Abstract
Recently, we have discovered an endogenous cholinergic pathway for angiogenesis mediated by endothelial nicotinic acetylcholine receptors (nAChRs). Since angiogenesis plays a major role in wound repair, we hypothesized that activation of nAChRs with nicotine would accelerate wound healing in a murine excisional wound model. In genetically diabetic and control mice full-thickness skin wounds (0.8 cm) were created on the dorsum and topically treated over 7 days with either vehicle (phosphate-buffered saline, PBS) or nicotine (10−8 mol/L, 10−9 mol/L; each, n = 5). Wound size was measured over 14 days followed by resection, histological analysis, and quantitation of vascularity. In diabetic animals an agonist (epibatidine, 10−10 mol/L) or antagonist (hexamethonium, 10−4 mol/L) of nAChRs as well as the positive control basic fibroblast growth factor (bFGF, 25 μg/kg) were also tested. To further study the role of endothelial nAChRs in angiogenesis, we used an ex vivo vascular explant model. In diabetic mice wound healing was markedly impaired. Nicotine significantly accelerated wound healing as assessed by closure rate and histological score. The effects of nicotine were equal to bFGF and were mimicked by epibatidine and blocked by hexamethonium. Histomorphometry revealed increased neovascularization in animals treated with nicotine. Furthermore, capillary-like sprouting from vascular explants was significantly enhanced by nicotine. In conclusion, agonist-induced stimulation of nAChRs accelerates wound healing in diabetic mice by promoting angiogenesis. We have discovered a cholinergic pathway for angiogenesis that is involved in wound healing, and which is a potential target for therapeutic angiogenesis.
Impaired wound healing is a major source of morbidity in diabetic patients. 1 Poor outcome has, in part, been related to microvascular disease, peripheral neuropathy, altered blood cell rheology, glycation of cell membrane components, and impaired angiogenesis. Of these, angiogenesis plays a pivotal role as it is required for wound repair. 2 Neovascularization is regulated by a finely tuned balance of angiogenic and anti-angiogenic factors. Consequently, recent research has focused on the potential role of stimulators and inhibitors of angiogenesis.
A variety of angiogenic growth factors including platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), transforming growth factor α (TGF-α), insulin-like growth factor (IGF), and epidermal growth factor (EGF) have been shown to accelerate wound healing in diabetic animal models. 3-7 Conversely, inhibitors of angiogenesis, such as TNP-470, delay wound repair in diabetic mice. 8 In humans, recombinant PDGF improved wound healing in patients suffering from cutaneous diabetic ulcers or pressure sores. 9,10
We have recently demonstrated that nicotine, a major constituent of tobacco smoke, promotes angiogenesis in vitro and in vivo. 11 Nicotine increased proliferation and tube formation of endothelial cells in an in vitro assay. Furthermore, in a murine model of hind-limb ischemia, intramuscular injections of nicotine increased capillary density, enhanced collateral size and number, and augmented blood flow. 11 The angiogenic effects of nicotine are mediated by nicotinic acetylcholine receptors (nAChRs). Stimulation of these endothelial receptors induces changes in the growth, morphology, and function of cultured endothelial cells that are characteristic of the response to angiogenic factors. 12,13 Based on our previous work, we hypothesized that administration of nicotine might enhance angiogenesis and accelerate wound repair in a diabetic murine excisional wound model.
Materials and Methods
Animals
Eight- to twelve-week-old female BKS.Cg-m +/+ Leprdb mice (n = 65; 40 to 50 g, stock number 000642, Jackson Laboratories, Bar Harbor, ME) were used. Homozygous mutant mice are polyphagic, polydipsic and polyuric, and display similar metabolic perturbations as observed in human type II diabetes such as obesity, hyperglycemia, insulin resistance, and impaired wound healing. The genetic background is characterized by an autosomal recessive point mutation in the leptin receptor gene on chromosome 4. 14 This mutation promotes abnormal splicing creating a stop codon that shortens the intracellular domain of the receptor. 15 Age-matched, female control mice were obtained from the same colony but were heterozygous for the diabetes allele (n = 25; 20 to 25 g). All mice had free access to tap water and rodent chow and were housed individually in a temperature-controlled animal facility with a 12-hour light/dark cycle. The study protocol was approved by the Administrative Panel on Laboratory Animal Care of Stanford University.
Wound Model
Mice were anesthetized using an intraperitoneal injection of ketamine (80 mg/kg, Abbott Laboratories, Chicago, IL) and xylazine (16 mg/kg, Ben Venue Laboratories, Bedford, OH). The dorsal surface was shaved, washed with povidone-iodine solution (Professional Disposables Inc., Mount Vernon, NY), and rinsed with an alcohol swab (Kendall Health Care, Mansfield, MA). Then a disposable 0.8-cm diameter skin punch biopsy tool (Acuderm Inc., Fort Lauderdale, FL) was used to create a full-thickness excisional wound down to the fascia. To improve adherence of the wound dressing, tincture benzoin (Paddock Laboratories, Minneapolis, MN) was applied to the perimeter of the wound and allowed to dry. Finally, the wound was covered with a transparent, bioocclusive dressing (Opsite, Smith and Nephew Medical Limited, Hull, England) thereby creating a moist wound chamber environment. All animals were given 1.0 ml of 0.9% saline solution intraperitoneally at the end of the surgical procedure and cages were placed on a heating pad until mice fully recovered from anesthesia. Substances tested were superfused over the wound using a 30-gauge needle inserted through the Opsite dressing (total volume 0.1 ml). Solutions were prepared immediately before use and applied once daily for 7 days.
