Abstract
Oligoastrocytomas are heterogeneous tumors that have molecular features that overlap with either oligodendrogliomas or astrocytomas. Differences in the frequency of chromosomal losses of 1p and 19q in oligodendrogliomas are related to tumor location, with a low rate of allelic loss in tumors of the temporal and a high rate in tumors of the frontal, parietal, and occipital lobes. To test the possibility of regional molecular heterogeneity in oligoastrocytoma, we examined a series of 203 gliomas including 68 oligoastrocytomas and two control groups of 73 oligodendrogliomas and 62 astrocytomas for allelic losses of chromosomal arms 1p and 19q, and TP53 mutations, and compared these data with tumor localization. Common molecular alterations were found in oligodendrogliomas and oligoastrocytomas arising in extratemporal sites. In respect to the molecular parameters analyzed, temporal oligoastrocytomas were either indistinguishable from astrocytoma or similar to temporal oligodendrogliomas. Oligodendroglial neoplasms can thus be separated into three molecular subsets, two of which include lesions with the morphological features of oligodendrogliomas and oligoastrocytomas and one resembling temporal oligoastrocytoma. Molecular subclassification thus unifies previous findings about prognosis, behavior, response to therapy, genotype, and location in oligodendroglial tumors.
Oligodendroglial tumors, including oligodendrogliomas and oligoastrocytomas, constitute between 5% and 18% of all primary human brain tumors. 1-4 Advances in diagnostic recognition and clinical management have succeeded in a dramatic improvement of the clinical outcome of patients with anaplastic oligodendroglioma. Distinct molecular subsets of oligodendroglial tumors also have highly differential responses to therapy. 5,6 Therefore, the further identification and evaluation of diagnostic parameters with high prognostic power is of great interest in oligodendroglial neoplasms.
Somatic deletions on the short arm of chromosome 1 [loss of heterozygosity (LOH) 1p] and the long arm of chromosome 19 (LOH 19q) are typical of oligodendroglioma and oligoastrocytoma. 7-12 To date, however, neither the 1p nor 19q gene has been identified. Although LOH 19p is also frequently seen in anaplastic astrocytomas and may be associated with tumor progression, LOH 1p is more closely associated with oligodendroglial gliomas. 13 The combination of LOH 1p and LOH 19q is observed only rarely in gliomas other than oligodendroglioma and oligoastrocytoma. 11
Oligoastrocytomas exhibit both astrocytic and oligodendroglial morphologies. 14 Because of the rather vague criteria for defining oligoastrocytoma, the incidence of oligoastrocytoma varies considerably between different studies. Based on molecular findings, oligoastrocytomas occupy an intermediate position between oligodendrogliomas and astrocytomas. From 30 to 70% of oligoastrocytomas show LOH 1p and LOH 19q 8-12,15 thus genetically resembling oligodendrogliomas, whereas ∼30% show mutations in the TP53 gene or LOH 17p 10,16 suggesting a relation to astrocytomas. Significantly, LOH 1p and LOH 19q are inversely associated with TP53 mutations. 16 On microdissection, identical genetic alterations have been identified in astrocytic and oligodendroglial portions, indicating a clonal origin of oligoastrocytomas. 11 Although one might assume that in oligoastrocytomas the astrocytic component implies a less favorable prognosis, most studies could not confirm differences in outcome between oligodendroglioma and oligoastrocytoma. 17-19
LOH 1p and LOH 19q have been associated with chemosensitivity and durable responses to chemotherapy in patients with anaplastic oligodendrogliomas. 5,6 In addition, patients with oligodendrogliomas and anaplastic oligodendrogliomas exhibiting these molecular lesions have longer overall survival from the time of diagnosis. 5,6,20-22 Further, LOH 1p may indicate a better response to chemosensitivity and prolonged survival in a small group of astrocytomas and oligoastrocytomas. In oligoastrocytomas, a subset has been shown to respond favorably to procarbacine lomustine (CCNU)/vincristine-based chemotherapy. 23,24
Recently, an association was identified between the incidence of genetic lesions in oligodendroglioma and tumor location. 25 Anaplastic oligodendrogliomas located in the frontal, parietal, and occipital lobes were significantly more likely to harbor LOH 1p and LOH 19q, than those arising in the temporal lobe, insula, and diencephalon. In addition, LOH1p and LOH 19q were significantly correlated with a bilateral growth pattern. Because of the strong predictive value of LOH 1p and LOH 19q, these findings may argue for differential therapy approaches in patients with oligodendrogliomas depending on gross tumor localization. The morphological and genetic similarities between oligodendrogliomas and oligoastrocytomas naturally raise the question of whether molecular subsets of oligoastrocytomas correlate with tumor location. The present study was thus conducted to clarify and extend molecular subclassification of oligoastrocytomas. To this end, we analyzed a series of 203 gliomas, including 68 oligoastrocytomas and two control groups of 73 oligodendrogliomas and 62 astrocytomas, for LOH 1p, LOH 19q, and TP53 mutations, with respect to tumor location.
