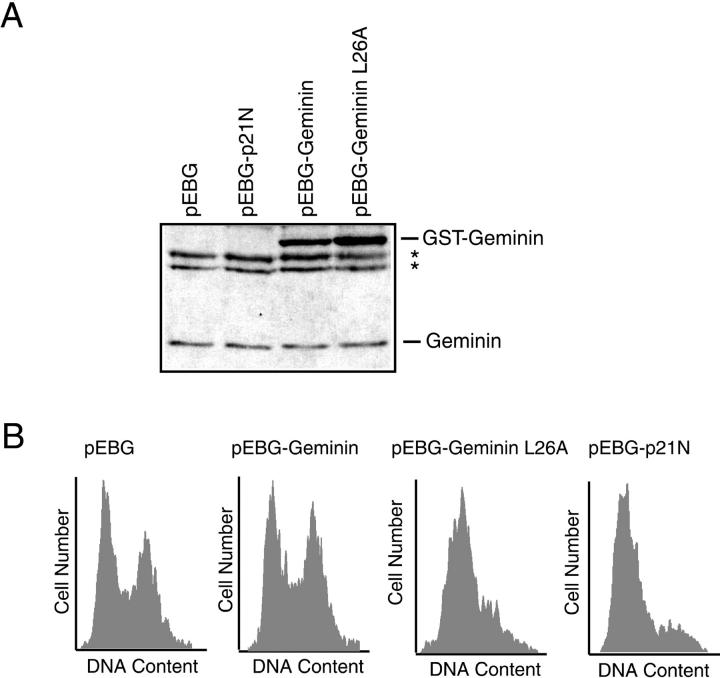
Figure 6.
Overexpression of geminin L26A but not wild-type geminin blocks cell cycle progression in U2OS cells. A: GST-geminin expression in cells in culture. Whole cell extracts were prepared from U2OS cells transfected with pEBG (lane 1), pEBG-p21N (lane 2), pEBG-geminin WT (lane 3), or pEBG-geminin L26A (lane 4) and immunoblotted with anti-geminin antibodies (1:2000 dilution). *, indicates non-specific cross-reacting proteins. B: Effect of geminin overexpression on cell cycle progression. U2OS cells were co-transfected with a plasmid encoding farnesylated GFP and pEBG, pEBG-geminin WT, pEBG-geminin L26A, or pEBG-p21N. The cell cycle profile of the GFP-positive transfected cells was determined 60 hours after transfection.
Immunohistochemistry for Geminin, p21/CIP1/Waf1 and Ki-67
For studies of the 24 large B-cell lymphomas from the files of Brigham and Women’s Hospital pathology, tissue arrays were constructed by obtaining five representative 0.6-mm cores from diagnostic areas of each formalin-fixed, paraffin-embedded block using a tissue arrayer (Beecher Instruments, Silver Spring, MD). Immunohistochemistry was performed using 5-μm thick, formalin-fixed, paraffin-embedded tissue sections. All staining was performed by standard immunoperoxidase methods. Briefly, slides were deparaffinized and pre-treated in 1 mmol/L EDTA (pH 8.0) for 20 minutes at 95°C using steam heat. All further steps were performed at room temperature in a hydrated chamber. Slides were pre-treated with peroxidase block (DAKO, USA) for 5 minutes to quench endogenous peroxidase activity, and a 1:5 dilution of goat serum in 50 mmol/L Tris-Cl (pH 7.4), for 20 minutes to block non-specific binding sites. Either rabbit anti-geminin antibody at a 1:1000 dilution in 50 mmol/L Tris-Cl (pH 7.4) with 3% goat serum or murine anti-Ki-67 antibody (MIB-1; AMAC, Inc., Westbrook, ME) at a 1:100 dilution in 50 mmol/L Tris-Cl (pH 7.4), with 3% goat serum was applied for 1 hour. After washing in 50 mmol/L Tris-Cl (pH 7.4), secondary goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated antibody (Envision detection kit, DAKO) was applied for 30 minutes. After further washing, immunoperoxidase staining was developed using a DAB chromogen kit (DAKO) per the manufacturer’s instuctions and counterstained with hematoxylin. For the large-cell lymphoma array studies, immunoperoxidase staining for geminin and Ki-67 within the malignant cell population for each five cores in every case was scored in a blinded fashion as a percentage of the total malignant cell population by two experienced hematopathologists (J.L.K. and A.P.W.), averaged, and graphically represented using kaleidagraph. The staining for p21/WAF1 was with the HE12 murine monoclonal anti-p21 antibody (Santa Cruz) according to conditions previously published. 13, 14
Plasmid Constructs
The coding region of the geminin gene was excised from pET14b-hsgeminin 9 using SpeI and NotI and ligated to the mammalian GST-expression vector, pEBG, cut with the same enzymes. The EBG-geminin L26A mutant was constructed using the Stratagene Quikchange system. Expression plasmids encoding p21 were described previously. 15
