Abstract
Both mRNA and protein expression of the chemokines IP-10/CXCL10 and Mig/CXCL9, as well as of their receptor, CXCR3, were assessed in the thyroid glands of 16 patients suffering from Graves’ disease (GD). In addition, IP-10/CXCL10 levels were measured in the serum of 50 GD patients. Expression of IP-10/CXCL10, Mig/CXCL9, and CXCR3 was poor or absent in normal thyroid tissue from patients undergoing thyroidectomy because of primary localized thyroid tumors, while both the chemokines and their receptor were present in most thyroid glands of patients affected by GD. IP-10/CXCL10 and Mig/CXCL9 localized to infiltrating lymphocytes and macrophages, as well as to resident epithelial follicular cells, whereas CXCR3 was mainly found at the level of infiltrating inflammatory cells and endothelial cells from large and small vessels. Of note, maximal expression of IP-10/CXCL10 and Mig/CXCL9 was found in the thyroid gland of patients with recent-onset GD and was correlated with interferon (IFN)-γ. Accordingly, high levels of IP-10/CXCL10 could be measured in the serum of patients with short-duration GD. Taken together, the results of this study demonstrate that the CXCR3-binding chemokines IP-10/CXCL10 and Mig/CXCL9 play an important role in the recruitment of cells and in the amplification of inflammation in GD. They also suggest that the production of these chemokines by resident follicular epithelial cells may contribute to the recruitment of CXCR3-expressing type 1 T-helper cells in the initial phases of GD.
Hyperthyroidism and tissue stimulation are characteristic of Graves’ disease (GD), a common autoimmune disease of the thyroid. One of the hallmarks of thyroid affected by GD is lymphocyte infiltration, predominantly composed by TCRαβ CD4+ or CD8+ cells, most of which are activated memory cells expressing activation markers, with a relatively high proportion of TCRγδ cells in some cases. 1,2 T cells are critical in the induction, development, and maintenance of GD, even if both clinical symptoms and outcome are mediated by thyroid-stimulating antibodies that induce the hyperfunction of the thyroid gland. 1,2 T lymphocytes are the effectors of tissue damage in many autoimmune diseases and are also involved in the induction of autoimmune responses, presumably mediated by the production of cytokines. 3 The trafficking and in situ maintenance of immune cells are a complex phenomena that involves the participation of several adhesion molecules and the interaction of chemoattractant cytokines (chemokines) with their receptors. 4-6 Chemokines can be divided into major subfamilies, based on the position of the first two cysteine amino acid residues in the molecule. 4 There are at least four families of chemokines, but only two have been extensively characterized. 4-6 In general, CXC chemokines attract neutrophils and lymphocytes, whereas the chemokines belonging to the CC family act primarily on monocytes, but can also attract lymphocytes, basophils, and eosinophils. 4-6 Recently, a new classification system for the different families of chemokines has been proposed. 6 As far as GD is concerned, little information is available on the recruitment by chemokines of the various lymphocyte subsets and their persistence at thyroid level.
In this study, both mRNA and protein expression of interferon (IFN)-γ-inducible protein of 10-kDa IP-10/CXCL10 and monokine induced by IFN-γ Mig/CXCL9 were assessed in the thyroid gland of 16 patients suffering from GD. As is known, these chemokines are mainly induced by IFN-γ and share the property to bind CXCR3, which is expressed by type 1 T helper (Th1), natural killer, B, T cell receptor (TCR) γδ, 4-9 lymphocytes, dendritic cells, 10 and monocytes/macrophages. 11 CXCR3 and its ligands, IP-10/CXCL10 and Mig/CXCL9, were detected in perifollicular infiltrates and in follicular epithelia from the thyroids of patients affected by GD, whereas they could not be found in the glands of patients affected by nonautoimmune thyroid disorders. Interestingly, IP-10/CXCL10 and Mig/CXCL9 were mainly expressed in thyroid tissue specimens from patients affected by recent-onset GD and their mRNA levels were strictly associated with the levels of IFN-γ, as demonstrated by both in situ hybridization and quantitative reverse transcription-polymerase chain reaction (RT-PCR). Accordingly, levels of IP-10/CXCL10 were found to be elevated in the serum of patients affected by recent-onset GD in comparison with healthy controls. Furthermore, the reduction of IP-10/CXCL10 levels was associated with a slight increase of serum levels of macrophage-derived chemokine (MDC)/CCL22, a chemokine previously associated with a type 2 Th (Th2) immune response, 12,13 an inversion of the MDC/IP-10 ratio being detectable with the progression of the disease. Taken together, these data suggest a pathogenic role of these chemokines in the recruitment of activated T cells in the inflamed thyroid tissue and suggest that the detection of IP-10/CXCL10 may represent a useful clinical marker in GD.
Materials and Methods
Tissue Specimens and Sera
Thyroid tissue specimens were obtained from patients undergoing thyroid surgery for different pathological conditions. In detail, 16 patients were affected by GD (age range, 25 to 63 years; mean age, 46 years), diagnosed on the basis of its clinical, biochemical, and immunological features and thyroid scintiscans. They had been affected by the disease for a median time of 21.5 months (range, 12 to 60 months). In detail, nine patients had a short duration time of 18 months (range, 12 to 23 years), whereas seven of them had long-standing disease of 45 months (range, 33 to 60 months). Seven patients suffered from nonautoimmune hyperthyroidism (age range, 39 to 60 years; mean age, 47), diagnosed as unifocal thyroid autonomy by a Tc 99-pertechnatate thyroid scintiscan that showed a single hot nodule with a low uptake in the surrounding parenchyma [toxic adenomas (TAs)]. All of the patients were euthyroid at the moment of surgical intervention. Because of difficulties in obtaining normal thyroid tissue, the control group (age range, 39 to 64 years; mean age, 49) consisted of nine surgical specimens obtained from the contralateral disease-free lobe of five patients with TA and four patients with a histologically confirmed diagnosis of primary localized thyroid tumor. Serum samples were collected from 50 patients affected by GD, as well as from 25 healthy volunteers from hospital staff and their relatives. Among patients suffering from GD whose serum IP-10 levels were analyzed, only 2 of 50 were hyperthyroid at the moment of serum analysis. All patients affected by GD had been treated with methymazole at variable doses. Corticosteroid therapy was an exclusion criterion for the enrollment of all of the patients. The procedures used in this study were in accordance with the Regional Ethical Committee on Human Experimentation.
