Abstract
Human immunodeficiency virus-associated nephropathy (HIVAN) is etiologically related to the viral infection, but the mechanisms of virus-induced renal injury remain undetermined. Peculiar histopathological features of HIVAN are the enhanced proliferation and the loss of differentiation markers of glomerular epithelial cells (podocytes). We found that podocytes were not permissive to HIV-1 replication. In this study we investigated the effects of the HIV-1 regulatory protein Tat on primary cultures and on a continuous line of podocytes. Our results demonstrated that Tat induced hyperproliferation of these cells in a dose-dependent manner. This activity was primarily mediated by the basic domain of the viral protein. Proteoglycans were required for this phenomenon because Tat-induced increase of podocyte growth was significantly impaired by inhibition of proteoglycan synthesis with β-d-xyloside. In podocyte cultures Tat promoted both the transcription and the release of basic fibroblast growth factor, which contributed to the enhanced cell proliferation. Moreover, Tat deregulated the podocyte phenotype causing down-regulation of maturity markers such as WT-1 and synaptopodin, alteration of cytoarchitecture, and impairment of permselectivity. Together, these results demonstrate that the interaction of extracellular Tat with podocytes can induce alterations that mimic the pathological changes of podocytes detected in HIVAN.
A relevant increase in kidney disease incidence has been recently reported in human immunodeficiency virus (HIV)-infected patients. 1 HIV-associated nephropathy (HIVAN) is the prominent renal complication of HIV infection, characterized by heavy proteinuria and progressive renal failure with severe prognosis. 2,3 If untreated, HIVAN may progress to end-stage renal disease in few months. In African-American adults, HIVAN is the third leading cause of end-stage renal disease. 1 HIVAN is considered a pan-nephropathy 2 characterized, on the pathological point of view, by a collapsing form of glomerulosclerosis associated with severe tubulointerstitial injury (microcystic dilatation and apoptotic changes of tubuli and mononuclear infiltration). It has been recently shown that glomerular lesions of HIVAN involve striking alterations of glomerular epithelial cells (podocytes), which include hyperplasia and dysregulation of mature podocyte phenotypic markers. 4 Clinical and experimental data indicate that HIVAN is etiologically related to HIV infection, but the pathogenesis of virus-induced renal injury is not clearly understood. 5 We recently reported that tubular epithelial cells and glomerular mesangial cells (MCs) could sustain viral productive replication with distinct pathological effects that mimic the alterations detected in HIVAN. In cultured tubular epithelial cells, HIV-1 caused apoptosis, 6 whereas in MCs HIV-1 established persistent infection and stimulated the synthesis of cytokines involved in the pathogenesis of glomerulosclerosis. 7 Conversely, podocytes were not permissive to virus replication, possibly because they did not express HIV-1 receptors. 7 A HIVAN-like disease characterized by podocyte dysregulation occurred in transgenic mice with intrarenal expression of gag-pol-deleted HIV provirus. In these transgenic mice the synthesis of unspliced and multiply spliced viral regulatory proteins (Tat, Rev, and Nef) occurred in renal cells in the absence of a productive viral replication. 8,9 Thus, podocyte alterations could be caused by the expression of these viral proteins. In the present study, we investigated the effects of Tat on human cultured podocytes. HIV-1 Tat is a transactivating factor of 86 to 101 amino acids, which is encoded by two exons and is translated from multiply spliced 2-kb mRNAs. 10 Tat is essential for HIV-1 replication and it is released by HIV-infected cells, as demonstrated by the detection of Tat in the sera of HIV patients. 11,12 Extracellular Tat exerts pleiotropic effects on uninfected cells either entering into the cells or interacting with cell-surface receptors. In fact, Tat has been shown to stimulate proliferation, migration, and production of growth factors and cytokines in several cell types. 13-16 Tat can also elicit apoptotic effects on T cells and neurons 12,17,18 whereas it may induce cell survival in other cell types such as endothelial and Kaposi’s sarcoma cells. 19 Remarkably, Tat can induce opposite effects (promotion versus inhibition) on cell survival on the same cell type, depending on its concentration. 11 Thus, there is an increasing body of evidence for the role of extracellular Tat in AIDS-associated immune dysfunction and pathological manifestations. 20 Herein we report that HIV-1 Tat affects the podocyte phenotype and functions inducing cell proliferation, down-regulation of mature phenotypic markers, and alteration of cytoarchitecture.
Materials and Methods
Reagents
Reagents were obtained from the following sources: HIV-1 Tat, rabbit anti-Tat serum from Intracel (London, UK); [11-24], [46-60] Tat peptides from Neosystem (Strasbourg, France); [61-86] Tat peptide from Tecnogen (Piana di Monte Verna, Italy); monoclonal antibody (mAb) against Ki-67 antigen, biotinylated anti-mouse IgG, biotinylated anti-rabbit IgG, and peroxidase-conjugated streptavidin from DAKO (Glostrup, Denmark); mAb anti-synaptopodin, (kindlyprovided by Dr. P. Mundel, Division of Nephrology, Albert Einstein College of Medicine, Bronx, NY, USA); anti-Wilms’ tumor antigen (WT-1) rabbit polyclonal Ab, anti-Flk-1 rabbit polyclonal Ab, and anti-Flt-1 rabbit polyclonal Ab from Santa Cruz Biotechnology (Santa Cruz, CA); mAb anti-basic fibroblast growth factor (bFGF) from R&D (Minneapolis, MN); mAb anti-human integrin α3β1, mAb anti-human integrin αvβ3, and mAb anti-human integrin β1 from Chemicon Int. (Temecula, CA); fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse and anti-rabbit IgG, FITC-labeled phalloidin, β-d-xyloside, (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxy-anilide inner salt) sodium salt (XTT), and other immune reagents and chemicals were from Sigma Chemical Co. (St. Louis, MO), unless otherwise specified. Tissue culture media were from Euroclone (Paignton, UK) and fetal calf serum (FCS) from Hyclone (Logan, UT); molecular biology reagents were from Perkin-Elmer Cetus (Norwalk, CT); plastic-ware from Falcon (Becton-Dickinson, San Jose, CA).