Cell Cycle Analysis
pEBG, pEBG-geminin, and pEBG-p21N were co-transfected with a plasmid expressing farneslyated-GFP (Clontech) into U20S cells. Cells were harvested 48 hours after transfection and analyzed by flow cytometry as described. 15 Briefly, transfected cells were trypsinized, stained with propidum iodide, and then analyzed by flow cytometry. The DNA content was determined for 5000 GFP-positive cells (thus selectively studying the transfected cells).
Results
Expression of Geminin in Normal Tissues
Immunostaining with anti-geminin antibody reveals strong nuclear staining in the proliferating cell populations. For example, geminin was detected in the germinal centers within secondary follicles of tonsils and reactive lymph nodes, within the bases of the colon and small intestinal crypts, within the developing spermatocytes in the seminiferous tubules of the testis, within the basal layers of the squamous epithelium of the skin and the uterine cervix (Figure 1 ▶ and data not shown), and within eccrine glands and hair follicles of the skin. This pattern of expression coincides exactly with cells that are in the active cell cycle and suggests that instead of being associated with cell quiescence, geminin is, in fact, expressed specifically in cycling cells. In contrast, tissues with minimal cell proliferation, eg, heart, nerve, the cerebral cortex, cerebellum, prostate, kidney, lung, skeletal muscle, blood vessels, and adrenal glands fail to stain for geminin (see Figure 1 ▶ and data not shown). Some tissues demonstrated only rare scattered epithelial cells that were positive for geminin in the nuclei, eg, ductal and lobular cells of the breast (Figure 1C) ▶ , acinar cells of the pancreas, and hepatocytes of the liver. In addition, lymphocytes primarily within the white pulp of the spleen also demonstrated scattered positive cells.
Figure 1.
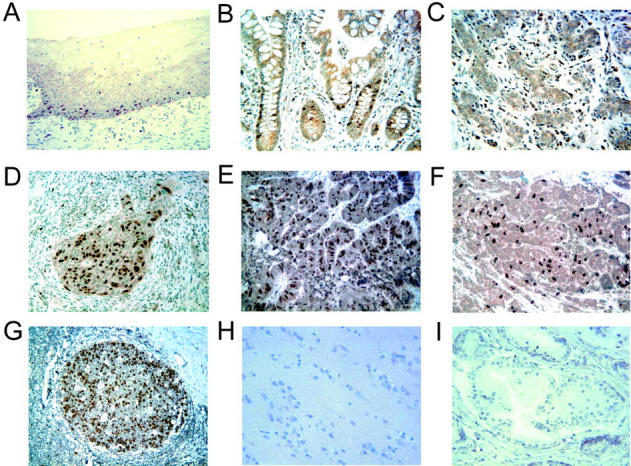
Expression of geminin in representative human tissue by immunohistochemistry. Sections measuring 5 μm from paraffin-embedded tissues were pre-treated in 1 mmol/L EDTA (pH 8.0), for 20 minutes at 95°C using steam heat, blocked in 20% normal goat serum, and incubated with rabbit antibody to geminin followed by HRP-conjugated goat anti-rabbit IgG. Binding of the primary antibody was revealed by addition of 3′3′-diaminobenzidine followed by counterstaining with hematoxylin. Nuclear expression was seen in scattered cells within the basal layers of the squamous mucosa from normal uterine cervix (A), crypts of normal colon (B), and rare ductal and lobular cells in normal breast (C). In contrast, frequent cells demonstrated nuclear staining in carcinomas of the uterine cervix (D), colon (E), and breast (F). High levels of nuclear staining were present in the actively proliferating cells of reactive secondary follicles of the tonsil (G), whereas no staining was present in the cerebrum (H) or prostate (I). Magnification, ×200.