In Vivo Protocols
Effect of Nicotine on Wound Healing
The impact of nicotine (10−8 mol/L and 10−9 mol/L) on wound healing was analyzed in diabetic and control mice and compared to vehicle (phosphate-buffered saline, PBS). Each group consisted of five animals. Solutions of nicotine (free base) were prepared in PBS. Nicotine concentrations of 10−8 mol/L and 10−9 mol/L were chosen based on our previous observations that intramuscular injections of these doses stimulated the maximum angiogenic response in an ischemic hind-limb model. 11 In diabetic animals basic fibroblast growth factor (bFGF, 25 μg/kg; equals 1.5 × 10−9 mol/kg) was used as a positive control to compare the effects of nicotine with an established angiogenic growth factor.
Role of nAChRs in Wound Healing
In diabetic animals the effect of hexamethonium (10−4 mol/L), an antagonist of nAChRs, was tested in the presence of nicotine 10−8 mol/L (n = 5). In addition, we studied whether the effects of nicotine were mimicked by epibatidine (10−10 mol/L), an agonist with a 100-fold greater affinity for nAChRs than nicotine (n = 5). All drugs mentioned were purchased from Sigma Chemicals, St. Louis, MO.
Effect of Nicotine on Wound Vascularity
To determine the effects of nicotine on wound vascularity over time, the following experiment was performed. After wounding, diabetic mice (n = 30) were topically treated with either PBS or nicotine 10−8 mol/L (each, n = 15). Animals were euthanized at day 5, 9, or 14 (each, n = 5) and wounds were resected for further analysis. Before sacrifice, space-filling carboxylate-modified fluorescent microspheres (FluoSpheres, 0.2 μm, 5.3 × 1012 particles/ml, Molecular Probes, Eugene, OR) were injected into the left ventricle to visualize neovascularization in the healing wound.
Analysis of Wound Closure and Vascularity
Histomorphometry
Wound closure was documented with a digital camera (Nikon Coolpix 995, Nikon, Japan) on day 0, 5, 9, and 14. Images were analyzed using National Institute of Health (NIH) image 1.60 software by tracing the wound margin with a fine resolution computer mouse and calculating pixel area. A circular filter paper the same diameter as the original wound served as a reference on every image for assessment of wound healing. Measurements were performed in duplicate and mean values of consecutive tracings were computed and expressed as percentage of closure from the original wound.
Animals were euthanized on day 14 and the entire wound, as well as a ∼5 mm margin of surrounding normal skin, was excised to the level of the fascia and placed in 10% formalin. Tissue blocks were embedded in paraffin, and 5 μm sections from the mid-portion of the wound were stained with hematoxylin-eosin and Masson’s trichrome stain (Sigma, St. Louis, MO). Histological scoring was performed in a blinded fashion by two surgical pathologists according to the method described by Greenhalgh et al. 3 Each slide was given a histological score ranging from 1 to 12: 1–3, none to minimal cell accumulation, no granulation tissue or epithelial travel; 4–6, thin, immature granulation that is dominated by inflammatory cells but has few fibroblasts, capillaries, or collagen deposition, minimal epithelial migration; 7–9, moderately thick granulation tissue, can range from being dominated by inflammatory cells to more fibroblasts and collagen deposition, extensive neovascularization, epithelium can range from minimal to moderate migration; 10–12, thick, vascular granulation tissue dominated by fibroblasts and extensive collagen deposition, epithelium partially to completely covering the wound.
Fluorescence Microscopic Evaluation of Wound Vascularity
Resected wounds from diabetic mice sacrificed at different time-points (day 5, 9, or 14) were embedded in Tissue Tek (Sakura Finetek Inc., Torrance, CA) and stored at −80°C. Cross-sections (10 μm) were made from the mid-portion of each wound using a cryostat. Tissue slides were fixed in acetone and analyzed in blinded fashion by fluorescence microscopy (magnification, ×200; Laborlux S, Leitz, Wetzlar, Germany). Slides were scanned from the margin to the center of the wound. For each slide, three images with the greatest signal intensity from different areas of the cross-section were captured with a digital camera (Nikon Coolpix 995). Image analysis software (Image-Tool 2.02, University of Texas Health Sciences Center at San Antonio (UTHSCSA),San Antonio, TX) was used to quantitate fluorescence intensity. The mean percentage of fluorescent pixels of three images served as an index of angiogenic response.
In Vitro Protocol
A modified angiogenesis assay previously described by Chen et al 16 was used to examine the effects of nicotine on angiogenesis in vitro. Briefly, thoracic aortae were harvested from control mice and diabetic animals (each, n = 10). After removal of fatty tissue and the adventitia under microscopic visualization the aorta was cut into thin rings. The ends of each aorta were discarded because they were manipulated during preparation and cutting. Vascular segments (∼2.0 mm2) were placed in four chamber slides (Nalge Nunc Int. Corp., Naperville, IL) covered with growth factor reduced Matrigel (100 μl/well, Becton Dickinson, Franklin Lakes, NJ). The endothelium faced the bottom of each chamber. Explants were sealed in place with an overlay of 20 μl of Matrigel. Rosswell Park Memorial Institute medium (RPMI 1640, GIBCO, Carlsbad, CA) containing 2% fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 μg/ml) was added to each chamber (750 μl/well). Tissues were incubated for 8 days at 37°C in a 95% O2/5% CO2 atmosphere, culture medium was changed daily. Explants were either treated with vehicle (medium), or medium containing nicotine (10−8 mol/L).