Preparation of Human Glomerular Cells
Primary cultures of glomerular mesangial and epithelial cells were established as described. 21 Briefly, decapsulated glomeruli were isolated by differential sieving from renal cortex fragments taken from surgically removed kidneys of six Caucasian patients, treated with collagenase and seeded in Dulbecco’s modified Eagle’s medium (DMEM) containing 20% FCS and d-valine. After 2 weeks MCs were collected by digestion with trypsin-ethylenediaminetetraacetic acid and grown in DMEM-10% FCS. Cultured cells were characterized according to established criteria. 21-23 Primary cultures of podocytes were obtained by plating at high-density glomeruli untreated with collagenase. After a 10 day-incubation in DMEM-20% FCS, the cultures were trypsinized and the outgrowing podocytes were expanded. Phenotypic characterization was performed according to cell morphology (polyhedral cells with cobblestone-like appearance); positive staining for synaptopodin, WT-1, cytokeratins, vimentin, and laminin; negative staining for smooth muscle-type myosin, FVIIIr:Ag, and CD45; and cytotoxicity in response to 50 μg/ml of puromycin aminonucleoside. Established lines of differentiated MCs and podocytes were obtained by infection of pure primary cultures with a hybrid Adeno5/SV40 virus, as described. 21 Individual foci of immortalized cells were subcultured and cloned. The selected clones were used between passages 30 and 45. The podocyte line was characterized according to the phenotype, following the criteria mentioned above.
Cell Growth Assays
MCs and podocytes of primary cultures and continuous lines were seeded at the concentration of 2 × 104 cells/cm2 and rested for 5 hours in DMEM-2% FCS. Then, the cells were washed and stimulated with different doses of Tat (0.2 to 200 ng/ml) in medium containing 0.1% bovine serum albumin (BSA). To evaluate the kinetics of the cell growth, the cultures were incubated in medium without phenol red containing 200 μg/ml of XTT and 0.2% phenazine methosulphate and the absorbance at 450 nm was monitored at different times. 24 The results were confirmed by viable cell counting with the Trypan blue exclusion test. Podocyte proliferation was also measured by the [3H]-thymidine uptake method. Each culture condition was tested in triplicate incubating the cells for 48 hours in 96-well plates as described. During the last 6 hours 1 μCi/well of [3H]-thymidine (Amersham Pharmacia Biotech, Little Chalfont, UK) was added to the cultures. The harvested samples were processed for β-scintillation counting.
Experiments were performed by stimulating podocytes with Tat peptides at concentrations equimolar to those of Tat protein. Proliferation tests were also performed by plating podocytes into 96-well plates coated with Tat [1 μg/ml in phosphate-buffered saline (PBS), pH 7.4, for 12 hours at 4°C]. After washing the plates with PBS, the podocytes were seeded at the usual concentration and their growth was evaluated as described. Blocking experiments were done by preincubating podocyte cultures with antibodies (Abs) specific to surface molecules (5 μg/ml in DMEM-0.1% BSA for 1.5 hours at 37°C). To inhibit the synthesis of proteoglycans, β-d-xyloside (25 mmol/L) was added to podocyte cultures 24 hours before stimulation with Tat or Tat peptides. To evaluate the influence of soluble factors in Tat-induced hyperproliferation, podocyte cultures were stimulated with 10 ng/ml of Tat for 1 hour at 37°C or incubated with medium alone, washed for three times and then incubated with DMEM-0.1% BSA. After a 24-hour incubation the supernatants of Tat- and mock-stimulated cultures were taken and centrifuged at 15,000 rpm for 15 minutes at 4°C, then the media were absorbed overnight at 4°C into microtiter plates coated with anti-bFGF mAb (10 μg/ml in PBS pH 7.4 for 12 hours at 4°C) or with anti-Ki67 mAb, used as control antibody. Podocyte cultures were incubated for 24 hours at 37°C with 50% DMEM-0.1%BSA and 50% conditioned media. Cell proliferation was evaluated by viable cell counting and the XTT assay.
Immunostaining Studies
Podocytes were cultured in chamber slides and stimulated with 10 ng/ml of Tat. After a 24-hour incubation the cells were fixed with cold methanol for 15 minutes and washed with PBS. The monolayers were incubated with 1 μg/ml of anti-Ki-67 mAb for 1 hour at room temperature, washed in PBS, stained with FITC-conjugated goat anti-mouse IgG, and examined with an epifluorescence microscope. Control experiments included incubation of the cells with irrelevant mouse isotypic Igs or omission of primary Abs followed by the appropriate labeled secondary Abs. Immunoperoxidase tests were performed on podocyte cultures fixed in 3.5% paraformaldehyde for 15 minutes at room temperature. The monolayers were incubated with 3% hydrogen peroxide to quench endogenous peroxide activity and with PBS-1% BSA to prevent aspecific reactions. Then, the specimens were incubated with primary Abs (2 μg/ml of anti-WT-1 Igs; 1:10 diluted supernatant of hybridoma specific to synaptopodin; 2 μg/ml of irrelevant mouse and rabbit isotypic Igs) for 1 hour at room temperature After washing with Tris-buffered saline, pH 7.4, and 2% Triton X-100, the cells were incubated at room temperature for 10 minutes with biotinylated anti-mouse or anti-rabbit Igs and then for 10 minutes with streptavidin conjugated to horseradish peroxidase. The specimens were stained with 0.03% benzidine and counterstained with hematoxylin and eosin.
The expression of integrins (α3β1, αvβ3, β1) and vascular endothelial growth factor (VEGF)-receptors (VEGFRs: Flk-1, Flt-1) in podocytes was evaluated by flow cytometry. Briefly, 106 cells were incubated for 30 minutes at room temperature with 1 μg of the specific Ab and then with FITC-labeled goat anti-mouse or anti-rabbit IgG. After washings with PBS, the cells were analyzed (FACScan, Becton Dickinson).
Cell Cytoskeleton Studies
Cytoskeleton alterations were studied on fixed podocytes after permeabilization with 0.1% Triton X-100 in PBS and stained for F-actin with 2 μg/ml of FITC-conjugated phalloidin. The modification of podocyte shape was monitored as described. 25 The cells were plated in a hermetically sealed Plexiglas Nikon NP-2 incubator (Nikon Co., Tokyo, Japan) at 37°C, rested for 5 hours with medium containing 2% FCS, stimulated with Tat (1 to 10 ng/ml) and then observed throughout a 2-hour period by Nikon Diaphot inverted microscope with a ×10 phase-contrast objective. The imagines were recorded by a JVC-1CCD video camera (video card Targa 2000; Truevision, Santa Clara, CA) and evaluated by MicroImage-analysis system (Casti Imaging, Venice, Italy).