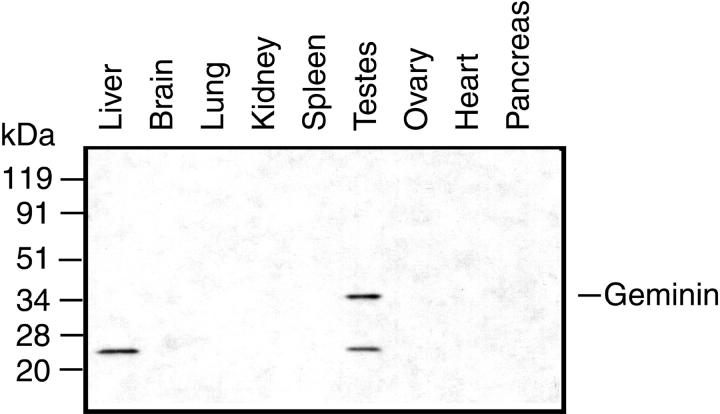
Immunoblotting of selected tissue extracts with anti-geminin antibody revealed that the authentic 33-kd geminin protein was expressed in testis but not in liver, brain, lung, kidney, spleen, ovary, heart, or pancreas (Figure 2) ▶ . Of all of the tissues on the blot, testis is the only one with proliferating cells. Thus detection of geminin exclusively in the testis confirms that it is expressed only in proliferating cells. This pattern of geminin protein expression mimics the tissue distribution of geminin mRNA previously described. 16 Interestingly, a smaller protein of approximately 24-kd is recognized by the anti-geminin antibodies in both testis and liver. We are not certain whether this indicates the existence of other geminin isoforms or is a non-specific cross-reacting protein.
Figure 2.
Geminin protein is present in testis but not in tissues containing predominantly quiescent cells. Protein extracts prepared from the indicated tissues were blotted with affinity purified anti-geminin antibodies (1:1000 dilution).
Expression of p21/CIP1/WAF1 in Normal Tissues
To compare geminin expression with that of another inhibitor of DNA replication, we stained normal tissues with HE12 monoclonal antibody to p21, 17, 18 a protein that interacts with and inhibits cyclin dependent kinases involved in the G1-S transition. As demonstrated in Figure 3 ▶ , p21 staining was best detected in the pancreas, adrenal, the suprabasal layers of the squamous epithelium of the skin, sebaceous glands, and the cells at the surface of the colon epithelium (as opposed to the bases of the crypts). p21 is also detected in the suprabasal layers of the squamous epithelium of the cervix and in very rare cells in the mammary ductal epithelium (data not shown). Staining for Ki-67 shows that most of these cells are in quiescence (data not shown). Thus, geminin expression in normal tissues is distinctly different from that of a bona fide cell cycle inhibitor like p21.
Figure 3.