Cloning and Sequencing of IP-10/CXCL10 and Mig/CXCL9 Probes
IP-10/CXCL10 and Mig/CXCL9 mRNA probes were prepared as previously described. 9 mRNA was extracted from human epithelial cells stimulated with tumor necrosis factor-α and IFN-γ and reversed to first-strand cDNA by oligo dT primer, using a MMLV reverse transcriptase kit (Life Technologies, Berlin, Germany). The following primers were used: IP-10/CXCL10: forward, TGATTTGCTGCCTTATCTTTCTGA; reverse, CAGCCTCTGTGTGGTCCATCCTTG; and Mig/CXCL9: forward, CAGCAGATGTGAAGGAACTG; reverse, GCATGATGAAATTCAACTGG. Amplification of the first-strand products is performed in a Thermal cycler (Mastercycler gradient; Eppendorf; Hamburg, Germany). The samples are subjected to 35 cycles of amplification using 400 nmol/L of each primer and 2.5 U of Taq-DNA polymerase (Polymed, Florence, Italy). The DNA fragments of 335 bp (IP-10) and 362 bp (Mig) amplified by PCR were subcloned in pGEM-T (Promega Co., Madison, WI), according to the manufacturer’s instructions. Sequencing of the amplified product was performed as previously described. 9
In Situ Hybridization
In situ hybridization was performed according to a technique described elsewhere. 9 Briefly, 10-μm frozen sections were cut and fixed in 4% paraformaldehyde for 20 minutes. After a prehybridization treatment (0.2 N HCl, 0.125 mg/ml pronase, 4% paraformaldehyde, acetic anhydride 1:400 in 0.1 mol/L triethanolamine buffer, pH 8) sections were dehydrated in increasing ethanol concentrations. Thirty μl of hybridization solution (4× standard saline citrate, 1× Denhardt’s solution, 10% dextran sulfate, 0.1 mg/ml sheared herring sperm DNA, and 1 mg/ml yeast tRNA) containing 8 × 105 counts/minute of 35S-labeled human IP-10/CXCL10 and Mig/CXCL9 RNA probe were applied on each section and covered with Parafilm. Human IP-10/CXCL10 and Mig/CXCL9 RNA probes 9 were synthesized as described. cDNAs were subcloned in PGEM-4Z plasmid vector, by incubating with T4 ligase (Gibco-BRL, Life Technologies, Berlin, Germany) at 15°C for 4 hours. The cDNA was subsequently linearized with HindIII or BamHI restriction enzymes followed by phenol-chloroform extraction and ethanol precipitation. Thereafter, sense and anti-sense RNA-radiolabeled probes were synthesized using SP6 or T7 RNA polymerases as appropriate (Riboprobe Gemini System, Promega), in the presence of α-35S-thioUTP (1300 mCi/mmol; NEN-Du Pont, Paris, France). RNA probes were extracted with phenol-chloroform, ethanol precipitated, and subsequently subjected to alkaline digestion. Hybridization was performed at 52°C for 16 hours. After that, sections were washed and autoradiography was performed. Exposure time varied from 15 to 20 days. Sections were developed in D19, fixed in Kodak fixative, counterstained with hematoxylin-eosin-phloxine and mounted with Permount. Negative controls consisted of sections hybridized to a sense RNA probe.
Quantitative Image Analysis
Quantitative evaluation of IP-10/CXCL10 and Mig/CXCL9 mRNA was performed by two independent observers blinded with regards to patient diagnosis, by means of a computerized video image analysis system (Quantimet Q500 ML; Leica Corp., Cambridge Ltd., Cambridge, UK). 14 For the quantitation of in situ hybridization results, the three most positive fields were chosen from each section and analyzed under a dark-field microscope equipped with a ×100 lens. The autoradiographic signal corresponding to the specific hybridization was acquired by a charge-coupled device video camera connected to the microscope, converted to digital, and transformed into pixel units. The threshold of specific detection was calibrated on control sections hybridized with the corresponding sense probes. The results, evaluated in terms of number of pixels per defined area occupied by the IP-10/CXCL10 and Mig/CXCL9 autoradiographic signal are expressed as the mean.
Real-Time Quantitative RT-PCR (Taq-Man)
Total RNA was extracted using the RNeasy kit (Qiagen, Hilden, Germany) and treated with DNase I (Qiagen) to eliminate possible genomic DNA contamination. Taq-Man RT-PCR has been described elsewhere. 15 The primers and probes were designed using Primer Express version 1.0 software (Applied Biosystems, Warrington, UK). CXCL10/IP-10 and IFN-γ quantitation were performed using predeveloped Taq-Man assay reagent target kits (Applied Biosystems). CXCL9/Mig quantitation was performed using the following primers and probes: FAM probe, 5′-AAGGGTCGCTGTTCCTGCATCAGC-3′; forward 5′-TGCAAGGAACCCCAGTAGTGA-3′; reverse 5′-GGTGGATAGTCCCTTGGTTGG-3′. mRNA levels were quantitated by comparing experimental levels to standard curves generated using serial dilutions of mRNA obtained from human microvascular endothelial cells stimulated with IFN-γ (1000 U/ml) plus tumor necrosis factor-α (8 ng/ml) or peripheral blood-activated T lymphocytes.
Immunohistochemistry
Immunohistochemical staining was performed on 5-μm cryostat sections fixed in 4% paraformaldehyde at room temperature for 20 minutes. Sections were subsequently exposed to 0.3% hydrogen-peroxide-methanol solution to quench the endogenous peroxidase activity. After a 30-minute preincubation with normal horse serum (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), sections were layered for 30 minutes with rabbit anti-human IP-10 or Mig polyclonal antibodies (Abs), followed by biotinylated anti-rabbit goat Ab, and the avidin-biotin-peroxidase complex (Vectastain ABC kit), as described. 9 As a peroxidase substrate, 3-amino-9-ethylcarbazole (Sigma, St. Louis, MO) was used. Finally, sections were counterstained with Gill’s hematoxylin (Merck, Darmstadt, Germany) and mounted with Kaiser’s glycerol gelatin (Merck). All incubations were performed at room temperature. As a negative control, primary Ab was omitted or replaced with mouse ascites fluid.
IP-10/CXCL10 or Mig/CXCL9 and TSH receptor (TSHr), α-smooth muscle actin, pan-cytokeratin (CK), von Willebrand (vWF) factor, CD3, CD4, CD8, CD68 (monocytes/macrophages), CD56 (natural killer cells), CD20 (B cells), CD11c (dendritic cells), and CD83 (dendritic cells) were co-localized on the same sections by double-label immunohistochemistry, according to the method detailed elsewhere. 9,16 Double-label immunohistochemistry for all of these markers was performed and all of the 16 tissue specimens analyzed for IP-10/CXCL10, Mig/CXCL9 and CXCR3 expression, and an average of 20 sections for each patients was analyzed. Briefly, the anti-vWf (rabbit anti-human polyclonal Ab; DAKO, Glostrup, Denmark) or anti-TSHr (clone 4C1/E1/G8; Novocastra, Newcastle, UK), anti-CK (clone C11; Sigma, Milan, Italy), anti-α-smooth muscle actin (clone 1A4, Sigma), anti-CD68 (clone EBM11, DAKO), anti-CD3 (clone UCHT-1; Cymbus Byotechnology, Chandlers-Ford, UK), anti-CD4 (clone MT310, DAKO), anti-CD8 (clone DK25, DAKO), anti-CD20 (clone L26, DAKO), anti-CD56 (clone MOC-1, DAKO), anti-CD11c (S-HCL-3; Becton Dickinson, San Jose, CA), anti-CD83 (clone HB15e; Pharmingen, Europe), monoclonal antibodies (mAbs) were applied first and Vector SG (bluish-gray color, Vector Laboratories) was used as peroxidase substrate. Sections were subsequently exposed to anti-IP-10 or anti-Mig rabbit anti-human polyclonal antibodies and 3-amino-9-ethyl-carbazole was used as a chromogen. No counterstain was applied. To avoid crossreaction between the detection system for the second mAb and the first mAb, the biotinylated anti-rabbit IgG goat Ab was used at lower concentration in the second step of the double-staining protocol. 16 Furthermore, to ascertain that the detection system for the second Ab did not cross-react with the first, double-label immunohistochemistry was performed by omitting the second primary antibody or replacing it with an isotype-matched control mAb with irrelevant specificity, which resulted in a single signal. 16 Blocking of immunostaining through preincubation with the specific peptide (100 μg/ml of IP-10 and 100 μg/ml Mig; Peprotech, UK) for 1 hour at 4°C, was performed as previously described, 17 and completely abolished the signal.