Materials and Methods
Tissue Samples
Two hundred three gliomas, consisting of 37 oligodendrogliomas World Health Organization (WHO) grade II (O II), 36 anaplastic oligodendrogliomas WHO grade III (O III), 38 oligoastrocytomas WHO grade II (OA II), 30 anaplastic oligoastrocytomas WHO grade III (OA III), 28 astrocytomas WHO grade II (A II), and 34 anaplastic astrocytomas WHO grade III (A III), and corresponding blood samples were obtained from patients treated at the Charité Hospital in Berlin, the Helios Klinikum in Buch, the University Hospital in Würzburg, the Neukölln Hospital in Berlin, the University Hospital in Bonn, the University Hospital in Tübingen and the Massachusetts General Hospital in Boston between 1992 and 2001. Because no clearly defined parameters for the diagnosis of oligoastrocytoma have been established, we required for this diagnosis the lesser represented component to amount to at least 20% of the material examined. Among the 203 tumors, 103 were located in the frontal, 53 in the temporal, 17 in the frontotemporal, 11 in the parietal, 6 in the parietotemporal, 7 in the ventricular, 3 in the occipitotemporal, and 1 in the occipital region; 2 were from the spinal cord. All tumors were classified graded by neuropathologists according to the 2000 WHO criteria and all cases were reviewed by one neuropathologist (AvD). 26 Thirty-one patients with oligoastrocytomas reported on in an earlier study were included. 16 Before extraction of DNA from tumor tissues and leukocytes by standard methods, all tumor samples were examined by frozen sections to exclude contaminating nontumorous portions. 11
Microsatellite Analysis for LOH
The microsatellite markers D1S1608 (1p36.31), D1S548 (1p36.23), D1S1597 (1p36.21), D1S1592 (1p36.13), and D1S1161 (1p35.1) were used to identify LOH 1p. For determining LOH 19q, the markers D19S431 (19q12), D19S433 (19q12), D19S559 (D19q13.2), and D19S601 (19q13.33) were used. Amplification conditions and primer sequences are based on corresponding Genome Database entries (www.gdb.org). Polymerase chain reaction products were separated on 8% denaturing acrylamide gels and visualized by silver staining. LOH was scored as previously described. 27
Single-Strand Conformation Polymorphism Analysis and Direct Sequencing
For analysis of the TP53 gene, a set of previously published primers for exons 5 to 8 were used. Polymerase chain reaction was performed in a volume of 10 μl containing 10 ng of DNA, 50 mmol/L KCl, 10 mmol/L Tris-HCl, 200 mmol/L of each dNTP, 0.1% gelatin, 20 pmol of each primer, 1.0 to 2.0 mmol/L MgCl2, and 0.025 U Taq polymerase. Initial denaturation at 94°C for 3 minutes was followed by 30 cycles on an automated thermal cycler (Biometra, Göttingen, Germany). These included denaturation at 94°C for 30 seconds, annealing at 57°C for 40 seconds, and extension at 72°C for 40 seconds. A final extension step at 72°C for 10 minutes was added. Single-strand conformation polymorphism analysis was performed on a sequencing apparatus (BlueSeq 400; Serva, Marburg, Germany) using 8% and 14% acrylamide gels and electrophoresis at 3 to 6 W and variable temperatures for 15 hours. Silver staining of the gels was performed as previously described. 28,29 Aberrantly migrating single-strand conformation polymorphism bands were excised and the DNA was extracted as described. 30 After reamplification with the same set of primers the polymerase chain reaction products were sequenced on a semiautomated sequencer (model 373A; Applied Biosystems, Foster City, CA) using a Taq cycle sequencing kit (Applied Biosystems). Each amplicon was sequenced bidirectionally.
Statistical Analysis
For statistical analysis Statview 4.0 (SAS, Cary, NC) was used. For the analysis of nominal and independent variables, chi-square and Fisher’s exact tests, were applied. The distribution of age and nominal variables was analyzed by t-test.