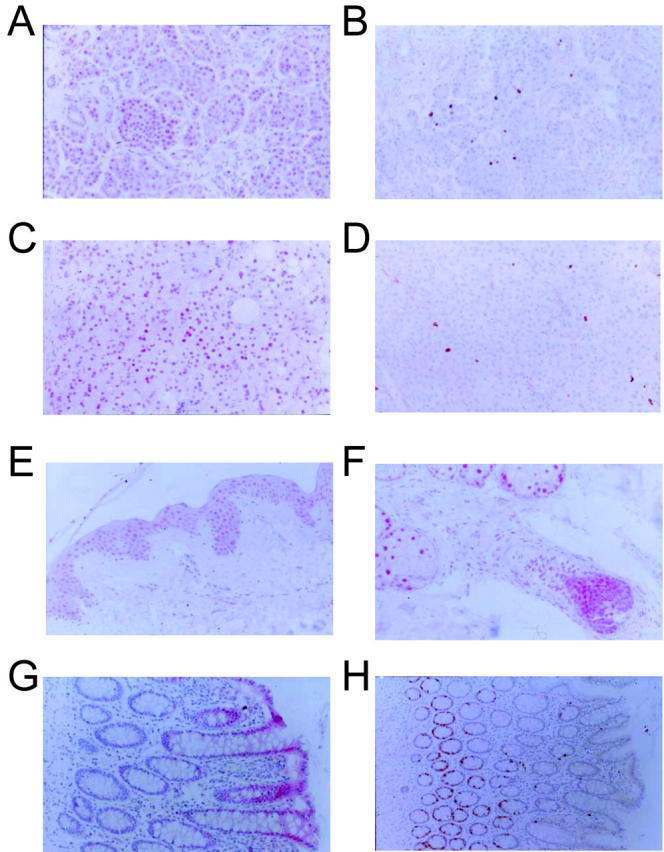
The cell cycle inhibitor p21 is expressed in quiescent cells. Sections from pancreas (A and B), adrenal cortes (C and D), skin (E), skin appendages (F) and colon mucosa (G and H) were stained with either anti-p21 antibody (A, C, E, F, and G) or with anti-Ki-67 antibody (B, D, and H). Magnification, ×100.
Expression of Geminin in Cancers
In contrast to the situation in normal tissues, where the expression of geminin was very restricted in distribution, cancers showed a remarkable up-regulation of the protein. For example, although very little geminin was detected in normal mammary epithelium, the protein was easily detected in invasive carcinoma of the breast. (Figure 1F) ▶ The same was true for invasive carcinoma of the cervix and the colon (Figure 1, D and E ▶ , respectively).
Correlation of Geminin Expression with Cell Proliferation in Lymphomas
To quantitatively correlate geminin expression with cell proliferation, and to rule out the possibility that a reasonable fraction of tumors down-regulate the putative cell cycle inhibitor, we decided to survey a selection of lymphomas. Normal germinal center B cells (as in the tonsil) are positive for geminin, so that it is conceivable that if geminin is indeed a bona fide tumor suppressor, the protein would be down-regulated in some fraction of the tumors.
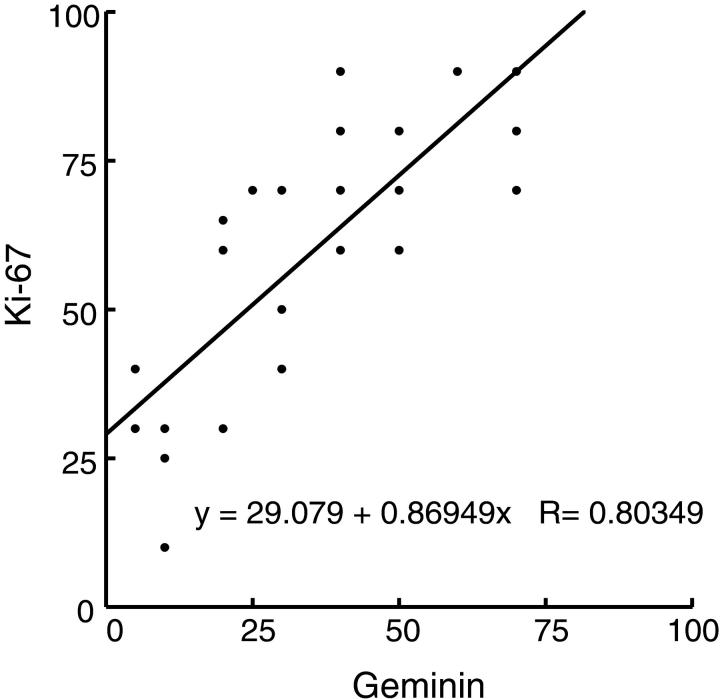
Twenty-four non-Hodgkin’s lymphomas of the diffuse large-cell type, B-cell phenotype, were surveyed for both expression of geminin and for the proliferation marker Ki-67 (Figure 4 and 5) ▶ ▶ . Robust expression of geminin in varying degrees was detected in all 24 tumors (Figure 4) ▶ . Expression of both geminin and Ki-67 were scored on a semi-quantitative scale (see legend to Figure 5 ▶ ). Plotting of the geminin staining index with the Ki-67 staining index revealed that the two were directly correlated with each other, suggesting that geminin expression is related to increased cell proliferation.