Serum Assays
Tg Abs (normal range, <100 U/ml) were measured by a commercial immunoradiometric assay kit (Biochem Immunosystem, Bologna, Italy) whereas TPO Abs (normal range, <10 U/ml) were tested by RIA kit set (DiaSorin, Inc., Saluggia, Italy). The detection limit of the assays, the intra- and interassay coefficients of variation were: 5.0 U/ml, 3.9% and 6.9%, for Tg Ab and 0.7 U/ml, 2.5% and 6.6%, for TPO Abs, respectively. Quality control pools at low, normal, and high concentrations for all parameters were present in each assay. Samples were assayed in duplicate. Serum IP-10/CXCL10 levels were assayed by a quantitative sandwich immunoassay using a commercially available kit (R&D Systems, Minneapolis, MN), with a sensitivity ranging from 0.41 and 4.46 pg/ml and a mean minimum detectable dose of 1.67 pg/ml. The intra- and interassay coefficients of variation were 3% and 6.9%.
Statistical Analysis
Statistical analyses were performed by SSPS, Inc. (Chicago, IL). The comparison of IP-10/CXCL10 and Mig/CXCL9 mRNA expression levels among patients with different thyroid pathology was performed by Mann Whitney U-test for unpaired data. Similarly, IP-10/CXCL10, Mig/CXCL9, and IFN-γ mRNA expression levels were compared between patients with recent (<2 years) or late-onset GD. Correlation between two variables was ascertained by linear regression analysis and Spearman’s correlation test. A P value <0.05 was considered statistically significant.
Results
High IP-10/CXCL10 and Mig/CXCL9 mRNA Expression in the Thyroid of Patients with Recent-Onset GD
The expression of mRNA for IP-10/CXCL10 and Mig/CXCL9 in normal thyroids, as well as in thyroids of patients suffering from GD, was first assessed by using in situ hybridization. In normal thyroid tissue obtained from patients undergoing surgery for TAs or primary thyroid tumors, as well as in TAs, a nonautoimmune disorder of the thyroid, IP-10/CXCL10 and Mig/CXCL9 were not, or poorly, detectable, and only at level of infiltrating inflammatory cells (Figure 1 ▶ ; A, B, F, and G). The difference in the levels of both IP-10/CXCL10 and Mig/CXCL9 mRNA expression between normal thyroids and TAs, as determined by quantitative image analysis, was not significant (Figure 2, A and B) ▶ . Sections hybridized with sense IP-10/CXCL10 or Mig/CXCL9 RNA probe showed virtually no signal (Figure 1D ▶ and data not shown). In thyroids from patients with GD, a high number of infiltrating inflammatory cells exhibited intense signal all over the tissue specimen, part of positive cells being identifiable as mononuclear infiltrating cells, which in many cases surrounded vessels and follicles (Figure 1, C and H) ▶ . However, resident epithelial follicular cells also exhibited high levels of IP-10/CXCL10, but lower levels of Mig/CXCL9, mRNA expression (Figure 1, E and I) ▶ . The quantitative evaluation of IP-10/CXCL10 and Mig/CXCL9 mRNA in all thyroid specimens examined supported the concept that patients affected by GD express high levels of chemokines. Of note, maximal expression of IP-10/CXCL10 and Mig/CXCL9 mRNA was detected in thyroids of patients with recent-onset (<2 years) disorder (Figure 2C) ▶ .
Figure 1.

IP-10/CXCL10 and Mig/CXCL9 expression in thyroid tissue specimens from patients with GD. A: Section of normal thyroid hybridized with anti-sense IP-10/CXCL10 mRNA probe showing virtually no signal. B: Section of a TA hybridized with anti-sense IP-10/CXCL10 mRNA probe showing virtually no signal. C: High signal in the thyroid specimen from a patient with GD hybridized with anti-sense IP-10/CXCL10 mRNA probe. D: Absence of signal in a consecutive section hybridized with a sense IP-10/CXCL10 mRNA probe. E: High-power magnification of the thyroid follicular epithelium from the same patient with GD hybridized with anti-sense IP-10/CXCL10 mRNA probe. F: Section of normal thyroid hybridized with anti-sense Mig/CXCL9 mRNA probe showing virtually no signal. G: Section of a TA hybridized with anti-sense Mig/CXCL9 mRNA probe showing virtually no signal. H: High signal in the thyroid specimen from a patient with GD hybridized with anti-sense Mig/CXCL9 mRNA probe. I: High-power magnification of the thyroid follicular epithelium from the same patient with GD hybridized with anti-sense Mig/CXCL9 mRNA probe. Dark-field original magnifications: ×100 (A–D, F–H); ×1000 (E, I).
Figure 2.