Results
Two hundred three gliomas and corresponding blood samples were analyzed for LOH 1p and LOH 19q and for mutations in exons 5 to 8 of the TP53 tumor suppressor gene. The informative cases had the following alterations: 97 tumors showed LOH 1p (24 O II, 25 O III, 21 OA II, 19 OA III, 2 A II, 6 A III) and 121 cases had LOH 19q (28 O II, 27 O III, 27 OA II, 21 OA III, 4 A II, 14 A III). Combined LOH 1p and LOH 19q was detected in 91 cases (24 O II, 25 O III, 20 OA II, 17 OA III, 2 A II, 3 A III). In 46 tumors a TP53 mutation was detected (1 O II, 3 O III, 10 OA II, 7 OA III, 13 A II, 12 A III). Data are summarized in Table 1 ▶ .
Table 1.
Molecular Alterations in Oligodendrogliomas, Oligoastrocytomas, and Astrocytomas
| Histology | Molecular alteration | Frequency | Frequency for distinct tumor sites | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | Parietal | Occipital | Ventricular | Temporal + other lobe | Temporal | Spinal | |||
| O | LOH 1p | 49/70 | 36/44 | 2/2 | 0/1 | 2/2 | 6/8 | 3/13 | – |
| LOH 19q | 55/73 | 38/46 | 2/2 | 1/1 | 2/2 | 8/9 | 4/13 | – | |
| LOH1p/19q | 49/70 | 36/44 | 2/2 | 0/1 | 2/2 | 6/8 | 3/13 | – | |
| TP53 mut | 4/56 | 3/33 | 0/2 | – | 0/2 | 0/6 | 1/13 | – | |
| OA | LOH1p | 40/64 | 26/33 | 3/5 | – | – | 4/5 | 7/21 | – |
| LOH 19q | 48/67 | 27/32 | 3/5 | – | – | 5/6 | 13/24 | – | |
| LOH1p/19q | 37/63 | 24/32 | 3/5 | – | – | 3/5 | 7/21 | – | |
| TP53 mut | 17/61 | 5/29 | 2/5 | – | – | 0/5 | 10/22 | – | |
| A | LOH1p | 8/53 | 5/21 | 0/3 | – | 0/5 | 2/10 | 1/12 | 0/2 |
| LOH 19q | 18/58 | 8/21 | 0/4 | – | 3/5 | 4/11 | 2/15 | 1/2 | |
| LOH1p/19q | 5/49 | 4/18 | 0/3 | – | 0/5 | 1/10 | 0/11 | 0/2 | |
| TP53 mut | 25/53 | 11/20 | 3/4 | – | 1/4 | 6/11 | 4/12 | 0/2 | |
O, Oligodendrogliomas; OA, oligoastrocytoma; A, astrocytoma; LOH, loss of heterozygosity; TP53 mut, detection mutation in exons 5 to 8 of TP53;–, no data available. Numbers of cases with alterations are given in respect to informative cases (for LOH data) and in respect to cases examined (for TP53 mutation data)
Oligodendrogliomas from nontemporal sites had significantly more LOH 1p and LOH 19q than those situated in the temporal lobes. Within the respective regions, LOH frequencies in O II did not differ from those in O III. LOH 1p occurred in 36 of 44 frontal, 2 of 2 ventricular, 0 of 1 occipital, 2 of 2 parietal, and only 3 of 13 temporal oligodendrogliomas (P = 0.0001, chi-square test). LOH 19q occurred in 38 of 46 frontal, 2 of 2 ventricular, 1 of 1 occipital, 2 of 2 parietal, and only 4 of 13 temporal oligodendrogliomas (P = 0.0004, chi-square test).
Because those tumors involving both the temporal and another lobe seemed to reflect the alterations seen in nontemporal tumors, we divided the oligodendrogliomas into the three categories: temporal, nontemporal, and temporal with another lobe. Indeed, 40 of 49 nontemporal O, 6 of 8 O involving the temporal and another lobe, and 3 of 13 temporal O exhibited LOH 1p (P = 0.0002, chi-square test). The data for LOH 19q were: 43 of 51 nontemporal O, 8 of 9 O involving the temporal with another lobe, and 4 of 13 nontemporal O exhibited LOH 19q (P = 0.0006, chi-square test). TP53 mutations were rare and not associated with specific brain regions.