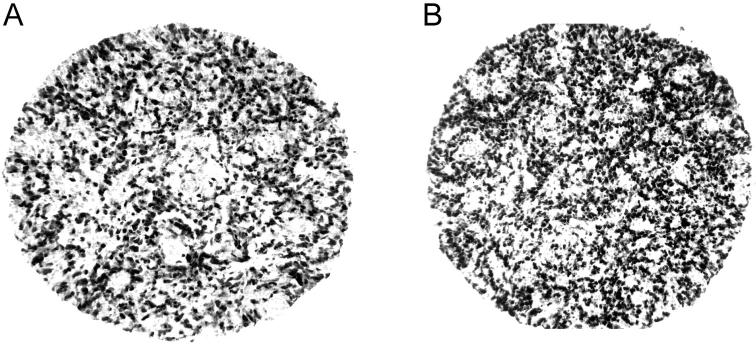
Figure 4.
Immunohistochemistry of lymphomas with anti-geminin and anti-Ki-67 antibodies. Tissue arrays from diffuse, large B-cell lymphomas were constructed by obtaining five representative 0.6-mm cores from diagnostic areas of each formalin-fixed, paraffin-embedded block using a tissue arrayer (Beecher Instruments, Silver Spring, MD). Representative cores immunostained with either anti-geminin (A), or the proliferation marker, anti-Ki-67 (B), demonstrate nearly an equivalent fraction of tumor cells with nuclear staining. Magnification, ×400.
Figure 5.
Correlation of Ki-67 staining with geminin staining. Plot of geminin versus Ki-67 staining expressed as a percentage of positive malignant cells/total malignant cells from 24 diffuse large B-cell lymphoma cases. Each point represents an average of five tissue array cores stained for either geminin or Ki-67 from each of the 24 cases scored in a blinded fashion.
Transient Expression of Geminin in Cancer Cells in Culture
The ability of geminin to potently inhibit DNA replication in Xenopus egg extracts in vitro was at odds with its natural pattern of high expression only in proliferating cells. This contradiction suggested that overexpressed geminin would not block cell cycle progression in cells in culture. Geminin fused at its N-terminus with glutathione S transferase (GST-geminin) effectively interacted with Cdt1 and inhibited replication in Xenopus egg extracts. 9 GST-geminin was expressed using a strong promoter (EF1α) in mammalian cells from the EBG-geminin plasmid vector. Transient transfection of EBG-geminin into U2OS cells, an osteosarcoma cell line, resulted in overexpression of GST-geminin protein relative to endogeneous geminin (Figure 6A) ▶ . Since only ∼3% of the cells were transfected with the geminin-expressing plasmid (data not shown), the ratio of GST-geminin to endogenous geminin in the transfected cells is very high (>30:1). Co-transfection of a plasmid expressing green fluorescent protein (GFP) allowed us to use flow cytometry to specifically identify and analyze only the transfected cells expressing geminin. Analysis of the DNA content of the geminin overexpressing cells showed that geminin did not block these cells in any phase of the cell cycle as compared to control cells transfected with an empty pEBG vector (Figure 6B) ▶ . Thus, even though GST-geminin is highly overexpressed in these cells, there was no significant difference in cell cycle progression. Identical results were obtained by overexpressing an untagged version of geminin (data not shown). The inability of wild-type geminin to inhibit cell cycle progression was not limited to U2OS cells and identical results were also attained for 293T, HeLa, and HCT116 cells. It is important to note, however, that we have only performed these geminin overexpression studies in cancer cell lines and do not know what effect, if any, geminin overexpression might have on untransformed diploid fibroblasts. As a positive control, we overexpressed the N terminal half of p21 (p21N) that is known to interact with and inhibit cyclin-dependent kinases. 19 p21N strongly blocks cells in G1 phase of the cell cycle as seen in Figure 6B ▶ .