Permeability Assay
Podocytes were seeded on polycarbonate filters (pore size, 0.4 μm) of Transwell chambers (Costar, Cambridge, MA) at the concentration of 1.5 × 105/well in DMEM-2% FCS. The permeability of the monolayers was measured by diffusion of Trypan blue-albumin complexes. 26 After overnight incubation, 0.2 ml of medium without phenol red and FCS were added in the upper chamber and the cells were stimulated for 1 hour at 37°C with 10 ng/ml of Tat. Then, the upper chamber was filled with 0.3 ml of DMEM-200 mmol/L Trypan blue-albumin. The chambers were incubated at 37°C with continuous agitation. After 5 minutes the monolayers showing leakage of dye were discarded. Transport of albumin across monolayers was determined by sampling aliquots at different times (15 to 120 minutes) and measuring absorbance at 590 nm in the outer and inner wells in duplicate. 26
Analysis of bFGF Synthesis by Podocytes
The expression of bFGF was assessed by reverse transcriptase-polymerase chain reaction (PCR) using total RNA taken from untreated or Tat-stimulated podocytes. cDNA was synthesized by priming RNA (1, 0.1, or 0.01 μg) with oligo-dT (Clontech). PCR was performed using 30 pmol/L of mRNA-specific primers (forward 5′GGCTTCTTCCTGCGCATCC3′; reverse, 5′CCGTACTTTGCTTGACCCG3′) synthesized by Genset (Paris, France). 27 PCR was performed for only 20 cycles (30 seconds at 94°C, 30 seconds at 57°C, and 30 seconds at 72°C) to detect amplicons of bFGF transcripts (283 bp) under conditions of linear amplification. 21 The production of bFGF by podocytes was evaluated by measuring the bFGF content of podocyte culture supernatants, taken in different experimental conditions, by a commercial enzyme-linked immunosorbent assay kit following the manufacturer’s recommendations (R&D Systems, Minneapolis, MN).
Statistical Analysis
All data are given as mean values ± SD. Differences among multiple groups were evaluated by one-way analysis of variance in combination with Tukey’s multiple comparison test. A P value of <0.05 was considered significant.
Results
HIV-1 Tat Enhanced Proliferation of Podocytes
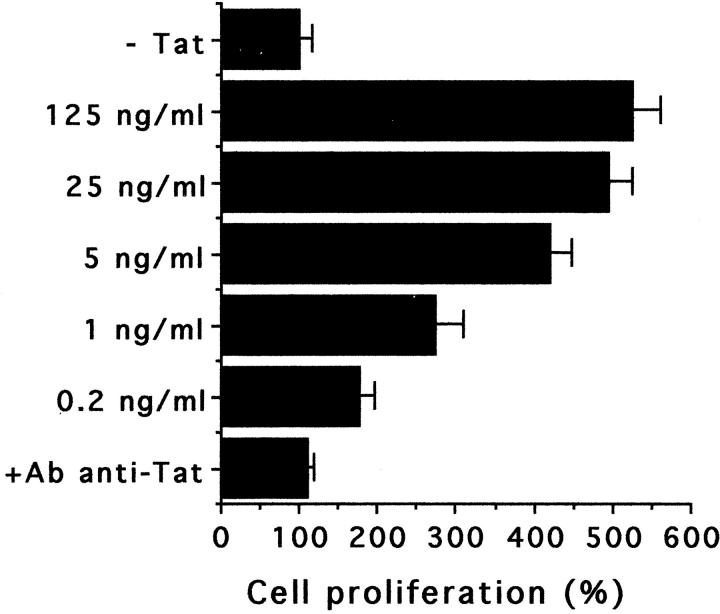
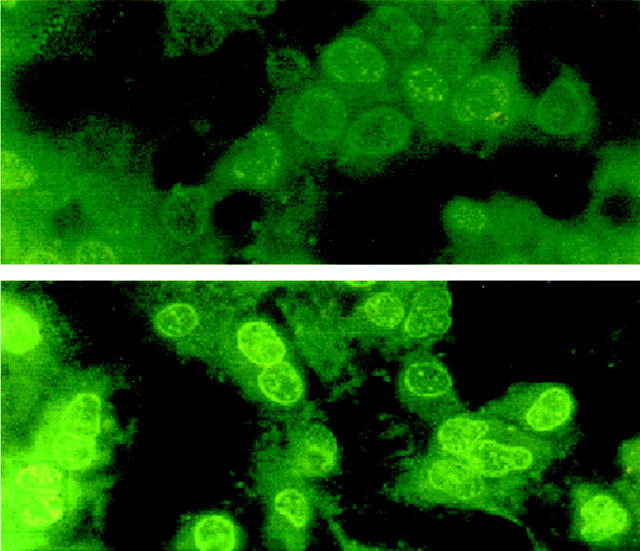
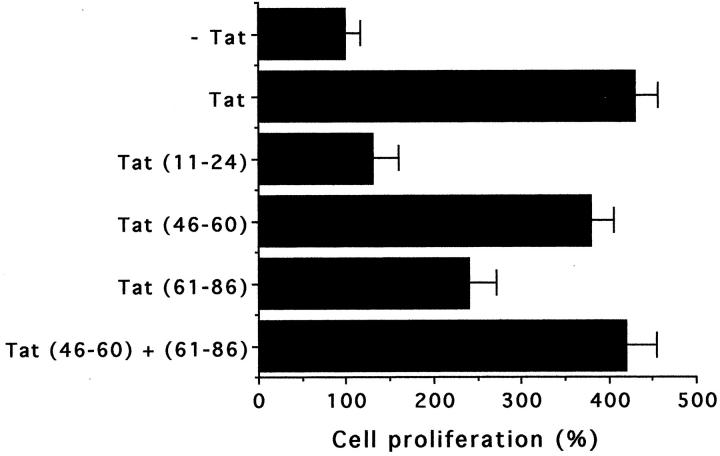
The treatment of podocytes with exogenous Tat induced a relevant increase of cell proliferation. Figure 1 ▶ shows the time-dependent increase in cell proliferation of a continuous line of podocytes after stimulation with Tat at 2 ng/ml. This Tat concentration is comparable to that detected in sera of AIDS patients. 12 Tat also stimulated the growth of MCs, but to a lower extent. Similar results were obtained with primary cultures of podocytes and MCs derived from six different donors (data not shown). The proliferative effect of Tat was also detected by [3H]-thymidine uptake assay (data not shown). The Tat-induced proliferation was dose-dependent and occurred at a concentration of Tat as low as 0.2 ng/ml (Figure 2) ▶ . Heat-treated or neutralized Tat was inactive, demonstrating the specificity of the observed phenomenon. The neutralization of Tat was performed by preincubation with the specific Ab (5 μg/ml for 1 hour at 37°C): the addition of Ab alone did not affect podocyte growth (not shown). By detection of Ki-67 antigen, a nuclear protein expressed by all proliferating cells, a striking increase of the number of cycling cells was observed after the treatment with Tat (vehicle alone, 15 ± 7%; Tat, 90 ± 5%; Figure 3 ▶ ). To elucidate the mechanisms of Tat activity, we tried to identify the protein domains required for this phenomenon. Cell growth experiments were performed using the basic [46-60] peptide and the [61-86] peptide that encompasses the RGD region of Tat. The [11-24] peptide considered biologically unrelevant was used as control. 28 As shown in Figure 4 ▶ , the results indicated that the basic domain of Tat exerted the prominent effect in enhancing podocyte proliferation, but also the RGD region contributed to the activity of the whole protein, as shown by the additive effect obtained by incubating cells with both [46-60] and [61-86] peptides. In contrast, the [11-24] peptide was ineffective on cell proliferation.