After 2 to 3 days, capillary-like sprouting into the Matrigel layer was observed. The endothelial origin of the outgrowths was confirmed by CD31 staining (rat anti-mouse, Caltag Laboratories, Burlingame, CA). Sprouting was analyzed under an inverted microscope (magnification, ×50; Labovert FS, Leitz, Wetzlar, Germany) after 4, 6, and 8 days, and digitalized images were stored on a computer. Image analysis was performed using Image-Tool 2.02 software by modifying a method described by Stiffey-Wilusz et al. 17 After system calibration and image pre-processing (smoothing with Gaussian filter) thresholding was performed until all sprouts were clearly outlined. Thresholding converted the grayscale picture into a binary image containing black and white pixels. Sprouting was calculated as the sum of black pixels in the traced area. The explant itself was subtracted from the pixel calculation. Pixel counts were then related to the explant area and expressed as kilopixels/mm2. All measurements were performed in duplicate and mean values were computed.
Statistical Analysis
All data are given as mean ± SEM. Statistical significance was tested using analysis of variance or two-tailed Student’s t-test for unpaired or paired comparisons between groups. Pearson correlation coefficients were calculated when indicated. Statistical significance was accepted at P < 0.05 after correcting for multiple comparisons with the Bonferroni procedure.
Results
Effect of Nicotine on Wound Healing
At day 14 there was no difference in wound closure between vehicle- and nicotine-treated control mice (90.4 ± 2.9% vs. 94.3 ± 1.0% vs. 95.9 ± 0.9%, vehicle vs. nicotine 10−9 mol/L vs. nicotine 10−8 mol/L) (Figure 1a) ▶ . The average histological score of wounds was similar in control mice (10.7 ± 1.0 vs. 11.0 ± 1.0 vs. 11.0 ± 0.6, vehicle vs. nicotine 10−9 mol/L or 10−8 mol/L (Figure 1c) ▶ .
Figure 1.
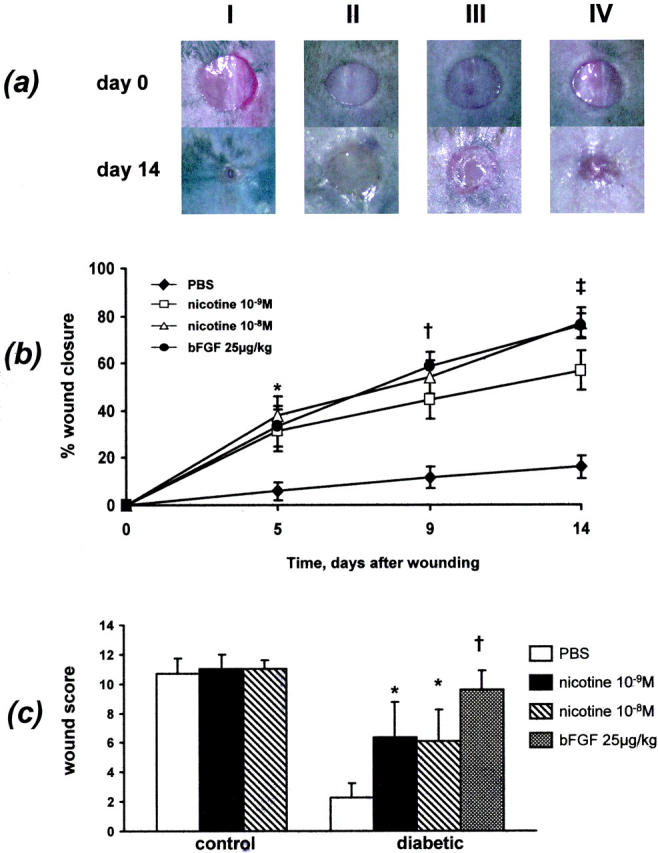
Effects of nicotine on wound closure. a: Macroscopic aspect of wounds under different treatment. I, control animal treated with vehicle; II, diabetic animal treated with vehicle; III, diabetic animal treated with nicotine 10−8 mol/L; IV, diabetic animal treated with bFGF 25 μg/kg. b: Wound closure analysis. In diabetic mice nicotine (10−8 mol/L or 10−9 mol/L) dose dependently accelerated wound closure compared to vehicle (each group, n = 5). The effect of nicotine on wound healing was comparable to that of bFGF. c: Histological grading of resected wounds. Nicotine-treated diabetic mice had a significantly higher histological score compared to vehicle (PBS)-treated animals (n = 5). * P < 0.05, †, P < 0.01, ‡, P < 0.001 (vs. PBS)
In contrast, wound healing was markedly impaired in diabetic animals. Vehicle-treated mice only achieved 15.5 ± 4.4% wound closure after 14 days (Figure 1, a and b) ▶ . This observation corresponded with a low histological score of 2.2 ± 1.0 (Figure 1c) ▶ . Application of nicotine (10−8 mol/L or 10−9 mol/L) significantly accelerated wound healing after 5 days. By day 14, this effect was most significant (15.5 ± 4.4% vs. 56.7 ± 6.2% vs. 77.0 ± 6.3%, vehicle vs. nicotine 10−9 mol/L and 10−8 mol/L, P < 0.001, Figure 1, a and b ▶ ). Consistent with these findings the histological score of wounds from mice treated with nicotine was significantly higher (2.2 ± 1.0 vs. 6.3 ± 2.4 vs. 6.1 ± 2.1, vehicle vs. nicotine 10−9 mol/L and 10−8 mol/L, P < 0.05, Figure 1c ▶ ). The degree of wound closure elicited by nicotine was similar to the positive control bFGF (Figure 1, a and b) ▶ . By day 14, wound closure in bFGF-treated animals was 75.7 ± 5.3% and histological score was 9.6 ± 1.3 (Figure 1, a–c) ▶ .
