Abstract
Metastasis occurrence in the course of hepatocellular carcinoma (HCC) severely affects prognosis and survival. We have shown that HCC invasive cells express α3β1-integrin whereas noninvasive cells do not. Here we show that transforming growth factor (TGF)-β1 stimulates α3-integrin expression at a transcriptional level in noninvasive HCC cells, causing transformation into a motile and invasive phenotype. Such activities are inhibited by neutralizing anti-α3- but not anti-α6-integrin monoclonal antibodies. HCC invasive cells secrete abundant levels of active TGF-β1 in comparison with noninvasive cells, but in the latter, addition of active matrix metalloproteinases-2 increases the concentration of active TGF-β1. In this way, the cells express α3-integrin at a transcriptional level and acquire motility on Ln-5. By contrast, an anti-TGF-β1-neutralizing antibody reduces α3-integrin expression and the invasive ability of HCC invading cells. In HCC patients, TGF-β1 serum concentrations and α3-integrin expression are strongly correlated. The integrin, absent in normal and peritumoral liver parenchyma, is abundantly expressed in HCC primary and metastatic tissue. In particular, patients with metastasis show higher levels of TGF-β1 serum concentrations and stronger expression of TGF-β1 and α3-integrin in HCC tissues. In conclusion, TGF-β1 may play an important role in HCC invasiveness by stimulating α3-integrin expression, and could therefore be an important target for new therapies.
Hepatocellular carcinoma (HCC) is the most frequent epithelial malignancy of the liver. 1 Although great improvements have been made in the diagnosis and therapy of HCC, survival is still poor even for those patients with better clinical and pathological features. 2,3 This is mainly because of recurrence of the HCC after surgery, or to the presence of disseminated micronodules that rapidly grow and grossly invade the remaining liver parenchyma, affecting prognosis, survival, and life expectation. 2,4
HCC cells interact with several different extracellular matrix (ECM) components to migrate and invade surrounding tissues. Such interactions are ensured by integrins, a class of heterodimeric transmembrane receptors composed of one α and one β chain. 5,6 Integrins are polarized at cellular surfaces, and are involved in a number of cell functions such as adhesion, migration, invasion, proliferation, and survival. 7,8 In several physiological and pathological conditions, such as embryogenesis, development, wound healing, psoriasis, lichen planus, and skin and oral cancer, a rearrangement or de novo expression of integrins has been reported. 9-12 Recently, we have shown that α3β1-integrin is essential for HCC cell migration on laminin-5 (Ln-5) and invasion through three-dimensional ECM structures. 13 Ln-5 is an α3β3γ2 component of the Ln family, that has been shown to be implicated in cancer metastasis. 14-17
The mechanisms whereby α3β1-integrins modulate cancer cell migration and invasion are not entirely known, but we and other groups have shown that this integrin is implicated in the production and/or activation of matrix metalloproteinase (MMP)-9 and MMP-2. 13,18 These two enzymes are members of the MMP family, provided with proteolytic activity, secreted as proenzymes, and activated at the cellular surface by a membrane-type 1 MMP (MT1-MMP), implicated in cancer invasion and metastasis. 19,20 Regulation of this proteolytic activity is important to allow HCC cells to penetrate through the surrounding tissues enriched by ECM components as a consequence of the underlying cirrhosis: 21 breakdown of such tissue boundaries is crucial for HCC penetration through peritumoral tissues and spread. 22 α3β1-integrin expression has been shown to have a role during embryogenesis, when it is abundantly expressed by fetal hepatocytes, whereas it is absent in normal adult hepatocytes, probably as a result of tissue differentiation or a different specific microenvironment. 23-25 However, the mechanisms that modulate integrin expression on hepatocytes during tissue morphogenesis and neoplastic degeneration are still unclear.
Transforming growth factor (TGF)-β1 is a potent cytokine involved in a number of different functions such as epithelial mesenchymal transition, tissue morphogenesis, angiogenesis, and hence tumor progression, invasion, and metastasis. 26,27 In liver tissues, TGF-β1 has a profibrotic activity, stimulating ECM production, and is involved in the pathogenesis of liver cirrhosis. 28 In other tissues, it stimulates integrin expression in different types of both normal and cancer cells, although no studies have reported stimulation of integrin α3β1. 24
The goal of this study was to investigate how TGF-β1 modulates the invasiveness of HCC cells in vitro and in vivo, focusing particularly on integrin expression.
Materials and Methods
Cell Cultures
The human HCC cell lines Chang, Hep3B, Alexander, HepG2, and SK-Hep1, and the murine fibroblast cell line NIH3T3, were obtained from the American Type Culture Collection (Rockville, MD). Other HCC cell lines: ie, HLE and HLF, were obtained from the Japanese Cancer Resources Bank. HepG2.2.15, a HepG2-transfected with the hepatitis B virus, was kindly provided by A. McLachalan (The Scripps Research Institute, La Jolla, CA). Chang, Hep3B, SK-Hep1, and NIH3T3, cells were maintained in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) supplemented with 10% (v/v) fetal calf serum (Mascia Brunelli, Italy), 2 mmol/L l-glutamine, penicillin (20 U/ml), and streptomycin (20 μg/ml) (Life Technologies, Inc.). HLE, HLF, HepG2, HepG2.2.15, and Alexander, were cultured in RPMI (Life Technologies, Inc.), supplemented with 10% (v/v) fetal calf serum, l-glutamine, penicillin, and streptomycin. All cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Reagents
TGF-β1 was purchased from Sigma (St. Louis, MO); monoclonal antibodies BE12 and AA3 (against human β4) were kindly provided by V. Quaranta (The Scripps Research Institute, La Jolla, CA); 29 MIG1, TR1, and CM6 (against rat Ln-5) were prepared as described. 30 Gi9, monoclonal antibody (to human α2), was purchased from Immunotech (Marseille, France); F2, F4, and F1 (to human α3) were kindly provided by L. Zardi (IST, Genoa, Italy). 31 MAR6 and MAR4 (to human α6 and β1, respectively) 32 were a gift from S. Menard (INT, Milan, Italy). 32,33 Function-blocking antibodies against human α3- and β1-integrin (P1B5, and P4C10, respectively) were purchased from Life Technologies, Inc. (Gaithersburg, MD); antibody against α6 (GoH3) was purchased from Pharmingen (San Diego, CA). The neutralizing antibody against TGF-β1 was purchased from R&D Systems (Minneapolis, MN). The BB-94 MMP inhibitor was kindly provided by British Biotech Technology, Ltd., and diluted in dimethyl sulfoxide, following the manufacturer’s instructions. Rat Ln-5 was purified as previously described. 34 Fluorescein isothiocyanate-labeled goat anti-mouse secondary antibody was purchased from Zymed (San Francisco, CA). A reduced growth factor (GFR) Matrigel matrix was purchased from Becton Dickinson (Bedford, MA). The conditioned serum-free medium of NIH/3T3 cells was collected after 24 hours as previously described. 13 Human recombinant MMP-2 35 was kindly supplied by W. G. Stetler-Stevenson (National Institutes of Health, Bethesda, MD). 35
Fluorescence-Activated Cell Sorting (FACS) Analysis
Cells were cultured at low density in serum-free conditions in the presence or absence of TGF-β1 (3 ng/ml). In some experiments HCC noninvading cells were cultured in the presence or absence of active MMP-2 (1 μg/ml) for 48 hours, whereas HCC invading cells were cultured for 7 days in serum-free conditions in the presence of anti-TGF-β1-neutralizing antibody (50 μg/ml). Cells were then trypsinized and gently washed with ice-cold Tris-buffered saline (pH 7.4) containing 2% fetal calf serum, and centrifuged at low speed (400 × g). The cells were incubated with monoclonal antibodies against α2 (P1E6), α3 (F1, F2, F4, and P1B5), α6 (MAR6), β1 (MAR4), and β4 (AA3, BE12) integrins diluted in Tris-buffered saline (10 μg/ml) for 30 minutes on ice, washed, and centrifuged. Finally, they were incubated with a fluorescein isothiocyanate-labeled goat anti-mouse secondary antibody diluted in Tris-buffered saline (1:30) for 30 minutes on ice, and analyzed on a Beckman Coulter (Fullerton, CA) FACScan flow cytometer. As a negative control, cells were incubated with an antibody directed against human thyroglobulin (Tγ) or against αIIbβ3 (CP3) followed by a secondary antibody.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction Analyses
Total RNA was extracted from 2 × 106 HCC cells using the RNeasy minikit (Qiagen, Valencia, CA) and 2 μg of total RNA were reversed-transcribed using the RETROscript kit (Ambion, Austin TX), following the manufacturer’s instructions.