Figure 1.
Tat-induced increase in the proliferation of glomerular cells. Continuous lines of podocytes and MCs were stimulated with Tat (2 ng/ml) and incubated in DMEM-0.1% BSA. Cell proliferation was assessed by measuring at 450 nm absorbance the culture medium after bioreduction of XTT, in three independent experiments each in triplicate. The results are expressed as the percentage of cell proliferation in comparison with that of untreated cell cultures at 48 hours of incubation, corresponding to the maximum proliferation rate detected in preliminary experiments. Viable cells were counted by Trypan blue exclusion staining. After a 48-hour incubation the number of podocytes increased from 2 × 104 cells/cm2 up to 4 × 104/cm2 or 8.5 × 104/cm2 on average, in the absence or presence of Tat, respectively.
Figure 2.
Dose-dependence of the increase of podocyte growth induced by Tat. The results are expressed as the percentage of cell proliferation in comparison with that of untreated cell cultures. Cell growth evaluation was performed after a 48-hour incubation with the XTT assay in five independent experiments, each in triplicate. Similar results were obtained by viable cell counting (100% increase = increase from 3 × 104 cells/cm2 to 5.5 × 104 cells/cm2, on average).
Figure 3.
Expression of the proliferation marker Ki-67 in podocytes after a 24-hour incubation. In cultures of untreated podocytes (top) the average number of Ki-67-positive cell cycle-engaged cells was 15 ± 7% of the cells incubated in DMEM-0.1% BSA. After treatment with 10 ng/ml of Tat (bottom), 90 ± 5% of the cultured podocytes expressed the Ki-67 nuclear antigen. Original magnification, ×400.
Figure 4.
Evaluation of the involvement of different Tat domains in podocyte hyperproliferation. The experiments were performed by using Tat (10 ng/ml) or equimolar concentrations of Tat peptides: [11-24] Tat and [46-60] Tat including the basic domain [61-86] Tat encompassing the RGD region. The data (from five experiments, each in triplicate) refer to the percentage of cell growth after a 48-hour incubation in comparison with that of untreated cells. Podocyte growth was evaluated by the XTT assay (by viable cell counting, 100% increase = increase from 4 × 104 cells/cm2 to 7.5 × 104 cells/cm2, on average).
Proteoglycans Were Required for Tat-Induced Increase of Podocyte Proliferation
To evaluate whether Tat stimulated podocytes as a result of surface receptor interaction or as a consequence of its internalization, podocyte proliferation was studied by seeding the cells on 96-well plates coated with Tat. After a 48-hour incubation, immobilized Tat stimulated cell proliferation in a way comparable to that of soluble Tat (immobilized Tat, 265 ± 35% increase; 10 ng/ml Tat, 290 ± 30% increase). With other cell types Tat has been shown to modulate cell functions by engaging integrins or VEGFRs via its RGD and basic regions, respectively. 13,15,29 By cytofluorimetric analysis podocytes expressed α3β1 and αvβ3 integrins and VEGFR-1 and -2 (data not shown). Therefore, experiments with specific blocking Abs were performed to evaluate the involvement of these cell receptors in Tat-induced hyperproliferation of podocytes. As shown in Figure 5 ▶ , pretreatment of podocytes with both anti-VEGFR-1 and -2 Abs, at concentrations previously shown to block proliferation of endothelial cells, 30 did not inhibit proliferation of podocytes induced by [46-60] Tat peptide. Conversely, anti-integrin Abs significantly reduced the activity of [61-86] peptide (Figure 5) ▶ .
Figure 5.
Evaluation of the involvement of different cell-surface molecules in podocyte hyperproliferation promoted by Tat peptides. Podocytes were preincubated with vehicle alone or with 5 μg/ml of Abs specific to integrins (Abs anti-α3β1 + anti-αvβ3 + anti-β1) and/or to VEGFRs (Abs anti-Flk-1 + anti-Flt-1) for 1.5 hours at 37°C and then stimulated with [46-60] Tat peptide (2 ng/ml) or [61-86] Tat peptide (2.5 ng/ml). The data (from three experiments, each in triplicate) were evaluated by the XTT assay. The results are expressed as the percentage of cell proliferation in comparison with that of untreated cell cultures after a 48-hour incubation. By viable cell counting, 100% increase of podocyte growth was equivalent to an increase from 3.9 × 104 cells/cm2 to 7.8 × 104 cells/cm2, on average.
It is known that HIV-1 Tat protein binds to heparan sulfate proteoglycans (HSPGs) of the cell surface and extracellular matrix. 31,32 Figure 6 ▶ shows that the treatment of podocytes with β-d-xyloside, an inhibitor of proteoglycan biosynthesis, 33 caused a significant reduction of Tat-induced cell proliferation. Remarkably, similar results were obtained by using [46-60] peptide. In contrast, β-d-xyloside did not affect proliferation induced by [61-86] peptide or basal proliferation of podocytes. This suggests that the interaction of Tat basic domain with proteoglycans plays a key role in Tat-induced biological activity on podocytes.
Figure 6.