Oligoastrocytomas of nontemporal origin had significantly more LOH 1p than those situated in the temporal lobes. Within the respective regions, LOH frequencies in OA II did not differ from those in OA III. LOH 1p occurred in 26 of 33 frontal, 3 of 4 frontotemporal, 3 of 5 parietal, 1 of 1 parietotemporal, and only 7 of 21 temporal oligoastrocytomas (P < 0.0001, chi-square test). LOH 19q occurred in 27 of 32 frontal, 3 of 4 frontotemporal, 1 of 1 occipitotemporal, 3 of 5 parietal, 1 of 1 parietotemporal, and 13 of 24 temporal oligoastrocytomas. We also divided oligoastrocytomas into the three categories: temporal, nontemporal, and temporal with another lobe. Twenty-nine of 38 nontemporal OA, 4 of 5 OA involving the temporal with another lobe, and 7 of 21 temporal OA exhibited LOH 1p (P < 0.004, chi-square test). The data for LOH 19q were: 30 of 37 nontemporal OA, 5 of 6 OA involving the temporal with another lobe, and 13 of 24 nontemporal OA exhibited LOH 19q (P = 0.06, chi-square test). TP53 mutations were seen in 7 of 34 nontemporal OA, in 0 of 5 OA affecting the temporal with another lobe, but in 10 of 22 temporal OA (P < 0.05, chi-square test). Figure 1 ▶ depicts a representative case of temporal oligoastrocytoma with TP53 mutation. In 57 oligoastrocytomas with both LOH 1p and TP53 data, a significant inverse association was detected between LOH 1p and TP53 mutation (P < 0.0001, Fisher’s exact test). This inverse association was also seen for LOH 19q and TP53 mutation (P = 0.0004, Fisher’s exact test). Because 31 cases from an earlier study had been included that showed a similar distribution, the analyses were repeated excluding those cases: the remaining 26 oligoastrocytomas again demonstrated that LOH 1p (P < 0.005, Fisher’s exact test) and LOH 19q (P < 0.005, Fisher’s exact test) tend not to occur with TP53 mutations. Interestingly, patients with tumors of the temporal lobe (mean, 36 years) were younger (P < 0.05, t-test) than patients with nontemporal tumors (mean, 41 years). Within the temporal tumor group, patients with TP53 mutations (mean, 33 years) were younger than those without this alteration (mean, 38 years).
Figure 1.

Temporal oligoastrocytoma WHO grade II (case 3744) with TP53 mutation. Left: Oligodendroglial differentiation (H&E; original magnification, ×200). Right: Astrocytic differentiation. The tumor DNA carries a somatic C → T transition resulting in an Arg → Cys change in codon 273 in exon 8 of TP53.
Astrocytomas did not exhibit different frequencies of either LOH 1p and LOH 19q or TP53 mutations with respect to tumor localization. LOH 19q occurred more frequently in A III than in A II. Only 4 of 26 A II but 14 of 32 A III exhibited LOH 19q (P < 0.025, Fisher’s exact test). The LOH 1p frequencies did not differ for A II (2 of 25) and A III (6 of 28). TP53 mutations occurred in 13 of 26 A II and 12 of 27 A III.
Discussion
To clarify the nosological position of oligoastrocytoma among the diffuse gliomas, we analyzed a series of 203 gliomas, including 68 oligoastrocytomas, and two control groups of 73 oligodendrogliomas and 62 astrocytomas, for LOH 1p, LOH 19q, and TP53 mutations, with particular emphasis on correlations with tumor location. Both control groups—the pure astrocytomas and oligodendrogliomas—displayed molecular genetic features similar to those reported in the literature. Oligodendrogliomas and oligoastrocytoma had LOH 1p and LOH 19q in the majority of the cases. 7-12 The overall low frequency of TP53 mutations in oligodendrogliomas also confirmed previous studies. 10,16,31 Although LOH 1p was a rare event in astrocytomas, evenly distributed among WHO II and III grades, LOH 19q was significantly associated with higher grade, thereby further supporting the suggestion that a progression-associated tumor suppressor gene resides on this chromosomal arm. 13
We next correlated these molecular findings with tumor location. Astrocytomas did not exhibit any associations between molecular genetic features and location. Furthermore, in oligodendrogliomas, TP53 mutations did not correlate with tumor site. Nonetheless, as previously reported, temporal lobe oligodendrogliomas have significantly less frequent LOH 1p and LOH 19q than their morphologically indistinguishable nontemporal counterparts. 25 Although such differences can be assessed easily for tumors occupying a single site, such as the temporal lobe, a considerable percentage of lesions involve both the temporal lobe and portions of either the frontal, parietal, or occipital lobes. We therefore placed those oligodendrogliomas involving more than one lobe into a separate category; these tumors had genetic features similar to the group of nontemporal oligodendrogliomas. The findings indicate that those oligodendrogliomas without LOH 1p and LOH 19q predominantly arise in the temporal lobes.