cDNA were diluted and one-twentieth of each sample was used for subsequent polymerase chain reaction analysis using Taq polymerase (DyNAzyme II; Finnzymes, Finland), and processed in a MWG-biotech thermocycler. Amplification was performed in sequential cycles including a45-second denaturation step at 94°C, followed by a 45-second annealing step at 60°C and 1-minute extension step at 72°C. The specific number of cycles (27 for GAPDH and 29 for α3-integrin, respectively), was determined after preliminary linear range-finding experiments to avoid the saturation phase (data not shown). The reaction was also performed in the absence of cDNA and in the presence of untreated RNA as template to test any possible contamination. After amplification, samples were separated on a 1.5% ethidium bromide-stained agarose gel, photographed, and quantified using an appropriate software image analysis program (Image Master 1D Prime; Pharmacia Biotech, UK). The relative intensity of the GAPDH bands was used to quantify the relative efficiency of the reverse transcriptase-polymerase chain reaction amplification. The oligonucleotides used as primer for amplification were purchased from Genset (Genset SA, Paris). Sequences were generated with DNAclub software in such a way that 5′ and 3′ primers would span different exons on the basis of the GenBank sequences (no. 7669491 for GAPDH and no. 6006010 for α3-integrin, respectively), and tested for specificity using the BLAST program available at the National Center for Biotechnology Information website. Sequences were as follows: for GAPDH: 5′-TCA CCA TCT TCT AGG AGC GAG A-3′, and 5′-CTT CTG GGT GGC AGT GAT G-3′ with a product of 337 pb; and for α3-integrin: 5′-AAG CCA AGT CTG AGA CTG TG-3′ and 5′-GTA GTA TTG GTC CCG AGT CT-3′ with a product of 656 pb.
Transwell Haptotactic Migration Assay
Migration assays were performed as previously described. 36 Briefly, transwell filters (8.0-μm pore size; Corning, NY) were coated on the bottom side with Ln-5 (1 μg/ml) or with blotto (5% nonfat dry milk in PBST) used as negative controls. After trypsinization, cells were plated (6 × 104) in the upper part of the chamber, under serum-free conditions. In some experiments MIG1 and TR1 (30 μg/ml) monoclonal antibodies against Ln-5 were added to the migration medium. In other experiments, cells migrated in the presence of the MMP inhibitor BB-94 (3 μg/ml) or in the presence of functional monoclonal antibodies directed against α3 (P1B5), α6 (GoH3), and β1 (P4C10) 10 μg/ml. Exogenous human recombinant MMP-2 was activated and added to the medium in the migration assay of the noninvasive HCC cells (concentrations ranging from 1 to 0.1 nmol/L). In other experiments MMP-2-treated noninvasive cells were abundantly washed before being used for migration assays. Filters were incubated for 16 hours at 37°C in a humidified incubator containing 5% CO2, fixed and stained with 0.5% crystal violet in methanol. Nonmigratory cells were removed with a cotton tip, and migratory cells were counted under a light microscope, with ×400 magnification. Each experiment was run in duplicate, and four microscopic fields from each of the two filters were counted in every case. Results were expressed as the mean number of cells counted in each field ± SD.
Chemoinvasion Through a Reconstituted Basement Membrane
The BM Matrigel preparation, extracted from the Engelbreth-Holm-Swarm mouse tumor, polymerizes at room temperature into a reconstituted three-dimensional structure. Eight-μm pore size (Nucleopore, CA) polycarbonate filters were coated with reconstituted BM GFR Matrigel (8 μg/cm2) according to the manufacturer’s instructions. 37 Briefly, the homogeneity of the coating was checked by protein staining. Filters were then reconstituted with serum-free medium, and placed in Boyden chambers. Cells, at concentrations of 3 × 105 resuspended in serum-free medium, were plated in the upper part of the chamber. In the lower compartment, NIH/3T3-conditioned serum-free medium was used as chemoattractant, and a nonconditioned serum-free medium was used as negative control. The Boyden chambers were incubated for 6 hours at 37C° in a humidified incubator with 5% CO2. The filters were then fixed and stained, and noninvasive cells were removed with a cotton tip, as described above. The total number of invasive cells was quantified by the image analysis software previously reported. 13 For each condition, three filters were run in each experiment. Results show the mean number of counted cells ± SD.
Detection of TGF-β1 by Enzyme-Linked Immunosorbent Assay
The invasive and noninvasive HCC cells were cultured in serum-free conditions, and conditioned media were collected after 72 hours of incubation. Protein concentrations were measured by the bicinchoninic acid method (Pierce Chemical Co., Rockford, IL). Samples were normalized for protein concentration, and active TGF-β1 levels were determined according to the manufacture’s instruction (R&D Systems). In addition, the TGF-β1 concentration was measured in the serum of 40 patients with HCC (28 metastatic and 12 nonmetastatic), and in 20 patients with liver cirrhosis as control. The clinical features of these patients have already been reported previously 22 and are not therefore repeated here.
Immunohistochemistry
This study was performed in accordance with the Helsinki Declaration and informed written consent was obtained from all patients. Tissue specimens were obtained from surgery and/or needle biopsy from the same HCC patients in whom we evaluated the TGF-β1 serum concentrations. Part of each specimen was included in 3.7% formaldehyde and processed for routine histology and the other part was immediately snap-frozen in liquid nitrogen. The tissues were included in Optimal Cutting Temperature 4583 (OCT) embedding compound (Miles Laboratories, Inc., Naperville, IL) and 5-μm-thick sections were serially cut with a microtome (Microtom, HM 505E; Carl Zeiss Oberkochen, Germany), collected on appropriate glass slides (Sigma Chemical Company) and processed for indirect alkaline phosphatase. As a control we used five liver tissues obtained from healthy patients who underwent surgical operations for accidental trauma.
Briefly, sections were fixed in a cold chloroform/acetone mixture for 10 minutes, air-dried, and incubated with different monoclonal antibodies directed against α3-integrin. All of the primary antibodies were diluted in RPMI medium with 10% added fetal calf serum, and after gentle washing, sections were then incubated with a proper secondary antibody (DAKO, Glostrup, Denmark) for 30 minutes in a humidified chamber. Sections were washed and incubated with alkaline phosphatase anti-alkaline phosphatase complexes. Staining was developed with red fuchsin chromogen, and abundantly washed for 20 minutes. Finally, sections were mounted with glycerol and examined with a Nikon Eclipse photomicroscope (Nikon, Corp., Tokyo, Japan).
Immunohistochemistry staining was quantified by counting the number of positive cells per microscopic field. Ten randomly chosen microscopic fields were evaluated for each tissue specimen, and the mean and SD were calculated.
Statistical Analysis
Student’s t-test was used to determine the 99% confidence intervals for the TGF-β1 serum levels and for quantification of the α3-integrin expression levels in HCC patients with and without metastasis. The correlation between these two factors was investigated with the Pearson correlation coefficient.
Results
All of the experiments in vitro were performed at least three times on all of the HCC cell lines: the invasive (HLE, HLF, Chang, SK-Hep1) and the noninvasive (HepG2, HepG2.2.15, Hep3B, Alexander) in accordance with our previous study. In vivo, we investigated the correlation between TGF-β1 serum concentrations and α3-integrin expression on the primary nodule, metastatic, and peritumoral tissues of all of the 40 patients with HCC.