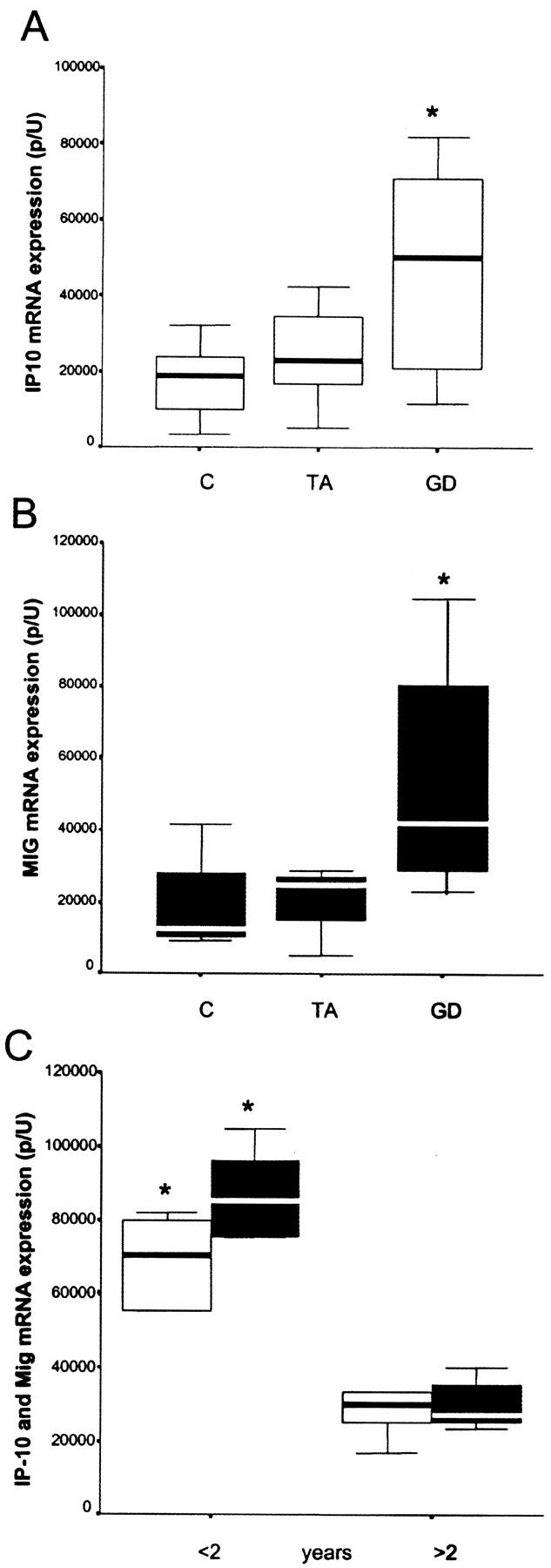
High expression of IP-10/CXCL10 and Mig/CXCL9 mRNA in thyroids of patients with recent-onset GD. A: High expression of IP-10/CXCL10 mRNA levels in the thyroid of patients with GD in comparison with patients with TA (P < 0.05) or normal thyroids (P < 0.01). B: High expression of Mig/CXCL9 mRNA levels in the thyroid of patients with GD in comparison with patients with TAs (P < 0.05) or normal thyroids (P < 0.005). The difference in the levels of both IP-10/CXCL10 and Mig/CXCL9 mRNA expression between normal thyroids and TAs, as determined by quantitative image analysis, was not significant. C: Significant differences in IP-10/CXCL10 and Mig/CXCL9 mRNA expression in GD-affected patients in relation to recent or late onset (P < 0.05 and P < 0.005 for IP-10/CXCL10 and Mig/CXCL9, respectively). Quantitative evaluation of IP-10/CXCL10 and Mig/CXCL9 mRNA was performed by means of a computerized video image analysis system. Results are expressed as medians and percentiles.
IFN-γ mRNA Levels Are Related to IP-10/CXCL10, Mig/CXCL9, and Disease Duration in Thyroid of Patients Affected by GD
The assessment by quantitative RT-PCR of IFN-γ, IP-10/CXCL10, and Mig/CXCL9 mRNA levels revealed that their expression was highly heterogeneous. In agreement with data obtained using in situ hybridization, IP-10/CXCL10 (Figure 3A ▶ , P < 0.001) and Mig/CXCL9 (P < 0.01, Figure 3B ▶ ) mRNA levels were strictly related to IFN-γ mRNA levels, and IFN-γ mRNA levels were strikingly higher in patients with recent-onset (<2 years) disorder (P < 0.01) (Figure 3C) ▶ .
Figure 3.
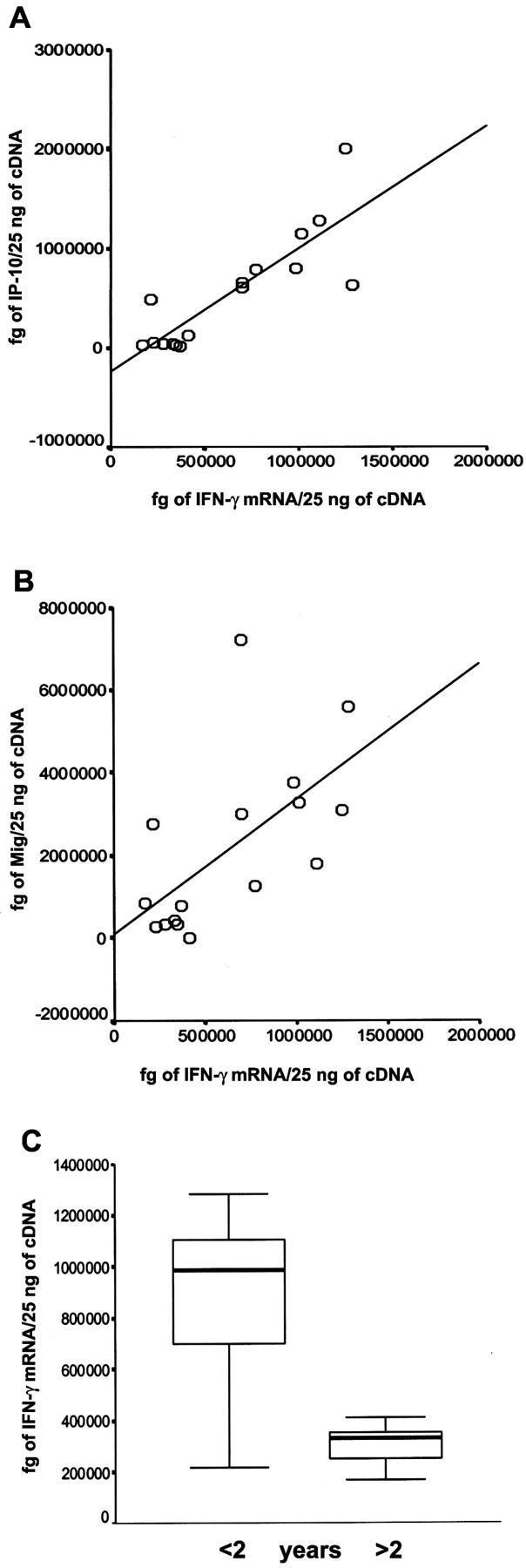
IFN-γ mRNA levels are related to IP-10/CXCL10, Mig/CXCL9, and disease duration in thyroids of patients affected by GD. IFN-γ mRNA levels are strictly associated with IP-10/CXCL10 (A) and Mig/CXCL9 (B) mRNA expression (R2 = 0715, P < 0.001; R2 = 0376, P < 0.01). C: Statistically significant difference in IFN-γ mRNA levels in GD in relation to recent or late onset (P < 0.01).
IP-10/CXCL10, Mig/CXCL9, and CXCR3 Protein Localization in the Thyroid of Patients with GD
The expression of IP-10/CXCL10 and Mig/CXCL9 proteins was then assessed by using immunohistochemistry. Virtually no staining was observed in normal thyroid and only rarely in scattered infiltrating inflammatory cells of thyroids from patients with TAs (data not shown), whereas immunoreactivity was widely present in thyroids of most patients with GD (Figure 4) ▶ . Furthermore, blocking of immunostaining through preincubation with the specific peptides completely abolished the signal, demonstrating the specificity of the staining (Figure 4B) ▶ . To better characterize the nature of IP-10/CXCL10- and Mig/CXCL9-producing cells, double immunostaining for IP-10/CXCL10 or Mig/CXCL9 and TSH receptor (TSHr), CK, vWf, CD3, CD68, CD56, CD20, CD11c, and CD83 was performed. The results showed that among inflammatory cells, monocytes/macrophages, and T lymphocytes were the main source of both IP-10/CXCL10 and Mig/CXCL9 (Figure 4 ▶ ; D, E, I, and L). However, co-staining with both CK and TSHr demonstrated that even resident epithelial follicular cells expressed IP-10/CXCL10 (Figure 4C) ▶ and Mig/CXCL9 protein (Figure 4G) ▶ . Negative controls consisted of sections stained with anti-TSHr antibody, revealed, and then stained with an isotype control Ab and finally the peroxidase substrate (Figure 4H) ▶ . By using immunohistochemistry, high CXCR3 was detected in mononuclear cells surrounding follicular structures in the thyroid of patients affected by GD, but neither in normal thyroids nor in TAs (Figure 5 ▶ and data not shown). No staining was found in any tissues by omitting the primary Ab or replacing it with an isotype-matched control Ab with irrelevant specificity (Figure 5B) ▶ . Furthermore, CXCR3 was highly expressed by endothelial cells of several large and small vessels, consistently with their state of activation, in thyroid tissue of GD and TAs. (Figure 5D) ▶ . Double immunostaining for CXCR3 and α-smooth muscle actin, CK, vWf, CD3, CD4, CD8, CD68, CD56, CD20, CD11c, and CD83 revealed that the majority of mononuclear cells expressing CXCR3 were T lymphocytes of the CD4 (Figure 5E) ▶ and CD8 (Figure 5F) ▶ subtype, macrophages (Figure 5G) ▶ , and dendritic cells (Figure 5, H and I) ▶ .