Evaluation of the involvement of proteoglycans in Tat-induced podocyte hyperproliferation. Cultures of podocytes untreated (white columns) or treated with 25 mmol/L of β-d-xyloside (black columns) were incubated in the presence of absence of Tat (10 ng/ml) [46-60], Tat peptide (2 ng/ml), or [61-86] Tat peptide (2.5 ng/ml). The data (from three experiments, each in duplicate) were evaluated by XTT assay and expressed as percentage of cell proliferation in comparison with the values obtained with the untreated podocytes after a 48-hour incubation.
bFGF Synthesis Stimulated by Tat Is Involved in Tat-Induced Podocyte Proliferation
Numerous studies have implicated bFGF in the pathogenesis of kidney disease as a mitogenic factor for renal cells. 34,35 bFGF has been also involved in Tat-induced proliferation of endothelial and Kaposi’s sarcoma cells. 36,37 Thus, we evaluated the possible role of this growth factor in our experimental model. We found that both primary and immortalized cultures of podocytes produced low levels of bFGF (10 to 20 pg/ml). Podocyte stimulation with Tat for 24 hours promoted an increased expression of specific mRNA and a concomitant bFGF release (140 to 150 pg/ml), suggesting an enhanced bFGF synthesis (Figure 7) ▶ . However, because increased levels of bFGF (40 to 45 pg/ml) were detected also in podocyte cultures pulsed for 1 hour with Tat, it is conceivable that a retrieval of preformed bFGF may also concur. The later mechanism may be because of either the release of intracellular store of bFGF or to its mobilization from the cell surface. It is known that up to 30% of the synthesized bFGF binds to cell-surface and extracellular matrix-associated HSPGs. 31,38 The basic region of Tat can displace preformed HSPG-bound bFGF by competing for heparin-binding sites. 31 Indeed, an augmented concentration of bFGF in the culture supernatants (33 to 39 ng/ml) was observed when podocytes were treated with 30 μg/ml of heparin, in the absence of an enhanced mRNA transcription monitored by reverse transcriptase-PCR. The increase of cell proliferation also detected in the latter experimental conditions (not shown) remarked the possible role of bFGF in podocyte proliferation. Indeed, when podocytes were incubated with the conditioned media of Tat-treated podocytes, an enhanced cell growth was observed (170 ± 15% after a 24-hour incubation, assumed as 100% the growth of untreated podocytes). The absorption of conditioned media on plates coated with anti-bFGF mAb, but not with an irrelevant mAb, caused a significant reduction of the proliferative activity (120 ± 11% in the same experimental conditions, P < 0.01).
Figure 7.
Reverse transcriptase-PCR analysis of bFGF-mRNA expression in untreated and Tat-stimulated podocytes. RNA samples were taken from untreated (lane 1, 1 μg; lane 3, 0.1 μg; lane 5, 0.01 μg) and Tat-treated podocytes (lane 2, 1 μg; lane 4, 0.1 μg; lane 6, 0.01 μg) after a 24-hour incubation. The figure shows amplicons obtained with 20 PCR cycles to compare the amounts of specific transcripts under conditions of linear amplification (DNA size markers, 50- to 2000-bp ladder; Sigma).
Tat Induced Dysregulation and Functional Alterations of Podocytes
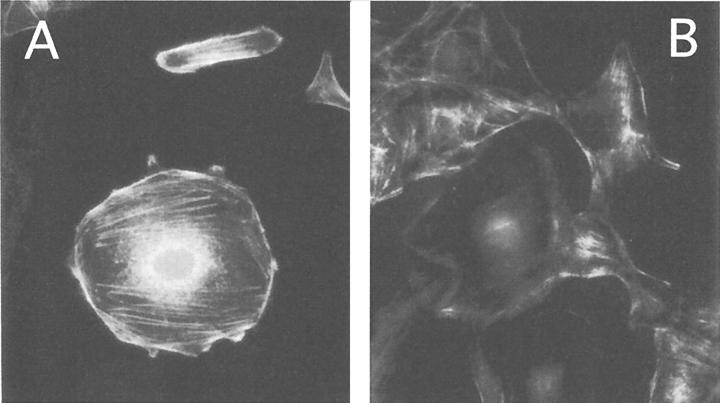
Glomerular pathology of HIVAN is characterized by deregulated podocyte phenotype with morphological alterations of podocyte cytoarchitecture and loss of specific markers of mature podocytes, such as WT-1 and synaptopodin. 39,40 The stimulation of podocytes with Tat caused a relevant impairment of the expression of synaptopodin in cultured cells after a 24-hour incubation (Figure 8) ▶ . Similar results were obtained testing WT-1 positivity in the same experimental conditions (data not shown). Because of the association of synaptopodin with the cytoskeleton, we investigated the effect of Tat onto podocyte cytoskeletal organization. We observed changes of the podocyte shape early after exposure of the cells to 10 ng/ml of Tat (Figure 9) ▶ . In the same experimental conditions, modifications of the normal distribution of actin-containing stress fibers were revealed. After a 2-hour incubation of podocytes with Tat, stress fibers tended to rearrange, retract, and depolymerize (Figure 10) ▶ . In all these experiments the viability of treated podocytes did not vary from that of untreated (90 to 95%). Tat-induced alterations were reversible after washing and culturing podocytes for 24 hours (not shown).
Figure 8.
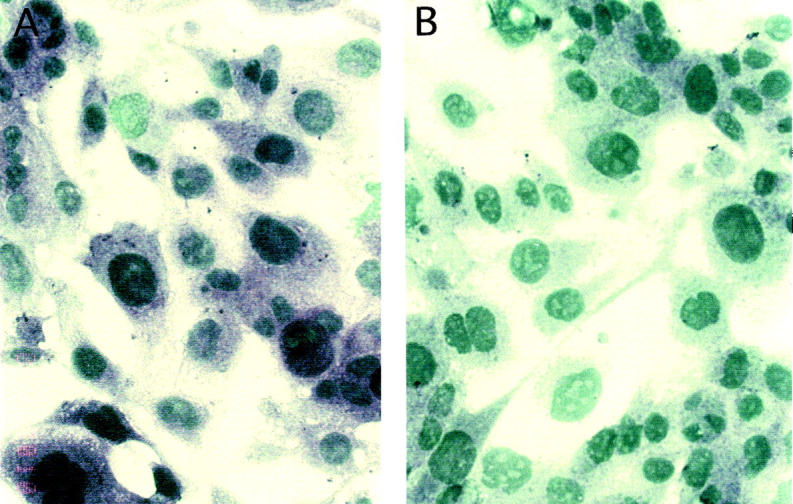
Expression of the differentiation marker synaptopodin in podocytes. A: Expression of synaptopodin in untreated podocytes. B: Reduction of staining for synaptopodin in podocytes treated with 10 ng/ml of Tat for 24 hours. Original magnification, ×400.