TGF-β1 Stimulates Integrin Expression
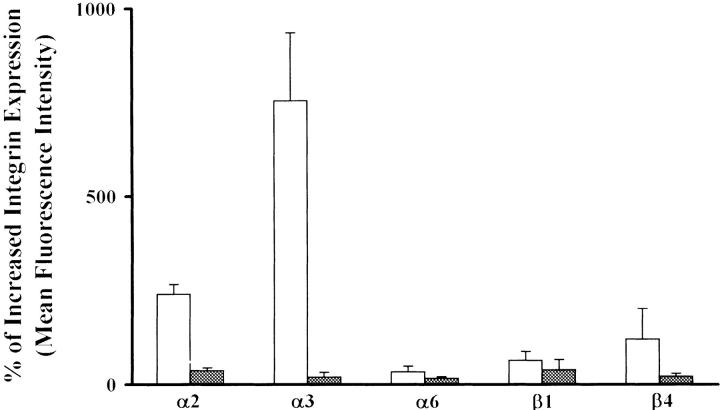
Exogenous TGF-β1 was added to the HCC cells in serum-free conditions, and integrin expression was evaluated by FACS analysis after 48 hours. As previously reported, 13 the integrin subunit α3 was virtually absent in HepG2 cells, whereas it became strongly expressed after 48 hours of stimulation with TGF-β1 in serum-free conditions (Figure 1) ▶ . Overlapping results were reproduced by using four different anti-α3 (F1, F2, F4, P1B5) monoclonal antibodies. Furthermore, we also investigated other integrin subunits such as α2, α6, β1, and β4, and we observed that after TGF-β1 stimulation they were slightly increased (Figure 1) ▶ ; consistent, similar results were observed in all of the other noninvasive HCC cells (Figure 1) ▶ . Instead, the addition of TGF-β1 to the invasive cells did not substantially affect the expression levels of any integrin subunits (Figure 1) ▶ .
Figure 1.
TGF-β1 stimulates integrin expression in noninvasive but not in invasive cells. TGF-β1 increases the expression of integrins and in particular of α3 by HepG2 noninvasive cells (open bars), but not in the SK-Hep1 invasive cells (filled bars). The percentage of increased expression is relative to untreated cells.
In conclusion, TGF-β1 stimulates α3 expression on the noninvasive but not on the invasive HCC cell lines that are already α3-positive.
TGF-β1-Stimulated HepG2 Cells Acquire Migration on Ln-5
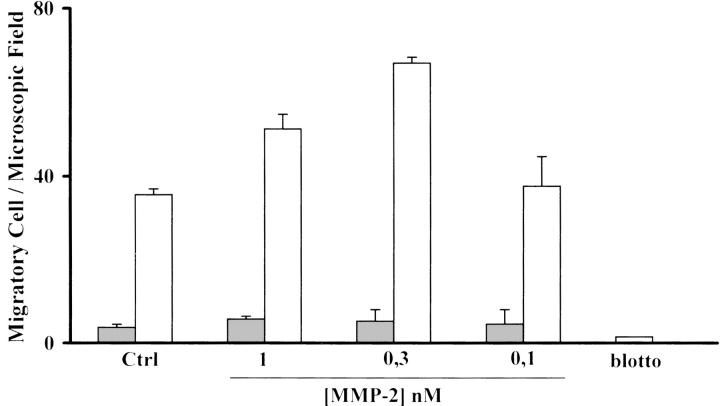
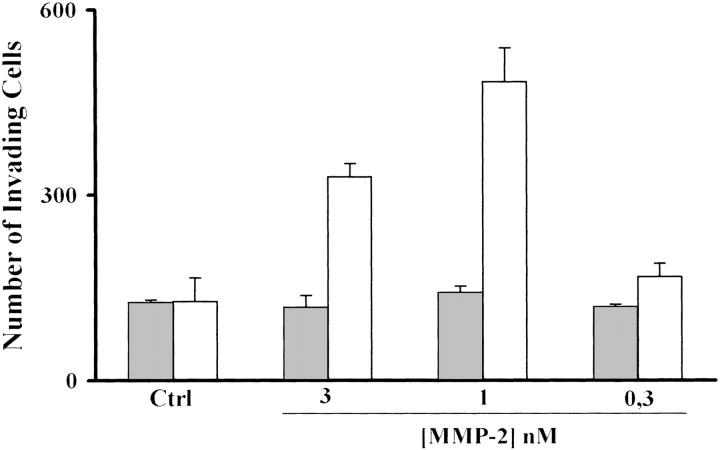
HepG2 cells, as well as the other noninvasive HCC cells, are not constitutively migratory on Ln-5, as previously reported. 13 However, as shown in Figure 2 ▶ , after TGF-β1 stimulation they become motile on Ln-5, and this ability is further enhanced in the presence of endogenously added MMP-2, whereas HepG2 unstimulated cells still remain immobile even in the presence of MMP-2. Migration in the presence of MMP-2 was dose-dependent at the same concentration range needed to stimulate other cell line migration on Ln-5. In all of the migration experiments blotto-coated filters were used as a negative control.
Figure 2.
TGF-β1 HepG2-treated cells acquire migration on Ln-5. Treated HepG2 cells (open bars) migrate on Ln-5 but untreated cells (filled bars) do not. This migration is further stimulated in a dose-dependent manner by exogenously added MMP-2, whereas untreated cells remain nonmigratory. Blotto is used as a negative control.
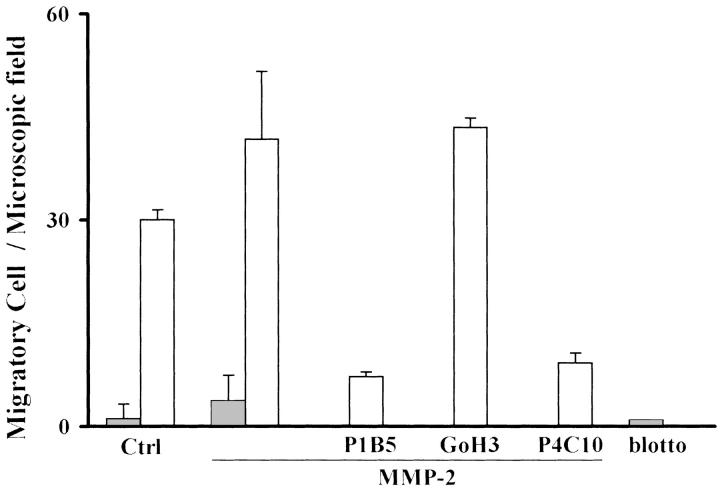
To test the hypothesis that HepG2 cells acquire migration after TGF-β1 stimulation because of the acquisition of α3 expression, functional blocking anti-integrin antibodies were added during migration assays in the presence of the MMP-2 concentration we had previously found to maximally increase migration. As reported in Figure 3 ▶ , antibodies directed against α3 (P1B5) and β1 (P4C10) blocked migration on Ln-5, whereas in the presence of antibodies directed against α6 (GoH3), stimulated HepG2 cells migrated in the same way as the control.
Figure 3.
TGF-β1 HepG2-treated cell migration on Ln-5 is α3-dependent. The α3-integrin expressed by TGF-β1 HepG2-treated cells (open bars) is functionally active because cell migration on Ln-5 in the presence of MMP-2 is blocked by neutralizing antibodies against α3 but not against α6-integrin (untreated cells shown by filled bars).