Figure 4.
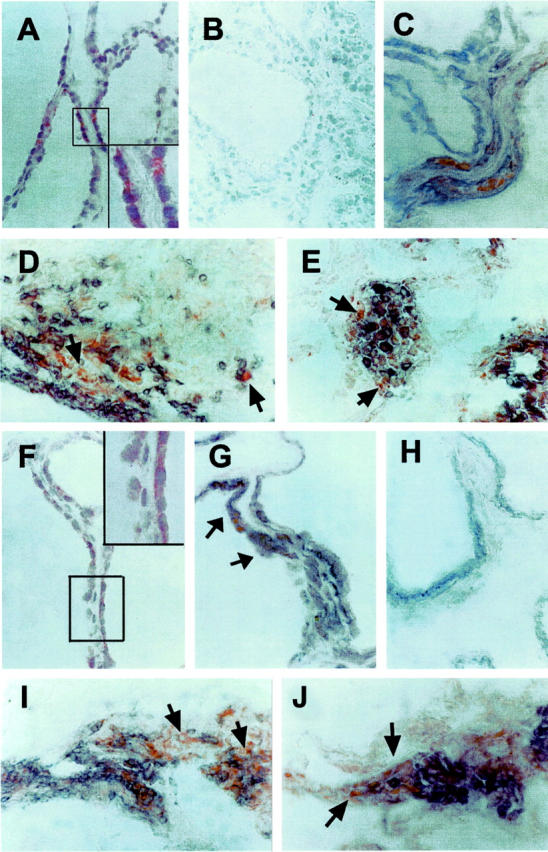
IP-10/CXCL10 and Mig/CXCL9 protein expression and localization in thyroid tissue specimens from patients with GD. A: High immunoreactivity for IP-10/CXCL10 protein in the thyroid tissue specimen from the same patient with GD shown in Figure 1 ▶ (red color). Inset: High-power magnification of a portion of the same section shown in A. B: Absence of staining for IP-10/CXCL10 when immunohistochemical staining was performed after preadsorption with 100 μg/ml of IP-10/CXCL10 in the same tissue specimen shown in A. C: Double immunostaining for IP-10/CXCL10 (red) and TSH receptor (bluish gray) in the thyroid follicular epithelium from the same patient with GD. No counterstain was applied. D: Double immunostaining for IP-10/CXCL10 (red) and CD3 (bluish gray) in many mononuclear infiltrating cells in the thyroid tissue specimen from the same patient with GD. No counterstain was applied. Arrows point to cells expressing only IP-10/CXCL10. E: Double immunostaining for IP-10 (red) and CD68 (bluish gray) in many mononuclear infiltrating cells in the thyroid tissue specimen from the same patient with GD. No counterstain was applied. Arrows point to cells expressing only IP-10/CXCL10. F: High immunoreactivity for Mig/CXCL9 protein in the thyroid tissue specimen from the same patient with GD (red). Inset: High-power magnification of a portion of the same section shown in F. G: Double immunostaining for Mig/CXCL9 (red) and TSH receptor (bluish gray) in the thyroid follicular epithelium from the same patient with GD (arrows). No counterstain was applied. H: A section from the same patient was stained with the anti-TSHr and revealed using vector SG as a peroxidase substrate. I: Double immunostaining for Mig/CXCL9 (red) and CD3 (bluish gray) in many mononuclear infiltrating cells in the thyroid tissue specimen from the same patient with GD. No counterstain was applied. Arrows point to cells expressing only Mig/CXCL9. J: Double immunostaining for Mig/CXCL9 (red) and CD68 (bluish gray) in many mononuclear infiltrating cells in the thyroid tissue specimen from the same patient with GD. No counterstain was applied. Arrows point to cells expressing only Mig/CXCL9. Original magnifications: ×400 (A–J); ×1000 (insets in A and F).
Figure 5.
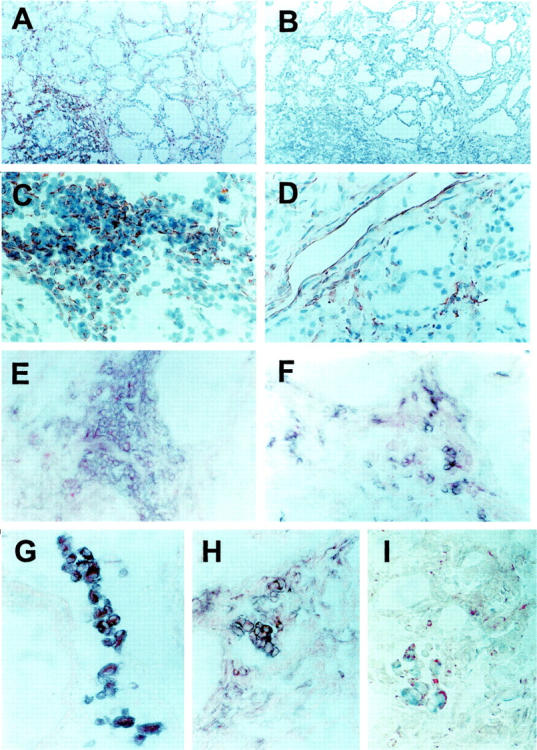
CXCR3 expression and distribution in thyroid tissue specimens from patients affected by GD. A: High CXCR3 expression in the thyroid of the same patient shown in Figures 1 and 2 ▶ ▶ affected by GD (red color). Section was counterstained with Gill’s hematoxylin. B: Absence of CXCR3 signal in an adjacent section immunostained with an isotype control Ab with irrelevant specificity. C: Higher power magnification showing CXCR3 expression in infiltrating inflammatory cells (red color) in the thyroid of the same patient affected by GD. Section was counterstained with Gill’s hematoxylin. D: Higher power magnification showing CXCR3 expression in endothelial cells (red color) in the thyroid of the same patient affected by GD. Section was counterstained with Gill’s hematoxylin. E: Double immunostaining for CXCR3 (red color) and CD4 (blue color) in the thyroid of the same patient affected by GD demonstrating CXCR3 expression by infiltrating T cells of the CD4 phenotype. No counterstain was applied. F: Double immunostaining for CXCR3 (red color) and CD8 (blue color) in the thyroid of the same patient affected by GD demonstrating CXCR3 expression by infiltrating T cells of the CD8 phenotype. No counterstain was applied. G: Double immunostaining for CXCR3 (red color) and CD68 (blue color) in the thyroid of the same patient affected by GD demonstrating CXCR3 expression by infiltrating monocytes/macrophages. No counterstain was applied. H: Double immunostaining for CXCR3 (red color) and CD11c (blue color) in the thyroid of the same patient affected by GD demonstrating CXCR3 expression by infiltrating dendritic cells. No counterstain was applied. I: Double immunostaining for CXCR3 (red color) and CD83 (blue color) in the thyroid of the same patient affected by GD demonstrating CXCR3 expression by infiltrating dendritic cells. No counterstain was applied. Original magnifications: ×100 (A–B); ×400 (C–I).