Histology further revealed increased cellular infiltration and granulation tissue in nicotine-treated wounds (Figure 2) ▶ . In addition, nicotine-augmented collagen deposition as shown by trichrome staining. Finally, nicotine increased the number of capillary vessels per high power field.
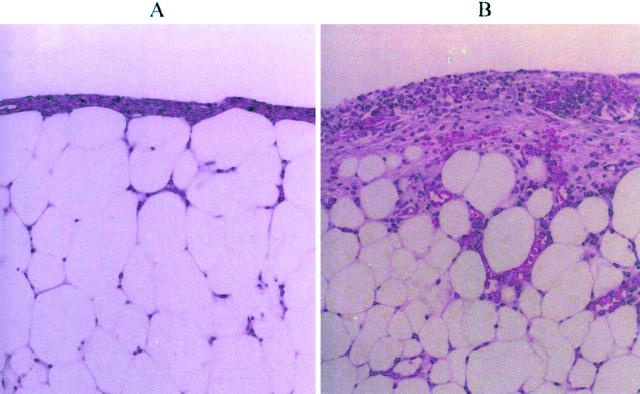
Figure 2.
Effects of nicotine on wound histology (day 14). A: Diabetic mouse treated with vehicle. The wound bed shows adipose tissue covered by a thin layer of collagen. No granulation tissue or epithelium is observed (H&E stain, ×10). B: Diabetic mouse treated with nicotine (10−8 mol/L). Notice a thick layer of granulation tissue and infiltration of upper adipose tissue by neutrophils and microvessels (H&E stain, ×10).
Role of nAChRs in Wound Healing
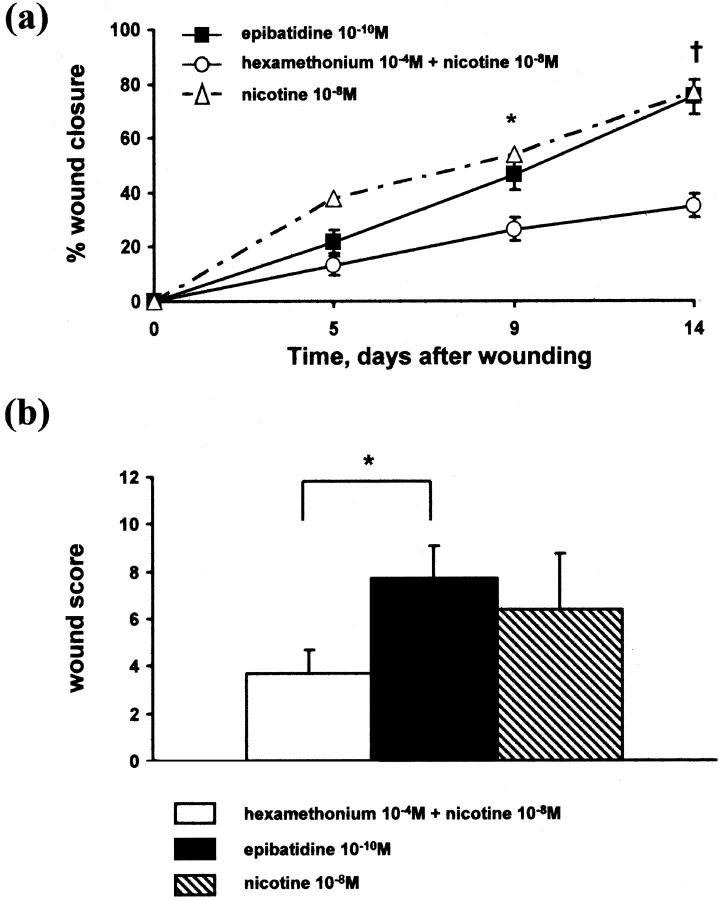
Hexamethonium (10−4 mol/L) significantly inhibited the effect of nicotine (10−8 mol/L) on wound healing (Figure 3a) ▶ . Wound closure achieved after 14 days was only 35.0 ± 4.4% compared to 77.0 ± 6.3% in animals treated with nicotine alone (P < 0.01). The histological score with hexamethonium treatment also tended to be lower compared to nicotine treatment alone (3.7 ± 1.0 vs. 6.1 ± 2.1, Figure 3b ▶ ).
Figure 3.
Role of nAChRs in nicotine-induced wound healing. a: Wound closure. Hexamethonium (10−4 mol/L) significantly attenuated nicotine-induced wound healing whereas epibatidine (10−10 mol/L) mimicked the effects of nicotine (n = 5). b: Histological score. There was a significant difference in the histological score of wounds from diabetic mice treated with epibatidine (10−10 mol/L) compared to animals treated with hexamethonium (10−4 mol/L) and nicotine (10−8 mol/L) (n = 5). *, P < 0.05, †, P < 0.01
Epibatidine (10−10 mol/L) significantly accelerated wound healing in diabetic animals (Figure 3a) ▶ . Wound closure rate after 14 days was 75.0 ± 6.2%, histological score was 7.7 ± 1.4 (Figure 3b) ▶ . Overall, the impact of epibatidine on wound healing was similar to either nicotine 10−8 mol/L or bFGF.