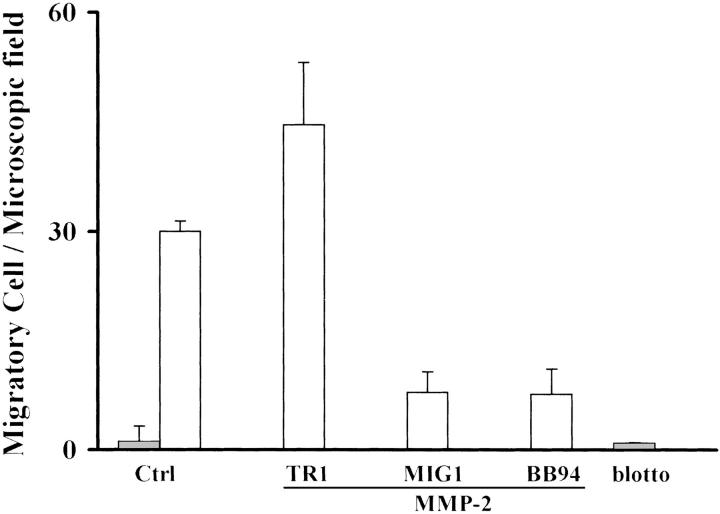
Furthermore, we investigated the role of MMP-2 on the TGF-β1-stimulated HepG2 cells using an MMP inhibitor, BB-94, and a monoclonal antibody directed against Ln-5, MIG-1, that as we have previously reported, specifically inhibits migration on MMP-cleaved Ln-5. 36 As shown in Figure 4 ▶ , migration of HepG2-stimulated cells in the presence of MMP-2 was blocked by MIG-1 monoclonal antibody, whereas TR1 antibody, a nonfunctional monoclonal antibody directed against Ln-5, did not affect motility. Addition of BB-94 into the migration medium caused inhibition of the migratory activity of HepG2-stimulated cells.
Figure 4.
TGF-β1 HepG2-treated cells migrate on MMP-cleaved Ln-5. HepG2-stimulated cells (open bars) migrate efficiently only on cleaved Ln-5 because the acquired migration is inhibited by the MMP inhibitor, BB94, and a monoclonal antibody that specifically blocks migration only on cleaved Ln-5. TR1 is used as the antibody control (untreated cells shown by filled bars).
In conclusion, TGF-β1-stimulated HepG2 cells acquire a motile phenotype on Ln-5 because of the α3 expression on the cellular surface; this migration is enhanced in the presence of MMP-2.
TGF-β1-Stimulated HepG2 Cells Invade Through a Reconstituted BM in the Presence of MMP-2
TGF-β1-treated and untreated HepG2 cells did not show any invasive ability except in the presence of exogenous MMP-2, that stimulates invasion through BM preparations in a dose-dependent manner in TGF-β1-stimulated HepG2 but not in unstimulated cells, as shown in Figure 5 ▶ . This suggests that TGF-β1 stimulation is required, but is not sufficient to cause HepG2 invasion through a three-dimensional structure like the BM.
Figure 5.
TGF-β1 HepG2-treated cells require MMP-2 to invade through a reconstituted BM. In the presence of MMP-2, HepG2-stimulated cells (open bars) invade through a reconstituted BM in a dose-dependent manner, whereas untreated cells remain noninvasive even in the presence of MMP-2 (filled bar).
Invasive But Not Noninvasive HCC Cells Secrete Active TGF-β1
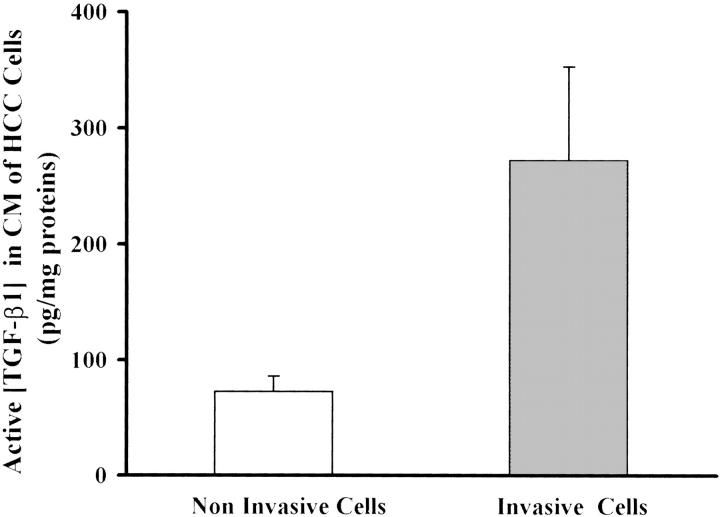
After having been stimulated with TGF-β1, HepG2 cells acquired a motile phenotype similar to that of invasive HCC cell lines on Ln-5, as did all of the other noninvasive cells. Therefore, we explored the possibility that the high levels of α3-integrin expression on the invasive cellular surface might be caused by endogenous production of active TGF-β1. To this end, we collected the serum-free conditioned media from all of the invasive and noninvasive cell lines, and measured the concentration of active TGF-β1 by an enzyme-linked immunosorbent assay test. As shown in Figure 6 ▶ , the invasive cell lines secrete much higher levels of active TGF-β1 (272 ± 80 pg/mg proteins) compared with the noninvasive cells (72 ± 13 pg/mg proteins), and this difference was statistically significant (P < 0.01), whereas the total TGF-β1 levels were similar between invasive and noninvasive cells (6271.2 ± 2211.4 versus 4526.6 ± 1418.9; P = 0.1). This suggests that the autogenous production of active TGF-β1 might be responsible for α3-integrin expression and for the acquired motile phenotype of the noninvasive cells.
Figure 6.
Active TGF-β1 levels in conditioned medium of HCC cells. Active TGF-β1 concentrations were higher in the conditioned medium of invasive than noninvasive cells (272 ± 80 versus 72 ± 13 pg/mg proteins; P < 0.001) measured by an enzyme-linked immunosorbent assay.
To test this hypothesis, noninvasive HepG2 cells were cultured in serum-free medium for 48 hours in the presence of active MMP-2, that has recently been shown to activate latent TGF-β1. 38 In accordance with this, the amount of active TGF-β1 in the conditioned medium of MMP-2-cultured HepG2 cells was significantly higher than in controls (47.7 ± 2.5 versus 108.3 ± 18.9 pg/mg protein; P = 0.002) as the mean of three distinct experiments (Figure 7A) ▶ .
Figure 7.
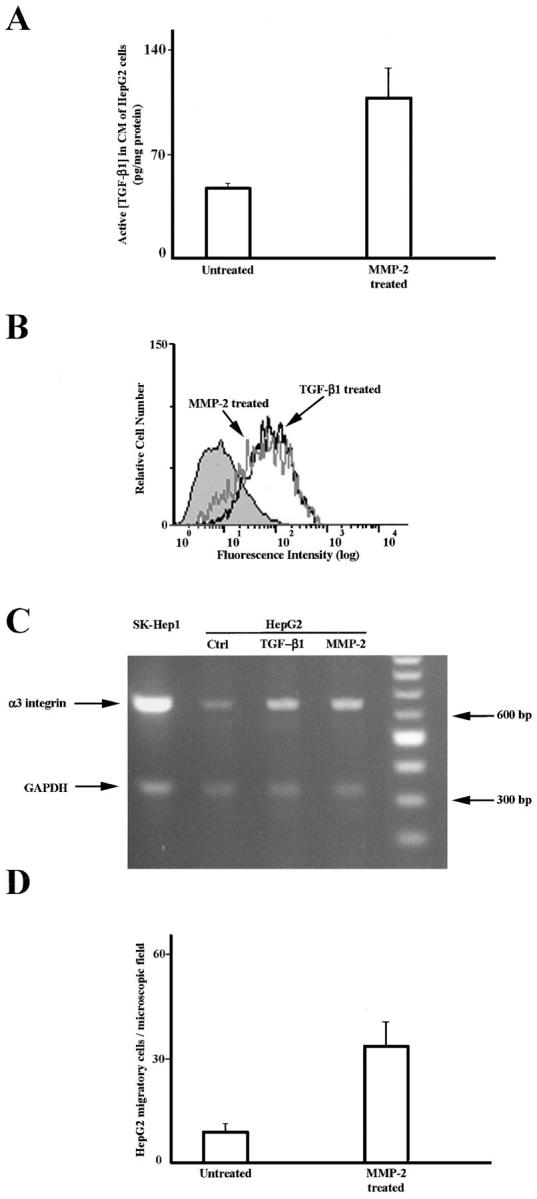
MMP-2 activates endogenous TGF-β1 and stimulates α3-integrin expression and migratory activity on HepG2-noninvading cells. The active TGF-β1 concentration is increased in the conditioned media of HepG2-noninvading cells cultured in the presence of MMP-2 (A). Each bar represents the mean of three different experiments. HepG2-α3-integrin expression is increased after incubation with TGF-β1 or with active MMP-2, as evaluated by FACS analysis (B). α3-integrin RNA is expressed in Sk-Hep1 invasive cells but not in HepG2-noninvading cells. After incubation with TGF-β1 or with active MMP-2 HepG2 cells express α3-integrin RNA (C). HepG2 migration on Ln-5 occurs only in MMP-2- and TGF-β1-cultured cells (D).