High IP-10/CXCL10 Serum Levels in Patients Affected by Recent-Onset GD
To support the possibility that IP-10/CXCL10 and Mig/CXCL9 production in the thyroid tissue of GD was related to the initial phases of inflammation, IP-10/CXCL10 levels were measured in the serum of 50 patients affected by GD and of 25 healthy patients. Mean IP-10/CXCL10 serum levels in GD were significantly higher (P < 0.05) in comparison with age- and sex-matched healthy patients (P < 0.05), although in a large number of GD IP-10/CXCL10 levels overlapped between the two groups, with high SD (Figure 6A) ▶ . Of note, however, in the GD group, IP-10/CXCL10 levels seemed to be a property of patients with recent-onset GD (R2 = 0.146, P < 0.05, Figure 6B ▶ ). By contrast, no correlation between IP-10/CXCL10 serum levels and other parameters such as sex, age, or levels of circulating anti-Tg or anti-TPO antibodies was observed (data not shown). Interestingly, the reduction of IP-10/CXCL10 levels was associated with a slight increase of serum levels of MDC/CCL22, a chemokine previously associated with a Th2 immune response, 12,13 with an inversion of the MDC/IP-10 ratio with the progression of the disease (Figure 6C) ▶ .
Figure 6.
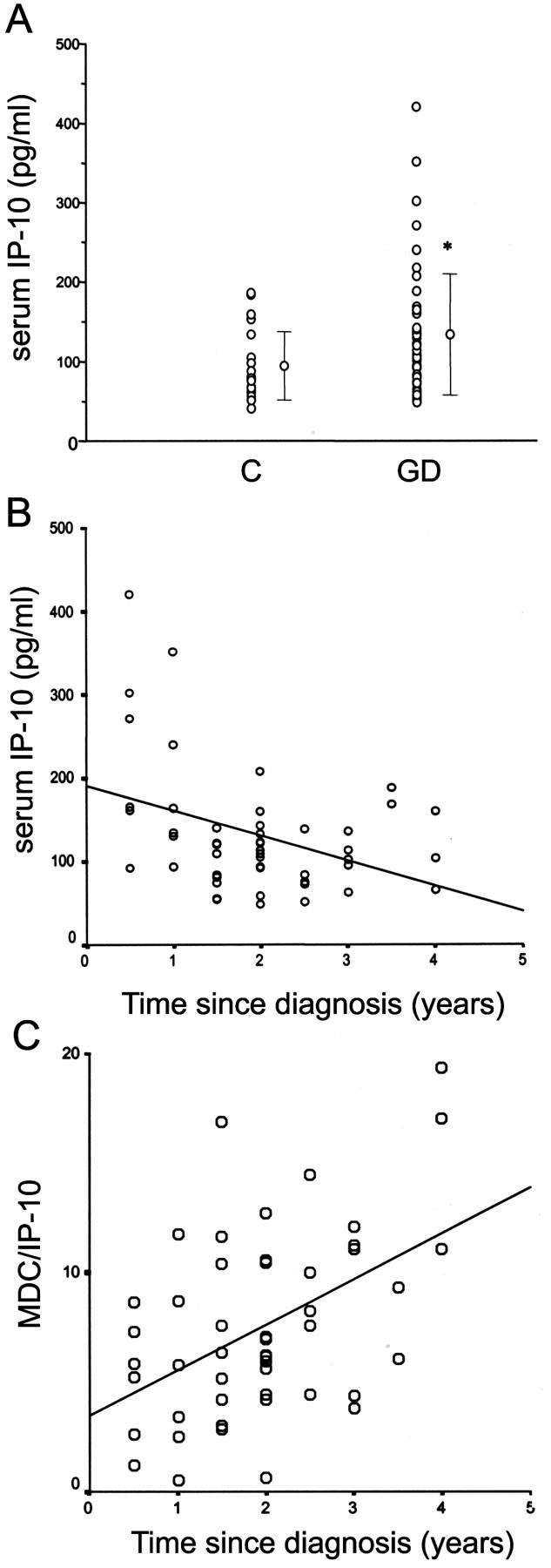
IP-10/CXCL10 serum levels are related with disease duration in patients affected by GD. A: Mean ± SD. IP-10/CXCL10 serum levels in GD are significantly higher (P < 0.05) in comparison with age- and sex-matched healthy patients, although in a large number of GD IP-10/CXCL10 levels overlaps with controls with high SD. B: Analysis of IP-10/CXCL10 serum levels in patients with different duration of GD. IP-10/CXCL10 serum levels are inversely correlated with the duration of the disease, the highest levels being a property of patients with recent-onset GD (R2 = 0.146, P < 0.05). C: Analysis of MDC/IP-10 ratio serum levels in patients with different duration of GD. The ratio between MDC/CCL22 and IP-10/CXCL10 serum levels is strictly correlated with the duration of the disease, the highest levels being a property of patients with late-onset GD (R2 = 0.2198, P < 0.005).