Figure 9.
Micrographs representative of the shape change of podocytes as observed by comparison of time 0 (A and C) and 1-hour frames (B and D). A and B: Podocytes stimulated with 10 ng/ml of Tat and incubated at 37°C in DMEM-0.1% BSA. The arrows indicate cells with modified shape. C and D: Untreated podocytes in the same experimental conditions. The micrographs were obtained by time-lapse analysis of podocyte shape modification performed by digital saving of imagines at 15-minute intervals. Three experiments were performed with similar results. Original magnification, ×100.
Figure 10.
F-actin distribution in podocytes. A: F-actin distribution in fixed and permeabilized podocytes after incubation for 2 hours at 37°C. B: F-actin distribution in podocytes stimulated with 10 ng/ml of Tat for 2 hours at 37°C. Five experiments were performed with similar results. Original magnification, ×400.
Because the cytoarchitecture of podocytes influences glomerular permselectivity, 39 we tested the possibility that Tat could alter this function by evaluating its effect on the permeability to albumin of podocyte monolayers. The results indicated that after a 1-hour incubation, the diffusion of Trypan blue-albumin complexes through podocyte monolayers treated with 2 ng/ml of Tat was doubled (absorbance of 630 ± 35 at 590 nm in the inner wells) in respect to that of untreated cultures (absorbance of 295 ± 42).
Discussion
Podocytes are specialized cells with essential functions in renal glomerular physiology. 39,41 Podocyte differentiation is characterized by the block of cell proliferation associated with the expression of cyclin D1, cyclin-dependent kinase inhibitors, and distinctive markers such as WT-1 and synaptopodin. 42 Primary podocyte injury has been indicated to cause certain forms of focal segmental glomerulosclerosis, but the nature of the damage is unknown. 43,44 Glomerulopathy of HIVAN is mainly represented by severe focal segmental glomerulosclerosis with peculiar alterations of podocytes (hyperproliferation, loss of maturity markers, and cytoarchitecture changes). 2,4 Clinical studies have recently shown that HIV infection of the kidney is etiologically related to the development of nephropathy. 45,46 Experimental findings demonstrated that pathological features of HIVAN could be caused by virus infection of the renal cells or by their exposure to HIV proteins. 5-9 However, the direct effects of HIV-1 on podocytes are still undetermined. It has been previously shown that podocytes are not permissive to HIV-1 replication, 7,47 probably because of the lack of virus receptors. 7 In the present study we show that HIV-1 Tat induced changes on cultured human podocytes similar to those detected in HIVAN. Tat protein is a powerful transcriptional activator of the viral gene expression required for HIV replication. Tat also induces pleiotropic effects on different cell types, mainly transactivating specific cellular genes or acting as a secreted growth factor. 13-16 Indeed, Tat can be released and enter cells freely, or it can interact with cell-surface molecules activating various signaling pathways. 28 In this study we found that extracellular Tat enhanced proliferation of podocytes in a dose-dependent manner. Of note, this effect occurred also at a concentration of Tat similar to that detected in sera of infected patients. 12 After stimulation with Tat, podocytes showed an enhanced expression of the proliferation marker Ki-67 and a disappearance of the differentiation markers such as synaptopodin and WT-1. This evidence is consistent with the histopathological changes 4 and the cell cycle re-entry of podocytes observed in HIVAN glomerulopathy. 42-44
Extracellular Tat has been found to mimic both matrix molecules via its RGD region (C-terminal sequence, amino acids 73 to 86) and angiogenic growth factor through its basic domain (amino acids 46 to 60 28 ). We report herein that Tat activity on podocytes was exerted through its interaction with cell-surface molecules rather than its internalization because the proliferative activity was retained by immobilized Tat. Both peptides containing basic and RGD domains contributed to the Tat-induced increase of podocyte proliferation as shown by the concomitant stimulation with these peptides. However, the prominent effect was accounted to the basic domain. Preincubation with blocking Abs demonstrated that the effect of [61-86] Tat peptide was dependent on the interaction with integrins, whereas that of [46-60] Tat peptide could not be related to the engagement of VEGFR-1 and -2 receptors. Moreover, we found that Tat-induced increase of podocyte growth was significantly impaired by the inhibition of proteoglycan biosynthesis. It has been recently proved that cell-membrane HSPGs may interact with the extracellular Tat. 48 Tat binds to the negatively charged sulfate groups of HSPGs through its arginine-rich basic domain. 31 Indeed, HSPGs are present on the surface and in the extracellular matrix of podocytes, and play a role in the pathophysiology of the glomerular cells. 49 Thus, the interaction of Tat via its basic region with HSPGs may be critical to promote podocyte proliferation. This interaction could also represent a novel target for therapeutic intervention in HIV infection. In fact, the use of polysulfated compounds blocking the basic domain of Tat, such as suramin, has been shown to antagonize Tat activity and synergize with other anti-HIV drugs. 50 Moreover, HSPGs are known to play a critical role in glomerular permselectivity. 49 Therefore, Tat interaction with podocyte HSPGs may affect the function of these cells in the regulation of glomerular permeability. Indeed, Tat significantly enhanced albumin translocation in vitro across the podocyte monolayer.
We found that extracellular Tat increased bFGF release in podocyte cultures both by enhancing the synthesis of specific mRNA and mobilizing the sequestered growth factor. Our data also indicated that bFGF was involved, at least in part, in Tat-induced proliferation of podocytes, as previously seen in other cell types. 36,37 A body of evidence indicates that bFGF is implicated in the pathogenesis of renal disease, because it is mitogenic for both MCs and proximal tubular epithelial cells and stimulates MC extracellular matrix production. 34,35 Recent studies demonstrated that bFGF also has a proliferative effect on podocytes and that it is involved in the development of segmental glomerulosclerotic lesions. 51,52 Interestingly, increased expression of bFGF was shown in a transgenic model of HIVAN, 53 and high levels of bFGF were detected in the kidneys of HIV-infected children. 54 Our results suggest that the production of bFGF in the glomeruli of the HIV-1-infected patients may be triggered by Tat.
The complex functions of podocytes depend on a highly differentiated and unique cytoarchitecture. 39 We found that Tat caused alteration of the podocyte cytoskeleton associated with a rapid modification of cell shape. Moreover, Tat caused a reduced expression of synaptopodin, a proline-rich actin-associated protein that influences the actin-based shape and motility of podocyte foot processes. 40 Such alterations of cytoskeleton may explain the observed increase of the podocyte monolayer permeability induced by Tat at a concentration detectable in HIV patients. Because HIVAN is a clinicopathological entity characterized by heavy proteinuria, Tat may contribute to the enhanced glomerular permeability.