As shown in Figure 7B ▶ , α3-integrin expression was strongly increased after MMP-2 incubation, reaching comparable levels to those observed after TGF-β1 stimulation, as evaluated by FACS analysis. Furthermore, we examined RNA messengers of the noninvasive HepG2 cells cultured in the presence of MMP-2, TGF-β1, or control medium, using the SK-Hep1 invading cells as cell culture control. TGF-β1 as well as MMP-2 stimulate HepG2 α3 expression at a transcriptional level, whereas the SK-Hep1 cells already expressed the RNA in basal conditions (Figure 7C) ▶ . Finally, MMP-2 but not control cultured HepG2 cells showed strong migratory activity on Ln-5 (Figure 7D) ▶ .
These results suggest that MMP-2 activates the latent TGF-β1 secreted by the noninvasive HCC cells and this stimulates, at the transcriptional level, α3-integrin expression that then modulates cell migration.
Anti-TGF-β1-Neutralizing Antibody Inhibits α3-integrin Expression and Cell Invasion Through a Reconstituted BM
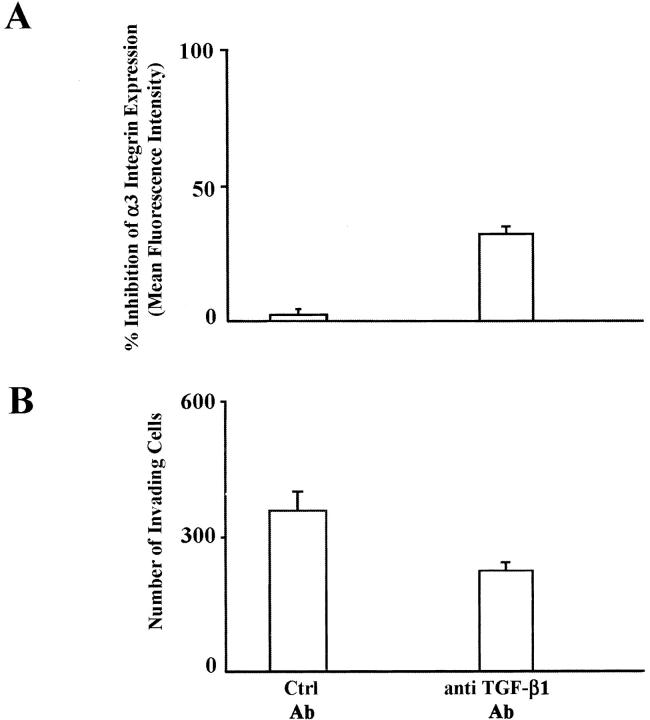
SK-Hep1-invading cells were cultured for 7 days in the presence of an anti-TGF-β1-neutralizing antibody or of a control immunoglobulin. As evaluated by FACS analysis, α3-integrin expression was reduced by the presence of anti-TGF-β1-neutralizing antibody (Figure 8A) ▶ . The invasive ability of anti-TGF-β1-neutralizing antibody SK-Hep1-treated and untreated cells was measured by chemoinvasion through Matrigel. As shown in Figure 8B ▶ , the number of invading SK-Hep1-treated cells was significantly lower than that of untreated cells (235.0 ± 12.9 versus 368.7 ± 50.2, P = 0.001).
Figure 8.
Anti-TGF-β1-neutralizing antibody reduces α3-integrin expression on SK-Hep1 invasive cells and inhibits invasion through a reconstituted BM. The SK-Hep1 expression of α3-integrin and the invasion ability through a reconstituted BM is reduced by the anti-TGF-β1-neutralizing antibody but not by control antibody (A and B, respectively).
TGF-β1 Serum Concentrations and α3-integrin Expression Levels in HCC Patients with and without Metastasis
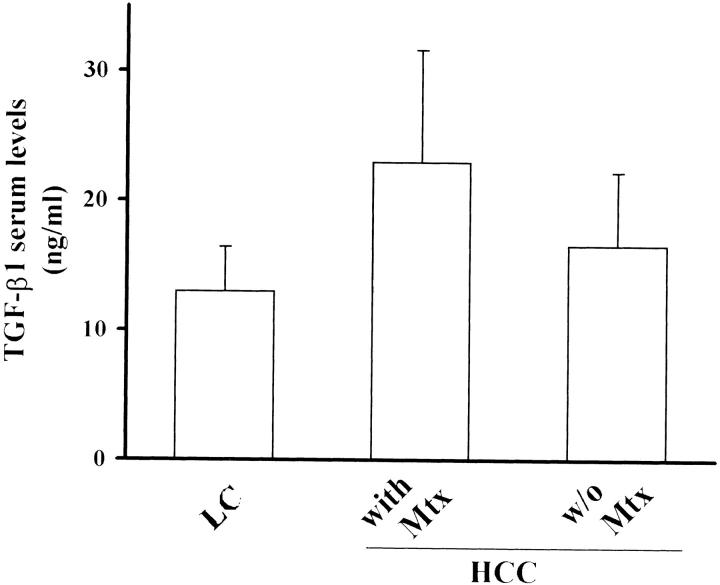
TGF-β1 concentration was evaluated in the serum of 40 HCC patients with and without metastasis and of 20 patients affected by liver cirrhosis as a control group. In agreement with other studies, 39 patients with HCC showed higher levels of TGF-β1 compared to patients with liver cirrhosis (20.6 ± 8.3 versus 12.9 ± 3.4 ng/ml; P < 0.001) (Figure 9) ▶ . Furthermore, a statistically significant difference (P < 0.02) was observed between patients with (22.9 ± 8.6 ng/ml) and without metastasis (16.6 ± 5.6). This suggests the involvement of TGF-β1 in HCC invasion and metastasis.
Figure 9.
TGF-β1 serum concentrations in patients with liver cirrhosis and HCC. TGF-β1 serum concentrations were higher in patients with HCC than with liver cirrhosis (P < 0.001); and further increased in patients with metastasis (Mtx) compared with those without Mtx (P < 0.02).
In the same patients we investigated the expression of α3-integrin in the HCC tumoral, peritumoral, and metastatic tissues by immunohistochemistry, part of the tissues being examined by regular histology to confirm the presence of metastasis (data not shown). Sections were processed under the same experimental conditions, to avoid staining heterogeneity, and four different monoclonal antibodies (F1, F2, F4, P1B5) directed against α3-integrin were used. Results obtained with the different antibodies were similar in all of the staining experiments (shown in Figure 10 ▶ ). α3-integrin is absent in the normal and peritumoral liver parenchyma, whereas it is expressed in the blood vessels present in the portal spaces. On the contrary, it becomes evident in the HCC tissues, and even more strongly expressed in the parenchyma of HCC patients with metastasis. The distribution pattern of α3-integrin was similar, although with different intensity, in metastatic and nonmetastatic patients, the integrin being mainly localized at the cellular surface of HCC cells, and in some cases also intracellularly (Figure 10) ▶ . Furthermore, the metastatic nodules showed an α3-integrin staining distribution and intensity comparable to that of the corresponding HCC primary lesions. The metastatic nodule was defined by histology (not shown).