Discussion
Two polarized forms of CD4+ T helper (Th) cell-mediated-specific immune responses, based on their profile of cytokine production, have been described. 18,19 Th1 cells, which produce interleukin (IL)-2, IFN-γ, and tumor necrosis factor-β, activate macrophages and are responsible for cell-mediated immunity, preferentially develop during infections sustained by intracellular bacteria, and are involved in the pathogenesis of organ-specific autoimmune disorders. By contrast, Th2 cells, which produce IL-4, IL-5, IL-6, IL-9, and IL-13, induce strong antibody responses by B cells, as well as eosinophil activation, and predominate during infestations by gastrointestinal nematodes or in allergic inflammation. 18,19 Several data suggest that chemokines may be crucial for the recruitment and/or homing of Th1 or Th2 cells in the inflamed tissues, inasmuch as distinct chemokine receptors seem to be preferentially associated with one or the other T-cell subset. 20-24 Th1 cells indeed usually express CXC chemokine receptor 3 (CXCR)3 and CC chemokine receptor (CCR)5, 20-24 whereas Th2 cells preferentially express CCR3, CCR4, and CCR8. 20-24
Thyroid glands affected by GD show striking lymphocytic infiltration, mainly composed by CD45RO(+) memory T cells. 25 Evidence based on the assessment of cytokine profile of T-cell clones generated from T cells infiltrating the thyroid gland of patients with GD suggested a predominance of Th1 cells. 25,26 However, other studies based on PCR analysis revealed the expression of both IFN-γ and IL-4 in the bulk infiltrates of GD. 27 Recently, it has been reported that T cells involved in GD might change throughout the course of the disease, the predominant subtype of CD4+ T cells of patients with short duration hyperthyroidism being Th1, whereas T-cell clones from patients with longer duration disease predominantly showing the Th2 phenotype. 28 The mechanisms by which the different lymphocytic subsets are recruited and arrested in the thyroid tissue are unknown. However, recent studies have reported the existence of a correlation between mRNA levels of RANTES/CCL5, macrophage inflammatory protein-1alpha/CCL3 (MIP-1α) and -1beta/CCL4 (MIP-1β) and the degree of T-cell infiltration in GD, but not in TA, patients. 29,30 RANTES/CCL5, MIP-1α/CCL3 and MIP-1β/CCL4, 29,30 which are expressed mainly by lymphocytes, are perhaps involved in the maintenance of lymphocytic infiltration, 29,30 whereas stromal-derived factor (SDF)-1/CXCL12 seems to be involved in thyroid tissue homeostasis in TAs, but not in the genesis of lymphocytic infiltration in GD. 31 Furthermore, high expression of IP-10/CXCL10 and Mig/CXCL9 was recently reported in thyroid tissue obtained from patients suffering from Hashimoto’s thyroiditis. 32
In this study, we provide evidence that the chemokines IP-10/CXCL10 and Mig/CXCL9, as well as their common receptor, CXCR3, are present in human tissue specimens obtained from patients affected by GD. Thyroids from these patients expressed higher levels of IP-10/CXCL10, Mig/CXCL9, and CXCR3, if compared not only with normal thyroids, but also with TAs, a nonautoimmune thyroid disorder. In GD, IP-10/CXCL10, Mig/CXCL9, and CXCR3 were widely expressed by infiltrating inflammatory cells, the main producers of chemokines being macrophages and T lymphocytes. CXCR3 was expressed in T lymphocytes, monocytes/macrophages, and dendritic cells, as well as in some vascular structures, in agreement with the existence of endothelial cell activation. 33 Interestingly, as previously described in papillary carcinoma of the thyroid, 34 resident epithelial follicular cells also expressed IP-10/CXCL10 and Mig/CXCL9 mRNA and protein, suggesting a possible role for the inflamed thyroid epithelium in the recruitment of infiltrating lymphocytes. However, the most important finding emerging from this study was the demonstration of a clear difference in the level of IP-10/CXCL10 and Mig/CXCL9 expression in thyroid tissue obtained from patients with short or long duration GD. Similar results were obtained by analyzing IP-10/CXCL10 and Mig/CXCL9 mRNA expression by both in situ hybridization and quantitative RT-PCR. Furthermore, IP-10/CXCL10 and Mig/CXCL9 expression was also strictly related to IFN-γ mRNA levels detectable in the same thyroid tissue specimens.
These results were confirmed by the analysis of IP-10/CXCL10 serum levels in 50 patients affected by different duration GD and in 25 control patients. Mean serum IP-10/CXCL10 levels were significantly higher in patients affected by GD in comparison with control patients, even if in a large number of patients affected by GD serum IP-10/CXCL10 levels overlapped with those of controls. Of note however, a significant inverse correlation was observed between serum levels of IP-10/CXCL10 and disease duration, but not with sex, age, or levels of circulating Tg or TPO autoantibodies. Interestingly, a longer duration of the disease was also associated with an increase of MDC/IP-10 ratio in the sera of the same patients. MDC/CCL22 is a chemokine that has been found to be related to Th2 immune responses in the sera of patients affected by different types of immune disorders, 12,13 and the increase in the MDC/IP-10 ratio in the sera of GD patients is another signal of a possible shift from a Th1 to a Th2 immune response with the increase of disease duration.
Taken together, these data suggest a possible pathogenic role of chemokines IP-10/CXCL10 and Mig/CXCL9 in the initiation of GD. Recently, it has been shown that IP-10/CXCL10 produced by resident cells of the inflamed tissue has a pivotal role in initiating responses, which are characterized by the infiltration of CXCR3-expressing activated Th1 cells. 35-37 Moreover, after IP-10/CXCL10 production and the recruitment of CXCR3-expressing Th1 cells, other chemokines are produced that influence the subsequent events associated with leukocyte migration, such as cell spreading, diapedesis, and extravasation, thus contributing to the maintenance of lymphocytic infiltration. 35 Because IP-10/CXCL10 and Mig/CXCL9 are powerful inducers of Th1 cell recruitment through the interaction with their cognate receptor CXCR3, 8,20,21 the data reported in this study can account for the recruitment of these cells in the early phase of GD, as well as provide additional evidence to the view that Th1-dominated responses, or at least the production of IFN-γ and tumor necrosis factor-α, may play an important pathogenic role in the initial events of the inflammatory process in this disease. 25,26 On the other hand, the finding that IP-10/CXCL10 production declines at level of both thyroid gland and serum, together with the reduction of IFN-γ levels in tissue and with the observation of an increase of the MDC/IP-10 ratio in the serum in the later phases of GD, may be consistent with the results of another study suggesting a progressive switch from Th1- to Th2-polarized profile in the course of the disease. 28 This shift probably reflects a counter regulatory mechanism against inflammation, which has been observed even in other diseases. 37,38 Finally, the finding here reported that the CXCR3-binding chemokines are not only produced by infiltrating T cells and macrophages, but also by resident follicular epithelial cells themselves, suggests that these latter may play a previously unrecognized role in the recruitment of infiltrating cells and therefore in the triggering of thyroid gland inflammation. Additional experiments are required to identify the stimulatory agents (viral infections?) that may enable thyrocytes to produce IP-10/CXCL10 and Mig/CXCL9 in the initial stages of GD.
Footnotes
Address reprint requests to Paola Romagnani, M.D., Department of Clinical Pathophysiology, Endocrinology Unit, University of Florence, Viale Pieraccini 6, 50139, Firenze, Italy. E-mail: promagnani@iol.it.
Supported by funds from the Associazione Italiana per la Ricerca sul cancro (fellowship to E. L.), the Azienda Ospedaliera of Florence, and the Italian Ministery of Health.