Effect of Nicotine on Wound Vascularity
Wound angiogenesis was analyzed in 30 diabetic mice treated with either vehicle (PBS) or nicotine 10−8 mol/L (each, n = 15) (Figure 4) ▶ . Animals were sacrificed on day 5, 9, or 14 (each, n = 5) after injection of fluorescent microspheres to visualize neovascularization in the resected wounds. Again, nicotine accelerated the degree of wound healing compared to mice treated with vehicle and this effect became significant by day 9 after wounding (day 5: 9.9 ± 3.1% vs. 20.4 ± 4.3%, p = n.s.; day 9: 18.7 ± 4.3% vs. 49.1 ± 6.5%, P = 0.013; day 14: 24.2 ± 5.3% vs. 64.5 ± 7.8%, P = 0.003).
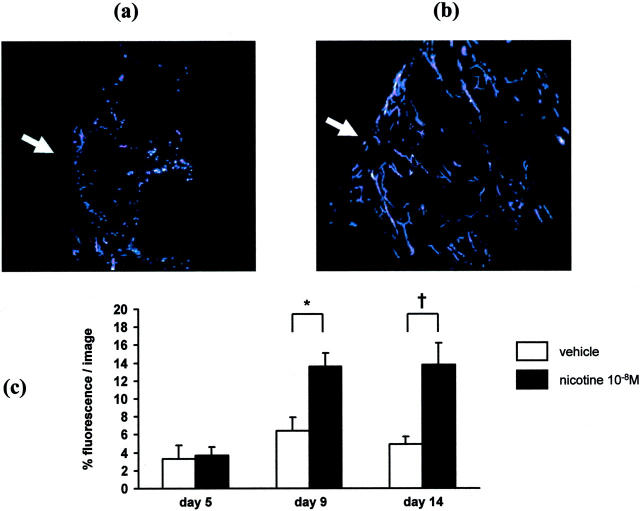
Figure 4.
Effects of nicotine on wound vascularity. a: Wound vascularity in vehicle (PBS)-treated diabetic mouse after 14 days. b: Wound vascularity in nicotine (10−8 mol/L)-treated diabetic mouse after 14 days. Note the centripetal spreading of newly formed blood vessels into the center of the wound (arrow indicates the wound margin; magnification, ×200). c: Effect of nicotine on wound vascularity over time. Nicotine significantly enhanced the amount of blood vessels in the wounded area as assessed by microsphere fluorescence (each bar, n = 5). *, P < 0.05, †, P < 0.01
Nicotine significantly enhanced wound vascularity as assessed by the percentage of the pixels in each image that were fluorescent (day 5: 3.31 ± 1.51% vs. 3.66 ± 0.97%, p = n.s.; day 9: 6.39 ± 1.57% vs. 13.62 ± 1.47%, P = 0.015; day 14: 4.94 ± 0.82% vs. 13.78 ± 2.43%, P = 0.007, Figure 4, a–c ▶ ). The percentage of fluorescence significantly correlated with the degree of wound closure in animals sacrificed after 9 or 14 days (r = 0.79, P = 0.002 and r = 0.63, P < 0.05).
Effects of Nicotine on Angiogenesis in Vitro
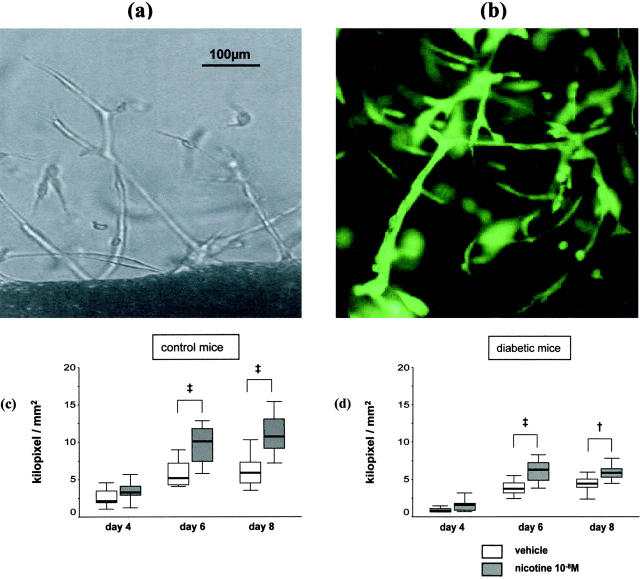
Capillary-like sprouting (CLS) of thoracic aortic explants was analyzed in control animals and diabetic mice (each, n = 10) (Figure 5) ▶ . Two segments were taken from each animal and treated once daily with either vehicle (medium) or medium containing nicotine 10−8 mol/L. The average explant area was 2.2 ± 0.2 mm2 and did not differ between treatment groups. By day 4, CLS into the Matrigel layer was documented (Figure 5a) ▶ . CD31 staining confirmed the endothelial origin of the outgrowths (Figure 5b) ▶ . Nicotine significantly enhanced CLS of vascular explants from control animals by day 6 of treatment compared to vehicle (day 4: 3.6 ± 0.5 vs. 2.6 ± 0.4 kilopixel/mm2, P = 0.095; day 6: 9.8 ± 0.6 vs. 5.7 ± 0.5 kilopixel/mm2, P < 0.001; day 8: 11.1 ± 0.9 vs. 6.2 ± 0.7 kilopixel/mm2, P < 0.001, Figure 5c ▶ ). Outgrowth was markedly impaired in vascular explants from diabetic animals that were treated with vehicle (P < 0.05 vs. vehicle-treated explants from control animals for all time-points). Again, treatment with nicotine significantly enhanced CLS from day 6 on (Figure 5d) ▶ .
Figure 5.