Figure 10.
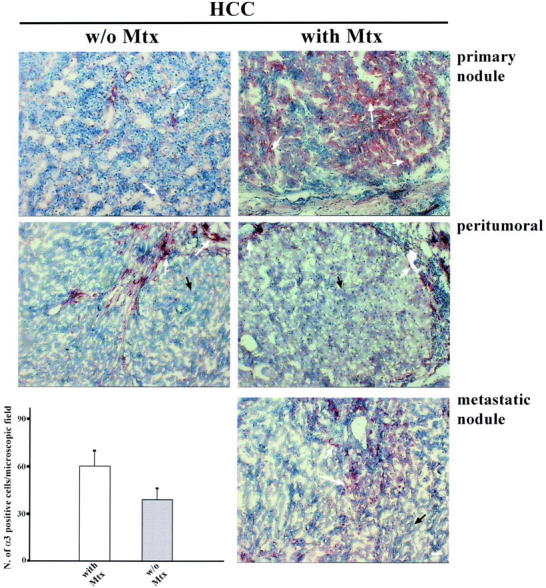
α3-integrin expression in tumoral, peritumoral, and metastatic HCC tissue. α3-integrin is localized in the parenchyma of metastatic (28 patients) and nonmetastatic (12 patients) HCC primary nodules (white arrows) with a similar distribution pattern, but is more strongly expressed in the metastatic tissue. In the peritumoral tissue of HCC patients with and without metastasis, α3-integrin is expressed by the blood vessel endothelial cells present in the stroma (white arrows) but is completely absent in the parenchyma (black arrows). In the metastatic nodule, α3-integrin is distributed as in the primary nodule. In the metastatic tissue, α3-integrin is expressed to the same extent as in the HCC primary nodule. α3-integrin expression level is higher in patients with metastasis than in those without (P < 0.001). Scale bar, 5 μm.
To quantify the levels of α3-integrin expression, we counted the number of positive cells in 10 randomly chosen microscopic fields and the mean ± SD reported in Figure 10 ▶ . As shown, the number of positive cells was higher in HCC patients with metastasis (60.5 ± 6.2) than without metastasis (39.7 ± 10.9), the difference being statistically significant (P < 0.001). These data suggest involvement of α3-integrin in HCC invasiveness and in metastasis formation.
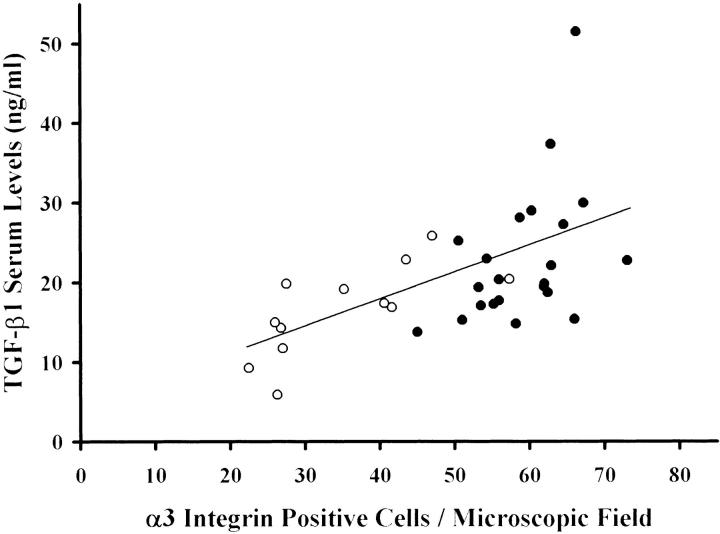
Furthermore, in HCC patients a strong correlation was found between TGF-β1 concentrations and α3-integrin levels (r = 0,57, P < 0.001) as shown in Figure 11 ▶ . In particular, patients with metastatic HCC showed higher concentrations of TGF-β1 and α3-integrin levels compared with those without metastasis (r = 0.44, P < 0.05, versus r = 0.72, P < 0.01). This suggests that the expression of α3-integrin in HCC tissue might be caused by TGF-β1.
Figure 11.
TGF-β1 serum concentrations and α3-integrin expression levels in patients with HCC. In patients with HCC, TGF-β1 serum concentrations and α3-integrin expression levels are strongly correlated (r = 0.57, P < 0.001). Patients with metastatic HCC (filled circle) have higher TGF-β1 serum concentrations and α3-integrin expression than patients with nonmetastatic HCC (open circle).
TGF-β1 Expression in HCC Patients with and without Metastasis
TGF-β1 expression was evaluated by immunohistochemistry using a polyclonal antibody against human TGF-β1 in the tumoral, peritumoral, and metastatic tissues of the 40 HCC patients. As shown in Figure 12 ▶ , TGF-β1 staining was more intense and diffuse in the tumoral tissues of HCC patients with than without metastasis, whereas no substantial differences were observed in the peritumoral tissues of both patient groups. TGF-β1 staining was mainly distributed around the parenchymal cells in the extracellular space. To quantify the TGF-β1 staining we counted the number of positive cells in 10 randomly chosen microscopic field and report the mean ± SD in Figure 12 ▶ . As shown in Figure 12 ▶ , the number of positive cells is higher in HCC patients with (51.6 ± 11.3) than without (38.1 ± 9.2) metastasis (P = 0.005).
Figure 12.
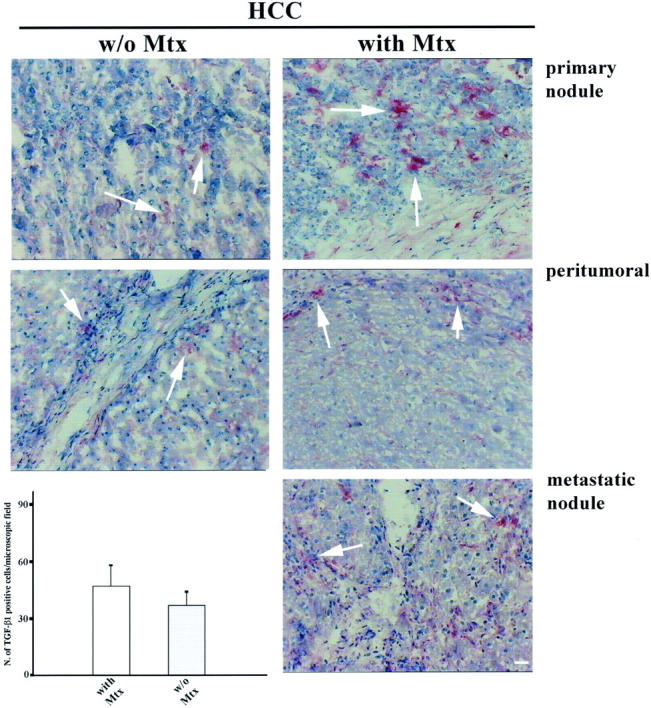
TGF-β1 expression in tumoral, peritumoral, and metastatic HCC tissue. TGF-β1 is distributed in the parenchymal tissues of tumoral, peritumoral, and metastatic HCC patients. In HCC metastatic patients the staining is more evident, and the number of positive cells is significantly higher (P = 0.005) than in nonmetastatic patients. Scale bar, 5 μm.