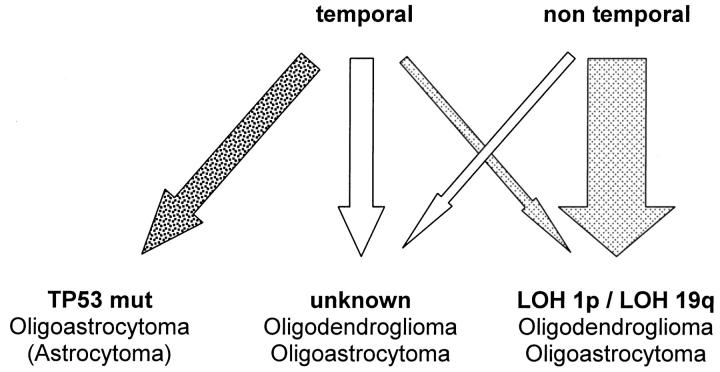
Oligoastrocytomas showed a similar distribution of LOH 1p and LOH 19q with respect to tumor location as that noted for oligodendrogliomas. Allelic losses of 1p and 19q were significantly less frequent in temporal oligoastrocytomas, whereas those oligoastrocytomas affecting temporal and additional lobes were similar to nontemporal oligoastrocytomas. However, oligoastrocytomas within the temporal lobe had significantly more frequent TP53 mutations than the oligoastrocytomas affecting other sites. This may reflect the general problem of separating mixed oligoastrocytomas from astrocytomas and may indicate that temporal oligoastrocytomas not only differ with respect to LOH 1p and LOH 19q, but are also enriched by a fraction of tumors possibly resembling astrocytoma. This line of argument is supported by the observation of an inverse association of LOH 1p and TP53 mutations in oligoastrocytomas. Although 32 of 57 oligoastrocytomas had LOH 1p without TP53 mutation and 13 of 57 had TP53 mutations without LOH 1p, only 2 of 57 exhibited both LOH 1p and TP53 mutations (P < 0.0001, Fisher’s exact test). This clearly demonstrates the existence of different pathogenetic pathways in the genesis of oligoastrocytomas and also confirms our previous study. 16 Analysis excluding 31 cases already studied in the previous series 16 further demonstrated the same two molecular subsets in the remaining 21 samples (P < 0.005, Fisher’s exact test), thereby confirming the initial study 16 using an independent series of tumors. In fact, only one of those 10 temporal oligoastrocytomas with TP53 mutation had LOH 1p. Taken together, these data indicate extensive genetic overlap between oligodendrogliomas and oligoastrocytomas in nontemporal sites, raising the question of whether these tumors represent variants of the same entity. In the temporal lobe, approximately half of the oligoastrocytomas share genetic features with astrocytomas, ie, presence of TP53 mutation and absence of LOH 1p and 19q, suggesting that these tumors may indeed be astrocytomas with some histological features resembling oligodendroglioma. It is thus possible that there are three molecular subsets of oligodendroglial tumors, differing not by morphology but on molecular grounds. Such a model would include a set of predominately extratemporal oligodendroglial tumors with LOH 1p and LOH 19q, and a set of predominately temporal lesions without these alterations—with both sets including morphologically defined oligodendrogliomas as well as oligoastrocytomas. In addition, there appears to be a third set of oligoastrocytomas with TP53 mutations in the temporal lobe that are genetically similar to astrocytomas. However, it should be pointed out that oligoastrocytomas and astrocytomas may well differ for other genetic alterations frequently described in astrocytomas such as CDKN2A deletions or LOH 22q. A model for oligodendroglial tumors is shown in Figure 2 ▶ . Such a model may imply that these tumors arise from different progenitor cell populations. 32
Figure 2.