One possible explanation for the inability of wild-type geminin to block cell cycle progression is that the GST-geminin is being actively degraded during G1 phase of the cell cycle as was previously shown for endogenous geminin 8, 9 and is thus not present in high enough levels to block pre-RC assembly during G1. To test this possibility, we constructed a mutant version of geminin in which the conserved leucine from the destruction box motif of geminin was replaced with an alanine. This mutant, which we will refer to as geminin L26A, is expected to stabilize geminin during G1 phase of the cell cycle by preventing its association with the anaphase promoting complex (APC). 8 When geminin L26A is transfected into U2OS cells, we find that geminin L26A appears to be more abundant than wild-type geminin (Figure 6A) ▶ indicating that, as expected, it is likely more stable than wild-type geminin. The overexpression of geminin L26A also results in the arrest of the cells in G1 phase of the cell cycle strongly suggesting that the inability of wild-type geminin to block DNA replication was the result of its degradation during G1. With respect to the relative abundance of GST-geminin to GST-geminin L26A, it is important to note that only 50% of the wild-type GST-geminin expressing cells are in G1 phase of the cell cycle whereas greater than 90% of the GST-geminin L26A expressing cells are in G1 phase of the cell cycle. This indicates that the difference between GST-geminin and GST-geminin L26A seen in Figure 6A ▶ significantly underestimates the increase in GST-geminin L26A levels relative to wild-type GST-geminin occurring specifically in G1 phase cells.
Discussion
The pattern of geminin expression observed in this study in normal tissues and malignancies was unexpected. Protein expression by immunohistochemistry is barely detectable in most normal tissues but is up-regulated in malignancies, a pattern reminiscent of that seen with other factors that are essential for cell cycle progression, eg, cyclins A, E, and B. 13,14 This pattern alone suggests that instead of being an inhibitor of the cell cycle, geminin might play an essential role in cell proliferation.
The patttern of expression of geminin in normal tissues is exactly the opposite of that seen with another cell cycle inhibitor, the cdk inhibitor p21/WAF1/CIP1. Unlike geminin, p21 expression in normal tissues was always associated with cell quiescence. This contrast suggests that geminin, unlike p21, might not be involved in suppressing the normal cell cycle. Further evidence that geminin may not be involved in suppressing cell proliferation comes from our observation in cultured cells that overexpression of wild-type geminin has no effect on cell cycle progression while overexpression of p21 blocks cells in the G1 phase of the cell cycle.
What could be the explanation for this apparent resistance of cancer cells in culture to geminin? Because geminin is a robust inhibitor of DNA replication in a cell-free system from vertebrates and because basal life processes like DNA replication are unlikely to have significantly diverged between Xenopus and humans, we do not think that the result means that the human DNA replication initiation process does not use Cdt1. Instead we favor the simpler explanation that the concentration of geminin obtained by the transient transfection experiment in G1 was insufficient to titrate out enough functional Cdt1 in cancer cells in culture since the overexpressed wild-type geminin is likely not stable during G1 phase of the cell cycle. Consistent with this idea, the overexpression of a stable form of geminin (L26A mutant) was able to arrest cells in G1 or early S phase (2N DNA content by fluorescence activated cell sorting (FACS)) suggesting that DNA replication initiation was inhibited by the mutant geminin. Based on this observation, we believe that the inability of wild-type geminin to inhibit DNA replication in these cell lines results from its proteolytic degradation in G1 phase of the cell cycle.
Regardless of the mechanism for the apparent resistance of normal and malignant cells to DNA replication inhibition by geminin, the results make it unlikely that geminin will be a classic tumor suppressor. In contrast, the obligate up-regulation of geminin as cells enter into proliferation, suggests that geminin is a positive factor for the cell cycle. Although we present no evidence toward this end, it is possible that the inactivation of Cdt1 after DNA replication has been initiated is essential for cell cycle progression and that free Cdt1 present in the cell outside of G1 might be detrimental to the cells. Alternatively, geminin might have an entirely unsuspected essential role in cell cycle progression that is independent of its ability to inhibit pre-RC assembly. This idea that geminin might have distinct positive and negative functions with respect to cell cycle progression is not unprecedented and has previously been proposed for the Cdk inhibitor p21. 20 Future experiments directed at inactivating geminin will shed some light on this possibility.