Discussion
TGF-β1 is a multifunctional growth factor involved in tumor progression and increased in the course of HCC. 27,39,40 We have previously provided evidence that α3β1-integrin expression has a crucial role for HCC invasiveness. 13 This study suggests that in vitro and in vivo TGF-β1 promotes HCC cell invasion via α3β1-integrin expression. We base this conclusion on the following observations: 1) TGF-β1 specifically stimulates α3-integrin expression at the transcriptional level in noninvasive HCC cell lines so that the stimulated cells acquire a migratory and invasive phenotype; 2) anti-α3 but not anti-α6-integrin-blocking antibodies inhibit this acquired motile activity; 3) HCC invasive cells lines, intensely α3-integrin-positive, secrete abundant amounts of active TGF-β1, whereas noninvasive cells secrete very low levels of active TGF-β1; 4) MMP-2 activates latent TGF-β1 in noninvasive HCC cultured cells stimulating α3-integrin expression at the transcriptional level and migratory activity on Ln-5; 5) in HCC invasive cells, anti-TGF-β1-neutralizing antibody reduces α3-integrin expression and cell invasiveness through a reconstituted BM; 6) in vivo, α3-integrin is expressed only in the HCC but not in the peritumoral and normal liver tissue, moreover, the intensity of the staining strongly correlates with TGF-β1 serum levels, with the tissue expression of TGF-β1 and with the occurrence of metastasis.
It has also been reported by other authors, that HepG2, as well as the other noninvasive HCC cell lines, do not express integrin α3β1, and it has also been demonstrated that TGF-β1 stimulates the expression of several integrin chains on noninvasive HCC cells. 24,41 In this study, we confirm these data, but in addition we show that after TGF-β1 stimulation, integrin α3β1 is the integrin most strongly expressed. Furthermore, in these cells the finding that α3-integrin is absent although TGF-β1 is secreted is explained by the fact that the noninvasive HCC cells lack TGF-β1 activation. Recently, MMP-2 has been shown to activate TGF-β1 38 but in our system the noninvasive cells do not secrete MMP-2. 13 Instead, addition of active MMP-2 to HepG2 noninvasive cells activates TGF-β1 and stimulates α3-integrin expression and motility on Ln-5. This mechanism could be very important because even HCC cells not secreting MMP-2 could potentially become invasive because it has been reported that cells surrounding HCC lesions express MMP-2. 22 Also, in invasive HCC cells TGF-β1 is crucial because neutralizing antibodies directed against it reduce α3-integrin expression and invasive activity. Furthermore, this de novo-expressed α3β1-integrin is functionally active because neutralizing anti-α3- but not anti-α6-integrin antibodies inhibit the cells’ acquired migration on Ln-5.
We have shown that HCC migration on Ln-5, and invasion through BM requires not only the presence of α3β1-integrin, but also the proteolysis of ECM components. 13 In our experiments, HCC noninvasive cells do not secrete gelatinases, as previously reported, 13 even after TGF-β1 treatment (data not shown), but the HCC-treated cells migrate on Ln-5, this motility being further increased by the presence of exogenously added MMP-2. This seems to be in apparent contrast with other studies reporting increased proteolytic activity in keratinocytes, 42 mesangial, 43 and pancreatic cells 44 but consistent with others showing decreased MMP activity after TGF-β1 stimulation. 45 In particular, in the liver TGF-β1 has a potent fibrogenetic effect stimulating ECM deposition, and suppressing proteolytic enzymes favoring the development of cirrhosis. 28 The migration of TGF-β1-treated cells on Ln-5 in the absence of MMP-2 likely occurs because of MT1-MMP, whose expression we have demonstrated at the cellular surface of all of the noninvasive HCC cells. 13 Motility on Ln-5 could be promoted by MT1-MMP, that has been shown to cleave the γ2 chain of Ln-5 as MMP-2 does, 34 although the increased migration induced by addition of MMP-2 could be caused by a synergistic effect on the proteolytic remodeling of Ln-5. The role of proteolytic activity in our experimental model is proved by the fact that migration is inhibited by the MMP inhibitor BB-94 and a specific antibody, MIG1, that we have previously found blocks migration on cleaved Ln-5. Moreover, MMP-2 is needed to allow HCC invasion through a reconstituted BM, because MT1-MMP proteolytic activity is not sufficient by itself. 13 All these data suggest that TGF-β1 is responsible for a more aggressive and invasive phenotype of several malignancies including HCC, 46-48 as confirmed by the fact that invasive HCC cells secrete abundant levels of active TGF-β1 whereas noninvasive cells do not. This finding is in agreement with other studies in which an autocrine stimulatory effect on HCC cells has been shown to be involved in malignant tumor progression. 40 In vivo, TGF-β1 involvement has been widely documented in the progression of several malignancies, and it has recently been proposed as a marker for prostate cancer. 49 In Western countries HCC develops in cirrhotic liver, and TGF-β1 levels are reported to be correlated with progression of the liver damage, as also with the occurrence of HCC. 28,39,40 As already reported in the literature, 38 we found higher TGF-β1 concentrations in patients with HCC than in those with liver cirrhosis, and a further increase in patients with a more aggressive and metastatic cancer phenotype. In addition, TGF-β1 serum concentrations strongly correlate with α3-integrin and TGF-β1 expression levels present in the HCC tissue and in the metastatic sites but not in the peritumoral parenchyma. Therefore, the fact that patients with metastatic HCC show higher TGF-β1 serum concentrations and α3-integrin expression levels, together with the in vitro data, suggest the idea that in HCC patients TGF-β1 triggers invasiveness by stimulating the expression of α3-integrin.
In conclusion, we propose a scenario in which TGF-β1 produced and activated by either cancer or surrounding cells stimulates α3-integrin expression at a transcriptional level, and this regulates the migratory and invasive ability of HCC cells. The de novo expression of α3-integrin in the HCC tissue, and in particular in the metastatic nodules, suggests that this integrin could be a marker of HCC development and metastasis. Because TGF-β1 is important for tumor invasion and metastasis it might be possible to block metastasis occurrence by blocking α3-integrin expression in HCC. This suggests new therapeutic strategies for blocking or preventing HCC spread.
Footnotes
Address reprint requests to Gianluigi Giannelli, M.D., Dipartimento di Clinica Medica, Immunologia, e Malattie Infettive, Sezione di Medicina Interna, Policlinico, Piazza G. Cesare 11, 70124 Bari, Italy. E-mail: g.giannelli@intmed.uniba.it.
Supported by the Ministry for University Technological and Scientific Research (grants to O. S.).