The potential relevance of Tat in the pathogenesis of HIVAN is strengthened by the immunohistochemical detection of Tat protein in the glomeruli and tubules of HIVAN biopsies, but not in other primary glomerular diseases occurring in HIV-infected patients. 55 Beside experimental studies, 6,7 clinical observations indicated that renal cells of HIVAN patients can harbor replicating virus and might function as a viral reservoir. 45,46 Thus, Tat released from infected renal cells in the glomerular microenvironment may cause the podocyte injury associated with HIV glomerulopathy. 2 Moreover, being that Tat is a low-molecular weight protein it is conceivable that Tat present in the plasma of HIV-infected patients 12 may filter through the glomerular capillary walls reaching podocytes.
In conclusion, we found that HIV-1 Tat can induce in vitro alterations of podocyte proliferation and differentiation similar to those detected in vivo in HIVAN. The interaction of Tat basic domain with cell-surface HSPGs and the increase of bFGF levels in podocytes seem to be critical events in this phenomenon. Based on our results we hypothesize that Tat-induced podocyte injury may contribute to the development of the glomerular lesions in HIVAN. Further studies aimed to elucidate the molecular mechanisms activated by Tat on podocytes might allow the development of novel therapeutic strategies to prevent the progression of this severe renal disease.
Acknowledgments
We thank Dr. Peter Mundel (Division of Nephrology, Albert Einstein College of Medicine, Bronx, NY, USA) for the generous gift of anti-synaptopodin monoclonal antibody.
Footnotes
Address reprint requests to Pier Giulio Conaldi, M.D., Ph.D., Laboratorio di Microbiologia e Biologia Molecolare, Ospedale di Busto Arsizio, Piazzale Solaro 3, 21052 Busto, Arsizio, Varese, Italy. E-mail: piergiulio.conaldi@uninsubria.it.
Supported by the Istituto Superiore di Sanità, Rome, Italy (grants 30C.21 and 30C.11) and by the Progetto Finalizzato Consiglio Nazionale delle Ricerche, Target Project on Biotechnology.
References
- 1.Winston JA, Burns GC, Klotman PE: The human immunodeficiency virus (HIV) epidemic and HIV-associated nephropathy. Semin Nephrol 1998, 18:373-377 [PubMed] [Google Scholar]
- 2.D’Agati V, Appel GB: Renal pathology of human immunodeficiency virus infection. Semin Nephrol 1998, 18:406-421 [PubMed] [Google Scholar]
- 3.Klotman PE: HIV-associated nephropathy. Kidney Int 1999, 56:1161-1176 [DOI] [PubMed] [Google Scholar]
- 4.Barisoni L, Kriz W, Mundel P, D’Agati V: The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 1999, 10:51-61 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz EJ, Klotman PE: Pathogenesis of human immunodeficiency virus (HIV)-associated nephropathy. Semin Nephrol 1998, 18:436-445 [PubMed] [Google Scholar]
- 6.Conaldi PG, Biancone L, Bottelli A, Wade-Evans A, Racusen LC, Boccellino M, Orlandi V, Serra C, Camussi G, Toniolo A: HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Invest 1998, 102:2041-2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conaldi PG, Bottelli A, Wade-Evans A, Biancone L, Baj A, Cataluppi V, Serra C, Dolei A, Toniolo A, Camussi G: HIV-persistent infection and cytokine induction in mesangial cells: a potential mechanism for HIV-associated glomerulosclerosis. AIDS 2000, 14:2045-2047 [DOI] [PubMed] [Google Scholar]
- 8.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE: Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest 1997, 100:84-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE: HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int 2000, 58:173-181 [DOI] [PubMed] [Google Scholar]
- 10.Vaishnav YN, Wong-Staal F: The biochemistry of AIDS. Annu Rev Biochem 1991, 60:577-630 [DOI] [PubMed] [Google Scholar]
- 11.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC: Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol 1993, 67:277-287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westendorp MO, Frank R, Ochsenbauer C, Striker K, Dhein J, Walczak H, Debatin KM, Krammer PH: Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995, 375:497-500 [DOI] [PubMed] [Google Scholar]
- 13.Barillari G, Gendelman R, Gallo RC, Ensoli B: The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci USA 1993, 90:7941-7945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotz M, Clark-Lewis I, Ganu V: HIV-1 transactivator protein Tat induces proliferation and TGF beta expression in articular chondrocytes. J Cell Biol 1994, 124:365-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, Modesti A, Nair BC, Cafaro A, Sturzl M, Ensoli B: The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the α5β1 and αvβ3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 1999, 94:663-672 [PubMed] [Google Scholar]
- 16.Del Sorbo L, De Martino A, Biancone L, Conaldi PG, Toniolo A, Camussi G: The synthesis of platelet-activating factor mediates chemotaxis of monocytes induced by HIV-1 Tat. Eur J Immunol 1999, 29:1513-1521 [DOI] [PubMed] [Google Scholar]
- 17.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB: Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 1995, 268:429-431 [DOI] [PubMed] [Google Scholar]
- 18.Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD: Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol 1996, 70:1475-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantaluppi V, Biancone L, Boccellino M, Doublier S, Benelli R, Carlone S, Albini A, Camussi G: HIV type 1 Tat protein is a survival factor for Kaposi’s sarcoma and endothelial cells. AIDS Res Hum Retroviruses 2001, 17:965-976 [DOI] [PubMed] [Google Scholar]
- 20.Cohen SS, Li C, Ding L, Cao Y, Pardee AB, Shevach EM, Cohen DI: Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc Natl Acad Sci USA 1999, 96:10842-10847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conaldi PG, Biancone L, Bottelli A, De Martino A, Camussi G, Toniolo A: Distinct pathogenic effects of group B coxsackieviruses on human glomerular and tubular kidney cells. J Virol 1997, 71:9180-9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Striker GE, Striker LJ: Glomerular cell culture. Lab Invest 1985, 53:122-131 [PubMed] [Google Scholar]
- 23.Delarue F, Virone A, Hagege J, Lacave R, Peraldi M-N, Adida C, Rondeau E, Feunteun J, Sraer J-D: Stable cell line of T-SV40 immortalized human glomerular visceral epithelial cells. Kidney Int 1991, 40:906-912 [DOI] [PubMed] [Google Scholar]
- 24.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL: An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods 1991, 142:257-265 [DOI] [PubMed] [Google Scholar]