Model for molecular subdivision of oligodendroglial tumors. Oligodendrogliomas and oligoastrocytomas with LOH 1p/LOH 19q (light-gray arrows) are located predominantly in nontemporal regions of the brain. Oligodendrogliomas and oligoastrocytomas without LOH 1p/LOH 19q (white arrows) are located predominantly in temporal regions of the brain. Oligoastrocytomas with TP53 mutations (dark-gray arrow) mostly arise in the temporal lobes and may resemble astrocytomas. The width of the arrows approximates the frequency of the three subsets in the present study.
Developmental studies on oligodendrocyte differentiation in the chick embryo suggests the existence of two distinct oligodendrocyte precursors emerging from the alar anterior entopeduncular area and from basal rhombomeric foci; however, this study also concluded that all telencephalic oligodendrocyte progenitors are derived from the alar anterior entopeduncular area. 33 Further evidence is provided by the identification of oligodendrocyte precursors with differential expression of PDGFR-α and plp/dm-20, respectively. 34 These observations point toward the possibility of human oligodendrogliomas arising from different progenitor cell populations. On the other hand, mouse models using controlled ectopic expression in either precursor or maturing astrocytic cells have modeled human glioblastomas, astrocytomas, oligodendrogliomas, or oligoastrocytomas, depending on the oncogenic stimuli. 35-37 Although these findings allow one to speculate that different tumors could arise from the same progenitor cell population, they also allow tumors of a similar origin to exhibit morphologically distinct appearances. 32 Another hypothesis may be to postulate stepwise occurrence of genetic alterations and different detection times for temporal and nontemporal tumors. However, earlier recognition of temporal tumors because of early onset of epilepsy seems not to be the cause. Although temporal tumor patients indeed are significantly younger, this is mainly because of the patient group of oligoastrocytomas with TP53 mutations. This rather supports the notion of initial genetic heterogeneity among these lesions.
On a practical level, the pathologist is left with the problem of how to classify oligodendroglial tumors. The classic distinction between oligodendrogliomas and oligoastrocytomas is problematic because of lack of clearly discriminating parameters. 26 Quantifying areas of astrocytic and oligodendroglial differentiation and applying threshold values is complicated by the insuperable problem of sampling bias, and is potentially aggravated by the tendency of reactive changes at tumor margins to mimic astrocytic proliferation. At a molecular level, the differences between morphologically defined oligodendrogliomas and oligoastrocytomas seem to disappear. Instead, a regional molecular heterogeneity emerges that may be used for molecular classification. Such an approach results in pooling oligodendroglial tumors based on the presence or absence of LOH 1p and LOH 19q, and, raise the radical possibility of dismissing temporal oligoastrocytomas with TP53 mutations as astrocytomas. Clinical reports are supportive for a molecular classification of oligodendroglial tumors. LOH 1p and LOH 19 q have been demonstrated as powerful tools to predict survival. 5,21,22 One study on anaplastic oligodendrogliomas demonstrated these molecular parameters to be the most powerful predictors of response to chemotherapy. 5 The regional heterogeneity of molecular parameters in oligodendroglial tumors also finds clinical support in the observation that patients with frontal oligodendrogliomas have better outcomes. 38 Taken together, a subclassification on molecular grounds provides a cogent approach to unifying previous findings about prognosis, behavior, response to therapy, genotype, and location in oligodendroglial tumors. The emerging clinicogenetic associations suggest that oligodendroglial tumors will require molecular subdivision in the near future.
Acknowledgments
We thank U. Lass and C. Spingies for skillful assistance.
Footnotes
Address reprint requests to Andreas von Deimling, M.D., Institut für Neuropathologie, Charité, Campus Virchow Klinikum, Augustenburgerplatz 1, D-13353 Berlin, Germany. E-mail: andreas.von_deimling@charite.de.
Supported by the Deutsche Krebshilfe (70-2385-Wi2), the Bundesministerium für Bildung und Forschung (01GS0101 and 01GS0151), the North American Treaty Organization (LST.CLG.977284), the National Institutes of Health (CA57683), and the Deutsche Forschungsgemeinschaft (SFB507-B10).