Footnotes
Address reprint requests to Anindya Dutta, Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, 78 Francis Street, Boston, MA 02115. E-mail: adutta@rics.bwh.harvard.edu.
Supported by National Institutes of Health grant CA89406 to A.D., a pre-doctoral fellowship to J.A.W. from the U.S. Army breast cancer research program, and the Brigham and Women’s Pathology Stanley Robbins Research Award (to A.P.W.).
J.A.W. and J.L.K. contributed equally to this work.
References
- 1.Evan GI, Vousden KH: Proliferation, cell cycle, and apoptosis in cancer. Nature 2001, 411:342-348 [DOI] [PubMed] [Google Scholar]
- 2.Moller MB: P27 in cell cycle control and cancer. Leuk Lymphoma 2000, 39:19-27 [DOI] [PubMed] [Google Scholar]
- 3.Sandhu C, Slingerland J: Deregulation of the cell cycle in cancer. Cancer Detect Prev 2000, 24:107-118 [PubMed] [Google Scholar]
- 4.Sherr CJ: Cancer cell cycles. Science 1996, 274:1672-1677 [DOI] [PubMed] [Google Scholar]
- 5.Dutta A, Bell SP: Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol 1997, 13:293-332 [DOI] [PubMed] [Google Scholar]
- 6.Kelly TJ, Brown GW: Regulation of chromosome replication. Annu Rev Biochem 2000, 69:829-880 [DOI] [PubMed] [Google Scholar]
- 7.Diffley JF: DNA replication: building the perfect switch. Curr Biol 2001, 11:R367-R370 [DOI] [PubMed] [Google Scholar]
- 8.McGarry TJ, Kirschner MW: Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 1998, 93:1043-1053 [DOI] [PubMed] [Google Scholar]
- 9.Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A: Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 2000, 290:2309-2312 [DOI] [PubMed] [Google Scholar]
- 10.Tada S, Li A, Maiorano D, Mechali M, Blow JJ: Repression of origin assembly in metaphase depends on inhibition of RLF- B/Cdt1 by geminin. Nat Cell Biol 2001, 3:107-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lygerou Z, Nurse P: Cell cycle. License withheld: geminin blocks DNA replication. Science 2000, 290:2271-2273 [DOI] [PubMed] [Google Scholar]
- 12.Lane D, Harlow E: Antibodies: A Laboratory Manual. 1998. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
- 13.Mashal RD, Lester S, Corless C, Richie JP, Chandra R, Propert KJ, Dutta A: Expression of cell cycle-regulated proteins in prostate cancer. Cancer Res 1996, 56:4159-4163 [PubMed] [Google Scholar]
- 14.Dutta A, Chandra R, Leiter LM, Lester S: Cyclins as markers of tumor proliferation: immunocytochemical studies in breast cancer. Proc Natl Acad Sci USA 1995, 92:5386-5390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wohlschlegel JA, Dwyer BT, Takeda DY, Dutta A: Mutational analysis of the Cy motif from p21 reveals sequence degeneracy and specificity for different cyclin-dependent kinases. Mol Cell Biol 2001, 21:4868-4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishitani H, Taraviras S, Lygerou Z, Nishimoto T: The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem 2001, 276:44905-44911 [DOI] [PubMed] [Google Scholar]
- 17.Cai K, Dynlacht BD: Activity and nature of p21(WAF1) complexes during the cell cycle. Proc Natl Acad Sci USA 1998, 95:12254-12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A: Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol 1996, 16:4673-4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Jackson PK, Kirschner MW, Dutta A: Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 1995, 374:386-388 [DOI] [PubMed] [Google Scholar]
- 20.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E: New functional activities for the p21 family of CDK inhibitors. Genes Dev 1997, 11:847-862 [DOI] [PubMed] [Google Scholar]