P. R. and M. R. contributed equally to this work.
References
- 1.Weetman AP: Graves’ disease. N Engl J Med 2000, 343:1236-1248 [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP: Determinants of autoimmune thyroid disease. Nat Immunol 2001, 2:769-770 [DOI] [PubMed] [Google Scholar]
- 3.Marrack P, Kappler J, Kotzin BL: Autoimmune disease: why and where it occurs. Nat Med 2001, 7:899-904 [DOI] [PubMed] [Google Scholar]
- 4.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA: International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 2000, 52:145-176 [PubMed] [Google Scholar]
- 5.Rossi D, Zlotnik A: The biology of chemokines and their receptors. Annu Rev Immunol 2000, 18:217-242 [DOI] [PubMed] [Google Scholar]
- 6.Zlotnik A, Yoshie O: Chemokines: a new classification system and their role in immunity. Immunity 2000, 12:121-127 [DOI] [PubMed] [Google Scholar]
- 7.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Lewis IC, Baggiolini M, Moser B: Chemokine receptor specific for IP-10 and Mig: structure, function and expression in activated T-lymphocytes. J Exp Med 1996, 184:963-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR: The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 1998, 101:746-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, Galli G, Francalanci M, Manetti R, Marra F, Vanini V, Maggi E, Romagnani S: Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus. Blood 2001, 97:601-607 [DOI] [PubMed] [Google Scholar]
- 10.Penna G, Sozzani S, Adorini L: Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol 2001, 167:1862-1866 [DOI] [PubMed] [Google Scholar]
- 11.Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM: Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J Exp Med 2001, 194:1375-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonecchi R, Sozzani S, Stine JT, Luini W, D’Amico G, Allavena P, Chantry D, Mantovani A: Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood 1998, 92:2668-2671 [PubMed] [Google Scholar]
- 13.Galli G, Chantry D, Annunziato F, Romagnani P, Cosmi L, Lazzeri E, Manetti R, Maggi E, Gray PW, Romagnani S: Macrophage-derived chemokine production by activated human T cells in vitro and in vivo: preferential association with the production of type 2 cytokines. Eur J Immunol 2000, 30:204-210 [DOI] [PubMed] [Google Scholar]
- 14.Marra F, DeFranco R, Grappone C, Parola M, Milani S, Leonarduzzi G, Pastacaldi S, Wenzel UO, Pinzani M, Dianzani MU, Laffi G, Gentilini P: Expression of monocyte chemotactic protein-1 precedes monocyte recruitment in a rat model of acute liver injury, and is modulated by vitamin E. J Invest Med 1999, 47:66-75 [PubMed] [Google Scholar]
- 15.Raggi CC, Maggi M, Renzi D, Calabro A, Bagnoni ML, Scaruffi P, Tonini GP, Pazzagli M, De Bernardi B, Bernini G, Serio M, Orlando C: Quantitative determination of sst2 gene expression in neuroblastoma tumor predicts patient outcome. J Clin Endocrinol Metab 2000, 85:3866-3873 [DOI] [PubMed] [Google Scholar]
- 16.Romagnani P, Beltrame C, Annunziato F, Lasagni L, Luconi M, Galli G, Cosmi L, Maggi E, Salvadori M, Pupilli C, Serio M: Role for interactions between IP-10/Mig and CXCR3 in proliferative glomerulonephritis. J Am Soc Nephrol 1999, 10:2518-2526 [DOI] [PubMed] [Google Scholar]
- 17.Romagnani P, Pupilli C, Lasagni L, Baccari MC, Bellini F, Amorosi A, Bertoni E, Serio M: Inducible nitric oxide synthase expression in vascular and glomerular structures of human chronic allograft nephropathy. J Pathol 1999, 187:345-350 [DOI] [PubMed] [Google Scholar]
- 18.Romagnani S: T helper subsets in human disease states. Annu Rev Immunol 1994, 12:227-257 [DOI] [PubMed] [Google Scholar]
- 19.Abbas AK, Murphy KM, Sher A: Functional diversity of helper T lymphocytes. Nature 1996, 383:787-793 [DOI] [PubMed] [Google Scholar]
- 20.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F: Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 1998, 187:129-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunziato F, Cosmi L, Galli G, Beltrame C, Romagnani P, Manetti R, Romagnani S, Maggi E: Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J Leukoc Biol 1999, 65:691-699 [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A: Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998, 187:875-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM: CCR5 is characteristic of Th1 lymphocytes. Nature 1998, 391:344-345 [DOI] [PubMed] [Google Scholar]
- 24.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M: The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol 1998, 161:547-551 [PubMed] [Google Scholar]
- 25.Mariotti S, del Prete GF, Mastromauro C, de Carli M, Romagnani S, Ricci M, Pinchera A: The autoimmune infiltrate of Basedow’s disease: analysis of clonal level and comparison with Hashimoto’s thyroiditis. Exp Clin Endocrinol 1991, 97:139-146 [DOI] [PubMed] [Google Scholar]
- 26.Romagnani S: Lymphokine production by human T cells in disease states. Annu Rev Immunol 1994, 12:227-257 [DOI] [PubMed] [Google Scholar]
- 27.Stassi G, Di Liberto D, Todaro M, Zeuner A, Ricci-Vitiani L, Stopacciaro L, Ruco L, Farina F, Zummo G, De Maria R: Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol 2000, 1:483-488 [DOI] [PubMed] [Google Scholar]
- 28.Aniszewski JP, Valyasevi RW, Bahn RS: Relationship between disease duration and predominant orbital T cell subset in Graves’ disease. J Clin Endocrinol Metab 2000, 85:776-780 [DOI] [PubMed] [Google Scholar]
- 29.Ashhab Y, Dominguez O, Sospedra M, Roura-Mir C, Lucas-Martin A, Pujol-Borrell R: A one-tube polymerase chain reaction protocol demonstrates CC chemokine overexpression in Graves’ disease glands. J Clin Endocrinol Metab 1999, 84:2873-2882 [DOI] [PubMed] [Google Scholar]
- 30.Simchen C, Lehmann I, Sittig D, Steinert M, Aust G: Expression and regulation of regulated on activation, normal T cells expressed and secreted in thyroid tissue of patients with Graves’ disease and thyroid autonomy and in thyroid-derived cell populations. J Clin Endocrinol Metab 2000, 85:4758-4764 [DOI] [PubMed] [Google Scholar]
- 31.Aust G, Steinert M, Kiessling S, Kamprad M, Simchen C: Reduced expression of stromal-derived factor 1 in autonomous thyroid adenomas and its regulation in thyroid-derived cells. J Clin Endocrinol Metab 2001, 86:3368-3376 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Lopez MA, Sancho D, Sanchez-Madrid F, Marazuela M: Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 and Mig and attract CXCR3+ lymphocytes. J Clin Endocrinol Metab 2001, 86:5008-5016 [DOI] [PubMed] [Google Scholar]
- 33.Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M: Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest 2001, 107:53-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarpino S, Stoppacciaro A, Ballerini F, Marchesi M, Prat M, Stella MC, Sozzani S, Allavena P, Mantovani A, Ruco LP: Papillary carcinoma of the thyroid: hepatocyte growth factor (HGF) stimulates tumor cells to release chemokines active in recruiting dendritic cells. Am J Pathol 2000, 156:831-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD: Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med 2001, 193:975-980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romagnani P, Lazzeri E, Lasagni L, Mavilia C, Beltrame C, Rotondi M, Annunziato F, Francalanci M, Maurenzig L, Cosmi L, Salvadori M, Maggi E, Serio M: IP-10 and Mig production by glomerular cells in human proliferative glomerulonephritis and their regulation by nitric oxide. J Am Soc Nephrol 2002, 13:53-64 [DOI] [PubMed] [Google Scholar]
- 37.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP: Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 2001, 7:563-568 [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto K, Miyake S, Yamamura T: A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 2001, 413:531-534 [DOI] [PubMed] [Google Scholar]