References
- 1.Mork SJ, Halvorsen TB, Lindegaard KF, Eide GE: Oligodendroglioma. Histologic evaluation and prognosis. J Neuropathol Exp Neurol 1986, 45:65-78 [DOI] [PubMed] [Google Scholar]
- 2.Russell DS, Rubinstein LJ: Pathology of Tumours of the Nervous System. 1989. Williams & Wilkins, Baltimore
- 3.Schiffer D: Brain Tumors. Biology, Pathology, and Clinical References. 1997. Springer, Berlin
- 4.Zulch K: Brain Tumors. Their Biology and Pathology. 1986. Springer Verlag, Berlin, Heidelberg
- 5.Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN: Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 1998, 90:1473-1479 [DOI] [PubMed] [Google Scholar]
- 6.Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, Ramsay DA, Cairncross JG, Louis DN: Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 2001, 7:839-845 [PubMed] [Google Scholar]
- 7.Ransom DT, Ritland SR, Jenkins RB, Scheithauer B, Kelly PJ, Kimmel DW: Loss of heterozygosity studies in human gliomas. Proc Annu Meet Am Assoc Cancer Res 1991, 32:302 [Google Scholar]
- 8.von Deimling A, Louis DN, von Ammon K, Petersen I, Wiestler OD, Seizinger BR: Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas and mixed gliomas. Cancer Res 1992, 52:4277-4279 [PubMed] [Google Scholar]
- 9.Bello MJ, Vaquero J, de Campos JM, Kusak ME, Sarasa JL, Saez-Castresana J, Pestana A, Rey JA: Molecular analysis of chromosome 1 abnormalities in human gliomas reveals frequent loss of 1p in oligodendroglial tumors. Int J Cancer 1994, 57:172-175 [DOI] [PubMed] [Google Scholar]
- 10.Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP: Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol 1994, 145:1175-1190 [PMC free article] [PubMed] [Google Scholar]
- 11.Kraus JA, Koopmann J, Kaskel P, Maintz D, Brandner S, Louis DN, Wiestler OD, von Deimling A: Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J Neuropath Exp Neurol 1995, 54:91-95 [DOI] [PubMed] [Google Scholar]
- 12.von Deimling A, Fimmers R, Schmidt MC, Bender B, Fassbender F, Nagel J, Jahnke R, Kaskel P, Duerr E-M, Koopmann J, Maintz D, Schild S, Vogel Y, Wick W, Platten M, Müller D, Przkora R, Waha A, Rollbrocker B, Wellenreuther R, Meyer-Puttlitz B, Schmidt O, Mollenhauer J, Poustka A, Stangl AP, Lenartz D, von Ammon K, Henson JW, Schramm J, Louis DN, Wiestler OD: Comprehensive allelotype and genetic analysis of 466 human nervous system tumors. J Neuropathol Exp Neurol 2000, 59:544-558 [DOI] [PubMed] [Google Scholar]
- 13.von Deimling A, Bender B, Jahnke R, Waha A, Kraus J, Albrecht S, Wellenreuther R, Fassbender F, Nagel J, Menon AG, Louis DN, Lenartz D, Schramm J, Wiestler OD: Loci associated with malignant progression in astrocytomas: a candidate on chromosome 19q. Cancer Res 1994, 54:1397-1401 [PubMed] [Google Scholar]
- 14.Hart MN, Petito CK, Earle KM: Mixed gliomas. Cancer 1973, 33:134-140 [DOI] [PubMed] [Google Scholar]
- 15.Smith JS, Alderete B, Minn Y, Borell TJ, Perry A, Mohapatra G, Hosek SM, Kimmel D, O’Fallon J, Yates A, Feuerstein BG, Burger PC, Scheithauer BW, Jenkins RB: Localization of common deletion regions on 1p and 19q in human gliomas and their association with histological subtype. Oncogene 1999, 18:4144-4152 [DOI] [PubMed] [Google Scholar]
- 16.Maintz D, Fiedler K, Koopmann J, Rollbrocker B, Nechev S, Lenartz D, Stangl AP, Louis DN, Schramm J, Wiestler OD, von Deimling A: Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol 1997, 56:1098-1104 [DOI] [PubMed] [Google Scholar]
- 17.Wallner KE, Gonzales M, Sheline GE: Treatment of oligodendrogliomas with or without postoperative irradiation. J Neurosurg 1988, 68:684-688 [DOI] [PubMed] [Google Scholar]
- 18.Kyritsis AP, Yung WKA, Bruner J, Gleason MJ, Levin VA: The treatment of anaplastic oligodendrogliomas and mixed gliomas. Neurosurgery 1993, 32:365-371 [DOI] [PubMed] [Google Scholar]
- 19.Streffer J, Schabet M, Banberg M, Grote E, Meyermann R, Voigt K, Dichgans J, Weller M: A role for preirradiation PCV chemotherapy for oligodendroglial brain tumors. J Neurol 2000, 247:297-302 [DOI] [PubMed] [Google Scholar]