Effects of nicotine on angiogenesis in vitro. a: Vascular explant of a thoracic aorta from a diabetic animal after a 6-day treatment with medium containing nicotine (10−8 mol/L) showing capillary-like sprouting into the Matrigel layer from the edges of the explant. b: Capillary-like sprouting from a thoracic aorta of a diabetic animal after 6 days treatment with nicotine 10−8 mol/L (CD31 staining). c: Capillary-like sprouting of thoracic aortic explants from control mice (n = 10). d: Capillary-like sprouting of thoracic aortic explants from diabetic mice (n = 10).
Discussion
The major findings of this study are as follows: nicotine accelerates wound healing in diabetic mice as assessed by the degree of wound closure and histological score; the effects of nicotine on wound healing are mediated by nAChRs as the nAChR agonist epibatidine mimics, whereas the antagonist hexamethonium blocks the action of nicotine; and nicotine-induced wound healing is mediated, at least in part, by its effects to increase wound angiogenesis.
Angiogenesis is a tightly regulated, dynamic process playing an essential role in wound healing. Proteases released from activated endothelial cells lead to degradation of extracellular matrix proteins paving the way for endothelial cells to migrate into the interstitial space. Recruited cells then proliferate and differentiate into mature blood vessels that sprout into the wound area. 1 In later stages of the wound healing process deposition of collagen in the granulation tissue reduces the density of blood vessels leading to scar formation. The angiogenic response is initiated, maintained, and terminated by a variety of factors such as cytokines, growth factors, matrix metalloproteinases, and adhesion molecules, to name but a few.
We recently discovered that nicotine is a potent angiogenic agent both in vivo and in vitro. 11 Nicotine stimulated migration, proliferation, and tube formation of endothelial cells in vitro, all of which are major steps of angiogenesis. 18 The findings in the present study are consistent with our previous work and reports from other investigators showing that nicotine stimulates DNA synthesis and proliferation of endothelial cells in vitro. 13,19 The mechanisms by which nicotine induces angiogenesis are likely multifactorial. Nicotine has been shown to directly alter the activity of endothelial nitric-oxide synthase and the release of prostacyclin in endothelial cells lines derived from dogs and humans. 20-22 Furthermore, nicotine is known to induce changes in the release of growth factors such as of bFGF and TGF-β1. 23,24 Most recently, it was shown that nicotine and its major metabolite cotinine up-regulate the expression of vascular endothelial growth factor (VEGF) in endothelial cells. 25 Changes in the expression of VEGF elicited by nicotine may help to explain the effects of this agent on atherosclerotic plaque neovascularization and tumor angiogenesis previously reported by our group.
In this study the nAChR agonist, nicotine, significantly accelerated wound healing in diabetic mice. Nicotinic AChRs are widely expressed in human skin and are representatives of a diverse superfamily of pentameric, ligand-gated ion channels. 26 Combinations of the various subunits offer a wide spectrum in both structure and function of the receptor. The endogenous ligand of the receptor is the nerve transmitter acetylcholine, which is abundant in human skin. Endothelial cells are also capable of making acetylcholine, 12,27 which may act in an autocrine or paracrine manner to stimulate endothelial cell proliferation and angiogenesis (unpublished observations from our group).
The effect of nicotine to accelerate wound healing in diabetic mice appears to be mediated through nAChRs, as its effects were mimicked by the nAChR agonist epibatidine, and inhibited by the nAChR antagonist, hexamethonium. Furthermore, the effect of nicotine is mediated, at least in part, by stimulation of wound angiogenesis. Histology revealed greater vascularity in nicotine-treated wounds. This was confirmed by the fluorescent microsphere studies. These microspheres (0.2 μm) fill the microvasculature; accordingly, the intensity of the fluorescent signal in the wound is a direct reflection of the intravascular space in the tissue. As further confirmation of the angiogenic effects of nicotine, we studied capillary sprouting from vascular explants ex vivo. Capillary sprouting was impaired in vascular segments of diabetic mice. Nicotine significantly increased capillary sprouting, consistent with an angiogenic effect. To date, transdermal applications of nicotine have been predominantly used for tobacco cessation, and have been under investigation for neurological disorders such as Alzheimer’s or Parkinson’s disease. 28-30 In addition, nicotine has been applied to the treatment of patients suffering from recurrent aphthous ulcers or active ulcerative colitis. 31,32 Case reports have described the use of nicotine for pemphigus and pyoderma gangrenosum. 33,34
From a historical point of view, leaves or extracts of tobacco plants have been used in the management of wound treatment by shamans and native healers. The French ambassador to Portugal, Jean Nicot de Villemain, introduced the plant in Europe and wrote about the medicinal properties of tobacco in 1560. 35 He described tobacco as a panacea and successfully treated an acquaintance’s face wound with the plant. His name was later given to the tobacco plant (Nicotiana tabacum) and to the stimulating alkaloid, nicotine.
Conversely, apart from these isolated reports and historical anecdotes, there has been a consensus in the medical and scientific community that tobacco use impairs wound healing. Indeed, a recently published clinical trial demonstrates that preoperative smoking intervention significantly reduces the occurrence of postoperative wound-related complications in smokers undergoing elective surgery. 36 This study would appear to conflict with our observations. However, nicotine is but one of over 4000 chemical constituents of cigarette smoke. The physiological effects of nicotine and cigarette smoke may, in fact, be very distinct. In a study of 80 healthy volunteers and 6 patients with peripheral vascular disease nicotine, chewing gum (2 mg chewed over 15 minutes) significantly increased cutaneous blood flow and skin temperature. 37 By contrast, in 24 smokers, a decrease in cutaneous blood flow and skin temperature was observed after they smoked two cigarettes each containing 1.1 mg nicotine. 38
Apart from enhancement of wound angiogenesis, other mechanisms may contribute to nicotine-induced wound healing. Nicotinic AChRs are expressed by epidermal keratinocytes and activation of these receptors by short-term exposure to nicotine or cholinergic agonists has been shown to stimulate keratinocyte proliferation, migration, and differentiation all of which are critical steps in the re-epithelialization of healing skin. 39,40 Furthermore, nicotine, and nAChRs agonists like epibatidine are well known for their potent anti-nociceptive and analgesic properties. 41 The level of pain and distress may well have an impact on wound healing as shown in animal studies in which nicotine prolonged tail-flick withdrawal latencies in rats challenged with noxious radiant heat stimuli. 42 In these experiments, nicotine-accelerated wound healing of experimentally induced blisters, an effect attributed to the analgesic properties of nicotine.