G. G. and E. F. contributed equally to this work.
References
- 1.Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M: Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet 1989, 2:1006-1008 [DOI] [PubMed] [Google Scholar]
- 2.Bergsland EK, Venook AP: Hepatocellular carcinoma. Curr Opin Oncol 2000, 12:357-361 [DOI] [PubMed] [Google Scholar]
- 3.El Serag HB, Mason AC, Key C: Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology 2001, 33:62-65 [DOI] [PubMed] [Google Scholar]
- 4.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I: Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994, 106:720-727 [DOI] [PubMed] [Google Scholar]
- 5.Hynes RO: Integrins: a family of cell surface receptors. Cell 1987, 48:549-554 [DOI] [PubMed] [Google Scholar]
- 6.Ruoslahti E, Giancotti FG: Integrins and tumor cell dissemination. Cancer Cells 1989, 1:119-126 [PubMed] [Google Scholar]
- 7.Loftus JC, Liddington RC: New insights into integrin-ligand interaction. J Clin Invest 1997, 100:S77-S81 [PubMed] [Google Scholar]
- 8.Ruoslahti E: Integrins. J Clin Invest 1991, 87:1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertle MD, Kubler MD, Leigh IM, Watt FM: Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest 1992, 89:1892-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannelli G, Savoia P, Schiraldi O, Lospalluti M, De Luca M, Marchisio PC, Quaranta V: Psoriatic lesions in patients with chronic liver disease are distinct from psoriasis vulgaris lesions, as judged on basis of integrin adhesion receptors. Hepatology 1994, 20:56-65 [DOI] [PubMed] [Google Scholar]
- 11.Giannelli G, Brassard J, Foti C, Stetler-Stevenson WG, Falk-Marzillier J, Zambonin-Zallone A, Schiraldi O, Quaranta V: Altered expression of basement membrane proteins and their integrin receptors in lichen planus: possible pathogenetic role of gelatinases A and B. Lab Invest 1996, 74:1091-1104 [PubMed] [Google Scholar]
- 12.McGuire RF, Bissell DM, Boyles J, Roll FJ: Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology 1992, 15:989-997 [DOI] [PubMed] [Google Scholar]
- 13.Giannelli G, Bergamini C, Fransvea E, Marinosci F, Quaranta V, Antonaci S: Human hepatocellular carcinoma (HCC) cells require both alpha3beta1 integrin and matrix metalloproteinases activity for migration and invasion. Lab Invest 2001, 81:613-627 [DOI] [PubMed] [Google Scholar]
- 14.Giannelli G, Antonaci S: Biological and clinical relevance of laminin-5 in cancer. Clin Exp Metastasis 2001, 18:439-443 [DOI] [PubMed] [Google Scholar]
- 15.Skyldberg B, Salo S, Eriksson E, Aspenblad U, Moberger B, Tryggvason K, Auer G: Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst 1999, 91:1882-1887 [DOI] [PubMed] [Google Scholar]
- 16.Sordat I, Rousselle P, Chaubert P, Petermann O, Aberdam D, Bosman FT, Sordat B: Tumor cell budding and laminin-5 expression in colorectal carcinoma can be modulated by the tissue micro-environment. Int J Cancer 2000, 88:708-717 [DOI] [PubMed] [Google Scholar]
- 17.Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K: Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res 1995, 55:4132-4139 [PubMed] [Google Scholar]
- 18.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A: The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer 2000, 87:336-342 [PubMed] [Google Scholar]
- 19.Kleiner DE, Stetler-Stevenson WG: Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 1999, 43(Suppl):S42-S51 [DOI] [PubMed] [Google Scholar]
- 20.Coussens LM, Werb Z: Matrix metalloproteinases and the development of cancer. Chem Biol 1996, 3:895-904 [DOI] [PubMed] [Google Scholar]
- 21.Bianchi FB, Biagini G, Ballardini G, Cenacchi G, Faccani A, Pisi E, Laschi R, Liotta L, Garbisa S: Basement membrane production by hepatocytes in chronic liver disease. Hepatology 1984, 4:1167-1172 [DOI] [PubMed] [Google Scholar]
- 22.Giannelli G, Bergamini C, Marinosci F, Fransvea E, Quaranta M, Lupo L, Schiraldi O, Antonaci S: Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer 2002, 97:425-431 [DOI] [PubMed] [Google Scholar]
- 23.Lora JM, Rowader KE, Soares L, Giancotti F, Zaret KS: Alpha3beta1-integrin as a critical mediator of the hepatic differentiation response to the extracellular matrix. Hepatology 1998, 28:1095-1104 [DOI] [PubMed] [Google Scholar]
- 24.Nejjari M, Hafdi Z, Dumortier J, Bringuier AF, Feldmann G, Scoazec JY: alpha6beta1 integrin expression in hepatocarcinoma cells: regulation and role in cell adhesion and migration. Int J Cancer 1999, 83:518-525 [DOI] [PubMed] [Google Scholar]
- 25.Scoazec JY, Flejou JF, D’Errico A, Fiorentino M, Zamparelli A, Bringuier AF, Feldmann G, Grigioni WF: Fibrolamellar carcinoma of the liver: composition of the extracellular matrix and expression of cell-matrix and cell-cell adhesion molecules. Hepatology 1996, 24:1128-1136 [DOI] [PubMed] [Google Scholar]
- 26.Hu PP, Datto MB, Wang XF: Molecular mechanisms of transforming growth factor-beta signaling. Endocr Rev 1998, 19:349-363 [DOI] [PubMed] [Google Scholar]
- 27.Oft M, Heider KH, Beug H: TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 1998, 8:1243-1252 [DOI] [PubMed] [Google Scholar]
- 28.Castilla A, Prieto J, Fausto N: Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N Engl J Med 1991, 324:933-940 [DOI] [PubMed] [Google Scholar]
- 29.Kajiji S, Tamura RN, Quaranta V: A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J 1989, 8:673-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plopper G, Falk-Marzillier J, Glaser S, Fitchmun M, Giannelli G, Romano T, Jones JC, Quaranta V: Changes in expression of monoclonal antibody epitopes on laminin-5r induced by cell contact. J Cell Sci 1996, 109:1965-1973 [DOI] [PubMed] [Google Scholar]
- 31.Bartolazzi A, Kaczmarek J, Nicolo G, Risso AM, Tarone G, Rossino P, Defilippi P, Castellani P: Localization of the alpha 3 beta 1 integrin in some common epithelial tumors of the ovary and in normal equivalents. Anticancer Res 1993, 13:1-11 [PubMed] [Google Scholar]
- 32.Pellegrini R, Bazzini P, Tosi E, Tagliabue E, Conforti G, Dejana E, Menard S, Colnaghi MI: Production and characterization of two monoclonal antibodies directed against the integrin beta 1 chain. Tumori 1992, 78:1-4 [DOI] [PubMed] [Google Scholar]
- 33.Bottini C, Miotti S, Fiorucci S, Facheris P, Menard S, Colnaghi MI: Polarization of the alpha 6 beta 4 integrin in ovarian carcinomas. Int J Cancer 1993, 54:261-267 [DOI] [PubMed] [Google Scholar]
- 34.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V: Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol 2000, 148:615-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridman R, Bird RE, Hoyhtya M, Oelkuct M, Komarek D, Liang CM, Berman ML, Liotta LA, Stetler-Stevenson WG, Fuerst TR: Expression of human recombinant 72 kDa gelatinase and tissue inhibitor of metalloproteinase-2 (TIMP-2): characterization of complex and free enzyme. Biochem J 1993, 289:411-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V: Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 1997, 277:225-228 [DOI] [PubMed] [Google Scholar]
- 37.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN: A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987, 47:3239-3245 [PubMed] [Google Scholar]
- 38.Yu Q, Stamenkovic I: Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 2000, 14:163-176 [PMC free article] [PubMed] [Google Scholar]
- 39.Sacco R, Leuci D, Tortorella C, Fiore G, Marinosci F, Schiraldi O, Antonaci S: Transforming growth factor beta1 and soluble Fas serum levels in hepatocellular carcinoma. Cytokine 2000, 12:811-814 [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki K, Date M, Furukawa F, Tahashi Y, Matsushita M, Sakitani K, Yamashiki N, Seki T, Saito H, Nishizawa M, Fujisawa J, Inoue K: Autocrine stimulatory mechanism by transforming growth factor beta in human hepatocellular carcinoma. Cancer Res 2000, 60:1394-1402 [PubMed] [Google Scholar]
- 41.Cai T, Lei QY, Wang LY, Zha XL: TGF-beta 1 modulated the expression of alpha 5 beta 1 integrin and integrin-mediated signaling in human hepatocarcinoma cells. Biochem Biophys Res Commun 2000, 274:519-525 [DOI] [PubMed] [Google Scholar]
- 42.Salo T, Lyons JG, Rahemtulla F, Birkedal-Hansen H, Larjava H: Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem 1991, 266:11436-11441 [PubMed] [Google Scholar]
- 43.Rocco MV, Chen Y, Goldfarb S, Ziyadeh FN: Elevated glucose stimulates TGF-beta gene expression and bioactivity in proximal tubule. Kidney Int 1992, 41:107-114 [DOI] [PubMed] [Google Scholar]
- 44.Miralles F, Battelino T, Czernichow P, Scharfmann R: TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol 1998, 143:827-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overall CM, Wrana JL, Sodek J: Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem 1991, 266:14064-14071 [PubMed] [Google Scholar]
- 46.Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ: Serum levels of transforming growth factor beta1 in patients with breast cancer. Arch Surg 2001, 136:937-940 [DOI] [PubMed] [Google Scholar]
- 47.Farina AR, Coppa A, Tiberio A, Tacconelli A, Turco A, Colletta G, Gulino A, Mackay AR: Transforming growth factor-beta1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. Int J Cancer 1998, 75:721-730 [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K: Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 2001, 91:964-971 [PubMed] [Google Scholar]
- 49.Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM: Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol 2001, 19:2856-2864 [DOI] [PubMed] [Google Scholar]