- 20.Bauman GS, Ino Y, Ueki K, Zlatescu MC, Fisher BJ, Macdonald DR, Stitt L, Louis DN, Cairncross JG: Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. Int J Radiat Oncol Biol Phys 2000, 48:825-830 [DOI] [PubMed] [Google Scholar]
- 21.Ino Y, Zlatescu M, Sasaki H, Macdonald D, Stemmer-Rachamimov A, Jhung S, Ramsay D, von Deimling A, Louis D, Cairncross G: Long survival and therapeutic responses in patients with histologically disparate high-grade gliomas demonstrating chromosome 1p loss. J Neurosurg 2000, 92:983-990 [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB: Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 2000, 18:636-645 [DOI] [PubMed] [Google Scholar]
- 23.Kim L, Hochberg FH, Thornton AF, Harsh GR, IV, Patel H, Finkelstein D, Louis DN: Procarbazine, lomustine, and vincristine (PVC) chemotherapy for grade III and grade IV oligoastrocytomas. J Neurosurg 1996, 85:602-607 [DOI] [PubMed] [Google Scholar]
- 24.Glass J, Hochberg FH, Gruber ML, Louis DN, Smith D, Rattner B: the treatment of oligodendrogliomas and mixed oligodendroglioma-astrocytomas with PCV chemotherapy. J Neurosurg 1992, 76:741-745 [DOI] [PubMed] [Google Scholar]
- 25.Zlatescu MC, TehraniYazdi A, Sasaki H, Megyesi JF, Betensky RA, Louis DN, Cairncross JG: Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res 2001, 61:6713-6715 [PubMed] [Google Scholar]
- 26.Kleihues P, Cavenee WK: Pathology and Genetics of Tumours of the Nervous System. 2000. IARC Press, Lyon
- 27.Louis DN, von Deimling A, Seizinger BR: A (CA)n dinucleotide repeat assay for evaluating loss of allelic heterozygosity in small and archival human brain tumor specimens. Am J Pathol 1992, 141:777-782 [PMC free article] [PubMed] [Google Scholar]
- 28.Bender B, Wiestler OD, von Deimling A: A device for processing large acrylamide gels. Biotechniques 1994, 16:204-206 [PubMed] [Google Scholar]
- 29.von Deimling A, Bender B, Louis DN, Wiestler OD: A rapid and non radioactive PCR based assay for the detection of allelic loss in human gliomas. Neuropathol Appl Neurobiol 1993, 19:524-529 [DOI] [PubMed] [Google Scholar]
- 30.Wellenreuther R, Kraus J, Lenartz D, Menon AG, Schramm J, Louis DN, Ramesh V, Gusella JF, Wiestler OD, von Deimling A: Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol 1995, 146:827-832 [PMC free article] [PubMed] [Google Scholar]
- 31.Ohgaki H, Eibl RH, Wiestler OD, Yasargil MG, Newcomb EW, Kleihues P: p53 mutations in nonastrocytic human brain tumors. Cancer Res 1991, 51:6202-6205 [PubMed] [Google Scholar]
- 32.Louis DN, Holland EC, Cairncross JG: Glioma classification: a molecular reappraisal. Am J Pathol 2001, 159:779-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivier C, Cobos I, Pere Zvillegas EM, Spassky N, Zalc B, Martinez S, Thomas JL: Monofocal origin of telencephalic oligodendrocytes in the anterior entopeduncular area of the chick embryo. Development 2001, 128:1757-1769 [DOI] [PubMed] [Google Scholar]
- 34.Spassky N, Olivier C, Perez-Villegas E, Goujet-Zalc C, Martinez S, Thomas J, Zalc B: Single or multiple oligodendroglial lineages: a controversy. Glia 2000, 29:143-148 [PubMed] [Google Scholar]
- 35.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN: Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 2000, 25:55-57 [DOI] [PubMed] [Google Scholar]
- 36.Holland EC, Li Y, Celestino J, Dai C, Schaefer L, Sawaya RA, Fuller GN: Astrocytes give rise to oligodendrogliomas and astrocytomas after gene transfer of polyoma virus middle T antigen in vivo. Am J Pathol 2000, 157:1031-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC: PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev 2001, 15:1913-1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kros JM, Pieterman H, van Eden CG, Avezaat CJ: Oligodendroglioma: the Rotterdam-Dijkzigt experience. Neurosurgery 1994, 34:959-966 [DOI] [PubMed] [Google Scholar]