To conclude, this paper is the first to demonstrate that nicotine enhances wound healing in genetically diabetic mice. The effects on wound healing are, in part, related to the stimulation of angiogenesis by nicotine, an effect which is mediated by nAChRs. Therapeutic stimulation of these receptors may represent a novel approach in the treatment of wounds, particularly in diabetic patients.
Footnotes
Address reprint requests to John P. Cooke, M.D., Ph.D., Division of Cardiovascular Medicine, Stanford University School of Medicine, 300 Pasteur Drive, Stanford, CA 94305. E-mail: john.cooke@stanford.edu.
Supported by grants from the National Heart, Lung and Blood Institute (R01 HL- HL63685, R01 HL00204, P01 AG18784, and PO1AI50153), the Tobacco Related Disease Research Program (7RT-0128), and the German Research Council (Ja 1043/1–1). Research described in this article was supported in part by Philip Morris Incorporated.
Dr. Cooke is an Established Investigator of the American Heart Association. Stanford University owns a patent on the use of nicotine for therapeutic angiogenesis. Two of the authors (J.J.J. and J.P.C.) are inventors of this patent, and may receive royalties from the license.
J. Jacobi and J.J. Jang contributed equally to this work.
References
- 1.Singer AJ, Clark RA: Cutaneous wound healing. N Engl J Med 1999, 341:738-746 [DOI] [PubMed] [Google Scholar]
- 2.Arnold F, West DC: Angiogenesis in wound healing. Pharmacol Ther 1991, 52:407-422 [DOI] [PubMed] [Google Scholar]
- 3.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R: PDGF and FGF stimulate healing in the genetically diabetic mouse. Am J Pathol 1990, 136:1235-1246 [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RL, Breeden MP, Greenhalgh DG: PDGF and TGF-α act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res 1994, 56:562-570 [DOI] [PubMed] [Google Scholar]
- 5.Tsuboi R, Rifkin DB: Recombinant basic fibroblast growth factor stimulates wound healing in healing-impaired db/db mice. J Exp Med 1990, 172:245-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuboi R, Shi CM, Sato C, Cox GN, Ogawa H: Co-administration of insulin-like growth factor (IGF)-I and IGF-binding protein-1 stimulates wound healing in animal models. J Invest Dermatol 1995, 104:199-203 [DOI] [PubMed] [Google Scholar]
- 7.Nagy A, Nagashima H, Cha S, Oxford GE, Zelles T, Peck AB, Humphreys-Beher MG: Reduced oral wound healing in the NOD mouse model for type 1 auto-immune diabetes and its reversal by epidermal growth factor supplementation. Diabetes 2001, 50:2100-2104 [DOI] [PubMed] [Google Scholar]
- 8.Klein SA, Bond SJ, Gupta SC, Yacoub OA, Anderson GL: Angiogenesis inhibitor TNP-470 inhibits murine cutaneous wound healing. J Surg Res 1999, 82:268-274 [DOI] [PubMed] [Google Scholar]
- 9.Steed DL: Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. J Vasc Surg 1995, 21:71-81 [DOI] [PubMed] [Google Scholar]
- 10.Robson MC, Phillips LG, Thomason A, Robson LE, Pierce GF: Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet 1992, 339:23-25 [DOI] [PubMed] [Google Scholar]
- 11.Heeschen C, Jang JJ, Pathak A, Kaji S, Hu BS, Tsao P, Johnson F, Cooke JP: Nicotine is an agent of angiogenesis: a pathophysiological link to cancer and atherosclerosis. Nat Med 2001, 7:833-839 [DOI] [PubMed] [Google Scholar]
- 12.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM: Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther 1998, 287:435-439 [PubMed] [Google Scholar]
- 13.Villablanca AC: Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol 1998, 84:2089-2098 [DOI] [PubMed] [Google Scholar]
- 14.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL: Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 1996, 271:994-996 [DOI] [PubMed] [Google Scholar]
- 15.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM: Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379:632-635 [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Cartwright J, Jr, Li Z, Lou S, Nguyen HH, Gotto AM, Jr, Henry PD: Inhibitory effects of hypercholesterolemia and Ox-LDL on angiogenesis-like endothelial growth in rabbit aortic explants: essential role of basic fibroblast growth factor. Arterioscler Thromb Vasc Biol 1997, 17:1303-1312 [DOI] [PubMed] [Google Scholar]
- 17.Stiffey-Wilusz J, Boice JA, Ronan J, Fletcher AM, Anderson MS: An ex vivo angiogenesis assay utilizing commercial porcine carotid artery: modification of the rat aortic ring assay. Angiogenesis 2001, 4:3-9 [DOI] [PubMed] [Google Scholar]
- 18.Folkman J, Shing Y: Angiogenesis. J Biol Chem 1992, 267:10931-10934 [PubMed] [Google Scholar]