- 25.Biancone L, Cantaluppi V, Boccellino M, Bussolati B, Del Sorbo L, Conaldi PG, Albini A, Toniolo A, Camussi G: Motility induced by human immunodeficiency virus-1 Tat on Kaposi’s sarcoma cells requires platelet-activating factor synthesis. Am J Pathol 1999, 155:1731-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camussi G, Turello E, Bussolino F, Baglioni C: Tumor necrosis factor alters cytoskeletal organization and barrier function of endothelial cells. Int Arch Allergy Appl Immunol 1991, 96:84-91 [DOI] [PubMed] [Google Scholar]
- 27.Jones SG, Morrisey K, Williams JD, Phillips AO: TGF-β1 stimulates the release of pre-formed bFGF from renal proximal tubular cells. Kidney Int 1999, 56:83-91 [DOI] [PubMed] [Google Scholar]
- 28.Jeang K-T, Xiao H, Rich EA: Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem 1999, 274:28837-22840 [DOI] [PubMed] [Google Scholar]
- 29.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F: The angiogenesis induced by HIV-1 Tat is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med 1996, 2:1371-1375 [DOI] [PubMed] [Google Scholar]
- 30.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A: Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol 2001, 159:993-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B: HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 1997, 11:1421-1431 [DOI] [PubMed] [Google Scholar]
- 32.Rusnati M, Tulipano G, Spillmann D, Tanghetti E, Oreste P, Zoppetti G, Giacca M, Presta M: Multiple interactions of HIV-1 Tat protein with size-defined heparin oligosaccharides. J Biol Chem 1999, 274:28198-28205 [DOI] [PubMed] [Google Scholar]
- 33.Ma YQ, Geng JG: Heparan sulfate-like proteoglycans mediate adhesion of human malignant melanoma A375 cells to P-selectin under flow. J Immunol 2000, 165:558-565 [DOI] [PubMed] [Google Scholar]
- 34.Klahr S, Morrissey JJ: The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int 2000, 57:S7-S14 [PubMed] [Google Scholar]
- 35.Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Muller GA: Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int 2000, 57:1521-1538 [DOI] [PubMed] [Google Scholar]
- 36.Ensoli B, Markham P, Kao V, Barillari G, Fiorelli V, Gendelman R, Raffeld M, Zon G, Gallo RC: Block of AIDS-Kaposi’s sarcoma (KS) cell growth, angiogenesis, and lesion formation in nude mice by antisense oligonucleotides targeting basic fibroblast growth factor. A novel strategy for the therapy of KS. J Clin Invest 1994, 94:1736-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barillari G, Sgadari C, Palladino C, Gendelman R, Caputo A, Morris CB, Nair BC, Markham P, Nel A, Stürzl M, Ensoli B: Inflammatory cytokines synergize with HIV-1 Tat protein to promote angiogenesis and Kaposi’s sarcoma via induction of basic fibroblast growth factor and αvβ3 integrin. J Immunol 1999, 163:1929-1935 [PubMed] [Google Scholar]
- 38.Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I: A heparin-binding angiogenic protein—basic fibroblast growth factor—is stored within basement membrane. Am J Pathol 1988, 130:393-400 [PMC free article] [PubMed] [Google Scholar]
- 39.Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol 1995, 192:385-397 [DOI] [PubMed] [Google Scholar]
- 40.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W: Synaptopodin, an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 1997, 139:193-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G: Nephrin redistribution on podocytes is a potential pathomechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 2001, 158:1723-1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagata M, Nakayama K, Terada Y, Hoshi S, Watanabe T: Cell cycle regulation and differentiation in the human podocyte lineage. Am J Pathol 1998, 153:1511-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P: Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int 2000, 58:137-143 [DOI] [PubMed] [Google Scholar]
- 44.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D’Agati V, Alpers CE: Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: role in podocyte proliferation and maturation. Kidney Int 2000, 58:674-683 [DOI] [PubMed] [Google Scholar]
- 45.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D’Agati V, Winston JA, Klotman ME, Klotman PE: Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 2000, 11:2079-2087 [DOI] [PubMed] [Google Scholar]
- 46.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati V, Klotman PE, Klotman ME: Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med 2001, 344:1979-1984 [DOI] [PubMed] [Google Scholar]
- 47.Green DF, Resnick L, Bourgoignie JJ: HIV infects glomerular endothelial and mesangial but not epithelial cells in vitro. Kidney Int 1992, 41:956-960 [DOI] [PubMed] [Google Scholar]
- 48.Tyagi M, Rusnati M, Presta M, Giacca M: Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J Biol Chem 2001, 276:3254-3261 [DOI] [PubMed] [Google Scholar]
- 49.Raats CJ, Van Den Born J, Berden JH: Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int 2000, 57:385-400 [DOI] [PubMed] [Google Scholar]
- 50.Rusnati M, Tulipano G, Urbinati C, Tanghetti E, Giuliani R, Giacca M, Ciomei M, Corallini A, Presta M: The basic domain in HIV-1 Tat protein as a target for polysulfonated heparin-mimicking extracellular Tat antagonist. J Biol Chem 1998, 273:16027-16037 [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi A, Yoshizawa N, Yamamoto M, Sawasaki Y, Oda T, Senoo A, Niwa H, Fuse Y: Basic fibroblast growth factor promotes proliferation on rat glomerular visceral epithelial cells in vitro. Am J Pathol 1992, 141:107-116 [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki T, Hatta H, Osawa G: Cytokines and podocytes injury: the mechanism of fibroblast growth factor 2-induced podocyte injury. Nephrol Dial Transplant 1999, 14:33-34 [DOI] [PubMed] [Google Scholar]
- 53.Ray PE, Bruggeman LA, Weeks BS, Kopp JB, Bryant JL, Owens JW, Notkins AL, Klotman PE: Basic FGF and its low affinity receptors in the pathogenesis of AIDS associated nephropathy in transgenic mice. Kidney Int 1994, 46:759-772 [DOI] [PubMed] [Google Scholar]
- 54.Ray PE, Liu XH, Xu L, Rakusan T: Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr Nephrol 1999, 13:586-593 [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T, Noble NA, Miller DE, Gold LI, Hishida A, Nagase M, Cohen AH, Border WA: Increased levels of transforming growth factor-β in HIV-associated nephropathy. Kidney Int 1999, 55:579-592 [DOI] [PubMed] [Google Scholar]