- 19.Carty CS, Huribal M, Marsan BU, Ricotta JJ, Dryjski M: Nicotine and its metabolite cotinine are mitogenic for human vascular smooth muscle cells. J Vasc Surg 1997, Apr;25:682-688 [DOI] [PubMed] [Google Scholar]
- 20.Tonnessen BH, Severson SR, Hurt RD, Miller VM: Modulation of nitric-oxide synthase by nicotine. J Pharmacol Exp Ther 2000, 295:601-606 [PubMed] [Google Scholar]
- 21.Zhang S, Day I, Ye S: Nicotine-induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis 2001, 154:277-283 [DOI] [PubMed] [Google Scholar]
- 22.Boutherin-Falson O, Blaes N: Nicotine increases basal prostacyclin production and DNA synthesis of human endothelial cells in primary cultures. Nouv Rev Fr Hematol 1990, 32:253-258 [PubMed] [Google Scholar]
- 23.Cucina A, Corvino V, Sapienza P, Borrelli V, Lucarelli M, Scarpa S, Strom R, Santoro-D’Angelo L, Cavallaro A: Nicotine regulates basic fibroblastic growth factor and transforming growth factor β1 production in endothelial cells. Biochem Biophys Res Commun 1999, 257:306-312 [DOI] [PubMed] [Google Scholar]
- 24.Carty CS, Soloway PD, Kayastha S, Bauer J, Marsan B, Ricotta JJ, Dryjski M: Nicotine and cotinine stimulate secretion of basic fibroblast growth factor and effect expression of matrix metalloproteinases in cultured human smooth muscle cells. J Vasc Surg 1996, 24:927-934 [DOI] [PubMed] [Google Scholar]
- 25.Conklin BS, Zhao W, Zhong DS, Chen C: Nicotine and cotinine up-regulate vascular endothelial growth factor expression in endothelial cells. Am J Pathol 2002, 160:413-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grando SA: Receptor-mediated action of nicotine in human skin. Int J Dermatol 2001, 40:691-693 [DOI] [PubMed] [Google Scholar]
- 27.Kawashima K, Watanabe N, Oohata H, Fujimoto K, Suzuki T, Ishizaki Y, Morita I, Murota S: Synthesis and release of acetylcholine by cultured bovine arterial endothelial cells. Neurosci Lett 1990, 119:156-158 [DOI] [PubMed] [Google Scholar]
- 28.Tonnesen P, Norregaard J, Simonsen K, Sawe U: A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med 1991, 325:311-315 [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Arrieta JM, Rodriguez JL, Sanz F: Nicotine for Alzheimer’s disease. Cochrane Database Syst Rev 2000, (2):CD001749. [DOI] [PubMed] [Google Scholar]
- 30.Vieregge A, Sieberer M, Jacobs H, Hagenah JM, Vieregge P: Transdermal nicotine in PD: a randomized, double-blind, placebo-controlled study. Neurology 2001, 57:1032-1035 [DOI] [PubMed] [Google Scholar]
- 31.Scheid P, Bohadana A, Martinet Y: Nicotine patches for aphthous ulcers due to Behcet’s syndrome. N Engl J Med 2000, 343:1816-1817 [DOI] [PubMed] [Google Scholar]
- 32.Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, Sawe U: Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994, 330:811-815 [DOI] [PubMed] [Google Scholar]
- 33.Grando SA, Dahl MV: Nicotine and pemphigus. Arch Dermatol 2000, 136:1269. [DOI] [PubMed] [Google Scholar]
- 34.Kanekura T, Usuki K, Kanzaki T: Nicotine for pyoderma gangrenosum. Lancet 1995, 345:1058. [DOI] [PubMed] [Google Scholar]
- 35.Haas LF: Jean Nicot 1530–1600. J Neurol Neurosurg Psychiatry 1992, 55:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Møller AM, Villebro N, Pedersen T, Tønnesen H: Effect of preoperative smoking intervention on postoperative complications: a randomized clinical trial. Lancet 2002, 359:114-117 [DOI] [PubMed] [Google Scholar]
- 37.Usuki K, Kanekura T, Aradono K, Kanzaki T: Effects of nicotine on peripheral cutaneous blood flow and skin temperature. J Dermatol Sci 1998, 16:173-181 [DOI] [PubMed] [Google Scholar]
- 38.Bornmyr S, Svensson H: Thermography and laser-Doppler flowmetry for monitoring changes in finger skin blood flow upon cigarette smoking. Clin Physiol 1991, 11:135-141 [DOI] [PubMed] [Google Scholar]
- 39.Grando SA, Horton RM, Pereira EF, Diethelm-Okita BM, George PM, Albuquerque EX, Conti-Fine BM: A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol 1995, 105:774-781 [DOI] [PubMed] [Google Scholar]
- 40.Grando SA, Horton RM, Mauro TM, Kist DA, Lee TX, Dahl MV: Activation of keratinocyte nicotinic cholinergic receptors stimulates calcium influx and enhances cell differentiation. J Invest Dermatol 1996, 107:412-418 [DOI] [PubMed] [Google Scholar]
- 41.Decker MW, Meyer MD: Therapeutic potential of neuronal nicotinic acetylcholine receptor agonists as novel analgesics. Biochem Pharmacol 1999, 58:917-923 [DOI] [PubMed] [Google Scholar]
- 42.Westerman RA, Carr RW, Delaney CA, Morris MJ, Roberts RG: The role of skin nociceptive afferent nerves in blister healing. Clin Exp Neurol 1993, 30:39-60 [PubMed] [Google Scholar]